Abstract
In recent years, extended ESBL and carbapenemase producing Gram negative bacteria have become widespread in hospitals, community settings and the environment. This has been triggered by the few therapeutic options left when infections with these multi-drug resistant organisms occur. The emergence of resistance to colistin, the last therapeutic option against carbapenem-resistant bacteria, worsened the situation. Recently, animals were regarded as potent antimicrobial reservoir and a possible source of infection to humans. Enteric Gram negative bacteria in animals can be easily transmitted to humans by direct contact or indirectly through the handling and consumption of undercooked/uncooked animal products. In the Mediterranean basin, little is known about the current overall epidemiology of multi-drug resistant bacteria in livestock, companion, and domestic animals. This review describes the current epidemiology of ESBL, carbapenemase producers and colistin resistant bacteria of animal origin in this region of the world. The CTX-M group 1 seems to prevail in animals in this area, followed by SHV-12 and CTX-M group 9. The dissemination of carbapenemase producers and colistin resistance remains low. Isolated multi-drug resistant bacteria were often co-resistant to non-beta-lactam antibiotics, frequently used in veterinary medicine as treatment, growth promoters, prophylaxis and in human medicine for therapeutic purposes. Antibiotics used in veterinary medicine in this area include mainly tetracycline, aminoglycosides, fluoroquinolones, and polymyxins. Indeed, it appears that the emergence of ESBL and carbapenemase producers in animals is not related to the use of beta-lactam antibiotics but is, rather, due to the co-selective pressure applied by the over usage of non-beta-lactams. The level of antibiotic consumption in animals should be, therefore, re-considered in the Mediterranean area especially in North Africa and western Asia where no accurate data are available about the level of antibiotic consumption in animals.
Keywords: ESBL, carbapenemase, mcr-1, Mediterranean, livestock
Background
Antimicrobial resistance is an emerging and rapidly evolving phenomenon. This phenomenon is currently observed in all bacterial species including clinically important Gram negative bacilli (GNB) (Rubin and Pitout, 2014). Gram negative bacilli, “enterobacteriaceae and non-fermenters” are normal inhabitants of the human intestinal microflora (Vaishnavi, 2013); they are responsible for the most common hospital and community acquired infections. Antibiotic resistance in GNB is mediated by target drug modification (Lambert, 2005), changes in bacterial cell permeability (Delcour, 2009) and, most importantly, the production of hydrolyzing enzymes, namely beta-lactamases. The most common beta-lactamases which are now widespread include the extended spectrum beta-lactamases (ESBL) (SHV, TEM, OXA, and CTX-M types), AmpC beta-lactamases, and carbapenemases (MBL, KPC, and class D oxacillinases) (Giedraitiene et al., 2011; Poirel et al., 2011). These enzymes provide the bacterium with resistance toward the majority of therapeutic options available in the clinical market. Furthermore, resistance determinants of these enzymes are often located on plasmids carrying resistance genes to other non-beta-lactam antibiotics, thus further limiting treatment options (Guerra et al., 2014).
The emergence of colistin resistance in GNB is another concern. Colistin belongs to the polymyxin group of polypeptide antibiotics (Olaitan et al., 2014a). Previously abandoned due to its nephrotoxicity and neurotoxicity, it is now in use once again and is considered to be the last resort antimicrobial agent against carbapenem resistant GNB (Kempf et al., 2013). Colistin resistance can be mediated either by the acquisition of the plasmid mediated “mcr” gene or by chromosomal mutations that lead to modification of the lipid A moiety of lipopolysaccharide (LPS), which is considered the primary target of colistin in Gram negative bacilli (Baron et al., 2016).
It is currently known that, in addition to the human intestinal microflora, resistant GNB can be found in water, soil, and fecal animal matter (Verraes et al., 2013). In fact, there is increasing evidence that animals constitute a potent reservoir of resistant GNB (Ewers et al., 2012). This is mainly due to the over- and misuse of antibiotics in veterinary medicine (Guerra et al., 2014): antibiotics are not only prescribed for treatment but are also administered for disease prevention and growth promotion (Economou and Gousia, 2015). Although studies have shown that the direct threat of resistant GNB to human health is still controversial (Olsen et al., 2014), the wide dissemination of these resistant organisms is worrying due to their ease of transmission (Rolain, 2013) and their high potential contribution to the spread of bacterial resistance across all ecosystems (Pomba et al., 2017). In this review, we attempt to describe the epidemiology of ESBL, AmpC and carbapenemase producing GNB of animal origin in the Mediterranean region. Colistin resistance in GNB in the same area is also described. The Mediterranean basin is a region of the world that compromises a wide diversity of populations. It includes five Asian countries (Cyprus, Israel, Lebanon, Syria, and Turkey), eleven European countries (Albania, Bosnia, Croatia, France, Greece, Herzegovina, Italy, Monaco, Montenegro, Slovenia, and Spain) and five African countries (Algeria, Egypt, Libya, Morocco, and Tunisia).
Distribution of ESBLs and AmpC producers in animals
Chicken and food of poultry origin
Poultry production is a complex system in the food and agricultural industry. It includes breeding chickens for meat and eggs. Chickens are kept either as a “breeding flock” or as a “broiler flock” for human consumption. Along with eggs, broilers are traded and transported across different countries around the world (Dierikx et al., 2013). This trade results in a vulnerable system that can be hacked by multi-drug resistant organisms (MDRO), i.e., once a MDRO is introduced into the production chain, it can be transferred internationally. This is why the dissemination of ESBL and AmpC-producing GNB, recently extensively reported in chicken farms (Blaak et al., 2015) is worrying, as these can contribute to not only local but global dissemination of antimicrobial resistance (Dierikx et al., 2013). Studies have shown that the carriage of ESBL and AmpC producers in chicken is persistent (Huijbers et al., 2016). ESBL and AmpC producers are isolated from grandparent breeding stock (Nilsson et al., 2014), broiler chickens (Reich et al., 2013), retail meat (Choi et al., 2015), and at the slaughterhouses (Maciuca et al., 2015).
In the Mediterranean basin, the first detection of ESBL in chicken dates back to 2000 in Greece, when a CTX-M-32 harboring Salmonella enterica was isolated from poultry end products (Politi et al., 2005). Since then, many studies have reported the emergence of ESBL in poultry in the Mediterranean area. In Italy for instance, the first ESBL reported was a case of SHV-12 detected in Salmonella spp (Chiaretto et al., 2008). Salmonella infantis species harboring CTX-M-1 were later isolated in 2011 from broiler chicken flocks. These strains led to human infection in Italy in 2013–2014 (Franco et al., 2015). In both studies, isolated strains were co-resistant to non-beta-lactam antibiotics, notably nalidixic acid, sulfonamide, trimethoprim, and tetracyclines. According to the European Food Safety Authority and the European Center for Disease Prevention and Control recent report, S. infantis is the fourth most common serovar detected in humans in the European Union and that is mostly being observed in the turkey and broiler chain. In this report, it has been stated that this serovar has been able to extensively disseminate along the broiler production chain (EFSA, 2017). Indeed it has been suggested that the consumption of contaminated chicken meat is among the most common sources of salmonellosis in humans (Antunes et al., 2016). Furthermore, in Italy, opportunistic pathogen such as Escherichia coli isolates producing CTX-M-32, CTX-M-1, and SHV-12 type beta-lactamases were also reported (Giufrè et al., 2012). These strains were retrieved from flocks which had no prior treatment with cephalosporins. It is proposed that the prescription of other antimicrobials such as enrofloxacin and tylosin is responsible for the co-selection of the aforementioned resistant organisms (Bortolaia et al., 2010). Reports on chicken feces (Giufrè et al., 2012), broiler chicken samples, and retail chicken meat (Ghodousi et al., 2016) showed that these latter carried E. coli producing CTX-M-grp-1, CTX-M-grp-2, and CTX-M-grp-9 enzymes in Italy. The co-existence of these enzymes with AmpC beta-lactamases was also reported, including CTX-M-1/CMY-2 (Accogli et al., 2013) and CIT-like/CTX-M (Ghodousi et al., 2015) in E. coli of poultry origin. CTX-M and AmpC beta-lactamase producers in the Italian poultry belong mostly to the A and B phylogroups with the genes being carried mainly on IncI1 plasmids. In France, the only report from poultry was the detection of two CTX-M-1-producing E. coli isolates (Meunier et al., 2006). CTX-M-1 was linked to the insertion sequence ISEcp1 (Meunier et al., 2006). This insertion sequence has been previously described as being a potent contributor to the mobilization and insertion of blaCTX-M genes (El Salabi et al., 2013). Although no studies described the emergence of ESBL in the Slovenian animal sector, one study reported the presence of CTX-M-1 and SHV-12-producing in Slovenian raw chicken meat samples sold on the Swiss market (Zogg et al., 2016).
In Spain, the Spanish Veterinary Antimicrobial Resistance Surveillance Network (VAV) monitored antimicrobial resistance of Salmonella enterica in healthy broilers in 2003–2004: two CTX-M-9 producers were isolated (Riaño et al., 2006). During the same period, ESBL-producing E. coli were also detected (Mesa et al., 2006; Moreno et al., 2007). Indeed, it seems that early monitoring systems often targeted resistance in Salmonella species, as these are common causative agents of human infections of food of animal origin (Antunes et al., 2016). Thereafter, as bacterial resistance became widely disseminated in all environments (Stoll et al., 2012), researchers began to think of poultry as a reservoir of resistance in enteric organisms. For instance, Egea et al. found that the prevalence of retail poultry meat colonized by CTX-M and/or SHV producing E. coli increased from 62.5% in 2007 to 93.3% in 2010 (Egea et al., 2012). During these three years, a significant increase was observed at the level of A0 and D1 phylogroups. Egea et al. suggested that the rise of meat colonization is muli-clonal since only 2 strains from the main phylogroup detected in this study showed genetic relatedness by PFGE typing. Thus, it appears that the diffusion of ESBL producers in retail chicken meat is related rather to successful spread of one or several plasmids carrying the blaCTX-M and blaSHV genes (Egea et al., 2012). Apart from E. coli, ESBL production in the poultry production system in Spain was also detected in Klebsiella pneumoniae, Enterobacter cloacae, Proteus mirabilis, and Serratia fonticola (Ojer-Usoz et al., 2013). In parallel, CMY-2 is the only AmpC beta-lactamase type reported in E. coli originating from chicken in this country (Blanc et al., 2006; Cortés et al., 2010; Solà-Ginés et al., 2015b). Apart from chicken, one study in Spain reported the detection of CTX-M-1, CTX-M-9, CTX-M-14 harboring E. coli strains in flies surrounding chicken farms (Solà-Ginés et al., 2015a). For instance, the detection of ESBL producers in flies reflects on one side the contamination status of the farm housing environment; and on the other side, it contributes to the colonization of other broilers with ESBL producing E. coli strains (Solà-Ginés et al., 2015a).
In Turkey, the first ESBL production in animals was detected in K. pneumoniae and Klebsiella oxytoca in 2007–2008 (Gundogan et al., 2011). In 2012–2014, E. coli producing CTX-M-1, CTX-M-3, CTX-M-15, CTX-M-8 as well as SHV-5 and SHV-12 were identified in raw chicken meat samples in different areas across the country (Perrin-Guyomard et al., 2016)-(Tekiner and Ozpinar, 2016). The A, D1 and D2 were the most common phylogroups detected. In the same aforementioned study, ESBL was also detected in E. cloacae, Citrobacter werkmanii, and K. pneumoniae (CTX-M-1) (Tekiner and Ozpinar, 2016). Similarly, CMY-2 type beta-lactamase was detected in E. coli (Pehlivanlar Onen et al., 2015) as well as in E. cloacae (Tekiner and Ozpinar, 2016). In Lebanon, CTX-M type beta-lactamase followed by CMY AmpC beta-lactamase appear to dominate the Lebanese chicken farms (Dandachi et al., 2018b). MLST typing of CTX-M positive E. coli strains revealed the presence of different sequence types across the territory. Furthermore, a significant resistance of ESBL producers toward gentamicin was observed. The spread of ESBL producers in Lebanon could be attributed in part to the co-selective pressure applied by the heavy usage of gentamicin in the veterinary sector as previously reported (Dandachi et al., 2018b). In Israel, only one study showed the presence of CTX-M-producing E. coli of A, B, and D phylogroups in liver samples of dead broiler chickens and ready-to-market chicken meat (Qabajah et al., 2014).
Concerning Africa, ESBL was first detected in E. coli strains isolated from foods of poultry origin in Tunisia in 2006. These harbored SHV-5, CTX-M-8, CTX-M-14, and CTX-M-1 type beta-lactamases (Jouini et al., 2007). It appears that in this country, blaCTX-M-1 and blaCMY-2 are the dominant genes responsible for ESBL and AmpC production in E. coli isolated from chicken samples (Ben Slama et al., 2010; Ben Sallem et al., 2012). This is in addition to blaCTX-M-15, blaCTX-M-14 (Maamar et al., 2016), and blaCTX-M-9 that were detected in E. coli isolated from the fecal samples of dead/diseased chickens (Grami et al., 2014). ESBL genes in Tunisia appear to be located on various plasmids carried by different E. coli phylogroups. These include mainly IncI1 followed by IncF and IncFIB (Table 2). blaCTX-M as well as CMYgenes in Tunisia were found to be also associated to the ISEcp1 insertion sequence. Furthermore, apart from the CMY gene, AmpC production in E. coli strains in this country was found to be also mediated via mutations in the promoter region of the chromosomal AmpC gene (Ben Slama et al., 2010). In Algeria, CTX-M-like enzymes were detected in E. coli (Mezhoud et al., 2015; Chabou et al., 2017) as well as in other species such as ST15 Salmonella Heidelberg (Djeffal et al., 2017). In their study, Djeffal et al. reported the detection of the same sequence type “ST15” of Salmonella spp isolated from both chicken and human. This emphasizes on the hypothesis that the poultry production system could constitute a potent contributor to the diffusion of multi-drug resistant Salmonella in the human population (Djeffal et al., 2017). In parallel, blaSHV-12 and CMY-2 genes were detected in E. coli strains recovered from slaughtered broilers' intestinal swabs (Belmahdi et al., 2016).
In Egypt, E. coli producing CTX-M-15 and CMY-2 were initially reported from blood samples from the hearts of septicemic broilers in 2011 (Ahmed and Shimamoto, 2013). CTX-M-15 and CTX-M-14 were further detected in E. coli, K. pneumoniae, K. oxytoca, and Enterobacter spp isolated from chicken carcasses in the north of Egypt (Abdallah et al., 2015; Ahmed and Shimamoto, 2015). E. coli isolates harboring SHV-12 have also been reported in Egypt; although they originated from liver and heart samples of chickens affected with colibacillosis (El-Shazly et al., 2017; Figure 1). Similarly to other countries in the Mediterranean basin, ESBL producers in the Egyptian poultry sector belong mainly to the A and B1 phylogroups with the blaCTX-M genes being associated with ISEcp1 (Table 2).
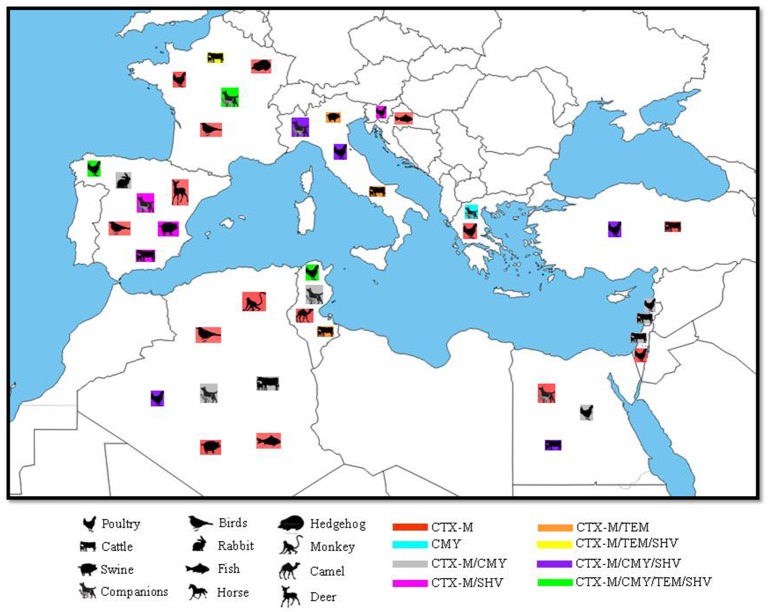
Figure 1.
Geographical distribution of ESBLs and their correspondent animal hosts in the Mediterranean Basin. N.B: only SHV and TEM genes confirmed by sequencing as ESBL were included.
Cattle and sheep
Cattle and sheep are essential members of the human food and agricultural system. For humans, cattle and sheep serve as a source of meat and milk. In agriculture, their feces are commonly used as manure for artificial fertilization (Nyberg et al., 2014). As it is now widely recognized that animals' intestines are a normal habitat for wild type and resistant micro-organisms (Nelson et al., 2013), it has been suggested that if resistant bacteria contaminated animal manures are used without prior treatment, there is a potential risk of transmitting this resistance to the surrounding environment and to the human population (Hruby et al., 2016). This transmission may occur through irrigation and drinking water without treatment (Hruby et al., 2016) or through animals grazing on contaminated lands (Bagge et al., 2009).
In France, the first identification of an ESBL producer in cattle dates back to 2004 when E. coli strains harboring CTX-M-1 and CTX-M-15 were isolated from cows (Meunier et al., 2006). E. coli producing the CTX-M-15 type ESBL were later isolated from the fecal sample of a dead calf (Valat et al., 2012) and from the feces of cattle located in 10 different geographical areas in France (Madec et al., 2012). In the aforementioned study, CTX-M-15 was carried on IncI1 plasmids but also on F31:A4:B1/IncFII and F2:A–:B–/IncFII plasmids which has been extensively reported in humans (Madec et al., 2012). Although CTX-M-15 appears to be dominant in French cattle, other ESBL types were also reported in E. coli (Hartmann et al., 2012) and Klebsiella species (Dahmen et al., 2013b; Haenni et al., 2014a) such as CTX-M-1, CTX-M-14, CTX-M-9, CTX-M-2, CTX-M-32, CTX-M-57, CTX-M-3 (Dahmen et al., 2013b; Haenni et al., 2014a), and TEM-71(Hartmann et al., 2012). These latter were carried by E. coli strains of different sequence types such as ST23, ST58, ST10, ST45, ST88, ST2210, ST2212-ST2215, ST2497, and ST2498 (Table 1); no epidemic clones such as ST101 were detected. Moreover, two studies in France detected AmpC-producing E. coli in calves. In both, AmpC beta-lactamase production was suggested as being due to highly conserved mutations in the promotor/attenuator region and to an over-expression of the chromosomal AmpC gene, respectively (Haenni et al., 2014a,c). In sheep, only one study was conducted in France in which one CTX-M-1 E. fergusonii and three K. pneumonia harboring both blaCTX-M-15 and DHA genes were detected (Poirel et al., 2013). The three K. pneumoniae were co-resistant to nalidixic acid, sulfonamides, trimethoprim-sulfamethoxazole and tetracycline and belonged to the same sequence type ST274. In Spain, ESBL-producing Gram-negative bacilli were isolated from beef samples collected from different geographical locations (Doi et al., 2010; Ojer-Usoz et al., 2013). In Italy, Stefani et al. reported the isolation of five Klebsiella ozaenae harboring CTX-M-1, CTX-M-1/TEM-24 and CTX-M-15 ESBL types from cattle (Stefani et al., 2014).
