Abstract
Currently, sorafenib-based therapy is the standard treatment for advanced hepatocellular carcinoma (HCC), and there is a strong rationale for investigating its use in combination with other agents to achieve better therapeutic effects. Aurora-A, a member of a family of mitotic serine/threonine kinases, is frequently overexpressed in human cancers and therefore represents a target for therapy. Here, we investigated a novel Aurora-A inhibitor, MLN8237, together with sorafenib in HCC cells in vitro and in vivo, and elucidated the possible molecular mechanism. Here, it was found that MLN8237 was strongly synergistic with sorafenib in inhibition of HCC progression by altering cell growth, cell-cycle regulation, apoptosis, migration, invasion, and angiogenesis. Mechanism dissection suggests that the combination of MLN8237 and sorafenib led to significant inhibition of the activation of phospho-Akt (p-Akt) and phospho-p38 mitogen-activated protein kinase (p-p38 MAPK) and their downstream genes including CDK4, cyclinD1, and VEGFA. The activators of p-Akt and p-p38 MAPK signaling partially reversed the synergistic inhibitory effects of sorafenib and MLN8237 on HCC progression. Subsequent in vivo studies further confirmed the synergistic effects of sorafenib and MLN8237. Collectively, the newly developed sorafenib-MLN8237 combination may be a novel therapy to better inhibit HCC progression.
Keywords: Aurora-A inhibitor, MLN8237, sorafenib, hepatocellular carcinoma, Akt
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy in the world, and approximately 600,000 new cases of HCC occur each year.1, 2 Surgical resection and liver transplantation are first-line curative options for patients with early-stage HCC, but most patients have advanced stage disease at diagnosis and no longer have the option of undergoing surgical resection. Treatments for advanced HCC include systemic chemotherapy, transarterial chemoembolization (TACE), and radiofrequency ablation. Although the above measures have relieved the disease, prognosis is not yet satisfactory.3
Sorafenib is the first multi-target, multi-kinase inhibitor that is systematically used in the treatment of advanced liver cancer and has been proven effective.4, 5 Through dual mechanisms, including the suppression of Raf kinase and the inhibition of vascular endothelial growth factor receptor (VEGFR), sorafenib simultaneously exerts anti-tumor cell proliferation and anti-angiogenic effects. In 2008, a randomized clinical trial (the SHARP trial) confirmed that sorafenib prolongs the overall survival of patients with advanced liver cancer from 7.9 to 10.7 months.6 Therefore, sorafenib has become the first-line drug in the treatment of advanced HCC. Importantly, however, many patients do not respond to sorafenib or develop drug resistance after several months of sorafenib treatment.7 Therefore, the identification of enhancers or synergistic agents of sorafenib has become an urgent need with regard to the clinical treatment of HCC.
The Aurora kinase family is a group of serine/threonine protein kinases consisting of Aurora-A kinase, Aurora-B kinase, and Aurora-C kinase.8 Aurora kinases participate in a variety of mitotic activities and maintain the integrity of the genome, thereby playing important roles during mitosis.9 Previously, we have reported that Aurora-A is highly expressed in HCC tissues and correlates with poor prognosis of patients.10 Importantly, we also showed that overexpression of Aurora-A could promote multiple malignant phenotypes of HCC cells.11, 12 Meanwhile, it was testified that hypoxia-inducible factor 1α and microRNA-129-3p play important roles in regulation of Aurora-A expression at either transcriptional or post-transcriptional level.13, 14 MLN8237 (Alisertib) is an oral small-molecule inhibitor that specifically inhibits Aurora-A kinase and is currently undergoing clinical testing in different tumor types.15 Aurora-A is expressed and activated only during mitosis, and normal cells during the non-proliferative phase will not be affected by Aurora-A inhibitors.16, 17 Therefore, inhibitors of Aurora-A likely target tumor tissues relatively specifically and cause less damage to normal tissues. In the present report, MLN8237 was evaluated in combination with sorafenib in two different HCC cell models both in vitro and in vivo. The results demonstrated that sorafenib-MLN8237 combination could exert the synergistic inhibitory effects on HCC progression by significant inactivation of Akt and MARK signaling. The findings suggest that sorafenib-MLN8237 combination represents a potential therapeutic strategy for the treatment of advanced HCC.