Table 1.
Non Beta-lactam resistance in MDR of animal origin vs. antibiotic consumption in the Mediterranean Basin.
| Country | Animal host | Species (number) | blagene Type (number) | Non beta-lactam Resistance | Antibiotic usage | References |
|---|---|---|---|---|---|---|
| Algeria | Poultry | E. coli (17) | CTX-M (17) | CMX,NAL,SXT | Unknown | Mezhoud et al., 2015 |
| Poultry | E. coli (16) | CTX-M (2), SHV (14), CMY (4) | AMK, CIP, KAN, NAL, STR, TOB | Belmahdi et al., 2016 | ||
| Poultry | Salmonella spp (11) | CTX-M (11) | CIP | Djeffal et al., 2017 | ||
| Cattle | A. baumannii (1) | NDM (1) | CIP | Chaalal et al., 2016; Yaici et al., 2016 | ||
| Cattle | E. coli (4) | NDM (4), CTX-M (4), CMY (4), | Yaici et al., 2016 | |||
| Birds | E. coli (11) | CTX-M (11) | CIP, NAL, NEO SXT, TET, | Meguenni et al., 2015 | ||
| Birds | A. baumannii (4) | OXA (4) | Morakchi et al., 2017 | |||
| Dogs | E. coli (1) | NDM (1) | FLU, TET | Yousfi et al., 2015 | ||
| Dogs | E. coli (15) | CTX-M (13), SHV (3) | CIP, GEN, NAL, SUL, SXT, TET, TMP, TOB | Yousfi et al., 2016b | ||
| Dogs | E. coli (3) | CTX-M (1), CMY (1), NDM (1), OXA-48 (2) | GEN, CIP, NAL, SXT, TEM, TOB, | Yousfi et al., 2016a | ||
| Cats | E. coli (2) | CMY (1), OXA-48 (2) | CIP, GEN, NAL, SXT, TEM, TOB | Yousfi et al., 2016a | ||
| Cats | E. coli (5) | CTX-M (5) | CIP, NAL, SUL, SXT, TET, TMP, TOB | Yousfi et al., 2016b | ||
| Fish | E. coli (22) | CTX-M (16), TEM (6) | AMK, CIP, CMX, GEN, KAN, NAL, NET, OFX | Brahmi et al., 2016 | ||
| Fish | A. baumannii (2) | OXA-23 (2) | CIP, GEN, KAN, SXT | Brahmi et al., 2016 | ||
| Macaques | K. pneumoniae (7) | CTX-M (7) | CIP, FOS, GEN, SXT | Bachiri et al., 2017 | ||
| Wild Boars | E. coli (30) | CTX-M (30) | AMK, CIP, FOS, GEN, SXT, TET | Bachiri et al., 2017 | ||
| K. pneumoniae (10) | CTX-M (10) | |||||
| Tunisia | Poultry | E. coli (13) | CTX-M (12), CMY (1) | CIP, CHL, GEN, NAL, SXT, SUL, STR, TET | Streptomycin, Tetracycline, Sulphonamides, Trimethoprim | Ben Slama et al., 2010; Ben Sallem et al., 2012 |
| Poultry | E. coli (67) | CTX-M (42), CMY (24) | AMK, GEN, NAL, NOR, SXT, TET | Mnif et al., 2012 | ||
| Poultry | E. coli (16) | CTX-M (16) | NAL, SXT, STR, SUL, TET | Kilani et al., 2015 | ||
| Poultry | E. coli (7) | CTX-M (7) | NAL, STR, TET, SUL, TMP | Grami et al., 2013 | ||
| Poultry | E. coli (10) | CTX-M (8), TEM (1), CMY (2) | NAL, SXT, SUL, TET, STR | Ben Sallem et al., 2012 | ||
| Poultry | E. coli (48) | CTX-M (35), CMY (13) | AMK, CIP, GEN, MIN, NAL, SXT, TET | Maamar et al., 2016 | ||
| Poultry | E. coli (5) | CTX-M (4), SHV (1) | Jouini et al., 2013 | |||
| Cattle | E. coli (1) | CTX-M (1) | GEN, TOB, TET | Grami et al., 2014 | ||
| Beef | E. coli (1) | CTX-M (1) | CIP, NAL, SXT, SUL, TET | Ben Slama et al., 2010 | ||
| Beef | E. coli (5) | CTX-M (5) | CHL, GEN, STR, SUL, SXT, TET, TOB | Jouini et al., 2013 | ||
| Sheep | E. coli (3) | CTX-M (5), TEM (1) | CIP, GEN, NAL, SXT, SUL, STR, TET | Ben Slama et al., 2010 | ||
| Dogs | E. coli (6) | CTX-M (6) | CHL, ENR. GEN, KAN, NAL, NET, SUL, STR, TET, TMP, TOB | Grami et al., 2013 | ||
| Dogs | E. coli (6) | CTX-M (5), CMY (1) | CIP, NAL, SXT, STR, SUL, TET | Sallem et al., 2013 | ||
| Cats | E. coli (1) | CTX-M (1) | NAL, STR, SUL, TET, TMP, | Grami et al., 2013 | ||
| Cats | E. coli (8) | CTX-M (8) | CIP, KAN, NAL, STR, SXT, SUL, TET | Sallem et al., 2013 | ||
| Dromedaries | E. coli (1) | CTX-M (1) | SUL, TET | Ben Sallem et al., 2012 | ||
| Egypt | Poultry | E. coli (18) | CTX-M (7), CMY (11) | CHL, CIP, KAN, NAL, SPX, STR, SXT, TET | Fluoroquinolones, Tetracyclines, Aminoglycosides, Cefotaxime | Ahmed and Shimamoto, 2013; Dahshan et al., 2015 |
| Poultry | E. coli (9) | CTX-M (2), SHV (1), TEM (1), CMY (1) | CIP, CMX, DOX, GEN, STR | El-Shazly et al., 2017 | ||
| Poultry | K. pneumoniae (15) | NDM (15), KPC (14), OXA (12) | - | Hamza et al., 2016 | ||
| Poultry | K. pneumoniae (11) , K. oxytoca (1) | NDM (12) | Abdallah et al., 2015 | |||
| E. coli (8) | CTX-M (8) | |||||
| K. pneumoniae (40) | CTX-M (40) | |||||
| K. oxytoca (2) | CTX-M (2) | |||||
| Enterobacter spp (9) | CTX-M (9) | |||||
| Cattle | E. coli (112) | CTX-M (106), OXA (6) | FOS, FLU, CMX, CHL, MLS, TET, | Tetracycline, quinolones | Braun et al., 2016 | |
| Cattle | E. coli (8) | CTX-M (2), SHV (5), CMY (1) | NAL, SXT, STR, TET | Ahmed et al., 2009 | ||
| Beef | E. coli (4) | CTX-M (1), SHV (1), CMY (2) | CHL, CIP, GEN, KAN, NAL, SPX, STR, SXT, TET | Fluoroquinolones | Ahmed and Shimamoto, 2015 | |
| Cats | E. coli (5) | CTX-M (5) | Abdel-Moein and Samir, 2014 | |||
| Dogs | E. coli (11) | CTX-M (11) | Abdel-Moein and Samir, 2014 | |||
| K. pneumoniae (3) | CTX-M (3) | |||||
| P. mirabilis (1) | CTX-M (1) | |||||
| Palestine | Cattle | E. coli (287) | CTX-M (287) | SXT, STR, TET | Chlortetracycline, doxycycline, Norfloxacin, Cephalexin, Ceftiofur, Sulfa agents, Gentamicin, Monensin | Adler et al., 2015 |
| K. pneumoniae (4) | SHV (4) | CHL, CIP, GEN | ||||
| Poultry | E. coli (9) | CTX-M (9) | Qabajah et al., 2014 | |||
| Lebanon | Poultry | E. coli (217), K. pneumoniae (8), P. mirabilis (3), E. albertii (2), E. fergusonii (1), E. cloacae (3), | CTX-M, CMY | CIP, GEN, SXT | Gentamicin, Tetracyclines | Dandachi et al., 2018a |
| Cattle | E. coli (27) | CTX-M (27) | CHL, ENR, GEN, KAN, NAL, STR, SUL, TET, TMP | Penicillin G - Streptomycin, Ampicillin, Amoxicillin Oxytetracycline, Gentamicin, | Gundogan et al., 2011; Diab et al., 2016 | |
| Fowl | A. baumannii (1) | OXA-48 (1) | AMK, GEN, TOB | Unknown | Al Bayssari et al., 2015b | |
| Horse | A. baumannii (1) | OXA-143 (1) | Rafei et al., 2015 | |||
| Rabbit | A. pitii (1) | OXA-24 (1) | ||||
| Turkey | Poultry | CTX-M (60), SHV (4), CMY (18) | CHL, KAN, NAL, STR, SUL, TET, TMP | Tetracycline, Quinolones | Politi et al., 2005; Pehlivanlar Onen et al., 2015 | |
| Cattle | E. coli (3) | CTX-M (2), CMY (1) | NAL, SXT, STR, TET | |||
| Poultry | E. coli (15) | CTX-M (15) | Tekiner and Ozpinar, 2016 | |||
| Cattle | E. coli (19) | CTX-M (19) | ||||
| Croatia | Mussel | Aeromonas. Caviae (25) | CTX-M (11), SHV (11), FOX (3) | Tetracycline, Amphenicol, Penicillins, Sulfonamides, Trimethoprim, Fluoroquinolones, Aminoglycosides, Polymixins | Maravić et al., 2013; EMA/ESVAC, 2014 | |
| A. Hydrophila (8) | CTX-M (8), SHV (2) | |||||
| Greece | Poultry | Salmonella enteric (2) | CTX-M (2) | CHL, KAN, STR, SUL, TMP, TET | Unknown | Politi et al., 2005 |
| Dogs | E. coli (8) | CMY (8) | FLU | Vingopoulou et al., 2014 | ||
| Slovenia | Poultry | E. coli (6) | CTX-M (2), SHV (4) | GEN, NAL, STR, SUL | Ceftiofur | Chiaretto et al., 2008 |
| Italy | Poultry, Cattle, Swine | Tetracyclines, Amphenicol, Penicillins, 3rd/4th Cephalosporins, Sulfonamides, Trimethoprim, Macrolides, Lincosamides, Fluoroquinolones, Aminoglycosides, Polymixins, Pleuromutilins, Tylosin, Flumequine, | ||||
| Poultry | E. coli (8) | CTX-M (7), SHV (1), | CIP | Giufrè et al., 2012 | ||
| Poultry | E. coli (60) | CTX-M (45), CIT-like (15) | CIP, GEN, SXT, TET | Ghodousi et al., 2015 | ||
| Poultry | E. coli (67) | CTX-M (24), SHV (43) | CIP, NAL, SUL, TMP, TET | Bortolaia et al., 2010 | ||
| Poultry | Salmonella spp (12) | SHV (12) | GENT, NAL, SUL, STR, TET | Chiaretto et al., 2008 | ||
| Poultry | Salmonella infantis (30) | CTX-M (30) | CIP, NAL, SUL, TMP, TET | Franco et al., 2015 | ||
| Swine | Salmonella infantis (2) | CTX-M (2) | ||||
| Cattle | K. ozaenae (5) | CTX-M (5), TEM (1) | Stefani et al., 2014 | |||
| Swine | E. coli (15) | CTX-M (10), TEM (7) | ||||
| Dogs | K. oxytoca (2) | SHV (2), DHA (2) | CIP, GEN, KAN, STR, SUL, TET, TMP | Donati et al., 2014 | ||
| K. pneumoniae (11) | CTX-M (11), SHV (5), DHA (1) | CIP, GEN, KAN, NAL, TET, TMP | ||||
| Dogs | K. pneumoniae (1) | CTX-M (1), SHV (1) | CIP, LEV | Bogaerts et al., 2015 | ||
| E. coli (1) | CMY (1) | CIP, LEV | ||||
| Cats | K. oxytoca (2) | CTX-M (2) | CIP, SUL, TMP, TET | Donati et al., 2014 | ||
| K. pneumoniae (4) | CTX-M (2), SHV (2), DHA (1), CMY (1) | CIP, KAN, NAL, SUL, TET, TMP | ||||
| Cats | E. coli (7) | CTX-M (7), CMY (2) | CHL, ENR, GEN, NAL, NIT, SPX, STR, SUL, TET, TMP. | Nebbia et al., 2014 | ||
| France | Poultry, Cattle, Swine | Tetracycline, Amphenicol, Penicillins, 1st/2nd/3rd/4th Cephalosporins, Sulfonamides, Trimethoprim, Macrolides, Lincosamides, Fluoroquinolones, Aminoglycosides, Polymixins, Pleuromutilins | EMA/ESVAC, 2014 | |||
| Cattle | E. coli (26) | CTX-M (21), TEM (5) | CHL, GENT, SXT | Hartmann et al., 2012 | ||
| Cattle | E. coli (3) | CTX-M (3) | CHL, ENR, FFC, GEN, KAN, NAL, STR, SUL, TET, TMP | Meunier et al., 2006 | ||
| Cattle | A. baumannii (9) | OXA-23 (9) | FOS, KAN, TET | Poirel et al., 2012 | ||
| Cattle | E. coli (9) | CTX-M (9) | CHL, ENR, GEN, KAN, NAL, NET, OFX, STR, SUL, TET, TOB, TMP | Madec et al., 2012 | ||
| Cattle | E. coli (5) | CTX-M (5) | APR, CHL, ENR, GEN, KAN, NAL, NET, OFX, STR, SUL, TET, TOB, TMP | Dahmen et al., 2013b | ||
| K. pneumoniae (1) | CTX-M (1) | |||||
| Sheep | K. pneumoniae (3) | CTX-M (3), DHA (3) | NAL, SUL, SXT, TET | Poirel et al., 2013 | ||
| E. fergusonii | CTX-M (1) | |||||
| Veal calves | E. coli (147) | CTX-M (147) | APR, CHL, ENR, FFC, GEN, KAN, NAL, NET, SUL, STR, TET, TOB, TMP | Haenni et al., 2014a | ||
| K. pneumoniae (3) | CTX-M (2), SHV (1) | FLU, SUL, STR, TET, TMP | ||||
| Swine | E. coli (3) | CTX-M (3) | CHL, NAL, STR, SUL, TET, TMP | Meunier et al., 2006 | ||
| Dog | E. cloacae (11) | CTX-M (10), SHV (1) | FLU, GEN, KAN, QUI, TET, SUL, STR, TMP | Haenni et al., 2016c | ||
| Dog | E. coli (47) | CTX-M (47), CMY (24) | CHL, GEN, KAN, STR, TOB ENR, FFC, NAL, NET, OFX, SUL, TET, TMP | Haenni et al., 2014a | ||
| Dog | E. coli (9) | CTX-M (8), TEM (1) | GEN, SUL, TET | Poirel et al., 2013 | ||
| K. pneumoniae (8) | CTX-M (8), DHA (1) | GEN, NAL, SUL, SXT, TET | ||||
| K. oxytoca (2) | CTX-M (2) | |||||
| Dog | P. mirabilis (14) | CTX-M (1), CMY (7), DHA (2), VEB (6) | APR, CHL, ENR, GEN, KAN, NAL, NET, STR, SUL, TOB, TMP | Schultz et al., 2017 | ||
| Dog | A. baumannii (2) | OXA-23 (2) | CIP, SXT | Hérivaux et al., 2016 | ||
| Dog | E. coli (3) | CMY (2), OXA-48 (1) | GEN, NAL | Melo et al., 2017 | ||
| Cat | A. baumannii (1) | OXA-23 (1) | GEN, NAL, SUL, STR, TET | Ewers et al., 2016 | ||
| Cat | K. pneumoniae (3) | CTX-M (3), DHA (3) | NAL, SUL, SXT, TET | Unknown | Poirel et al., 2013 | |
| E. coli (3) | CTX-M (3) | GEN, SUL, TET | Unknown | |||
| Cat | P. mirabilis (1) | CMY (1) | ENR, NAL, SUL, TMP | Schultz et al., 2017 | ||
| P. rettgeri (1) | CTX-M (1) | ENR, NAL, SUL, TMP | ||||
| Cat | E. coli (2) | CTX-M (2) | STR, TMP | Melo et al., 2017 | ||
| Cat | E. cloacae (11) | CTX-M (10), SHV (1) | FLU, GEN, KAN, QUI, SUL, STR, TET, TMP | Haenni et al., 2016c | ||
| Companions | E. coli (19) | CTX-M (19) | CIP, NAL, SUL, STR, TET | Dahmen et al., 2013a | ||
| Hedgehog | E. coli (1) | CTX-M (1), DHA (1) | NAL, SUL, SXT, TET | Unknown | Poirel et al., 2013 | |
| Tawny Owl | E. coli (1) | CTX-M (1) | ||||
| Domestic goose | E. coli (1) | CTX-M (1) | ||||
| Rock Pigeon | E. coli (1) | CTX-M (1) | ||||
| Horse | E. cloacae (14) | CTX-M (8), SHV (6) | FLU, GEN, KAN, QUI, SUL, STR, TET, TMP | Haenni et al., 2016c | ||
| Horse | P. mirabilis (14) | VEB (2) | ENR, CHL, KAN, NAL, NET, SUL, STR, TOB, TMP | Unknown | Schultz et al., 2017 | |
| Spain | Poultry, Cattle, Swine | Tetracycline, Amphenicol, Penicillins, 3rd/4th Cephalosporins, Sulfonamides, Trimethoprim, Macrolides, Lincosamides, Fluoroquinolones, Quinolones, Aminoglycosides, Polymixins, Pleuromutilins | Abreu et al., 2014; EMA/ESVAC, 2014 | |||
| Poultry | E. coli (64) | CTX-M (44), SHV (6), TEM (2), CMY (13) | CHL, CIP, FUR, GEN, KAN, NAL, SUL, SXT, TET, TOB, TMP | Blanc et al., 2006 | ||
| Poultry | S. enterica (2) | CTX-M (1), SHV (1) | NAL, SXT, STR, SUL, TET, | Riaño et al., 2006 | ||
| Poultry | E. coli (116) | CTX-M (116) | CIP, NAL, SXT | Abreu et al., 2014 | ||
| Poultry | E. coli (11) | CTX-M (6), SHV (2), CMY (2) | CHL, CIP, FFC, GEN, KAN, NAL, STR, SUL, TET, TMP | Solà-Ginés et al., 2015b | ||
| Poultry | E. coli (50) | CTX-M (40), CMY (10) | NAL | Cortés et al., 2010 | ||
| Poultry | E. coli (62) | CTX-M (20), SHV (42) | CIP, NAL | Egea et al., 2012 | ||
| Swine | E. coli (20) | CTX-M (20) | Solà-Ginés et al., 2015b | |||
| Swine | S. enteric (1) | SHV (1) | SUL, STR, TET | Riaño et al., 2006 | ||
| Swine | E. coli (39) | CTX-M (27), SHV(12) | CIP, CHL, FUR, GEN, KAN, NAL, SUL, SXT, TET, TMP, TOB | Blanc et al., 2006 | ||
| Swine | E. coli (20) | CTX-M (8), SHV (12) | APR, CIP, GEN, NAL, STR, SUL, TET, TMP | Escudero et al., 2010 | ||
| Dog | E. coli (1) | SHV (1) | CHL, CIP, NAL, SUL, TET, TMP | Teshager et al., 2000 | ||
| Dog | E. coli (1) | CMY (1) | Bogaerts et al., 2015 | |||
| P. mirabilis (2) | CMY (2) | DOX, MIN | ||||
| Dog | K. pneumoniae (2) | CTX-M (1), VIM (1), DHA (1) | González-Torralba et al., 2016 | |||
| E. cloacae (1) | SHV (1) | |||||
| Deer | E. coli (1) | CTX-M (1) | CIP, CHL, NAL, SXT, TET | Unknown | Alonso et al., 2016 | |
| Rabbit | E. coli (1) | CMY (1) | Unknown | Blanc et al., 2006 | ||
| E. cloacae (3) | CTX-M (3) |
*APR, refers to apramycin; AMK, amikacin; CIP, ciprofloxacin; CHL, chloramphenicol; CMX, co-trimoxazole; DOX, doxycycline; ENR, enrofloxacin; FFC, florfenicole; FLU, fluoroquinolones; FOS, fosfomycin; FUR, furazolidone; GEN, gentamicin; KAN, kanamycin; LEV, levofloxacin; MIN, minocycline; MLS, Macrolides; NAL, nalidixic acid; NET, netilmicin; NIT, nitrofurantoin; NOR, norfloxacin; OFX, oxofloxacin; QUI, quinolones; SPX, spectinomycin; SXT, trimethoprim-sulfamethoxazole; TEM, temocillin; TET, tetracycline; TMP, trimethoprim; TOB, tobramycin.
In Turkey, a study conducted in 2007–2008, showed the presence of ESBL-producing K. pneumoniae and K. oxytoca in raw calf meat (Gundogan et al., 2011). Later on, CTX-M-3 and CTX-M-15 harboring E. coli were isolated from beef samples sold in a market in the south of Turkey (Conen et al., 2015). Recently, a study conducted by Tekiner et al. reported the isolation of ESBL-producing E. coli, E. cloacae, and Citrobacter brakii from raw cows' milk collected from different cities of Turkey. In these areas, CTX-M-1 was dominant (Tekiner and Ozpinar, 2016). In Lebanon the situation differs, in that unlike Turkey but similarly to other Mediterranean countries, blaCTX-M-15, blaSHV-12, and blaCTX-M-14 are the dominant ESBL genes prevailing in E. coli in the Lebanese cattle (Diab et al., 2016). In this latter study, various sequence types were detected. Of special interest is the detection of ST10. ST10 was heavily reported in the literature as being shared between animal and human isolates all over the world: Chile (Hernandez et al., 2013), Denmark (Huijbers et al., 2014), Vietnam (Nguyen et al., 2015), Germany (Belmar Campos et al., 2014). Indeed, it has been suggested that ST10 became associated with the production and dissemination not only of CTX-M-type ESBLs but also of mcr-1 in animals, humans and environment (Monte et al., 2017). In Israel, Adler et al. reported the identification of CTX-M-1/CTX-M-9 and SHV-12 beta-lactamase producing E. coli and K. pneumoniae strains respectively, which were isolated from cattle farms situated in the main farming locations across the country (Adler et al., 2015).
In Egypt, SHV-12 (Ahmed et al., 2009) in addition to CTX-M-1/15 and CTX-M-9 were detected in E. coli strains isolated from cattle (Braun et al., 2016). On study targeting raw milk samples reported the detection of SHV-12 /CTX-M-3, in addition to CMY-2-producing E. coli strains (Ahmed and Shimamoto, 2015). In Tunisia, E. coli strains producing CTX-M-1 and TEM-20 were isolated from beef and sheep situated in different areas across the country (Jouini et al., 2007; Ben Slama et al., 2010). Furthermore, blaCTX-M-15 was detected in an ST10 E. coli isolate recovered from the milk sample of cattle affected with mastitis (Grami et al., 2014). Similarly, In Algeria, Yaici et al. reported the detection of four ST1284 E. coli strains carrying CTX-M-15, CMY-42, and NDM-5 in raw milk samples (Yaici et al., 2016).
Swine
Meat from pigs is used by humans for consumption and their feces are used as manure for land fertilization. Studies have shown that antibiotics are usually detected in higher concentrations in pig manures compared to that of other farm animals (Hou et al., 2015). This finding reflects high and uncontrolled antimicrobial usage in swine farms (Woolhouse et al., 2015). Heavy antibiotic usage creates a selective pressure that contributes to the emergence and spread of bacterial resistance; in this regard, pigs are suggested as a potential source of resistant bacteria.
Reports concerning the prevalence of ESBL of swine origin in the Mediterranean area are very scarce with the majority being reported from Spain where a blaSHV-12 positive Salmonella enterica was isolated in the early 2000s (Riaño et al., 2006). Furthermore, CTX-M-grp-9 (Doi et al., 2010; Ojer-Usoz et al., 2013), SHV-5 and CTX-M-grp-1 carried by A phylogroup E. coli strains and SHV-12 carried by B1 E. coli and blaSHV-5 were detected (Blanc et al., 2006; Cortés et al., 2010). One study conducted in 13 different Spanish provinces found seven AmpC-producing E. coli. In these cases, AmpC production was due to a mutation in the promoter region of the chromosomal AmpC gene (Escudero et al., 2010). In Italy, TEM-52, CTX-M-1, CTX-M-15, and CTX-M-1/TEM-201 carrying E. coli were reported in pigs (Stefani et al., 2014). Franco et al. reported also the presence of Salmonella infantis carrying CTX-M-1 in swine (Franco et al., 2015). In France, only one study conducted at the beginning of the Twenty-first century reported the detection of CTX-M-1-producing E. coli strains in pigs (Meunier et al., 2006). Similarly to what is widely observed in the Mediterranean basin, the CTX-M-1 was associated with the insertion sequence ISEcp1(Meunier et al., 2006). In Algeria, CTX-M-15 harboring E. coli and K. pneumoniae strains were isolated in 2014 from wild boars (Bachiri et al., 2017). MLST typing showed the K. pneumoniae belongs to the ST584 while on the other hand several sequence types (ST617, ST131, ST648, ST405, ST1431, ST1421, ST69, ST226) were observed among E. coli strains (Bachiri et al., 2017). The aforementioned study was the only one to investigate the epidemiology of ESBL-producing Gram-negative bacilli in the African and Asian countries lining the Mediterranean Sea.
Companion animals
Unlike food producing animals, companion animals are not used as consumption source of human food, nor are their feces used as manure for land fertilization. Instead, these animals are kept for the individual's protection, entertainment and company. The number of companion animals has significantly increased in modern society in recent decades (Pomba et al., 2017). Despite regular close contact with people, little attention has been given to the prevalence of antimicrobial resistance in these animals (Scott Weese, 2008). The close contact between companion animals such as dogs, cats, and horses and their owners makes the transmission of resistant organisms more likely to occur (Dierikx et al., 2012). As such, it is essential to investigate the prevalence of resistant bacteria in companion animals as well as to identify the possible risk factors for the transmission of resistant organisms to humans (Rubin and Pitout, 2014).
In the Mediterranean basin, the first detection of ESBL in companion animals was in Spain where an E. coli harboring SHV-12 was isolated from a dog with a urinary tract infection (Teshager et al., 2000). Subsequently, between 2008 and 2010, three strains carrying CMY-2 (one ST2171 E. coli and two P. mirabilis) were recovered from dogs infected with respiratory, urinary tract and skin and soft tissue infections, respectively (Bogaerts et al., 2015). In all three strains, the CMY-2 genes were associated with the ISEcp1. More recently, one K. pneumoniae and one E. cloacae producing CTX-M-15/DHA and SHV-12, respectively, were isolated from the fecal swabs of healthy dogs in this same country (González-Torralba et al., 2016).
In Italy, a study conducted by Donati et al. on 1,555 dog samples of clinical cases and necropsy specimens with suspicious bacterial infections, between the center and the north of Italy found two K. oxytoca harboring SHV-12/DHA-1 and 11 K. pneumoniae carrying the following genes: blaCTX-M-15 (six strains), blaCTX-M-15/DHA-1, blaCTX-M-15/SHV-28, blaCTX-M-1/SHV-28, and blaCTX-M-1 (Donati et al., 2014). In this same study, 429 cats' samples were also investigated revealing the presence two K. oxytoca producing CTX-M-9 and four K. pneumoniae producing CTX-M-15 (two isolates), CTX-M-15/ DHA-1 and SHV-28/CMY-2 beta-lactamases (Donati et al., 2014). The beta-lactamase and AmpC genes in K. oxytoca strains isolated from dogs and cats were located on different plasmid types: IncL/M versus IncHI2 respectively. This is unlike the K. pneumoniae strains where the blaCTX-M-15 was localized on the same plasmid IncR and both strains in dogs and cats shared the same ST340. ST15 and ST101 were also common between dogs and cats in this study. ST15 and ST101 are among the most international clones carrying ESBL as well as carbapenemase genes which became highly detected recently worldwide (Donati et al., 2014). Another study conducted reported the detection of CTX-M-1-producing K. pneumoniae was further reported from a dog with urinary tract infection and an E. coli carrying the CMY-2 type beta-lactamase associated to ISEcp1 also in a diseased cat with a urinary tract infection (Bogaerts et al., 2015). Infections in pets with E. coli strains carrying CTX-M-14 (three isolates), CTX-M-15, CTX-M-1, and CTX-M-14/CMY-2 (two isolates) were also reported in Italy (Nebbia et al., 2014). The strains also showed different sequence types and phylogroups (A “ST3848, ST3847,” B2 “ST131, ST155, ST555, ST4181,” B1 “ST602”) emphasizing that apparently the dissemination of ESBL and AmpC beta-lactamase producers is most likely due to the successful spread of various plasmids carrying these resistance genes (Nebbia et al., 2014).
In France, the highest number of studies addressing the prevalence of extended-spectrum-cephalosporin resistance in companion animals in the Mediterranean was conducted. In dogs, CTX-M-grp 1 (CTX-M-1, CTX-M-15, CTX-M-3, CTX-M-32) and CTX-M-grp 9 in addition to CMY-2 and TEM-52 prevail in E. coli (Dahmen et al., 2013a; Poirel et al., 2013; Haenni et al., 2014b; Bogaerts et al., 2015; Melo et al., 2017). These genes were mostly carried on IncI1, IncFII, and IncHI2 plasmid types and were harbored by strains of different sequence types and phylogroups. Furthermore, K. pneumoniae isolated from dogs showed to produce the CTX-M-15, CTX-M-32, SHV-12, and DHA-1 have been reported (Poirel et al., 2013; Haenni et al., 2014b). In parallel, P. mirabilis showed to produce CMY-2, DHA-16, VEB-6, and CTX-M-15 have been described (Schultz et al., 2017) and E. cloacae the CTX-M-15, CTX-M-14, CTX-M-3, and SHV-12 have been identified (Haenni et al., 2016c). In addition, CTX-M-15 and CMY-2 were also decribed in K. oxytoca and Salmonella enterica, respectively isolated from dogs in this same country (Poirel et al., 2013; Haenni et al., 2014b). On the other hand, in cats, the following distribution was observed: in E. coli (CTX-M-1, CTX-M-15, CTX-M-32, CTX-M-3, CTX-M-14) (Poirel et al., 2013; Melo et al., 2017), in K. pneumoniae (CTX-M-15/DHA) (Poirel et al., 2013), in E. cloacae (CTX-M-15, SHV-12) (Haenni et al., 2016c), in P. mirabilis (CMY-2) and in Proteus rettgeri (CTX-M-1) (Schultz et al., 2017). The dissemination of extended-spectrum-cephalosporin resistance in companion animals in France necessitates studies addressing the risk factors responsible for the acquisition of these strains in pets as well as novel approaches to control the spread of resistance in these animals. Furthermore, the contribution of the pet animals to the spread of resistance in the common population in France should be also investigated. Moreover, France is the only Mediterranean country in which studies reporting ESBL and/or AmpC-producing bacteria in horses are available. Between 2010 and 2013, E. cloacae harboring CTX-M-15, CTX-M-1, and SHV-12 were isolated from clinical samples of horses. These genes were located on IncHI2 and IncP plasmids and were harbored by strains of various sequence types such as ST127, ST372, ST145, ST114, ST135, ST118, ST268, ST107 (Haenni et al., 2016c). Later on, VEB-6 carrying P. mirabilis were isolated from healthy horses (Schultz et al., 2017). In Greece, CMY-2 carried on IncI1 plasmid and harbored by ST212 E. coli strains were isolated from diseased canines in 2011 (Vingopoulou et al., 2014). More recently, a study conducted in Greek households revealed the detection of extended-spectrum-cephalosporin-resistant E. coli isolates. The strains presented with different sequence types including the human pandemic ST131 clone which suggests a possible from humans to animals and vice-versa (Liakopoulos et al., 2018).
In Egypt, CTX-M beta-lactamases have been detected in E. coli recovered from cats' rectal swabs. In this same study, CTX-M-producing E. coli, K. pneumonia, and P. mirabilis were isolated from dogs (Abdel-Moein and Samir, 2014). In Algeria, only one study reported the detection of E. coli strains carrying blaCTX-M-1, blaCTX-M-15 in cats and blaCTX-M-1, blaCTX-M-15, blaSHV-12 in dogs (Yousfi et al., 2016b). In Tunisia, CTX-M-1 carrying E. coli were isolated from cats; while from dogs CTX-M-1, CTX-M-15, and CMY-2-producing E. coli were detected (Grami et al., 2013; Sallem et al., 2013). CTX-M-1 was mostly carried on IncI1 plasmid whereas CTX-M-15 on IncFII (Grami et al., 2013). The blaCTX-M-1 and CMY-2 genes were also found associated with the ISEcp1. Indeed it appears that the insertion sequence ISEcp1 might be also responsible for the dissemination of CMY-2 AmpC genes apart from the blaCTX-M ones.
Wild birds and domestic animals
Besides companion and food producing animals, scattered reports exist on the isolation of ESBL from domestic animals such as wild birds and dromedaries in the Mediterranean. For instance, CTX-M-producing E. coli was isolated from wild birds in Algeria (Meguenni et al., 2015), Turkey (Yilmaz and Guvensen, 2016), blaCTX-M-1 in addition to blaCTX-M-15 carrying E. cloacae in France (Bonnedahl et al., 2009). Furthermore, in France, CTX-M-1 and CTX-M-15 were detected in ST93, ST124, and ST10 E. coli strains recovered from tawny owls/rock pigeons and domestic geese, respectively. In addition, a CTX-M-15/DHA-producing ST274 K. pneumoniae was isolated from a hedgehog living in the same city (Poirel et al., 2013). Rooks carrying CTX-M-14 type ESBL in E. coli have been described in Italy and Spain (Jamborova et al., 2015). Furthermore, in Spain, E. coli and K. pneumoniae harboring CTX-M-14, CTX-M-1, CTX-M-32, CTX-M-9, CTX-M-15, CTX-M-14b, CTX-M-3, and CTX-M-8 were recovered from the fecal samples of gulls (Stedt et al., 2015). In rabbits, CMY-2-producing E. coli and CTX-M-14, CTX-M-9-producing E. cloacae were isolated (Blanc et al., 2006; Mesa et al., 2006). More recently, blaCTX-M-1 was identified in E. coli isolated from the fecal sample of a deer living in the Los Alcornocales natural park in southern Spain (Alonso et al., 2016). In Algeria, blaCTX-M-15 and blaCTX-M-9 genes were detected in E. coli isolated from the gut and gills of fish caught in the Mediterranean across Bejaia city (Brahmi et al., 2016). In this study, it has been suggested that the presence of beta-lactamase producers is due to contamination of the fish from river water and the rising amount of untreated waste that is released into the Mediterranean Sea from the agricultural as well as the industrial operations (Brahmi et al., 2016). These findings emphasizes on the importance of the natural environment in the dissemination of resistance from humans to animals and vice versa. Furthermore, Bachiri et al. also reported the detection of CTX-M-15-producing ST584 K. pneumoniae in Barbary macaques situated in national parks in the north of Algeria (Bachiri et al., 2017). In both Tunisia and Egypt, CTX-M beta-lactamases were detected in E. coli and Pseudomonas aeruginosa recovered from dromedaries and camels, respectively (Ben Sallem et al., 2012; Elhariri et al., 2017). In Croatia, the only study investigating the prevalence of ESBL in animals was conducted in 2009–2010 in mussels caught in the Adriatic Sea. In this study, 18 Aeromonas species carrying SHV-12, CTX-M-15, FOX-2, and PER-1 were identified (Maravić et al., 2013).
Prevalence of carbapenemase producers in livestock and domestic animals
Carbapenems are beta-lactam antibiotics often considered as the last resort antimicrobial agent against multi-drug resistant organisms (Temkin et al., 2014). Carbapenems are active against ESBL and AmpC-producing Gram negative bacilli. Due to the wide dissemination of multi-drug resistant organisms, these antimicrobials recently became heavily used in human medicine. As a result, the emergence of carbapenem resistance has accelerated and it is now a normal phenomenon encountered in hospital settings and, to a lesser extent, community settings. The production of hydrolyzing enzymes called “carbapenemases” is one of the mechanisms by which carbapenem resistance is mediated in Gram negative bacilli. These include (a) class A carbapenemases (KPC, GES, SME, IMI, NMC-A), (b) class B metallo beta-lactamases “MBL” (NDM, VIM, IMP and TMB), and (c) class D oxacillinases (Martínez-Martínez and Gonzalez-Lopez, 2014).
In the Mediterranean basin, in Egypt, OXA-48 and OXA-181 carbapenemases were detected in E. coli strains recovered from dairy cattle farms (Braun et al., 2016). In the poultry production system, one study reported the isolation of K. pneumonia and K. oxytoca harboring NDM metallo beta-lactamases (Abdallah et al., 2015). Another study described the identification of K. pneumoniae carrying OXA-48, NDM and KPC type carbapenemases. Isolated strains were recovered from the liver, lungs, and trachea of broiler chicken (Hamza et al., 2016). In Algeria, NDM-1 and NDM-5 were observed, respectively, in ST85 Acinetobacter baumannii and ST1284 E. coli originating from raw milk in the west and north of the country (Chaalal et al., 2016; Yaici et al., 2016). In E. coli, NDM-5 was located on an IncX3 plasmid (Yaici et al., 2016). In broilers, OXA-58 was identified (Chabou et al., 2017) while in pigeons, in addition to OXA-58 and OXA-23 were detected (Morakchi et al., 2017). In terms of companion animals, NDM-5 and OXA-48-producing E. coli were reported from healthy dogs Algeria (Yousfi et al., 2015, 2016a). The NDM-5 was harbored by an E. coli strain having the same sequence type ST1284 previously described in cattle (Yousfi et al., 2015; Yaici et al., 2016). OXA-48 was further detected in healthy and diseased cats in the same city (Yousfi et al., 2016a). Furthermore, in this same country, two A. baumannii producing OXA-23 were isolated from fish (Brahmi et al., 2016). In Lebanon, A. baumannii with different sequence types (ST294, ST491, ST492, ST493) were detected in a horse's mouth carrying OXA-143 (Rafei et al., 2015), and in pigs and cattle carrying OXA-23(Al Bayssari et al., 2015a). Furthermore, in cattle, a VIM-2-producing P. aeruginosa was isolated (Al Bayssari et al., 2015a). In fowl, Bayssari et al. reported the detection of OXA-23 and OXA-58 harboring A. baumannii and OXA-48-producing E. coli as well as VIM-2 producing P. aeruginosa (Al Bayssari et al., 2015b). VIM-2 producers in fowl and cattle were of different sequence types suggesting the presence of plasmid that is mediating the spread of this resistance gene. In France, OXA-23-producing Acinetobacter species were described in cows and dogs (Poirel et al., 2012; Hérivaux et al., 2016). Melo et al. reported the detection of OXA-48 located on an IncL plasmid and carried by an ST372 E. coli strain from dogs in France (Melo et al., 2017). In contrast, in Spain, only one study reported the isolation of a VIM-1-producing ST2090 K. pneumoniae from a dog's rectal swab (González-Torralba et al., 2016; Figure 2).