Results
MLN8237 Significantly Enhances the Cytotoxic Effect of Sorafenib in HCC Cells In Vitro
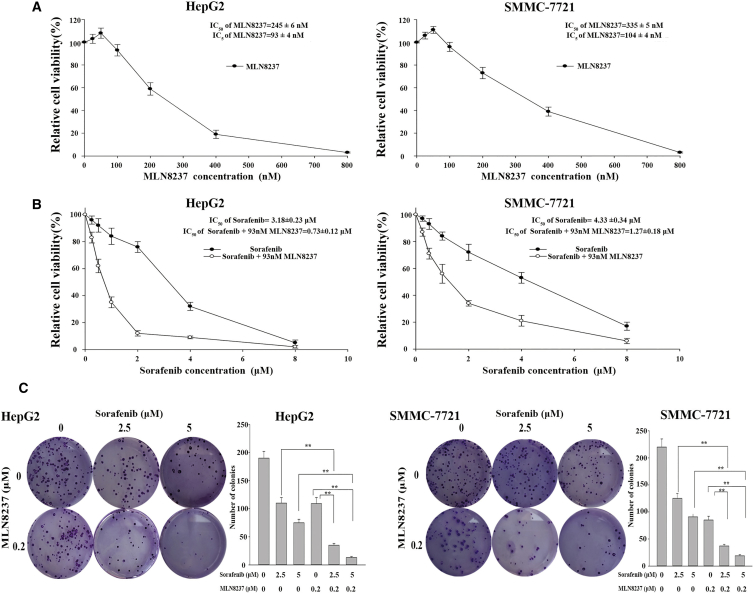
To elucidate the effect of MLN8237 on the cytotoxicity of sorafenib, we treated HepG2 and SMMC-7721 cells with various concentrations of MLN8237 (25–800 nM) for 48 hr, and the concentration-survival curves were plotted. The results clearly showed that MLN8237 inhibited cell growth in a concentration-dependent manner (Figure 1A). The 5% inhibitory concentrations (IC5) of MLN8237 were determined based on the concentration-survival curves, and the IC5 concentrations were selected for further investigation of the regulatory effect of MLN8237 on sorafenib. HepG2 and SMMC-7721 cells were then treated with various concentrations of sorafenib (0.25–8 μM). The resulting concentration-survival curves were compared with the corresponding curves obtained after co-treatment of the cells with MLN8237 (IC5 concentration) and sorafenib. The half maximal inhibitory concentrations (IC50) of sorafenib were calculated according to the fitted survival curves. The IC50 values of sorafenib in HepG2 and SMMC-7721 cells were 3.18 ± 0.23 and 4.33 ± 0.34 μM, respectively. Co-treatment with MLN8237 significantly enhanced the cytotoxicity of sorafenib. In the presence of MLN8237, the IC50 of sorafenib was reduced to 0.73 ± 0.12 μM in HepG2 cells (combination index [CI] = 0.61, moderate synergy) and 1.27 ± 0.18 μM in SMMC-7721 cells (CI = 0.60, moderate synergy; Figure 1B). Therefore, co-treatment with MLN8237 significantly reduced the IC50 of sorafenib for HepG2 cells (a 4.35-fold decrease) and SMMC-7721 cells (a 3.41-fold decrease). Furthermore, long-term cell growth was examined using clonogenic assay, and similar results were obtained. As shown in Figure 1C, the combination of sorafenib and MLN8237 exerts a more significant anti-proliferative effect on HepG2 and SMMC-7721 cells.
Figure 1.
The Effects of MLN8237, Sorafenib, and Their Combination on Proliferation of HepG2 and SMMC-7721 Cells
(A) The cytotoxicity of various concentrations of MLN8237 to HepG2 and SMMC-7721 cells. (B) The cytotoxicity of various concentrations of sorafenib to HepG2 and SMMC-7721 cells when administered alone or in combination with an IC5 concentration of MLN8237. (C) Examination of the clonogenicity of HepG2 and SMMC-7721 cells treated with MLN8237 alone, sorafenib alone, and their combination, as well as the results of the statistical analysis. Each experiment was performed at least three times. *p < 0.05; **p < 0.01.
MLN8237 Enhances the Anti-proliferative Activity of Sorafenib in HCC Cells by Blocking Cell-Cycle Progression and Increasing Apoptosis
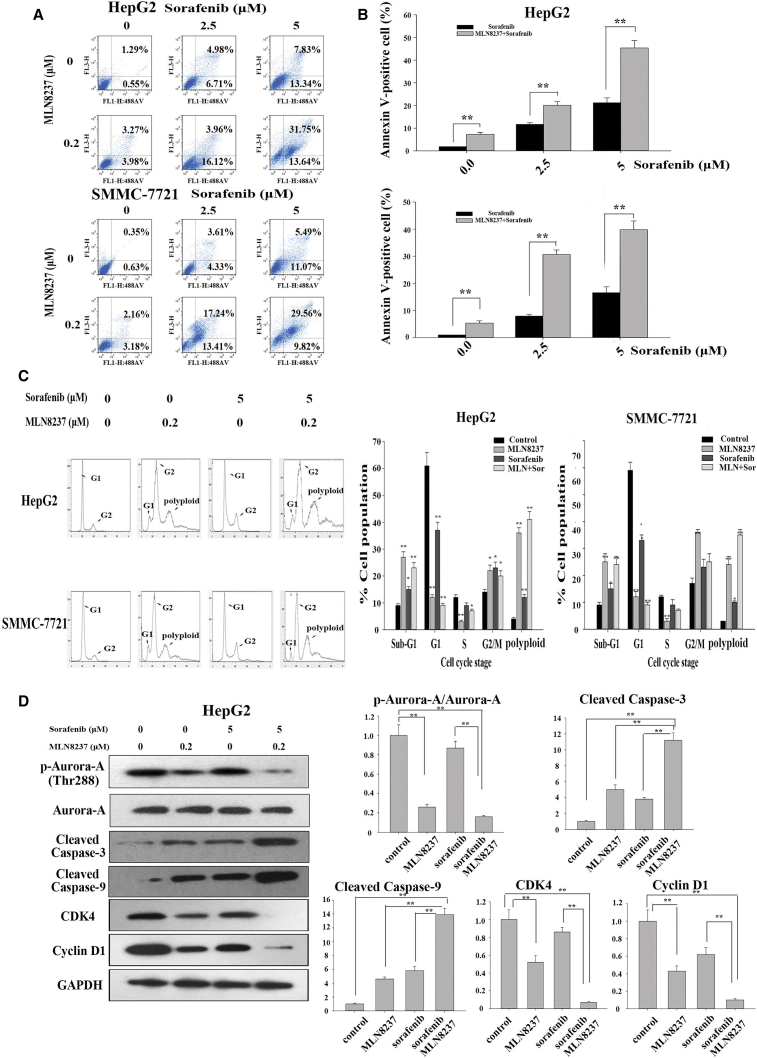
To explore the effects of MLN8237 and sorafenib on the apoptosis of tumor cells, we performed a flow cytometric analysis. The results showed that a low dose of MLN8237 or sorafenib alone had little effect on tumor cells. Combined treatment with MLN8237 and sorafenib significantly increased the apoptosis of HepG2 and SMMC-7721 cells (Figures 2A and 2B). The apoptosis-promoting effect was more pronounced when a slightly larger dose of sorafenib was administered in combination with MLN8237. In addition, cell-cycle analysis revealed that MLN8237 and/or sorafenib markedly reduced the percentage of G1 cells and significantly delayed the G2-M phase transition in HepG2 and SMMC-7721 cells. Moreover, the administration of MLN8237 alone or in combination with sorafenib resulted in a significantly increased percentage of polyploid cells, which was accompanied by a drastic reduction in the number of S-phase cells (Figure 2C; Figures S1A and S1B). Thus, combined treatment with MLN8237 and sorafenib enhanced cell death through delaying the G2-M phase transition, reducing the number of cells in the S phase, increasing the number of cells in the sub-G1 phase, and activating the pro-apoptotic mechanisms. Furthermore, the combined treatment with MLN8237 and sorafenib significantly enhanced the expression levels of cleaved caspase-3 and cleaved caspase-9 (two common apoptotic markers) in HepG2 cells compared with MLN8237 or sorafenib treatment alone. Compared with MLN8237 or sorafenib treatment alone, the combined treatment with MLN8237 and sorafenib markedly reduced the expression levels of two common cell-cycle-specific proteins, cyclin-dependent kinase 4 (CDK4) and cyclin D1 (Figure 2D). We tested p-Aurora-A in order to verify the effect of MLN8237 (Figure 2D). The same results were obtained using the SMMC-7721 cells (Figures S1A and S1B). The above results support our notion that MLN8237 enhances the anti-tumor effect of sorafenib through blocking cell-cycle progression and increasing apoptosis.
Figure 2.
The Effects of MLN8237, Sorafenib, and Their Combination on Apoptosis and Cell Cycles of HepG2 and SMMC-7721 Cells
(A) HepG2 and SMMC-7721 cells were treated with sorafenib (2.5 and 5 μM), MLN8237 (0.3 μM), or both for 48 hr. Subsequently, flow cytometric analysis was conducted to monitor apoptosis. (B) Statistical analysis of the apoptosis results obtained from three independent flow cytometry assays. (C) HepG2 and SMMC-7721 cells were treated with sorafenib, MLN8237, or both for 48 hr. Flow cytometric analysis was then conducted to examine the cell cycle. (D) HepG2 cells were treated with sorafenib, MLN8237, or both for 48 hr. Western blotting was then performed to monitor the expression of p-Aurora, cleaved caspase-3, cleaved caspase-9, CDK4, and cyclin D1. Each experiment was performed at least three times. *p < 0.05; **p < 0.01.