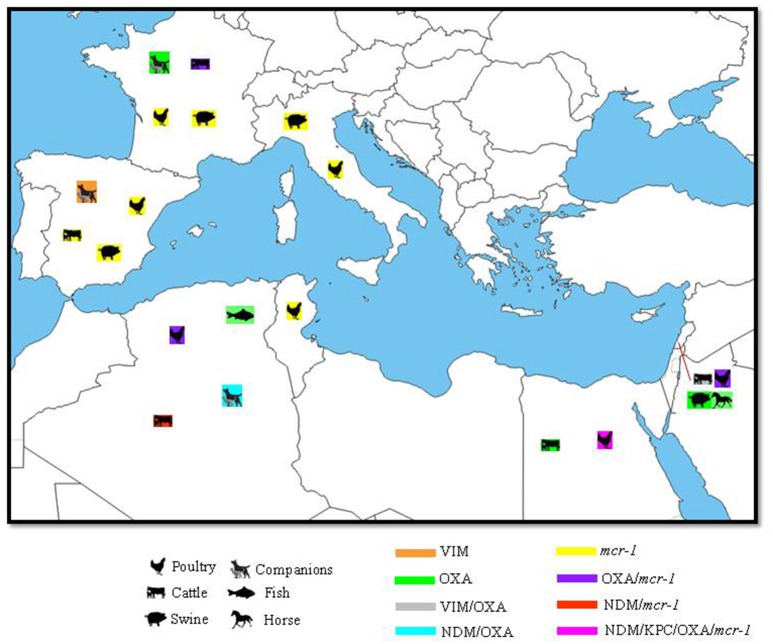
Figure 2.
Geographical distribution of carbapenemases and mcr colistin resistance gene with their hosts in the Mediterranean. N.B: only OXA genes confirmed by sequencing as carbapenemases were included.
Clonal relationship of beta-lactamase producers and plasmid types of beta-lactamase genes isolated from all animal sources
The different phylogroups and sequence types of beta-lactamase and mcr-1 positive strains as well as the type of plasmids carrying ESBL, AmpC, carbapenemase, and mcr-1 genes detected in all animal sources in the Mediterranean region are summarized in Table 2. In this area of the world, it appears that multi-drug resistance in the veterinary sector is mediated by the spread of different phylogroups and sequence types with the main ones being A, B, and D phylogroups (Table 2). The detection of ST10 in CTX-M producers in poultry, cattle, pets, and domestic animals in Algeria, Tunisia, Lebanon, and France is of special interest. ST10 was often described in the literature as being common to ESBL E. coli strains of human and avian origin worldwide such as in Germany (Belmar Campos et al., 2014), Denmark (Huijbers et al., 2014), Vietnam (Nguyen et al., 2015), and Chile (Hernandez et al., 2013). ST10 was suggested as being associated with the spread of CTX-M ESBL types and mcr-1 genes in humans, animals and environments (Monte et al., 2017). Another distinct finding is the detection of ST101 in dogs and cats in Italy. ST101 is an international sequence types frequently detected in pigs (El Garch et al., 2017), broilers (Solà-Ginés et al., 2015b) as well as in the clinical settings. In several countries, ST101 was associated to NDM-1 E. coli strains isolated from the clinical settings of Germany, Canada, Australia, UK, and Pakistan (Yoo et al., 2013) implying thus that ST101 is a candidate for the zoonotic transmission to the human population.
Table 2.
ST/phylogroups, IS and plasmid types associated with beta-lactamase and mcr genes in the Mediterranean.
| Country | Animal Host | Species | Bla and/or mcr genes | ST and/or phylogroup | Plasmid type | Associated IS | Reference |
|---|---|---|---|---|---|---|---|
| Algeria | Poultry | E. coli | CTX-M 1 | ST38, ST2179 | Belmahdi et al., 2016 | ||
| SHV-12 | ST1011, ST5086 | ||||||
| CMY-2 | ST744 | ||||||
| Poultry | S. Heidelberg | CTX-M-1 | ST15 | Djeffal et al., 2017 | |||
| Cattle | A. baumanii | NDM-1 | ST85 | Chaalal et al., 2016 | |||
| Cattle | E. coli | NDM-5/ CMY-42/ CTX-M-15 | ST1284 | IncX3 (NDM-5) | Yaici et al., 2016 | ||
| Swine | K. pneumoniae | CTX-M-15 | ST584 | Bachiri et al., 2017 | |||
| E. coli | CTX-M 15 | ST617, ST131, ST648, ST405, ST1431, ST1421, ST69, ST226 | |||||
| Dog | E. coli | CTX-M-15 | A, B1, E | Yousfi et al., 2016b | |||
| CTX-M-1/SHV-12 | E | ||||||
| SHV-12 | A, B1 | ||||||
| Dog | E. coli | NDM-5 | ST1284 | Yousfi et al., 2015 | |||
| Dog | E. coli | OXA-48 | A, D | Yousfi et al., 2016a | |||
| NDM-5/ CTX-M-15/ CMY-42 | A | ||||||
| Cat | E. coli | CTX-M-1 | B1 | Yousfi et al., 2016b | |||
| CTX-M-15 | A, U, E | ||||||
| Cat | OXA-48 / CMY-1 | U | Yousfi et al., 2016a | ||||
| OXA-48 | D | ||||||
| Barbary Macaques | K. pneumoniae | CTX-M-15 | ST584 | Bachiri et al., 2017 | |||
| Fish | A. baumanii | OXA-23 | ST2 | Brahmi et al., 2016 | |||
| Fish | E. coli | CTX-M-15 | ST471, ST132, ST398, ST37,ST477, ST131, ST31 | Brahmi et al., 2015 | |||
| CTX-M-9 | ST8 | ||||||
| TEM-24 | ST31, ST471, ST66, ST21, ST74 | ||||||
| Tunisia | Poultry | E. coli | CTX-M-1 | A, B1, D | ISEcp1 | Ben Sallem et al., 2012 | |
| CMY-2 | B2 | ISEcp1 | |||||
| D | ISEcp1D-IS10 | ||||||
| Poultry | CTX-M-1 | ISEcp1/IS26 | Jouini et al., 2007 | ||||
| Poultry | E. coli | CTX-M-1 | B1, A | Ben Slama et al., 2010 | |||
| CMY-2 | B1 | ||||||
| Poultry | E. coli | CTX-M-1 | A, B1, D, B2 | IncI1 | Mnif et al., 2012 | ||
| CTX-M-15 | A, B1 | ||||||
| CTX-M-1/CMY-2 | B2 | IncI1 | |||||
| CMY-2 | A, D, B1 | IncI1 | |||||
| Poultry | E. coli | CTX-M-1 | IncI1 | Grami et al., 2013 | |||
| CTX-M-9 | IncI1 | ||||||
| Poultry | E. coli | CTX-M-1 | A0, A1, D2, B2 | Kilani et al., 2015 | |||
| Poultry | E. coli | CMY-2 | A, B1, D | IncI1, IncF, IncFIB, IncFIA | Maamar et al., 2016 | ||
| CTX-M-14 | B1 | IncF | ISEcp1-IS903 | ||||
| CTX-M-1 | B1, D, A | IncI1, IncF, IncFIB, IncK, IncY, IncP, IncN | |||||
| CTX-M-15 | D | ISEcp1and ISEcp1-IS5 | |||||
| Poultry | E. coli | CTX-M-1/mcr-1 | D, H, K | IncHI2/ST4 | Grami et al., 2016 | ||
| Poultry | E. coli | CMY-2/mcr-1 | A (ST2197) | IncP (mcr-1) | ISApl1 | Maamar et al., 2018 | |
| IncI1 (CMY-2) | |||||||
| Cattle | E. coli | CTX-M-1 | A, B1 | Ben Slama et al., 2010 | |||
| CTX-M-1/ TEM-20 | B1 | ||||||
| Cattle | E. coli | CTX-M-1 | ISEcp1/IS26 | Jouini et al., 2007 | |||
| CTX-M-14 | ISEcp1 and IS903 | ||||||
| Cattle | E. coli | CTX-M-15 | ST10 | ISEcp1 | Grami et al., 2014 | ||
| Dog | E. coli | CTX-M-1 | IncI1 | Grami et al., 2013 | |||
| CTX-M-15 | IncFII | ||||||
| Dog | E. coli | CMY-2 | B1 | ISEcp1 | Sallem et al., 2013 | ||
| CTX-M-1 | D, B1, A | ISEcp1 | |||||
| Cat | E. coli | CTX-M-1 | B1, A, D | ISEcp1 | Sallem et al., 2013 | ||
| CTX-M-1/ TEM-135 | A | ISEcp1 (CTX-M-1) | |||||
| Cat | E. coli | CTX-M-1 | IncI1 | Grami et al., 2013 | |||
| Dromedaries | E. coli | CTX-M-1 | B1 | ISEcp1 | Ben Sallem et al., 2012 | ||
| Egypt | Poultry | E. coli | CTX-M-15 | clonal group O25b-ST131 | ISEcp1 | Ahmed and Shimamoto, 2013 | |
| Poultry | E. coli | CTX-M | A, B1, B2, D | Abdallah et al., 2015 | |||
| Poultry | E. coli | CTX-M-14 | D | El-Shazly et al., 2017 | |||
| SHV-12 | D | ||||||
| CMY-2 | A, B1, D | ||||||
| Poultry | E. coli | mcr-1 | phylotype A, F, B1 | IncFIB; IncI1; IncI2 | Lima Barbieri et al., 2017 | ||
| Cattle | E. coli | mcr-1 | ST10 | Khalifa et al., 2016 | |||
| Lebanon | Poultry | E. coli | CTX-M | ST156, ST5470, ST354, ST155, ST3224 | Dandachi et al., 2018a | ||
| Poultry | E. coli | mcr-1 | ST515 | Dandachi et al., 2018b | |||
| Cattle | E. coli | CTX-M-15 | A (ST1294, ST2325, ST1303, ST4623, ST5204) | Diab et al., 2016 | |||
| B1 (ST58, ST162, ST4252, ST155, ST196, ST540) | |||||||
| D (ST69) | |||||||
| CTX-M-14 | D (ST457) | ||||||
| CTX-M-15/SHV-12 | A (ST10, ST2450, ST5442) | ||||||
| CTX-M-14/SHV-12 | D (ST457) | ||||||
| SHV-12 | A (ST218, ST617, ST5204, ST1303,ST5728,ST1140, ST746) | ||||||
| Cattle | A. baumanii | OXA-23 | ST2 | Al Bayssari et al., 2015a | |||
| P. aeroginosa | VIM-2 | ST1762, ST1759 | |||||
| Swine | A. baumanii | OXA-23 | ST491 | Al Bayssari et al., 2015a | |||
| Fowl | A. baumanii | OXA-23 | ST492, ST493 | Al Bayssari et al., 2015b | |||
| OXA-58/OXA-23 | ST20 | ||||||
| P. aeroginosa | VIM-2 | ST1760, ST1761 | |||||
| Fowl | E. coli | OXA-48 | ST38 | Al Bayssari et al., 2015b | |||
| Horse | A. baumanii | OXA-143 | ST294 | Rafei et al., 2015 | |||
| Rabbit | A. pitii | OXA-24 | ST221 | Rafei et al., 2015 | |||
| Palestine | Poultry | E. coli | CTX-M | A, B, D | Qabajah et al., 2014 | ||
| Turkey | Poultry | E. coli | CMY-2 | A0, B2 D1, D2 | Pehlivanlar Onen et al., 2015 | ||
| CTX-M-1/CMY-2 | A0 | ||||||
| CTX-M-1 | A1, A0, D1, D2 | ||||||
| CTX-M-1/SHV-5 | D1 | ||||||
| CTX-M-3 | A0, D1 | ||||||
| CTX-M-15 | B1, D1, D2 | ||||||
| SHV-12 | D1 | ||||||
| CTX-M-15/SHV-12 | D2 | ||||||
| Italy | Poultry | E. coli | SHV-12 | IncI1, IncFIB | Bortolaia et al., 2010 | ||
| CTX-M-1 | IncI1, IncFIB, IncN | ||||||
| CTX-M-32 | IncN | ||||||
| Poultry | E. coli | CTX-M-1 | IncI1 | Accogli et al., 2013 | |||
| CMY-2 | IncI1 | ||||||
| Poultry | E. coli | CTX-M | A, B1, B2, D | Ghodousi et al., 2015 | |||
| CIT like | B1, B2, D | ||||||
| Poultry | E. coli | CTX-M | B2, ST131 | Ghodousi et al., 2016 | |||
| Swine | E. coli | OXA-181 | B1 (ST359), A (ST641) | IncX3 | Pulss et al., 2017 | ||
| mcr-1 | A (ST641) | IncX4 | |||||
| CMY-2 | A (ST641) | IncI1 | |||||
| Cat | E. coli | CMY | A | ISEcp1/IS26 | Bogaerts et al., 2015 | ||
| Dog | K. oxytoca | SHV-12, DHA-1 | N.I | IncL/M | Donati et al., 2014 | ||
| K. pneumoniae | CTX-M-15,DHA-1 | ST340 | IncR (CTX-M-15) | ||||
| CTX-M-15 | ST101 | ||||||
| SHV-28, | ST15 | ||||||
| CTX-M-15,SHV-28, | ST15 | ||||||
| CTX-M-1,SHV-28 | ST15 | CTX-M-1 in IncN and IncR | |||||
| CTX-M-1 | ST11 | ||||||
| Cat | K. oxytoca | CTX-M-9 | N.I | IncHI2 | Donati et al., 2014 | ||
| K. pneumoniae | CTX-M-15, DHA-1 | ST340 | CTX-M-15/DHA-1 on IncR | ||||
| SHV-28, CMY-2 | ST15 | CMY-2 on InCI1 | |||||
| CTX-M-15 | ST101 | ||||||
| Cat | E. coli | CTX-M-14/CMY-2 | A (ST3848, ST3847) | Nebbia et al., 2014 | |||
| CTX-M-14 | B2 (ST555, ST4181), B1 (ST602) | ||||||
| CTX-M-1 | B2 (ST155) | ||||||
| CTX-M-15 | B2 (ST131) | ||||||
| Slovenia | Poultry | E. coli | CTX-M-1 | D | Zogg et al., 2016 | ||
| SHV-12 | B1 and D | ||||||
| Spain | Poultry | E. coli | CTX-M-14 | ST101, ST156,ST165, ST350, ST889, ST1137 | IncK | Solà-Ginés et al., 2015b | |
| SHV-12 | ST350, ST533 | IncI1 | |||||
| CMY-2 | ST429, ST131 | IncK | |||||
| Poultry | E. coli | CMY-2 | A, D | Cortés et al., 2010 | |||
| CTX-M-14 | A, B1, B2 | ||||||
| CTX-M-32 | A | ||||||
| CTX-M-9 | B1 | ||||||
| SHV-12 | |||||||
| TEM-52 | B1 | ||||||
| Poultry | E. coli | CTX-M-9 | O25b:H4-B2-ST131. | Mora et al., 2010 | |||
| Poultry | E. coli | CTX-M, SHV | A, B1, D1 | Egea et al., 2012 | |||
| Poultry, Swine, Cattle | E. coli | CTX-M, SHV | B2, D | Doi et al., 2010 | |||
| cattle | E. coli | mcr-1 /mcr-3/ CTX-M-55 | ST533 | non mobilizable IncHI2 | Hernández et al., 2017 | ||
| Swine | E. coli | CTX-M-1 | A | Cortés et al., 2010 | |||
| SHV-5 | A | ||||||
| SHV-12 | B1 | ||||||
| Dog | E. coli (1) | CMY (1) | ST2171 | IncK | ISEcp1 | Bogaerts et al., 2015 | |
| P. mirabilis (2) | CMY (2) | ||||||
| Dog | K. pneumoniae | VIM-1 | ST2090 | González-Torralba et al., 2016 | |||
| Deer | E. coli | CTX-M-1 | ST224 | IncN | IS26 | Alonso et al., 2016 | |
| Croatia | Mussel | Aeromonas spp | CTX-M-15 | IncFIB | Maravić et al., 2013 | ||
| France | Poultry | E. coli | CTX-M-1 | ISEcp1 | Meunier et al., 2006 | ||
| Cattle | E. coli | CTX-M-1 | ISEcp1 | Meunier et al., 2006 | |||
| CTX-M-15 | ISEcp1 | ||||||
| Cattle | E. coli | CTX-M-15 | B1 | ISEcp1 | Valat et al., 2012 | ||
| Cattle | E. coli | CTX-M-1 | ST2497, ST2498 | Hartmann et al., 2012 | |||
| TEM-71 | ST178 | ||||||
| Cattle | E. coli | CTX-M-15, | ST2212, ST2213, ST2210, ST2214,ST2215, ST88 | F31:A4:B1/IncFII F2:A–:B–/IncFII and IncI1 | Madec et al., 2012 | ||
| Cattle | K. pneumoniae | CTX-M-14 | ST45 | F2:A-:B-IncFII | Dahmen et al., 2013b | ||
| E. coli | CTX-M-14 | ST23, ST58, ST10, ST45 | F2:A-:B-IncFII | ||||
| CTX-M-1 | ST23, ST58 | IncI1/ST3 | |||||
| Sheep | K. pneumoniae | CTX-M-15, DHA | all ST274 | Poirel et al., 2013 | |||
| Swine | E. coli | CTX-M-1 | ISEcp1 | Meunier et al., 2006 | |||
| Dogs | E. coli | CTX-M-15 | A (ST410, ST617) | IncFII | Dahmen et al., 2013a | ||
| CTX-M-1 | A (ST10), B1 (ST1303, ST1249) | IncFII | |||||
| IncFII | |||||||
| Dog | A. baumanii | OXA-23 | ST25 | Hérivaux et al., 2016 | |||
| Dogs | E. coli | CTX-M-1 | ST345, ST1001, ST124 | IncI1 | Poirel et al., 2013 | ||
| CTX-M-15 | NEW ST | N.T | |||||
| TEM-52 | ST359 | ||||||
| K. pneumoniae | CTX-M-15, DHA-1 | ST274 | |||||
| CTX-M-15, | ST15 | ||||||
| Dogs | E. coli | CTX-M-1 | A, B1,D | blaCTX-M-1/IncI1/ST3 | Haenni et al., 2014b | ||
| CTX-M-grp9 | B2 | ||||||
| CMY-2 | A, B1, B2, D | CMY-2/IncI1/ST2 | |||||
| Dog | E. cloacae | CTX-M-15 | ST114,ST136,ST270,ST100 | IncHI2 | Haenni et al., 2016c | ||
| CTX-M-14 | ST102 | N.T | |||||
| CTX-M-3 | ST408 | N.T | |||||
| SHV-12 | ST268 | IncHI2 | |||||
| Dog | E. coli | CMY | ST55 | N.T | Melo et al., 2017 | ||
| CMY | ST963 | N.T | |||||
| OXA-48 | ST372 | IncL | |||||
| Cat | K. pneumoniae | CTX-M-15, DHA | ST274 | Poirel et al., 2013 | |||
| E. coli | CTX-M-1 | ST124, ST641 | |||||
| CTX-M-14 | ST141 | ||||||
| Cats | E. coli | CTX-M-15 | A (ST617, ST410) | Dahmen et al., 2013a | |||
| CTX-M-32 | B1 (ST224) | ||||||
| CTX-M-3 | B2 (ST493) | ||||||
| CTX-M-14 | B1, (ST359), B2 (ST131) | ||||||
| Cat | E. cloacae | CTX-M-15 | 1 ST136, others ST114 | IncHI2 | Haenni et al., 2016c | ||
| SHV-12 | N.T | IncA/C | |||||
| Cat | E. coli | CTX-M-14 | ST68 | IncF | Melo et al., 2017 | ||
| CTX-M-1 | ST673 | IncFIB | |||||
| Cat | A. baumanii | OXA-23 | ST1/ST231 | Ewers et al., 2016 | |||
| Hedgehog | K. pneumoniae | CTX-M-15, DHA | ST274 | Poirel et al., 2013 | |||
| Tawny Owl | E. coli | CTX-M-1 | ST93 | Poirel et al., 2013 | |||
| Domestic goose | E. coli | CTX-M-15 | ST10 | Poirel et al., 2013 | |||
| Rock pigeon | E. coli | CTX-M-1 | ST124 | Poirel et al., 2013 | |||
| Horse | E. cloacae | CTX-M-15 | ST127, ST372, ST145, ST114 | IncHI2 | Haenni et al., 2016c | ||
| SHV-12 | ST135,ST145,ST118 | IncHI2 | |||||
| CTX-M-1 | ST268 | N.T | |||||
| ST107 | IncP | ||||||
| Greece | Dog | E. coli | CMY-2 | ST212 | IncI1/ST65 | Vingopoulou et al., 2014 |
Bla, beta-lactamase; ST, sequence type; IS, insertion sequence; N.T, non typeable.