MLN8237 Enhances the Anti-metastatic Activity of Sorafenib in HCC Cells by Inhibiting Migration, Invasion, and Angiogenesis
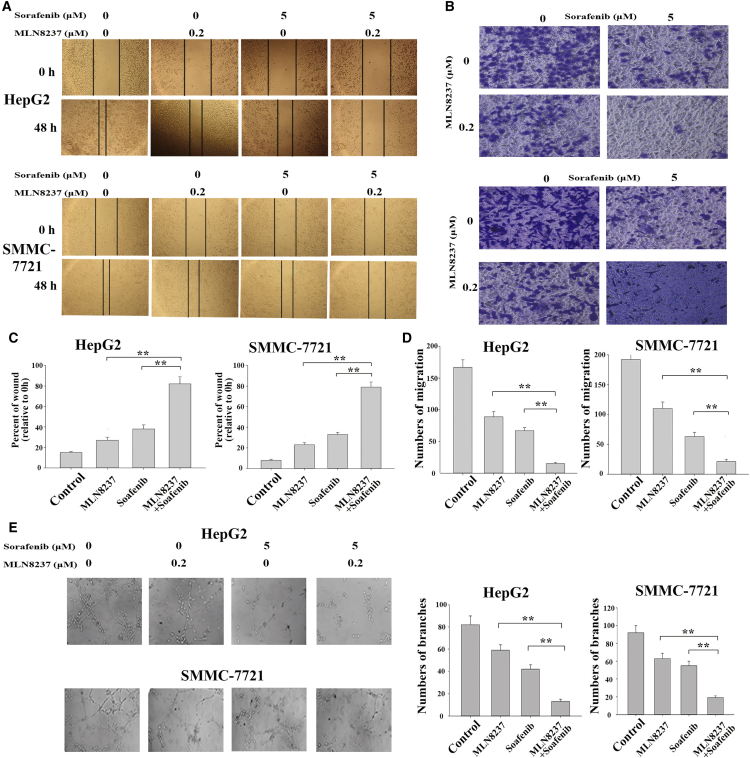
Although previous studies have shown that both MLN8237 and sorafenib inhibit tumor cell invasion and metastasis,18, 19 whether their combination could induce the synergistic anti-metastatic activity in HCC cells is unknown. The scratch wound healing and transwell assays of the present study revealed that the migratory and invasive capabilities of HepG2 cells were significantly reduced after treatment with the combination of MLN8237 and sorafenib compared with the treatment with MLN8237 or sorafenib alone (Figures 3A and 3C). Similar results were obtained using another cell line, SMMC-7721 (Figures 3B and 3D). The co-administration of MLN8237 and sorafenib significantly increased the expression level of E-cadherin and decreased the expression level of N-cadherin in HepG2 cells compared with the treatment with MLN8237 or sorafenib alone (Figure S2). E-cadherin and N-cadherin are a pair of epithelial-mesenchymal transition (EMT)-related markers. These results further indicated that the combined action of MLN8237 and sorafenib inhibits the invasion and metastasis of HCC cells. To examine the angiogenic ability of HCC following MLN8237 and/or sorafenib treatment, human umbilical vein endothelial cells (HUVECs) were cultivated in the presence of the culture supernatants collected from the HepG2 and SMMC-7721 cells that had been treated with the drugs for 24 hr. Compared with MLN8237 or sorafenib alone, the co-administration of MLN8237 and sorafenib significantly inhibited HUVEC tube formation (Figure 3E). Western blot analysis of vascular endothelial growth factor A (VEGFA, a member of the vascular endothelial growth factor family) showed that the combined action of MLN8237 and sorafenib significantly reduced the expression level of VEGFA (Figure S2). The above results demonstrate that MLN8237 enhances the anti-metastatic effect of sorafenib in HCC cells by inhibiting migration, invasion, and angiogenesis.
Figure 3.
The Effects of MLN8237, Sorafenib, and Their Combination on Invasion, Migration, and Angiogenesis of HepG2 and SMMC-7721 Cells
(A) HepG2 and SMMC-7721 cells were treated with sorafenib, MLN8237, or both for 48 hr. A scratch wound healing assay was then conducted to examine the invasive and metastatic capabilities of the cells. The results (from three independent experiments) were subjected to statistical analysis and are summarized in (C). (B) HepG2 and SMMC-7721 cells were treated with sorafenib, MLN8237, or both of the drugs for 48 hr. A transwell assay was then conducted to examine the invasive and metastatic capabilities of the cells. (D) Statistical results of the transwell assay (three independent experiments). (E) HepG2 and SMMC-7721 cells were treated with sorafenib, MLN8237, or both for 24 hr. Culture supernatants were collected and used in HUVEC tube formation assay. After culture of HUVECs in the presence of the supernatants for 48 hr, the number of tubules was counted. The results of the tube formation assay were statistically analyzed. Each experiment was performed at least three times. *p < 0.05; **p < 0.01.
Sorafenib-MLN8237 Combination Synergistically Inactivates Akt and MAPK Signaling in HCC Cells
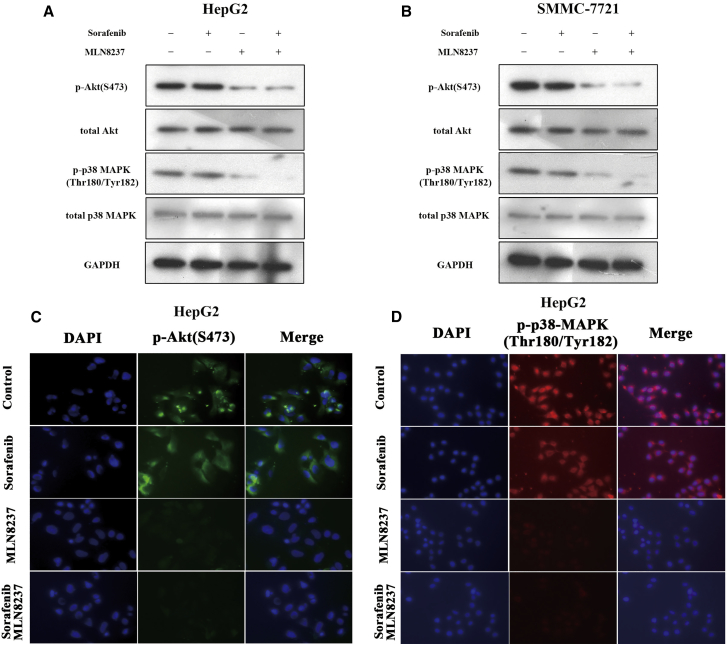
Subsequently, we explored the potential molecular mechanisms through which the combination of MLN8237 and sorafenib induced the synergistical anti-tumor activity of HCC cells. The present study first determined the effects of sorafenib and/or MLN8237 on the expression of p-Akt and phospho-p38 mitogen-activated protein kinase (p-p38 MAPK) proteins in HepG2 cells. Virtually no decrease was observed in p-Akt and p-p38 MAPK levels after the treatment of HepG2 cells with sorafenib alone. In contrast, MLN8237 inhibited the expression of p-Akt and p-p38 MAPK proteins (Figure 4A). Importantly, the combination of sorafenib and MLN8237 significantly inhibited the expression of p-Akt and p-p38 MAPK proteins in HepG2 cells. Similar results were obtained in SMMC-7721 cells (Figure 4B). Immunofluorescence analysis of the expression of p-Akt and p-p38 MAPK proteins yielded results consistent with the results of western blotting (Figured 4C and 4D; Figures S3A and S3B). The above results suggest that MLN8237 synergistically enhances the anti-tumor effect of sorafenib by inhibiting the p-AKT and p-p38 MAPK signaling in HCC cells.