More deeply speaking, ESBL and AmpC encoding genes were mostly carried on conjugative IncI1, IncFIB, IncN, and IncK plasmids (Table 1). ISEcp1 was the most common insertion sequence associated with the CTX-M ESBL types with the main ones being blaCTX-M-1 and blaCTX-M-15 genes. ISEcp1 has been previously described as a potent contributor to the mobilization and insertion of blaCTX-M genes worldwide (El Salabi et al., 2013). As for the carbapenemase encoding genes, these latter were found to be carried by IncX3 and IncL plasmids detected in E. coli strains isolated from cattle, swine and dogs in Algeria, Italy, and France, respectively. Overall, the detection of a variety of sequence types and phylogroups in ESBL and AmpC producers isolated from animals of all origins within and among countries's animals suggests that the dissemination of multi-drug resistance in the Mediterranean is multi-clonal and related rather to the diffusion of conjugative plasmids carrying beta-lactamase genes.
Prevalence of colistin resistance in livestock and domestic animals
Polymyxin E (colistin) and polymyxin B are polycationic antimicrobial peptides that are considered as the last-line antibiotic treatment for multi-drug resistant (MDR) Gram-negative bacterial infections (Olaitan and Li, 2016). From the 1960s until the 1990s, colistin was considered as an effective treatment for MDR-GNB (Olaitan et al., 2014b). However, due its nephrotoxicity within the human body, the clinical use of this antimicrobial was abandoned (Olaitan and Li, 2016). Recently, the emergence of carbapenem resistance in clinically important bacteria such as P. aeruginosa, A. baumannii, K. pneumonia, and Escherichia coli, necessitated the re-introduction of colistin into clinical practice as a last-resort treatment option (Olaitan and Li, 2016).
Colistin is not only administered in humans, its use has been also described in veterinary medicine. Indeed, it has been suggested that the uncontrolled use of colistin in animals has played an important role in the global emergence of colistin-resistant bacteria (Collignon et al., 2016). The World Health Organization recently added polymyxins to the list of critically important antibiotics used in food producing animals worldwide (Collignon et al., 2016). The main use for colistin in animals includes the treatment of gastrointestinal infections caused by E. coli in rabbits, pigs, broilers, veal, beef, cattle, sheep, and goats; and, in particular, gastrointestinal infections caused by E. coli (Poirel et al., 2017). Colistin is mainly administered orally using different formulations such as premix, powder and oral solutions (Catry et al., 2015). In European countries, several epidemiological studies reported the use of colistin in veterinary medicine. In fact, Kempf et al. reported that colistin is mainly used to inhibit infections caused by E. coli, a Gram-negative bacillus known as a common causative agent of diarrhea, septicemia, and colibacillosis in animals (Kempf et al., 2013). In Spain, Casal et al. revealed that colistin is among the most frequent administered drug for the treatment of digestive diseases in pigs (Casal et al., 2007).
Epidemiologically speaking, the worldwide prevalence of resistance to polymyxins accounts for 10% of Gram-negative bacteria with the highest rates being observed in Mediterranean countries and Southeast Asia (Al-Tawfiq et al., 2017). For many years, colistin resistance was thought to be mainly mediated by chromosomic mutations, with no possibility of horizontal gene transfer. However, the emergence of the mcr-1 plasmid mediated colistin resistance gene (Liu et al., 2016) has thoroughly altered the view of colistin resistance as a worldwide problem (Baron et al., 2016). The current epidemiology of colistin resistance is poorly understood.
In the Mediterranean area (Figure 2), the first detection of mcr-1 was in an E. coli strain isolated from chickens in Algeria (Olaitan et al., 2016). This same isolate was further detected in sheep in another region of this country in 2016 (Chabou et al., 2017). In Tunisia, Grami et al. reported a high prevalence of multi-clonal E. coli carrying the mcr-1 gene in three chicken farms imported from France (Grami et al., 2016). Isolated strains were found to co-harbor the blaCTX-M-1 ESBL gene along with mcr-1 on an IncHI2/ST4 plasmid (Table 1; Grami et al., 2016). Apart from colistin resistance, these strains were also co-resistant to tetracyclines, quinolones, fluoroquinolones, trimethoprim, and sulfonamides (Grami et al., 2016). The co-existence of ESBL and mcr-1 genes on the same plasmid facilitates the dissemination of colistin resistant strains by the co-selective pressure applied via the use of colistin as well as possibly the utilization of non-beta-lactam antibiotics. Molecular analysis targeting the co-localization of ESBL and mcr genes along with the ones mediating resistance toward non-beta-lactams is however warranted in order to validate this hypothesis. Also in Tunisia, two colistin resistant E. coli strains positive for mcr-1 and harboring the CMY-2 gene were recently detected in chicken. Both strains shared the same sequence type “ST2197” in addition to their PFGE patterns. The mcr-1 gene in these latter was associated with the ISApl1 and was carried by IncP plasmid while the CMY-2 gene was located on an IncI1 plasmid type (Maamar et al., 2018). Furthermore, in this same country, a recent study revealed the absence of mcr-1 and mcr-2 positive Gram-negative bacilli in camel calves in southern Tunisia (Rhouma et al., 2018). Likewise, in Egypt, mcr-1 was detected in E. coli isolated from diseased chickens as well as from cows displaying subclinical mastitis (Khalifa et al., 2016; Lima Barbieri et al., 2017). The emergence of mcr-1 in Egypt can be related to the use of colistin in animal agriculture, and its ready application as a therapeutic agent for colibacillosis as well as other infections, in rabbits and calves (Lima Barbieri et al., 2017). In Southeast Asia, Dandachi et al. reported the detection of the mcr-1 plasmid mediated colistin resistance gene in E. coli in poultry in the south of Lebanon (Dandachi et al., 2018a). This strain had a sequence type of ST515 that was not reported before in mcr-1 E. coli strains of poultry origin (Dandachi et al., 2018a).
Of the European countries bordering the Mediterranean, Spain was the first to report the detection of mcr-1 in E. coli and Salmonella enterica isolated from farm animals (Quesada et al., 2016). This could be related to the fact that Spain is one of the countries were colistin is extensively used in veterinary medicine (de Jong et al., 2013). More recently, mcr-1 co-existing with mcr-3 on the same non mobilizable IncHI2 plasmid was detected in an E. coli strain recovered from cattle feces in a slaughterhouse (Hernández et al., 2017). In France, as part of routine surveillance by the French agricultural food sector, mcr-1 was identified in four Salmonella spp isolated from sausage, food of poultry origin, and boot swabs taken from broiler farms (Perrin-Guyomard et al., 2016; Webb et al., 2016). E. coli harboring mcr-1 was also isolated in France from pig, broiler and turkey samples (Haenni et al., 2016a). Haenni et al. reported the identification of unique IncHI2/ST4 plasmid co-localizing mcr-1 and ESBL genes in an E. coli strain isolated from French veal calves (Haenni et al., 2016b). In Italy, Carnevali et al. reported the detection of mcr-1 in Salmonella spp strains isolated from poultry and pigs (Carnevali et al., 2016). Subsequently, mcr-1 was further detected in E. coli of swine origin. In the aforementioned report, mcr-1 was co-existent with the carbapenemase OXA-181 in the same bacterium and was carried on an IncX4 plasmid type (Pulss et al., 2017). In the Mediterranean basin, likewise ESBL producers, mcr positive strains belong to different phylogroups and appear to be not clonally related; however, they were not associated to a common plasmid or an insertion sequence type. This questions the molecular mechanism by which the mcr genes are being disseminating in this region of the world. More molecular work is warranted in this area especially that mcr genes are often located on plasmids carrying ESBL and/or carbapenemase genes.
Antibiotic use in animals and potential impact on public health
For many years, the use of antibiotics in the veterinary medicine has increased animal health via lowering mortality and the incidence of infectious diseases (Hao et al., 2014). However, in view of the heavy dissemination of resistant organisms namely ESBL, AmpC, and carbapenemase producers in addition to the emergence of colistin resistance in livestock and animals with frequent contacts with human; the efficiency of antibiotic administration to animals has been reconsidered. Indeed, antibiotic use in animals is not controlled, in that these latter are not only prescribed for treatment, but are also given for prophylaxis and as growth promoters (Economou and Gousia, 2015). In its recent publication, the world health organization recommended a reduction but an overall restriction of the use of medically important antibiotics for prophylaxis and growth promotion in farm animals (WHO, 2017). According to the world health organization list of Critically Important Antimicrobials for Human Medicine (WHO CIA list), these include mainly extended spectrum cephalosporins, macrolide, ketolides, glycopeptides and polymixins (WHO CIA, 2017). The control of antibiotic use in the veterinary sector aims to reduce the emergence of resistance in addition to preserving the efficacy of important classes for treatment in the human medicine.
In the Mediterranean region, tetracyclines, aminoglycosides, sulfonamides, fluoroquinolones, and polymixins are the most common antimicrobial classes prescribed in the veterinary sector (Table 1). The usage level of each antibiotic class in addition to its real purpose of administration apart from treatment is limited and not well understood in this area of the world. In fact, it is nowadays accepted that the over-use of antibiotics in animals is the main driven for the dissemination of multi-drug resistance (Barton, 2014). As shown in Table 1, ESBL, AmpC, and carbapenemase producers are often co-resistant to non-beta-lactam antibiotics with the most common being gentamicin, streptomycin, tetracycline, trimethoprim-sulfamethoxazole, nalidixic acid, and ciprofloxacin. One study conducted in healthy chicken in Tunisia showed the presence of tetA, tetB, sul1, and sul2 on the same plasmids carrying the blaCTX-M genes (Maamar et al., 2016). Another study in Egypt, reported the detection of tetB, qnrB2, qnrA1, aadA1 on the same gene cassette along with the blaCMY-2 AmpC beta-lactamase gene (Ahmed and Shimamoto, 2013). In Italy, strA/B, tetD, qnrB, aadA1, sulI genes were associated with the blaCTX-M and blaSHV ESBL genes types in companion animals (Donati et al., 2014). Furthermore, in this same country, aminoglycoside modifying enzymes (aadA1, aadA2), quinolone resistance genes (qnrS1), florfenicol/chloramphenicol resistance gene (floR), in addition to tetracycline and sulfonamide resistance genes (tetA, sul1, sul2, sul3) were found associated with OXA-48/181 and OXA-48/181/ CMY-2 /mcr-1 positive E. coli strains isolated from pigs (Pulss et al., 2017). In Salmonella enterica, Franco et al. reported the detection of a megaplasmid harboring the blaCTX-M-1 ESBL gene along with tetA, sulI, dfrA1, and dfrA14 conferring thus additional resistance toward tetracycline, sulfonamide, and trimethoprim (Franco et al., 2015). Beta-lactamase producing Gram-negative bacilli appear thus to be selected by the co-selective pressure applied by the use of non-beta-lactam antibiotics in livestock and companion animals. Surveillance studies addressing the types, purpose and level of antibiotic classes' administration in animals of the Mediterranean region are warranted in order to develop approaches that control the use of antibiotics while preserving animal's health. This is especially in Syria, Cyprus, Albania, Montenegro, Bosnia, Herzogovina, Monacco, Morocco, and Libya where even no data exists on the prevalence and epidemiology of multi-drug resistant organisms in animals.
The spread of multi-drug resistant organisms of animal origin is sparked by the concern of being transmitted to humans; these latter can then be causative agents for infections with limited therapeutic options (Bettiol and Harbarth, 2015). The transfer of resistant organisms from animals to humans can occur either via direct contact or indirectly via the consumption of under/uncooked animals products (Dahms et al., 2014). Recent studies have also highlighted the importance of the farms surrounding environment in the transmission chain. Air (von Salviati et al., 2015), dust (Blaak et al., 2015), contaminated waste waters (Guenther et al., 2011), and soil fertilized with animal manures (Laube et al., 2014) are all potential sources from which resistant organisms can be transferred to the general population. In their study, Olaitan et al. demonstrated the transfer of a colistin resistant E. coli strain from a pigs to its owner (Olaitan et al., 2015). This was documented by both strains (in the pig and its owner) having the same sequence types and sharing the same virulence as well as same PFGE patterns (Olaitan et al., 2015). The increased risk of ESBL fecal carriage in humans with frequent contact with broilers has been further taken as an evidence of transmission (Huijbers et al., 2014). Furthermore, sharing the same sequence types, virulence and PFGE patterns in addition to common plasmids/ESBL genes are all proofs for the possible transfer of resistant organisms and/or genes from the veterinary sector to the human population (Leverstein-van Hall et al., 2011). In Algeria, Djeffal et al. reported the detection of a common sequence type (ST15) in Salmonella spp producing ESBL isolated from both humans and avian isolates (Djeffal et al., 2017). In Egypt, Hamza et al. showed an abundance of carbapenemase genes namely blaOXA-48, blaKPC and blaNDM in chicken, drinking water, and farm workers suggesting a possible transmission of carbapenemase encoding genes from broilers to farmers and the surrounding environment (Hamza et al., 2016). Another study conducted in Italy reported the spread of a multi-drug resistant clone of “Salmonella enterica subsp. enterica serovar Infantis” that was first detected in 2011 in broiler farms and few years later led to human infections most likely via transmission from the broiler industry (Franco et al., 2015). In Spain, common blaCTX-M-grp1 and blaCTX-M-grp9 ESBL genes were detected in retail meat as well as in E. coli strains isolated from infected and colonized patients in the same region (Doi et al., 2010). In France, Hartmann et al. showed a clonal relationship among CTX-M carrying E. coli strains in cattle and farm cultivated soils (Hartmann et al., 2012). Another study in cattle, demonstrated that CTX-M-15 harboring plasmids in non-ST131 E. coli strains are highly similar to those detected in humans suggesting thus a multi-clonal plasmidic transmission of multi-drug resistant organisms from livestock to the humans (Madec et al., 2012). The detection of common genes and sequence types among animals and humans and the surrounding environment emphasizes the need to have a global intervention measures to avoid the dissemination of multi-drug resistance in the one health concept.
Conclusion
Antimicrobials have been used in veterinary medicine for more than 50 years. The use of antibiotics proved to be crucial for animal health by lowering mortality and incidence of diseases, in addition to controlling the transmission of infectious agents to the human population. Recently, the dissemination of ESBL, carbapenemase, and colistin resistant Gram negative bacteria in food producing animals brought into question the real efficacy of antibiotic administration in animals in terms of treatment, prophylaxis and growth promotion. Indeed, the emergence of MDR in food producing animals has been suggested to be largely linked to the over and misusage of antibiotics in veterinary medicine. The level of antibiotic consumption in animals varies between countries. Although, cephalosporins are not often prescribed in veterinary medicine, the use of other non-beta-lactams could account for the co-selection of multi-drug resistant bacteria. As shown in Table 1, ESBL and carbapenemase producers were frequently co-resistant to aminoglycosides, tetracyclines and fluoroquinolones, with these latter being mostly used in the veterinary field. Furthermore, the aforementioned antibiotics are classified by the World Health Organization as critically important antibiotics for human medicine that should be restricted in the animal field (Collignon et al., 2016). That said, the direct public health effect of the transmission of MDR bacteria from animals to humans is still controversial. Several studies have demonstrated a direct link of transmission between these two ecosystems. Resistant bacteria once transmitted to humans can be further selected by the over-use of antimicrobial agents in the clinical and community settings. This spread will promote the global dissemination of bacterial resistance across all ecosystems. The level of antibiotic consumption in animals in the European countries lining the Mediterranean is available in the European Surveillance of Veterinary Antimicrobial Consumption report (EMA/ESVAC, 2014), however this is not the case for the countries in North Africa and western Asia, where no accurate data are available. Therefore, surveillance studies investigating the levels of antibiotic prescription should be conducted in these areas. Antimicrobial prescriptions in animals should be re-considered and controlled to limit the spread of bacteria which are cross resistant to the antibiotics used in human medicine. In addition, a risk assessment of other factors contributing to the emergence of antimicrobial resistance in animals should be conducted in future studies. Poor sanitary conditions, overcrowding and poor infection control practices in animals are all possible contributors to the robust emergence of MDR in food-producing animals.
Author contributions
ID and SC wrote the review paper. ZD and J-MR corrected the manuscript. All authors approved and revised the final version of the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank TradOnline for English corrections.
Footnotes
Funding. This work was funded by the Lebanese Council for Research and the French Government under the Investissements d'avenir (Investments for the Future) program managed by the Agence Nationale de la Recherche (ANR, fr: National Agency for Research), (reference: Méditerranée Infection 10-IAHU-03).
References
- Abdallah H. M., Reuland E. A., Wintermans B. B., Al Naiemi N., Koek A., Abdelwahab A. M., et al. (2015). Extended-spectrum beta-lactamases and/or carbapenemases-producing enterobacteriaceae isolated from retail chicken meat in zagazig, egypt. PLoS ONE 10:e0136052. 10.1371/journal.pone.0136052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Moein K. A., Samir A. (2014). Occurrence of extended spectrum beta-lactamase-producing enterobacteriaceae among pet dogs and cats: An emerging public health threat outside health care facilities. Am. J. Infect. Control 42, 796–798. 10.1016/j.ajic.2014.03.020 [DOI] [PubMed] [Google Scholar]
- Abreu R., Castro B., Espigares E., Rodriguez-Alvarez C., Lecuona M., Moreno E., et al. (2014). Prevalence of CTX-M-type extended-spectrum beta-lactamases in escherichia coli strains isolated in poultry farms. Foodborne Pathog. Dis. 11, 868–873. 10.1089/fpd.2014.1796 [DOI] [PubMed] [Google Scholar]
- Accogli M., Fortini D., Giufre M., Graziani C., Dolejska M., Carattoli A., et al. (2013). IncI1 plasmids associated with the spread of CMY-2, CTX-M-1 and SHV-12 in Escherichia coli of animal and human origin. Clin. Microbiol. Infect. 19, E238–E240. 10.1111/1469-0691.12128 [DOI] [PubMed] [Google Scholar]
- Adler A., Sturlesi N., Fallach N., Zilberman-Barzilai D., Hussein O., Blum S. E., et al. (2015). Prevalence, risk factors, and transmission dynamics of extended-spectrum-beta-lactamase-producing enterobacteriaceae: a national survey of cattle farms in Israel in 2013. J. Clin. Microbiol. 53, 3515–3521. 10.1128/JCM.01915-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A. M., Shimamoto T. (2013). Molecular characterization of multidrug-resistant avian pathogenic Escherichia coli isolated from septicemic broilers. Int. J. Med. Microbiol. 303, 475–483. 10.1016/j.ijmm.2013.06.009 [DOI] [PubMed] [Google Scholar]
- Ahmed A. M., Shimamoto T. (2015). Molecular analysis of multidrug resistance in shiga toxin-producing Escherichia coli O157:H7 isolated from meat and dairy products. Int. J. Food Microbiol. 193:68–73. 10.1016/j.ijfoodmicro.2014.10.014 [DOI] [PubMed] [Google Scholar]
- Ahmed A. M., Younis E. E., Osman S. A., Ishida Y., El-Khodery S. A., Shimamoto T. (2009). Genetic analysis of antimicrobial resistance in Escherichia coli isolated from diarrheic neonatal calves. Vet. Microbiol. 136, 397–402. 10.1016/j.vetmic.2008.11.021 [DOI] [PubMed] [Google Scholar]
- Al Bayssari C., Dabboussi F., Hamze M., Rolain J. M. (2015a). Emergence of carbapenemase-producing Pseudomonas aeruginosa and Acinetobacter baumannii in livestock animals in lebanon. J. Antimicrob. Chemother. 70, 950–951. 10.1093/jac/dku469 [DOI] [PubMed] [Google Scholar]
- Al Bayssari C., Olaitan A. O., Dabboussi F., Hamze M., Rolain J. M. (2015b). Emergence of OXA-48-producing Escherichia coli clone ST38 in fowl. Antimicrob. Agents Chemother. 59, 745–746. 10.1128/AAC.03552-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso C. A., Gonzalez-Barrio D., Tenorio C., Ruiz-Fons F., Torres C. (2016). Antimicrobial resistance in faecal escherichia coli isolates from farmed red deer and wild small mammals. Detection of a multiresistant E. coli producing extended-spectrum beta-lactamase. Comp Immunol Microbiol Infect Dis 45:34–39. 10.1016/j.cimid.2016.02.003 [DOI] [PubMed] [Google Scholar]
- Al-Tawfiq J. A., Laxminarayan R., Mendelson M. (2017). How should we respond to the emergence of plasmid-mediated colistin resistance in humans and animals? Int. J. Infect. Dis. 54:77–84. 10.1016/j.ijid.2016.11.415 [DOI] [PubMed] [Google Scholar]
- Antunes P., Mourao J., Campos J., Peixe L. (2016). Salmonellosis: the role of poultry meat. Clin. Microbiol. Infect. 22, 110–121. 10.1016/j.cmi.2015.12.004 [DOI] [PubMed] [Google Scholar]
- Bachiri T., Bakour S., Ladjouzi R., Thongpan L., Rolain J. M., Touati A. (2017). High rates of CTX-M-15-producing Escherichia coli and Klebsiella pneumoniae in wild boars and barbary macaques in algeria. J. Glob. Antimicrob. Resist. 8, 35–40. 10.1016/j.jgar.2016.10.005 [DOI] [PubMed] [Google Scholar]
- Bagge E., Lewerin S. S., Johansson K. E. (2009). Detection and identification by PCR of Clostridium chauvoei in clinical isolates, bovine faeces and substrates from biogas plant. Acta Vet. Scand. 51:8. 10.1186/1751-0147-51-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron S., Hadjadj L., Rolain J. M., Olaitan A. O. (2016). Molecular mechanisms of polymyxin resistance: knowns and unknowns. Int. J. Antimicrob. Agents 48, 583–591. 10.1016/j.ijantimicag.2016.06.023 [DOI] [PubMed] [Google Scholar]
- Barton M. D. (2014). Impact of antibiotic use in the swine industry. Curr. Opin. Microbiol. 19, 9–15. 10.1016/j.mib.2014.05.017 [DOI] [PubMed] [Google Scholar]
- Belmahdi M., Bakour S., Al Bayssari C., Touati A., Rolain J. M. (2016). Molecular characterisation of extended-spectrum beta-lactamase- and plasmid AmpC-producing escherichia coli strains isolated from broilers in bejaia, algeria. J. Glob. Antimicrob. Resist. 6, 108–112. 10.1016/j.jgar.2016.04.006 [DOI] [PubMed] [Google Scholar]
- Belmar Campos C., Fenner I., Wiese N., Lensing C., Christner M., Rohde H., et al. (2014). Prevalence and genotypes of extended spectrum beta-lactamases in enterobacteriaceae isolated from human stool and chicken meat in hamburg, germany. Int. J. Med. Microbiol. 304, 678–684. 10.1016/j.ijmm.2014.04.012 [DOI] [PubMed] [Google Scholar]
- Ben Sallem R., Ben Slama K., Saenz Y., Rojo-Bezares B., Estepa V., Jouini A., et al. (2012). Prevalence and characterization of extended-spectrum beta-lactamase (ESBL)- and CMY-2-producing Escherichia coli isolates from healthy food-producing animals in tunisia. Foodborne Pathog. Dis. 9, 1137–1142. 10.1089/fpd.2012.1267 [DOI] [PubMed] [Google Scholar]
- Ben Slama K., Jouini A., Ben Sallem R., Somalo S., Saenz Y., Estepa V., et al. (2010). Prevalence of broad-spectrum cephalosporin-resistant escherichia coli isolates in food samples in tunisia, and characterization of integrons and antimicrobial resistance mechanisms implicated. Int. J. Food Microbiol. 137, 281–286. 10.1016/j.ijfoodmicro.2009.12.003 [DOI] [PubMed] [Google Scholar]
- Bettiol E., Harbarth S. (2015). Development of new antibiotics: taking off finally? Swiss Med. Wkly. 145:w14167. 10.4414/smw.2015.14167 [DOI] [PubMed] [Google Scholar]
- Blaak H., van Hoek A. H., Hamidjaja R. A., van der Plaats R. Q., Kerkhof-de Heer L., de Roda Husman A. M., et al. (2015). Distribution, numbers, and diversity of ESBL-producing E. coli in the poultry farm environment. PLoS ONE 10:e0135402. 10.1371/journal.pone.0135402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc V., Mesa R., Saco M., Lavilla S., Prats G., Miro E., et al. (2006). ESBL- and plasmidic class C beta-lactamase-producing E. coli strains isolated from poultry, pig and rabbit farms. Vet Microbiol 118, 299–304. 10.1016/j.vetmic.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Bogaerts P., Huang T. D., Bouchahrouf W., Bauraing C., Berhin C., El Garch F., et al. (2015). Characterization of ESBL- and AmpC-producing enterobacteriaceae from diseased companion animals in europe. Microb. Drug Resist. 21, 643–650. 10.1089/mdr.2014.0284 [DOI] [PubMed] [Google Scholar]
- Bonnedahl J., Drobni M., Gauthier-Clerc M., Hernandez J., Granholm S., Kayser Y., et al. (2009). Dissemination of Escherichia coli with CTX-M type ESBL between humans and yellow-legged gulls in the south of france. PLoS ONE 4:e5958. 10.1371/journal.pone.0005958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolaia V., Guardabassi L., Trevisani M., Bisgaard M., Venturi L., Bojesen A. M. (2010). High diversity of extended-spectrum beta-lactamases in Escherichia coli isolates from Italian broiler flocks. Antimicrob. Agents Chemother. 54, 1623–1626. 10.1128/AAC.01361-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmi S., Dunyach-Remy C., Touati A., Lavigne J. P. (2015). CTX-M-15-producing Escherichia coli and the pandemic clone O25b-ST131 isolated from wild fish in mediterranean sea. Clin. Microbiol. Infect. 21, e18–e20. 10.1016/j.cmi.2014.09.019 [DOI] [PubMed] [Google Scholar]
- Brahmi S., Touati A., Cadiere A., Djahmi N., Pantel A., Sotto A., et al. (2016). First description of two sequence type 2 Acinetobacter baumannii isolates carrying OXA-23 carbapenemase in pagellus acarne fished from the mediterranean sea near bejaia, algeria. Antimicrob. Agents Chemother. 60, 2513–2515. 10.1128/AAC.02384-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S. D., Ahmed M. F., El-Adawy H., Hotzel H., Engelmann I., Weiss D., et al. (2016). Surveillance of extended-spectrum beta-lactamase-producing Escherichia coli in dairy cattle farms in the nile delta, egypt. Front. Microbiol. 7:1020. 10.3389/fmicb.2016.01020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevali C., Morganti M., Scaltriti E., Bolzoni L., Pongolini S., Casadei G. (2016). Occurrence of mcr-1 in colistin-resistant Salmonella enterica isolates recovered from humans and animals in italy, 2012 to 2015. Antimicrob. Agents Chemother. 60, 7532–7534. 10.1128/AAC.01803-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J., Mateu E., Mejia W., Martin M. (2007). Factors associated with routine mass antimicrobial usage in fattening pig units in a high pig-density area. Vet. Res. 38, 481–492. 10.1051/vetres:2007010 [DOI] [PubMed] [Google Scholar]
- Catry B., Cavaleri M., Baptiste K., Grave K., Grein K., Holm A., et al. (2015). Use of colistin-containing products within the european union and european economic area (EU/EEA): Development of resistance in animals and possible impact on human and animal health. Int. J. Antimicrob. Agents 46, 297–306. 10.1016/j.ijantimicag.2015.06.005 [DOI] [PubMed] [Google Scholar]
- Chaalal W., Chaalal N., Bakour S., Kihal M., Rolain J. M. (2016). First occurrence of NDM-1 in Acinetobacter baumannii ST85 isolated from algerian dairy farms. J. Glob. Antimicrob. Resist. 7, 150–151. 10.1016/j.jgar.2016.09.002 [DOI] [PubMed] [Google Scholar]
- Chabou S., Leulmi H., Davoust B., Aouadi A., Rolain J. M. (2017). Prevalence of extended-spectrum beta-lactamase and carbapenemase-encoding genes in poultry feces from algeria and marseille, france. J. Glob. Antimicrob. Resist. 13, 28–32. 10.1016/j.jgar.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Chiaretto G., Zavagnin P., Bettini F., Mancin M., Minorello C., Saccardin C., et al. (2008). Extended spectrum beta-lactamase SHV-12-producing Salmonella from poultry. Vet. Microbiol. 128, 406–413. 10.1016/j.vetmic.2007.10.016 [DOI] [PubMed] [Google Scholar]
- Choi D., Chon J. W., Kim H. S., Kim D. H., Lim J. S., Yim J. H., et al. (2015). Incidence, antimicrobial resistance, and molecular characteristics of nontyphoidal Salmonella including extended-spectrum beta-lactamase producers in retail chicken meat. J. Food Prot. 78, 1932–1937. 10.4315/0362-028X.JFP-15-145 [DOI] [PubMed] [Google Scholar]
- Collignon P. C., Conly J. M., Andremont A., McEwen S. A., Aidara-Kane A., World Health Organization Advisory Group Bogota Meeting on Integrated Surveillance of Antimicrobial Resistance (WHO-AGISAR) et al. (2016). World health organization ranking of antimicrobials according to their importance in human medicine: A critical step for developing risk management strategies to control antimicrobial resistance from food animal production. Clin. Infect. Dis. 63, 1087–1093. 10.1093/cid/ciw475 [DOI] [PubMed] [Google Scholar]
- Conen A., Frei R., Adler H., Dangel M., Fux C. A., Widmer A. F. (2015). Microbiological screening is necessary to distinguish carriers of plasmid-mediated AmpC beta-lactamase-producing enterobacteriaceae and extended-spectrum beta-lactamase (ESBL)-producing enterobacteriaceae because of clinical similarity. PLoS ONE 10:e0120688. 10.1371/journal.pone.0120688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés P., Blanc V., Mora A., Dahbi G., Blanco J. E., Blanco M., et al. (2010). Isolation and characterization of potentially pathogenic antimicrobial-resistant Escherichia coli strains from chicken and pig farms in spain. Appl. Environ. Microbiol. 76, 2799–2805. 10.1128/AEM.02421-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmen S., Haenni M., Chatre P., Madec J. Y. (2013a). Characterization of blaCTX-M IncFII plasmids and clones of Escherichia coli from pets in france. J. Antimicrob. Chemother. 68, 2797–2801. 10.1093/jac/dkt291 [DOI] [PubMed] [Google Scholar]
- Dahmen S., Metayer V., Gay E., Madec J. Y., Haenni M. (2013b). Characterization of extended-spectrum beta-lactamase (ESBL)-carrying plasmids and clones of enterobacteriaceae causing cattle mastitis in france. Vet. Microbiol. 162, 793–799. 10.1016/j.vetmic.2012.10.015 [DOI] [PubMed] [Google Scholar]
- Dahms C., Hubner N. O., Wilke F., Kramer A. (2014). Mini-review: epidemiology and zoonotic potential of multiresistant bacteria and Clostridium difficile in livestock and food. GMS Hyg. Infect. Control 9:Doc21. 10.3205/dgkh000241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahshan H., Abd-Elall A. M., Megahed A. M., Abd-El-Kader M. A., Nabawy E. E. (2015). Veterinary antibiotic resistance, residues, and ecological risks in environmental samples obtained from poultry farms, Egypt. Environ. Monit. Assess. 187:2. 10.1007/s10661-014-4218-3 [DOI] [PubMed] [Google Scholar]
- Dandachi I., Salem E. S., Dahdouh E., Azar E., El-Bazzal B., Rolain J. M., et al. (2018b). Prevalence and characterization of multi-drug-resistant gram-negative Bacilli Isolated from lebanese poultry: a nationwide study. Front. Microbiol 9:550. 10.3389/fmicb.2018.00550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandachi I., Thongpan L., Daoud Z., Rolain J. M. (2018a). First detection of mcr-1 plasmid mediated colistin resistant E. coli in Lebanese poultry. J. Glob. Antimicrob. Resist. 12, 137–138. 10.1016/j.jgar.2018.01.004 [DOI] [PubMed] [Google Scholar]
- de Jong A., Thomas V., Klein U., Marion H., Moyaert H., Simjee S., et al. (2013). Pan-european resistance monitoring programmes encompassing food-borne bacteria and target pathogens of food-producing and companion animals. Int. J. Antimicrob. Agents 41, 403–409. 10.1016/j.ijantimicag.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Delcour A. H. (2009). Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta 1794, 808–816. 10.1016/j.bbapap.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diab M., Hamze M., Madec J. Y., Haenni M. (2016). High prevalence of non-ST131 CTX-M-15-producing Escherichia coli in healthy cattle in lebanon. Microb. Drug Resist. 23, 261–266. 10.1089/mdr.2016.0019 [DOI] [PubMed] [Google Scholar]
- Dierikx C. M., van der Goot J. A., Smith H. E., Kant A., Mevius D. J. (2013). Presence of ESBL/AmpC-producing Escherichia coli in the broiler production pyramid: a descriptive study. PLoS ONE 8:e79005. 10.1371/journal.pone.0079005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierikx C. M., van Duijkeren E., Schoormans A. H., van Essen-Zandbergen A., Veldman K., Kant A., et al. (2012). Occurrence and characteristics of extended-spectrum-beta-lactamase- and AmpC-producing clinical isolates derived from companion animals and horses. J. Antimicrob. Chemother. 67, 1368–1374. 10.1093/jac/dks049 [DOI] [PubMed] [Google Scholar]
- Djeffal S., Bakour S., Mamache B., Elgroud R., Agabou A., Chabou S., et al. (2017). Prevalence and clonal relationship of ESBL-producing salmonella strains from humans and poultry in Northeastern Algeria. BMC Vet. Res. 13:132. 10.1186/s12917-017-1050-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi Y., Paterson D. L., Egea P., Pascual A., Lopez-Cerero L., Navarro M. D., et al. (2010). Extended-spectrum and CMY-type beta-lactamase-producing Escherichia coli in clinical samples and retail meat from Pittsburgh, USA and Seville, Spain. Clin. Microbiol. Infect. 16, 33–38. 10.1111/j.1469-0691.2009.03001.x [DOI] [PubMed] [Google Scholar]
- Donati V., Feltrin F., Hendriksen R. S., Svendsen C. A., Cordaro G., Garcia-Fernandez A., et al. (2014). Extended-spectrum-beta-lactamases, AmpC beta-lactamases and plasmid mediated quinolone resistance in Klebsiella spp. from companion animals in Italy. PLoS ONE 9:e90564. 10.1371/journal.pone.0090564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economou V., Gousia P. (2015). Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 8:49–61. 10.2147/IDR.S55778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (2017). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2016. EFSA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea P., Lopez-Cerero L., Torres E., Gomez-Sanchez Mdel C., Serrano L., Navarro Sanchez-Ortiz M. D., et al. (2012). Increased raw poultry meat colonization by extended spectrum beta-lactamase-producing Escherichia coli in the south of spain. Int. J. Food Microbiol. 159, 69–73. 10.1016/j.ijfoodmicro.2012.08.002 [DOI] [PubMed] [Google Scholar]
- El Garch F., Sauget M., Hocquet D., LeChaudee D., Woehrle F., Bertrand X. (2017). mcr-1 is borne by highly diverse Escherichia coli isolates since 2004 in food-producing animals in Europe. Clin. Microbiol. Infect. 23, 51.e1–51.e4. 10.1016/j.cmi.2016.08.033 [DOI] [PubMed] [Google Scholar]