Figure 4.
MLN8237 Inhibits Activities of p-Akt and p-p38 MAPK in HepG2 and SMMC-7721 Cells
(A and B) HepG2 (A) and SMMC-7721 (B) cells were treated with sorafenib, MLN8237, or both for 48 hr. Western blotting was then conducted to monitor the expression of p-Akt, total Akt, p-p38 MAPK, and total p38-MAPK in the cells. (C and D) HepG2 cells treated with sorafenib, MLN8237, or both for 48 hr. Immunofluorescence to determine the expression of p-Akt (C) and p-p38 MAPK (D) in HepG2 cell.
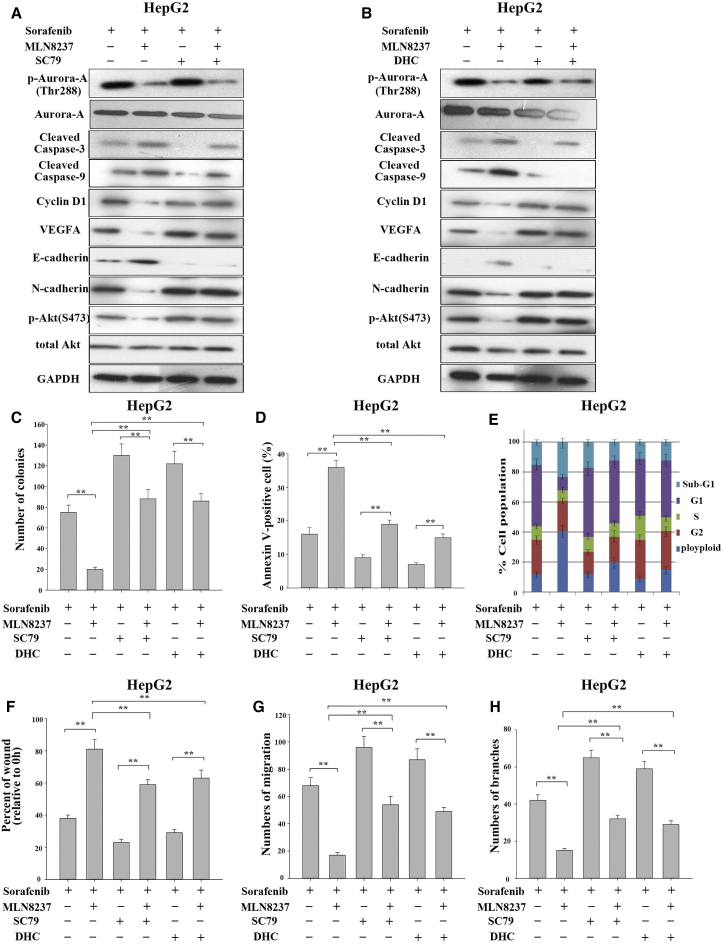
Activators of the p-Akt and p-p38 MAPK Signaling Interrupt the Synergistic Effect of MLN8237-Sorafenib Combination on HCC Cells
To further confirm that MLN8237 synergistically enhances the anti-tumor effect of sorafenib by inactivation of p-Akt and p-p38 MAPK signaling, we conducted blocking experiments using the activator of the p-Akt signaling pathway (SC79) and the activator of the p-p38 MAPK signaling pathway (dehydrocorydaline [DHC]).20, 21, 22, 23 First, the present study examined the effect of the activators SC79 and DHC. The expression of p-Akt protein increased in HepG2 cells as the duration of SC79 treatment and the concentration of SC79 increased. Similarly, DHC affected the expression of p-p38 MAPK protein in HepG2 cells in time- and concentration-dependent manners (Figure S4). As shown in Figure 5A, the treatment of HepG2 cells with a combination of sorafenib and MLN8237 markedly reduced the expression of p-Akt protein. In addition, the expression levels of the apoptotic marker proteins (cleaved caspase-3 and cleaved caspase-9) were significantly elevated, the expression levels of the cell-cycle proteins (CDK4 and cyclin D1) were markedly decreased, and the expression levels of VEGFA and the invasion and metastasis-related protein (N-cadherin) were also significantly reduced, whereas the expression of E-cadherin protein was increased. However, the administration of an increased amount of the activator of SC79 significantly reversed the above changes. The results demonstrated that the biological effects of the combination of sorafenib and MLN8237 on HCC are achieved through the inhibition of Akt phosphorylation. The same experiments were conducted using DHC, and similar results were obtained (Figure 5B). As expected, the simultaneous administration of SC79 or DHC with sorafenib and MLN8237 significantly reversed the effects of sorafenib-MLN8237 combination on malignant phenotypes of HCC cells (Figures 5C–5H; Figures S5A–S5F). The same results were obtained using the SMMC-7721 cells (Figures S6A–S6H). The above results further demonstrate that MLN8237 synergistically enhances the anti-tumor effects of sorafenib by inhibiting p-Akt and p-p38 MAPK signaling.
Figure 5.
Activators of p-Akt and p-p38 MAPK Partially Reverse the Synergistic Effects of MLN8237-Sorafenib Combination in HCC Cells
(A) HepG2 cells were treated with sorafenib alone or with the combination of sorafenib and MLN8237 in the presence or absence of the p-Akt activator SC79 (3 μM/24 hr). Western blotting was then performed to analyze and monitor the expression of p-Aurora, cleaved caspase-3, cleaved caspase-9, CDK4, cyclin D1, VEGFA, E-cadherin, N-cadherin, p-Akt, and total Akt in the cells. (B) HepG2 cells were treated with sorafenib alone or with the combination of sorafenib and MLN8237 in the presence or absence of the p-p38 MAPK activator DHC (3 μM/24 hr). Western blotting was then performed to analyze and monitor the expression of p-Aurora, cleaved caspase-3, cleaved caspase-9, CDK4, cyclin D1, VEGFA, E-cadherin, N-cadherin, p-p38 MAPK, and total p38 MAPK in the cells. (C) HepG2 cells were treated with sorafenib alone or with the combination of sorafenib and MLN8237 in the presence or absence of the p-Akt activator SC79 and the p-p38 MAPK activator DHC. A clonogenic assay was then conducted to examine the proliferative capability of the cells. (D) Apoptosis monitored via flow cytometry. (E) Cell cycle monitored via flow cytometry. (F) Examination of the invasive and metastatic capabilities of the cells via a scratch wound healing assay. (G) Examination of the invasive and metastatic capabilities of the cells via a transwell assay. (H) Examination of the angiogenic ability of HUVECs via a tube formation assay. Each experiment was performed at least three times. *p < 0.05; **p < 0.01.
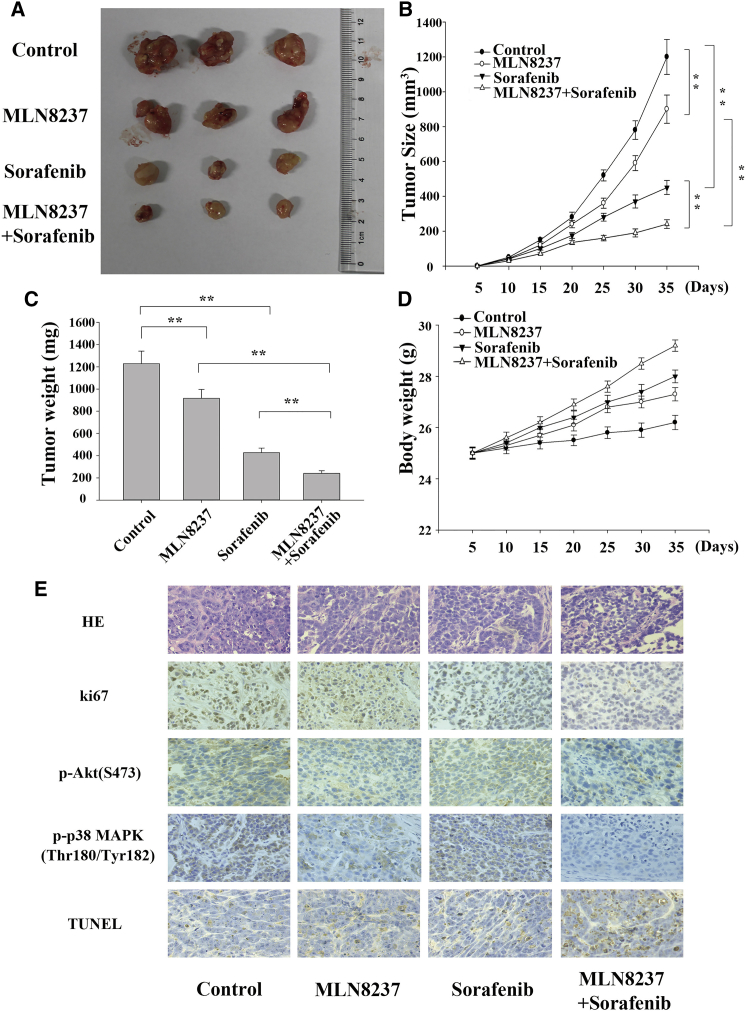
MLN8237 Significantly Enhances the Anti-proliferative and Pro-apoptotic Activities of Sorafenib in HCC Cells In Vivo
Xenograft tumors were generated by implanting HepG2 cells subcutaneously into mice. Starting on the 7th day after the inoculation of HepG2 cells, the mice were injected with the control medication, MLN8237, sorafenib, or MLN8237-sorafenib combination. The injections were administered every 3 days for 4 consecutive weeks. The volumes of the subcutaneous tumors were measured every 5 days, and the growth curves of the tumors were established. The growth of the xenograft tumors was inhibited by both the sorafenib and MLN8237 treatments. Interestingly, the tumor volume was significantly smaller in the group treated with the combination of sorafenib and MLN8237 compared with the groups treated with only one of the drugs (Figures 6A–6C). Also, drug treatments prevented the loss of body weight in nude mice (Figure 6D). Immunohistochemical analysis of the xenograft tumors showed that the combined treatment significantly inhibited the expression of p-Akt and p-p38 MAPK, and markedly reduced the level of ki-67 (a proliferating cell-associated nuclear antigen). The results of the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay showed that combined treatment with MLN8237 and sorafenib significantly enhances tumor apoptosis in mice (Figure 6E).