- El Salabi A., Walsh T. R., Chouchani C. (2013). Extended spectrum beta-lactamases, carbapenemases and mobile genetic elements responsible for antibiotics resistance in gram-negative bacteria. Crit. Rev. Microbiol. 39, 113–122. 10.3109/1040841X.2012.691870 [DOI] [PubMed] [Google Scholar]
- Elhariri M., Hamza D., Elhelw R., Dorgham S. M. (2017). Extended-spectrum beta-lactamase-producing Pseudomonas aeruginosa in camel in egypt: Potential human hazard. Ann. Clin. Microbiol. Antimicrob. 16:21. 10.1186/s12941-017-0197-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shazly D. A., Nasef S. A., Mahmoud F. F., Jonas D. (2017). Expanded spectrum beta-lactamase producing Escherichia coli isolated from chickens with colibacillosis in Egypt. Poult. Sci. 96, 2375–2384. 10.3382/ps/pew493 [DOI] [PubMed] [Google Scholar]
- Escudero E., Vinue L., Teshager T., Torres C., Moreno M. A. (2010). Resistance mechanisms and farm-level distribution of fecal Escherichia coli isolates resistant to extended-spectrum cephalosporins in pigs in spain. Res. Vet. Sci. 88, 83–87. 10.1016/j.rvsc.2009.05.021 [DOI] [PubMed] [Google Scholar]
- European Medicines Agency European Surveillance Of Veterinary Antimicrobial Consumption (EMA/ESVAC). (2014). Sales of Veterinary Antimicrobial Agentsin 29 EU/EEA Countries in 2014. Sixth ESVAC Report. European MedicinesAgency; p. 1–174. [Google Scholar]
- Ewers C., Bethe A., Semmler T., Guenther S., Wieler L. H. (2012). Extended-spectrum beta-lactamase-producing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: a global perspective. Clin. Microbiol. Infect. 18, 646–655. 10.1111/j.1469-0691.2012.03850.x [DOI] [PubMed] [Google Scholar]
- Ewers C., Klotz P., Scheufen S., Leidner U., Gottig S., Semmler T. (2016). Genome sequence of OXA-23 producing Acinetobacter baumannii IHIT7853, a carbapenem-resistant strain from a cat belonging to international clone IC1. Gut Pathog. 8:37. 10.1186/s13099-016-0119-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A., Leekitcharoenphon P., Feltrin F., Alba P., Cordaro G., Iurescia M., et al. (2015). Emergence of a clonal lineage of multidrug-resistant ESBL-producing Salmonella infantis transmitted from broilers and broiler meat to humans in italy between 2011 and 2014. PLoS ONE 10:e0144802. 10.1371/journal.pone.0144802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghodousi A., Bonura C., Di Carlo P., van Leeuwen W. B., Mammina C. (2016). Extraintestinal pathogenic Escherichia coli sequence type 131 H30-R and H30-rx subclones in retail chicken meat, Italy. Int. J. Food Microbiol. 228, 10–13. 10.1016/j.ijfoodmicro.2016.04.004 [DOI] [PubMed] [Google Scholar]
- Ghodousi A., Bonura C., Di Noto A. M., Mammina C. (2015). Extended-spectrum ss-lactamase, AmpC-producing, and fluoroquinolone-resistant Escherichia coli in retail broiler chicken meat, italy. Foodborne Pathog. Dis. 12, 619–625. 10.1089/fpd.2015.1936 [DOI] [PubMed] [Google Scholar]
- Giedraitiene A., Vitkauskiene A., Naginiene R., Pavilonis A. (2011). Antibiotic resistance mechanisms of clinically important bacteria. Medicina (Kaunas). 47, 137–146. 10.3390/medicina47030019 [DOI] [PubMed] [Google Scholar]
- Giufrè M., Graziani C., Accogli M., Luzzi I., Busani L., Cerquetti M., et al. (2012). Escherichia coli of human and avian origin: detection of clonal groups associated with fluoroquinolone and multidrug resistance in italy. J. Antimicrob. Chemother. 67, 860–867. 10.1093/jac/dkr565 [DOI] [PubMed] [Google Scholar]
- González-Torralba A., Oteo J., Asenjo A., Bautista V., Fuentes E., Alós J. -I. (2016). Survey of carbapenemase-producing enterobacteriaceae in companion dogs in Madrid, Spain. Antimicrob. Agents Chemother. 60, 2499–2501. 10.1128/AAC.02383-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grami R., Dahmen S., Mansour W., Mehri W., Haenni M., Aouni M., et al. (2014). blaCTX-M-15-carrying F2:A-:B- plasmid in Escherichia coli from cattle milk in Tunisia. Microb. Drug Resist. 20, 344–349. 10.1089/mdr.2013.0160 [DOI] [PubMed] [Google Scholar]
- Grami R., Mansour W., Dahmen S., Mehri W., Haenni M., Aouni M., et al. (2013). The blaCTX-M-1 IncI1/ST3 plasmid is dominant in chickens and pets in tunisia. J. Antimicrob. Chemother. 68, 2950–2952. 10.1093/jac/dkt258 [DOI] [PubMed] [Google Scholar]
- Grami R., Mansour W., Mehri W., Bouallegue O., Boujaafar N., Madec J. Y., et al. (2016). Impact of food animal trade on the spread of mcr-1-mediated colistin resistance, Tunisia, July 2015. Euro Surveill 21, 30144. 10.2807/1560-7917.ES.2016.21.8.30144 [DOI] [PubMed] [Google Scholar]
- Guenther S., Ewers C., Wieler L. H. (2011). Extended-spectrum beta-lactamases producing E. coli in wildlife, yet another form of environmental pollution? Front. Microbiol. 2:246. 10.3389/fmicb.2011.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra B., Fischer J., Helmuth R. (2014). An emerging public health problem: acquired carbapenemase-producing microorganisms are present in food-producing animals, their environment, companion animals and wild birds. Vet. Microbiol. 171, 290–297. 10.1016/j.vetmic.2014.02.001 [DOI] [PubMed] [Google Scholar]
- Gundogan N., Citak S., Yalcin E. (2011). Virulence properties of extended spectrum beta-lactamase-producing Klebsiella species in meat samples. J. Food Prot. 74, 559–564. 10.4315/0362-028X.JFP-10-315 [DOI] [PubMed] [Google Scholar]
- Haenni M., Chatre P., Madec J. Y. (2014c). Emergence of Escherichia coli producing extended-spectrum AmpC beta-lactamases (ESAC) in animals. Front. Microbiol. 5:53. 10.3389/fmicb.2014.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenni M., Chatre P., Metayer V., Bour M., Signol E., Madec J. Y., et al. (2014a). Comparative prevalence and characterization of ESBL-producing enterobacteriaceae in dominant versus subdominant enteric flora in veal calves at slaughterhouse, france. Vet. Microbiol. 171, 321–327. 10.1016/j.vetmic.2014.02.023 [DOI] [PubMed] [Google Scholar]
- Haenni M., Metayer V., Gay E., Madec J. Y. (2016a). Increasing trends in mcr-1 prevalence among extended-spectrum-beta-lactamase-producing Escherichia coli isolates from French calves despite decreasing exposure to colistin. Antimicrob. Agents Chemother. 60, 6433–6434. 10.1128/AAC.01147-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenni M., Poirel L., Kieffer N., Chatre P., Saras E., Metayer V., et al. (2016b). Co-occurrence of extended spectrum beta-lactamase and MCR-1 encoding genes on plasmids. Lancet Infect. Dis. 16, 281–282. 10.1016/S1473-3099(16)00007-4 [DOI] [PubMed] [Google Scholar]
- Haenni M., Saras E., Metayer V., Medaille C., Madec J. Y. (2014b). High prevalence of blaCTX-M-1/IncI1/ST3 and blaCMY-2/IncI1/ST2 plasmids in healthy urban dogs in France. Antimicrob. Agents Chemother. 58, 5358–5362. 10.1128/AAC.02545-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenni M., Saras E., Ponsin C., Dahmen S., Petitjean M., Hocquet D., et al. (2016c). High prevalence of international ESBL CTX-M-15-producing Enterobacter cloacae ST114 clone in animals. J. Antimicrob. Chemother. 71, 1497–1500. 10.1093/jac/dkw006 [DOI] [PubMed] [Google Scholar]
- Hamza E., Dorgham S. M., Hamza D. A. (2016). Carbapenemase-producing Klebsiella pneumoniae in broiler poultry farming in Egypt. J. Glob. Antimicrob. Resist. 7, 8–10. 10.1016/j.jgar.2016.06.004 [DOI] [PubMed] [Google Scholar]
- Hao H., Cheng G., Iqbal Z., Ai X., Hussain H. I., Huang L., et al. (2014). Benefits and risks of antimicrobial use in food-producing animals. Front. Microbiol. 5:288. 10.3389/fmicb.2014.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A., Locatelli A., Amoureux L., Depret G., Jolivet C., Gueneau E., et al. (2012). Occurrence of CTX-M producing Escherichia coli in soils, cattle, and farm environment in france (burgundy region). Front. Microbiol. 3:83. 10.3389/fmicb.2012.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hérivaux A., Pailhories H., Quinqueneau C., Lemarie C., Joly-Guillou M. L., Ruvoen N., et al. (2016). First report of carbapenemase-producing Acinetobacter baumannii carriage in pets from the community in France. Int. J. Antimicrob. Agents 48, 220–221. 10.1016/j.ijantimicag.2016.03.012 [DOI] [PubMed] [Google Scholar]
- Hernandez J., Johansson A., Stedt J., Bengtsson S., Porczak A., Granholm S., et al. (2013). Characterization and comparison of extended-spectrum beta-lactamase (ESBL) resistance genotypes and population structure of Escherichia coli isolated from Franklin's gulls (Leucophaeus pipixcan) and humans in Chile. PLoS ONE 8:e76150. 10.1371/journal.pone.0076150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández M., Iglesias M. R., Rodriguez-Lazaro D., Gallardo A., Quijada N., Miguela-Villoldo P., et al. (2017). Co-occurrence of colistin-resistance genes mcr-1 and mcr-3 among multidrug-resistant Escherichia coli isolated from cattle, spain, september 2015. Euro Surveill 22:31. 10.2807/1560-7917.ES.2017.22.31.30586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J., Wan W., Mao D., Wang C., Mu Q., Qin S., et al. (2015). Occurrence and distribution of sulfonamides, tetracyclines, quinolones, macrolides, and nitrofurans in livestock manure and amended soils of Northern China. Environ. Sci. Pollut. Res. Int. 22, 4545–4554. 10.1007/s11356-014-3632-y [DOI] [PubMed] [Google Scholar]
- Hruby C. E., Soupir M. L., Moorman T. B., Shelley M., Kanwar R. S. (2016). Effects of tillage and poultry manure application rates on Salmonella and fecal indicator bacteria concentrations in tiles draining des moines lobe soils. J. Environ. Manage. 171:60–69. 10.1016/j.jenvman.2016.01.040 [DOI] [PubMed] [Google Scholar]
- Huijbers P. M., Graat E. A., Haenen A. P., van Santen M. G., van Essen-Zandbergen A., Mevius D. J., et al. (2014). Extended-spectrum and AmpC beta-lactamase-producing Escherichia coli in broilers and people living and/or working on broiler farms: prevalence, risk factors and molecular characteristics. J. Antimicrob. Chemother. 69, 2669–2675. 10.1093/jac/dku178 [DOI] [PubMed] [Google Scholar]
- Huijbers P. M., Graat E. A., van Hoek A. H., Veenman C., de Jong M. C., van Duijkeren E. (2016). Transmission dynamics of extended-spectrum beta-lactamase and AmpC beta-lactamase-producing Escherichia coli in a broiler flock without antibiotic use. Prev. Vet. Med. 131:12–19. 10.1016/j.prevetmed.2016.07.001 [DOI] [PubMed] [Google Scholar]
- Jamborova I., Dolejska M., Vojtech J., Guenther S., Uricariu R., Drozdowska J., et al. (2015). Plasmid-mediated resistance to cephalosporins and fluoroquinolones in various Escherichia coli sequence types isolated from rooks wintering in europe. Appl. Environ. Microbiol. 81, 648–657. 10.1128/AEM.02459-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouini A., Slama K. B., Klibi N., Sallem R. B., Estepa V., Vinue L., et al. (2013). Lineages and virulence gene content among extended-spectrum beta-lactamase-producing Escherichia coli strains of food origin in Tunisia. J. Food Prot. 76, 323–327. 10.4315/0362-028X.JFP-12-251 [DOI] [PubMed] [Google Scholar]
- Jouini A., Vinue L., Slama K. B., Saenz Y., Klibi N., Hammami S., et al. (2007). Characterization of CTX-M and SHV extended-spectrum beta-lactamases and associated resistance genes in Escherichia coli strains of food samples in Tunisia. J. Antimicrob. Chemother. 60, 1137–1141. 10.1093/jac/dkm316 [DOI] [PubMed] [Google Scholar]
- Kempf I., Fleury M. A., Drider D., Bruneau M., Sanders P., Chauvin C., et al. (2013). What do we know about resistance to colistin in Enterobacteriaceae in avian and pig production in Europe? Int. J. Antimicrob. Agents 42, 379–383. 10.1016/j.ijantimicag.2013.06.012 [DOI] [PubMed] [Google Scholar]
- Khalifa H. O., Ahmed A. M., Oreiby A. F., Eid A. M., Shimamoto T. (2016). Characterisation of the plasmid-mediated colistin resistance gene mcr-1 in Escherichia coli isolated from animals in Egypt. Int. J. Antimicrob. Agents 47, 413–414. 10.1016/j.ijantimicag.2016.02.011 [DOI] [PubMed] [Google Scholar]
- Kilani H., Abbassi M. S., Ferjani S., Mansouri R., Sghaier S., Ben Salem R., et al. (2015). Occurrence of bla CTX-M-1, qnrB1 and virulence genes in avian ESBL-producing Escherichia coli isolates from tunisia. Front. Cell. Infect. Microbiol. 5:38. 10.3389/fcimb.2015.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. A. (2005). Bacterial resistance to antibiotics: modified target sites. Adv. Drug Deliv. Rev. 57, 1471–1485. 10.1016/j.addr.2005.04.003 [DOI] [PubMed] [Google Scholar]
- Laube H., Friese A., von Salviati C., Guerra B., Rosler U. (2014). Transmission of ESBL/AmpC-producing Escherichia coli from broiler chicken farms to surrounding areas. Vet. Microbiol. 172, 519–527. 10.1016/j.vetmic.2014.06.008 [DOI] [PubMed] [Google Scholar]
- Leverstein-van Hall M. A., Dierikx C. M., Cohen Stuart J., Voets G. M., van den Munckhof M. P., van Essen-Zandbergen A., et al. (2011). Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin. Microbiol. Infect. 17, 873–880. 10.1111/j.1469-0691.2011.03497.x [DOI] [PubMed] [Google Scholar]
- Liakopoulos A., Betts J., La Ragione R., van Essen-Zandbergen A., Ceccarelli D., Petinaki E., et al. (2018). Occurrence and characterization of extended-spectrum cephalosporin-resistant enterobacteriaceae in healthy household dogs in Greece. J. Med. Microbiol. 67, 931–935. 10.1099/jmm.0.000768 [DOI] [PubMed] [Google Scholar]
- Lima Barbieri N., Nielsen D. W., Wannemuehler Y., Cavender T., Hussein A., Yan S. G., et al. (2017). Mcr-1 identified in avian pathogenic Escherichia coli (APEC). PLoS ONE 12:e0172997. 10.1371/journal.pone.0172997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Y., Wang Y., Walsh T. R., Yi L. X., Zhang R., Spencer J., et al. (2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16, 161–168. 10.1016/S1473-3099(15)00424-7 [DOI] [PubMed] [Google Scholar]
- Maamar E., Alonso C. A., Hamzaoui Z., Dakhli N., Abbassi M. S., Ferjani S., et al. (2018). Emergence of plasmid-mediated colistin-resistance in CMY-2-producing Escherichia coli of lineage ST2197 in a Tunisian poultry farm. Int. J. Food Microbiol. 269:60–63. 10.1016/j.ijfoodmicro.2018.01.017 [DOI] [PubMed] [Google Scholar]
- Maamar E., Hammami S., Alonso C. A., Dakhli N., Abbassi M. S., Ferjani S., et al. (2016). High prevalence of extended-spectrum and plasmidic AmpC beta-lactamase-producing Escherichia coli from poultry in Tunisia. Int. J. Food Microbiol. 231:69–75. 10.1016/j.ijfoodmicro.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Maciuca I. E., Williams N. J., Tuchilus C., Dorneanu O., Guguianu E., Carp-Carare C., et al. (2015). High prevalence of Escherichia coli-producing CTX-M-15 extended-spectrum beta-lactamases in poultry and human clinical isolates in Romania. Microb. Drug Resist. 21, 651–662. 10.1089/mdr.2014.0248 [DOI] [PubMed] [Google Scholar]
- Madec J. Y., Poirel L., Saras E., Gourguechon A., Girlich D., Nordmann P., et al. (2012). Non-ST131 Escherichia coli from cattle harbouring human-like bla(CTX-M-15)-carrying plasmids. J. Antimicrob. Chemother. 67, 578–581. 10.1093/jac/dkr542 [DOI] [PubMed] [Google Scholar]
- Maravić A., Skocibusic M., Samanic I., Fredotovic Z., Cvjetan S., Jutronic M., et al. (2013). Aeromonas spp. simultaneously harbouring bla(CTX-M-15), bla(SHV-12), bla(PER-1) and bla(FOX-2), in wild-growing mediterranean mussel (Mytilus galloprovincialis) from Adriatic Sea, Croatia. Int J Food Microbiol 166, 301–308. 10.1016/j.ijfoodmicro.2013.07.010 [DOI] [PubMed] [Google Scholar]
- Martínez-Martínez L., Gonzalez-Lopez J. J. (2014). Carbapenemases in Enterobacteriaceae: types and molecular epidemiology. Enferm. Infecc. Microbiol. Clin. 32(Suppl. 4), 4–9. 10.1016/S0213-005X(14)70168-5 [DOI] [PubMed] [Google Scholar]
- Meguenni N., Le Devendec L., Jouy E., Le Corvec M., Bounar-Kechih S., Rabah Bakour D., et al. (2015). First description of an extended-spectrum cephalosporin- and fluoroquinolone- resistant avian pathogenic Escherichia coli clone in Algeria. Avian Dis. 59, 20–23. 10.1637/10804-022414-Reg.1 [DOI] [PubMed] [Google Scholar]
- Melo L. C., Boisson M. N., Saras E., Medaille C., Boulouis H. J., Madec J. Y., et al. (2017). OXA-48-producing ST372 Escherichia coli in a French dog. J. Antimicrob. Chemother. 72, 1256–1258. 10.1093/jac/dkw531 [DOI] [PubMed] [Google Scholar]
- Mesa R. J., Blanc V., Blanch A. R., Cortes P., Gonzalez J. J., Lavilla S., et al. (2006). Extended-spectrum beta-lactamase-producing Enterobacteriaceae in different environments (humans, food, animal farms and sewage). J. Antimicrob. Chemother. 58, 211–215. 10.1093/jac/dkl211 [DOI] [PubMed] [Google Scholar]
- Meunier D., Jouy E., Lazizzera C., Kobisch M., Madec J. Y. (2006). CTX-M-1- and CTX-M-15-type beta-lactamases in clinical Escherichia coli isolates recovered from food-producing animals in France. Int. J. Antimicrob. Agents 28, 402–407. 10.1016/j.ijantimicag.2006.08.016 [DOI] [PubMed] [Google Scholar]
- Mezhoud H., Boyen F., Touazi L. H., Garmyn A., Moula N., Smet A., et al. (2015). Extended spectrum beta-lactamase producing Escherichia coli in broiler breeding roosters: presence in the reproductive tract and effect on sperm motility. Anim. Reprod. Sci. 159, 205–211. 10.1016/j.anireprosci.2015.06.021 [DOI] [PubMed] [Google Scholar]