Figure 6.
The In Vivo Effects of MLN8237, Sorafenib, and Their Combination on HCC Cell Proliferation and Apoptosis
(A) Images of subcutaneous xenograft tumors. (B) The growth curves of the subcutaneous xenograft tumors. (C) The final weights of the subcutaneous xenograft tumors. (D) Changes in mouse body weights during the formation of subcutaneous xenograft tumors. (E) Representative images of H&E staining, immunohistochemical staining (for Ki67, p-Akt, and p-p38 MAPK), and TUNEL staining. Each experiment was performed at least three times. *p < 0.05; **p < 0.01.
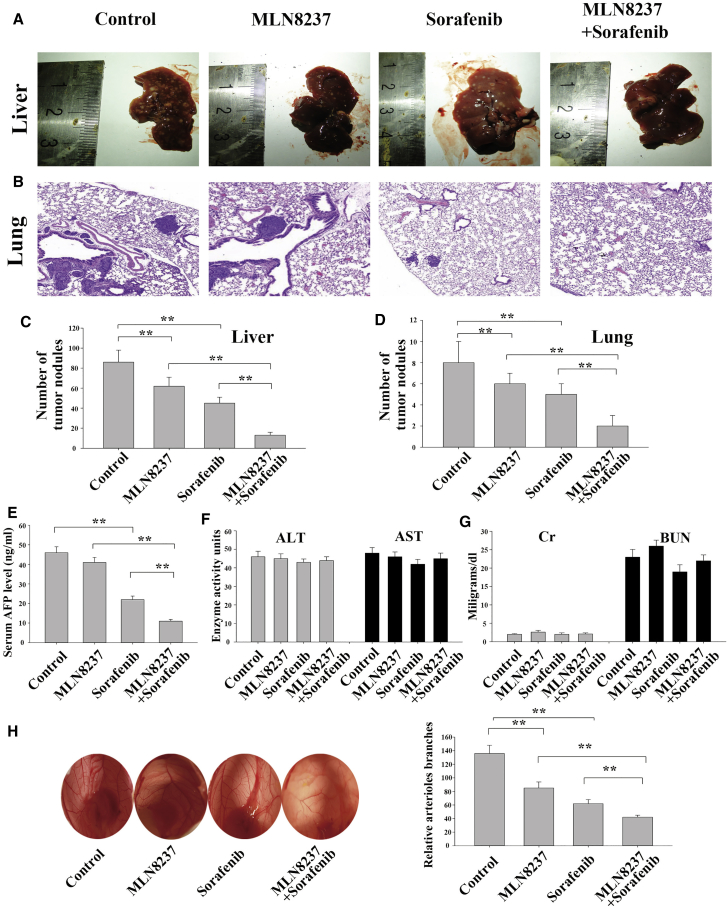
MLN8237 Significantly Enhances Anti-metastatic and Anti-angiogenetic Activities of Sorafenib in HCC Cells In Vivo
Xenograft tumors were generated by implanting HepG2 cells into mice via the tail vein. Starting on the 14th day after the inoculation of HepG2 cells, the mice were injected with the control medication, MLN8237, sorafenib, or MLN8237-sorafenib combination. The injections were administered every 3 days for 8 consecutive weeks. Subsequently, we executed the mice and collected their livers and lungs. The number of metastatic nodules in the livers was counted (Figures 7A and 7C). The lungs were subjected to H&E staining, and the number of metastatic nodules in the lungs was analyzed (Figures 7B and 7D). The number of metastases had clearly decreased in the single-medication group compared with the blank control group. Moreover, the number of metastases was markedly reduced in the combined medication group compared with the single-medication groups. An examination of the alpha-fetoprotein (AFP) level in mouse serum revealed that a significant difference existed between the combined medication group and the single-medication groups (Figure 7E). In contrast, no significant between-group difference was found in the levels of alanine aminotransferase, aspartate aminotransferase, creatinine, or blood urea nitrogen, indicating that the combination of MLN8237 and sorafenib had no significant hepatorenal toxicity (Figures 7F and 7G). The above results demonstrated that the combined treatment of MLN8237 and sorafenib inhibited the invasion and metastasis of HCC more effectively in mice than the single-drug treatment. HepG2 cells were treated with MLN8237 alone, sorafenib alone, or the combination of MLN8237 and sorafenib for 24 hr. The culture supernatants of the treated HepG2 cells were collected. Chicken embryos were exposed to the supernatants, and the number of angiogenic blood vessels in the chick embryo chorioallantoic membrane was determined. As shown in Figure 7H, the combined treatment with MLN8237 and sorafenib significantly reduced the number of angiogenic blood vessels in the embryo chorioallantoic membrane.