- Mnif B., Ktari S., Rhimi F. M., Hammami A. (2012). Extensive dissemination of CTX-M-1- and CMY-2-producing Escherichia coli in poultry farms in tunisia. Lett. Appl. Microbiol. 55, 407–413. 10.1111/j.1472-765X.2012.03309.x [DOI] [PubMed] [Google Scholar]
- Monte D. F., Mem A., Fernandes M. R., Cerdeira L., Esposito F., Galvao J. A., et al. (2017). Chicken meat as a reservoir of colistin-resistant Escherichia coli strains carrying mcr-1 genes in South America. Antimicrob. Agents Chemother. 61, e02718– e02716. 10.1128/AAC.02718-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora A., Herrera A., Mamani R., Lopez C., Alonso M. P., Blanco J. E., et al. (2010). Recent emergence of clonal group O25b:K1:H4-B2-ST131 ibeA strains among Escherichia coli poultry isolates, including CTX-M-9-producing strains, and comparison with clinical human isolates. Appl. Environ. Microbiol. 76, 6991–6997. 10.1128/AEM.01112-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morakchi H., Loucif L., Gacemi-Kirane D., Rolain J. M. (2017). Molecular characterisation of carbapenemases in urban pigeon droppings in France and Algeria. J Glob Antimicrob Resist 9:103–110. 10.1016/j.jgar.2017.02.010 [DOI] [PubMed] [Google Scholar]
- Moreno M. A., Teshager T., Porrero M. A., Garcia M., Escudero E., Torres C., et al. (2007). Abundance and phenotypic diversity of Escherichia coli isolates with diminished susceptibility to expanded-spectrum cephalosporins in faeces from healthy food animals after slaughter. Vet. Microbiol. 120, 363–369. 10.1016/j.vetmic.2006.10.032 [DOI] [PubMed] [Google Scholar]
- Nebbia P., Tramuta C., Odore R., Nucera D., Zanatta R., Robino P. (2014). Genetic and phenotypic characterisation of Escherichia coli producing cefotaximase-type extended-spectrum beta-lactamases: first evidence of the ST131 clone in cats with urinary infections in Italy. J. Feline Med. Surg. 16, 966–971. 10.1177/1098612X14527103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T. M., Rogers T. L., Brown M. V. (2013). The gut bacterial community of mammals from marine and terrestrial habitats. PLoS ONE 8:e83655. 10.1371/journal.pone.0083655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V. T., Carrique-Mas J. J., Ngo T. H., Ho H. M., Ha T. T., Campbell J. I., et al. (2015). Prevalence and risk factors for carriage of antimicrobial-resistant Escherichia coli on household and small-scale chicken farms in the mekong delta of Vietnam. J. Antimicrob. Chemother. 70, 2144–2152. 10.1093/jac/dkv053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O., Borjesson S., Landen A., Bengtsson B. (2014). Vertical transmission of Escherichia coli carrying plasmid-mediated AmpC (pAmpC) through the broiler production pyramid. J. Antimicrob. Chemother. 69, 1497–1500. 10.1093/jac/dku030 [DOI] [PubMed] [Google Scholar]
- Nyberg K. A., Ottoson J. R., Vinneras B., Albihn A. (2014). Fate and survival of Salmonella typhimurium and Escherichia coli O157:H7 in repacked soil lysimeters after application of cattle slurry and human urine. J. Sci. Food Agric. 94, 2541–2546. 10.1002/jsfa.6593 [DOI] [PubMed] [Google Scholar]
- Ojer-Usoz E., Gonzalez D., Vitas A. I., Leiva J., Garcia-Jalon I., Febles-Casquero A., et al. (2013). Prevalence of extended-spectrum beta-lactamase-producing Enterobacteriaceae in meat products sold in navarra, spain. Meat Sci. 93, 316–321. 10.1016/j.meatsci.2012.09.009 [DOI] [PubMed] [Google Scholar]
- Olaitan A. O., Chabou S., Okdah L., Morand S., Rolain J. M. (2016). Dissemination of the mcr-1 colistin resistance gene. Lancet Infect. Dis. 16, 289–290. 10.1016/S1473-3099(16)00067-0 [DOI] [PubMed] [Google Scholar]
- Olaitan A. O., Diene S. M., Kempf M., Berrazeg M., Bakour S., Gupta S. K., et al. (2014b). Worldwide emergence of colistin resistance in Klebsiella pneumoniae from healthy humans and patients in lao PDR, thailand, israel, nigeria and france owing to inactivation of the PhoP/PhoQ regulator mgrB: an epidemiological and molecular study. Int. J. Antimicrob. Agents 44, 500–507. 10.1016/j.ijantimicag.2014.07.020 [DOI] [PubMed] [Google Scholar]
- Olaitan A. O., Li J. (2016). Emergence of polymyxin resistance in gram-negative bacteria. Int. J. Antimicrob. Agents 48, 581–582. 10.1016/j.ijantimicag.2016.11.003 [DOI] [PubMed] [Google Scholar]
- Olaitan A. O., Morand S., Rolain J. M. (2014a). Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front. Microbiol. 5:643. 10.3389/fmicb.2014.00643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaitan A. O., Thongmalayvong B., Akkhavong K., Somphavong S., Paboriboune P., Khounsy S., et al. (2015). Clonal transmission of a colistin-resistant Escherichia coli from a domesticated pig to a human in laos. J. Antimicrob. Chemother. 70, 3402–3404. 10.1093/jac/dkv252 [DOI] [PubMed] [Google Scholar]
- Olsen R. H., Bisgaard M., Lohren U., Robineau B., Christensen H. (2014). Extended-spectrum beta-lactamase-producing Escherichia coli isolated from poultry: a review of current problems, illustrated with some laboratory findings. Avian Pathol. 43, 199–208. 10.1080/03079457.2014.907866 [DOI] [PubMed] [Google Scholar]
- Pehlivanlar Onen S., Aslantas O., Sebnem Yilmaz E., Kurekci C. (2015). Prevalence of beta-lactamase producing Escherichia coli from retail meat in turkey. J. Food Sci. 80, M2023–M2029. 10.1111/1750-3841.12984 [DOI] [PubMed] [Google Scholar]
- Perrin-Guyomard A., Bruneau M., Houee P., Deleurme K., Legrandois P., Poirier C., et al. (2016). Prevalence of mcr-1 in commensal Escherichia coli from french livestock, 2007 to 2014. Euro. Surveill 21, 1–3. 10.2807/1560-7917.ES.2016.21.6.30135 [DOI] [PubMed] [Google Scholar]
- Poirel L., Bercot B., Millemann Y., Bonnin R. A., Pannaux G., Nordmann P. (2012). Carbapenemase-producing Acinetobacter spp. in cattle, France. Emerg. Infect. Dis. 18, 523–525. 10.3201/eid1803.111330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirel L., Bernabeu S., Fortineau N., Podglajen I., Lawrence C., Nordmann P. (2011). Emergence of OXA-48-producing Escherichia coli clone ST38 in France. Antimicrob. Agents Chemother. 55, 4937–4938. 10.1128/AAC.00413-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirel L., Jayol A., Nordmann P. (2017). Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin. Microbiol. Rev. 30, 557–596. 10.1128/CMR.00064-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirel L., Nordmann P., Ducroz S., Boulouis H. J., Arne P., Millemann Y. (2013). Extended-spectrum beta-lactamase CTX-M-15-producing Klebsiella pneumoniae of sequence type ST274 in companion animals. Antimicrob. Agents Chemother. 57, 2372–2375. 10.1128/AAC.02622-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi L., Tassios P. T., Lambiri M., Kansouzidou A., Pasiotou M., Vatopoulos A. C., et al. (2005). Repeated occurrence of diverse extended-spectrum beta-lactamases in minor serotypes of food-borne Salmonella enterica subsp. enterica. J. Clin. Microbiol. 43, 3453–3456. 10.1128/JCM.43.7.3453-3456.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomba C., Rantala M., Greko C., Baptiste K. E., Catry B., van Duijkeren E., et al. (2017). Public health risk of antimicrobial resistance transfer from companion animals. J. Antimicrob. Chemother. 72, 957–968. 10.1093/jac/dkw481 [DOI] [PubMed] [Google Scholar]
- Pulss S., Semmler T., Prenger-Berninghoff E., Bauerfeind R., Ewers C. (2017). First report of an Escherichia coli strain from swine carrying an OXA-181 carbapenemase and the colistin resistance determinant MCR-1. Int. J. Antimicrob. Agents 50, 232–236. 10.1016/j.ijantimicag.2017.03.014 [DOI] [PubMed] [Google Scholar]
- Qabajah M., Awwad E., Ashhab Y. (2014). Molecular characterisation of Escherichia coli from dead broiler chickens with signs of colibacillosis and ready-to-market chicken meat in the west bank. Br. Poult. Sci. 55, 442–451. 10.1080/00071668.2014.935998 [DOI] [PubMed] [Google Scholar]
- Quesada A., Ugarte-Ruiz M., Iglesias M. R., Porrero M. C., Martinez R., Florez-Cuadrado D., et al. (2016). Detection of plasmid mediated colistin resistance (MCR-1) in Escherichia coli and Salmonella enterica isolated from poultry and swine in Spain. Res. Vet. Sci. 105, 134–135. 10.1016/j.rvsc.2016.02.003 [DOI] [PubMed] [Google Scholar]
- Rafei R., Hamze M., Pailhories H., Eveillard M., Marsollier L., Joly-Guillou M. L., et al. (2015). Extrahuman epidemiology of Acinetobacter baumannii in Lebanon. Appl. Environ. Microbiol. 81, 2359–2367. 10.1128/AEM.03824-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich F., Atanassova V., Klein G. (2013). Extended-spectrum beta-lactamase- and AmpC-producing enterobacteria in healthy broiler chickens, Germany. Emerging Infect. Dis. 19, 1253–1259. 10.3201/eid1908.120879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhouma M., Bessalah S., Salhi I., Theriault W., Fairbrother J. M., Fravalo P. (2018). Screening for fecal presence of colistin-resistant Escherichia coli and mcr-1 and mcr-2 genes in camel-calves in southern Tunisia. Acta Vet. Scand. 60:35. 10.1186/s13028-018-0389-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaño I., Moreno M. A., Teshager T., Saenz Y., Dominguez L., Torres C. (2006). Detection and characterization of extended-spectrum beta-lactamases in Salmonella enterica strains of healthy food animals in Spain. J. Antimicrob. Chemother. 58, 844–847. 10.1093/jac/dkl337 [DOI] [PubMed] [Google Scholar]
- Rolain J. M. (2013). Food and human gut as reservoirs of transferable antibiotic resistance encoding genes. Front. Microbiol. 4:173. 10.3389/fmicb.2013.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. E., Pitout J. D. (2014). Extended-spectrum beta-lactamase, carbapenemase and AmpC-producing Enterobacteriaceae in companion animals. Vet. Microbiol. 170, 10–18. 10.1016/j.vetmic.2014.01.017 [DOI] [PubMed] [Google Scholar]
- Sallem R. B., Gharsa H., Slama K. B., Rojo-Bezares B., Estepa V., Porres-Osante N., et al. (2013). First detection of CTX-M-1, CMY-2, and QnrB19 resistance mechanisms in fecal Escherichia coli isolates from healthy pets in Tunisia. Vector Borne Zoonotic Dis. 13, 98–102. 10.1089/vbz.2012.1047 [DOI] [PubMed] [Google Scholar]
- Schultz E., Cloeckaert A., Doublet B., Madec J. Y., Haenni M. (2017). Detection of SGI1/PGI1 elements and resistance to extended-spectrum cephalosporins in proteae of animal origin in France. Front. Microbiol. 8:32. 10.3389/fmicb.2017.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott Weese J. (2008). Antimicrobial resistance in companion animals. Anim. Health Res. Rev. 9, 169–176. 10.1017/S1466252308001485 [DOI] [PubMed] [Google Scholar]
- Solà-Ginés M., Cameron-Veas K., Badiola I., Dolz R., Majo N., Dahbi G., et al. (2015b). Diversity of multi-drug resistant avian pathogenic Escherichia coli (APEC) causing outbreaks of colibacillosis in broilers during 2012 in Spain. PLoS ONE 10:e0143191. 10.1371/journal.pone.0143191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solà-Ginés M., Gonzalez-Lopez J. J., Cameron-Veas K., Piedra-Carrasco N., Cerda-Cuellar M., Migura-Garcia L. (2015a). Houseflies (Musca domestica) as vectors for extended-spectrum beta-lactamase-producing Escherichia coli on spanish broiler farms. Appl. Environ. Microbiol. 81, 3604–3611. 10.1128/AEM.04252-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedt J., Bonnedahl J., Hernandez J., Waldenstrom J., McMahon B. J., Tolf C., et al. (2015). Carriage of CTX-M type extended spectrum beta-lactamases (ESBLs) in gulls across Europe. Acta Vet. Scand. 57:74. 10.1186/s13028-015-0166-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani S., Giovanelli I., Anacarso I., Condo C., Messi P., de Niederhausern S., et al. (2014). Prevalence and characterization of extended-spectrum beta-lactamase-producing Enterobacteriaceae in food-producing animals in Northern Italy. New Microbiol. 37, 551–555. Available online at: http://www.newmicrobiologica.org/PUB/allegati_pdf/2014/4/551.pdf [PubMed] [Google Scholar]
- Stoll C., Sidhu J. P., Tiehm A., Toze S. (2012). Prevalence of clinically relevant antibiotic resistance genes in surface water samples collected from Germany and Australia. Environ. Sci. Technol. 46, 9716–9726. 10.1021/es302020s [DOI] [PubMed] [Google Scholar]
- Tekiner I. H., Ozpinar H. (2016). Occurrence and characteristics of extended spectrum beta-lactamases-producing Enterobacteriaceae from foods of animal origin. Braz. J. Microbiol. 47, 444–451. 10.1016/j.bjm.2015.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin E., Adler A., Lerner A., Carmeli Y. (2014). Carbapenem-resistant Enterobacteriaceae: biology, epidemiology, and management. Ann. N. Y. Acad. Sci. 1323:22–42. 10.1111/nyas.12537 [DOI] [PubMed] [Google Scholar]
- Teshager T., Dominguez L., Moreno M. A., Saenz Y., Torres C., Cardenosa S. (2000). Isolation of an SHV-12 beta-lactamase-producing Escherichia coli strain from a dog with recurrent urinary tract infections. Antimicrob. Agents Chemother. 44, 3483–3484. 10.1128/AAC.44.12.3483-3484.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnavi C. (2013). Translocation of gut flora and its role in sepsis. Indian J. Med. Microbiol. 31, 334–342. 10.4103/0255-0857.118870 [DOI] [PubMed] [Google Scholar]
- Valat C., Haenni M., Saras E., Auvray F., Forest K., Oswald E., et al. (2012). CTX-M-15 extended-spectrum beta-lactamase in a shiga toxin-producing escherichia coli isolate of serotype O111:H8. Appl. Environ. Microbiol. 78, 1308–1309. 10.1128/AEM.06997-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verraes C., Van Boxstael S., Van Meervenne E., Van Coillie E., Butaye P., Catry B., et al. (2013). Antimicrobial resistance in the food chain: a review. Int. J. Environ. Res. Public Health 10, 2643–2669. 10.3390/ijerph10072643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingopoulou E. I., Siarkou V. I., Batzias G., Kaltsogianni F., Sianou E., Tzavaras I., et al. (2014). Emergence and maintenance of multidrug-resistant Escherichia coli of canine origin harbouring a blaCMY-2-IncI1/ST65 plasmid and topoisomerase mutations. J. Antimicrob. Chemother. 69, 2076–2080. 10.1093/jac/dku090 [DOI] [PubMed] [Google Scholar]
- von Salviati C., Laube H., Guerra B., Roesler U., Friese A. (2015). Emission of ESBL/AmpC-producing Escherichia coli from pig fattening farms to surrounding areas. Vet. Microbiol. 175, 77–84. 10.1016/j.vetmic.2014.10.010 [DOI] [PubMed] [Google Scholar]
- Webb H. E., Granier S. A., Marault M., Millemann Y., den Bakker H. C., Nightingale K. K., et al. (2016). Dissemination of the mcr-1 colistin resistance gene. Lancet Infect. Dis. 16, 144–145. 10.1016/S1473-3099(15)00538-1 [DOI] [PubMed] [Google Scholar]
- WHO (2017). WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals. Geneva: World Health Organization. [PubMed] [Google Scholar]
- WHO CIA (2017). WHO List of Critically Important Antimicrobials for Human Medicine (WHO CIA list). Geneva: World Health Organization. [Google Scholar]
- Woolhouse M., Ward M., van Bunnik B., Farrar J. (2015). Antimicrobial resistance in humans, livestock and the wider environment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370:20140083. 10.1098/rstb.2014.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaici L., Haenni M., Saras E., Boudehouche W., Touati A., Madec J. Y. (2016). blaNDM-5-carrying IncX3 plasmid in Escherichia coli ST1284 isolated from raw milk collected in a dairy farm in Algeria. J. Antimicrob. Chemother. 71, 2671–2672. 10.1093/jac/dkw160 [DOI] [PubMed] [Google Scholar]
- Yilmaz E. S., Guvensen N. C. (2016). In vitro biofilm formation in ESBL-producing Escherichia coli isolates from cage birds. Asian Pac. J. Trop. Med. 9, 1069–1074. 10.1016/j.apjtm.2016.10.003 [DOI] [PubMed] [Google Scholar]
- Yoo J. S., Kim H. M., Koo H. S., Yang J. W., Yoo J. I., Kim H. S., et al. (2013). Nosocomial transmission of NDM-1-producing Escherichia coli ST101 in a Korean hospital. J. Antimicrob. Chemother. 68, 2170–2172. 10.1093/jac/dkt126 [DOI] [PubMed] [Google Scholar]
- Yousfi M., Mairi A., Bakour S., Touati A., Hassissen L., Hadjadj L., et al. (2015). First report of NDM-5-producing Escherichia coli ST1284 isolated from dog in Bejaia, Algeria. New Microbes New Infect. 8, 17–18. 10.1016/j.nmni.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousfi M., Mairi A., Touati A., Hassissene L., Brasme L., Guillard T., et al. (2016b). Extended spectrum beta-lactamase and plasmid mediated quinolone resistance in Escherichia coli fecal isolates from healthy companion animals in Algeria. J. Infect. Chemother. 22, 431–435. 10.1016/j.jiac.2016.03.005 [DOI] [PubMed] [Google Scholar]
- Yousfi M., Touati A., Mairi A., Brasme L., Gharout-Sait A., Guillard T., et al. (2016a). Emergence of carbapenemase-producing Escherichia coli isolated from companion animals in Algeria. Microb. Drug Resist. 22, 342–346. 10.1089/mdr.2015.0196 [DOI] [PubMed] [Google Scholar]
- Zogg A. L., Zurfluh K., Nuesch-Inderbinen M., Stephan R. (2016). Characteristics of ESBL-producing enterobacteriaceae and methicillin resistant Staphylococcus aureus (MRSA) isolated from swiss and imported raw poultry meat collected at retail level. Schweiz. Arch. Tierheilkd. 158, 451–456. 10.17236/sat00071 [DOI] [PubMed] [Google Scholar]