Figure 7.
The Effects of MLN8237, Sorafenib, and Their Combination on HCC Cell Metastasis and Angiogenesis In Vivo
A mouse model of HCC was established via tail vein injection. The model mice was injected with the control medication, MLN8237, sorafenib, or their combination. (A) Representative images of the livers collected from the model mice. (B) Representative images of lung metastases in the model mice. (C) Statistical results of the liver nodules larger than 1 mm in the model mice harboring liver metastases. (D) Statistical results of the number of metastatic nodules (which were counted under a microscope) in the lungs of the model mice. (E) Examination of serum AFP level via ELISA. (F) Assessment of the hepatotoxicity of the drugs by analyzing the aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. (G) Assessment of renal toxicity by examining creatinine (Cr) and blood urea nitrogen (BUN) levels. (H) Examination of in vivo angiogenic capability using a chick embryo chorioallantoic membrane assay. Each experiment was performed at least three times. *p < 0.05; **p < 0.01.
Discussion
Worldwide, the mortality rate of HCC ranks third among all tumor types. The development and progression of HCC is closely related to abnormalities in various signaling pathways.24 The Akt signaling pathway is an important intracellular signal transduction pathway. Studies have shown that the Akt signaling pathway is usually dysregulated in human tumor cells. Therefore, this pathway has generated novel ideas for the targeted treatment of tumors and the prevention of tumor metastasis.25, 26 The Akt inhibitors GSK2110183 and MK2206 exhibit positive therapeutic effects on liver cancer and lung cancer.27, 28 The p38-MAPK pathway is another important intracellular signal transduction pathway that is abnormally activated in many tumors.29, 30 The p38-MAPK inhibitor SB203580 also displays a potent anti-tumor effect in a variety of tumors.31, 32, 33 Our previous study showed that the Aurora-A inhibitor MLN8237 significantly suppresses the activation of the Akt and p38 MAPK signaling pathways by inhibiting Aurora-A, thereby affecting a variety of the biological behaviors of HCC cells.12 Sorafenib is a multi-target kinase inhibitor that has been approved by the US FDA for use as a standard therapeutic drug for advanced HCC. Previous studies have found that sorafenib inhibits the growth and proliferation of HCC cells by downregulating the Akt signaling pathway. However, sorafenib exhibits a limited inhibitory effect on the phosphorylation of Akt, the mammalian target of rapamycin (mTOR), and other key enzymes in the Akt signaling pathway when acting alone.34 According to Dong’s study, the use of Akt inhibitor MK2206 can enhance the therapeutic effect of sorafenib by inhibiting EMT and multi-drug resistance (MDR).35 As proved by Roth’s experiment, another Akt inhibitor, ARQ092, can inhibit Akt signaling pathway in a cirrhotic rat model so as to strengthen the effect of sorafenib on HCC.36 The results of the study carried out by Mao et al.37 showed that silibinin can trigger the expression changes of downstream Mcl-1 and Bcl-2 genes through inhibiting the phosphorylation of Akt and STAT3, and then strengthen the effect of sorafenib. All these results have confirmed the importance of targeted Akt in HCC. At present, few studies have examined the effects of sorafenib on the p38-MAPK signaling pathway in HCC. In addition, epidemiological cohort studies showed that sorafenib prolongs the survival of patients with HCC by only approximately 3 months while causing a variety of adverse reactions.6 Therefore, the identification of enhancers or synergistic agents of sorafenib has become a need for clinical practice.
The present study examined the effects of the combination of MLN8237 and sorafenib on HCC cell proliferation, apoptosis, cell cycle, invasion, metastasis, and angiogenesis both in vitro and in vivo. The results clearly demonstrated that MLN8237 significantly enhances the anti-tumor effects of sorafenib. In addition, the present study confirmed that MLN8237 exerted its anti-tumor effects by inhibiting the activation of p-Akt and p-p38 MAPK. The main features of malignant tumors are their abnormal cell proliferation, apoptosis, and cell cycle, as well as their invasion, metastasis, and angiogenesis. The study conducted by Wilson et al.38 showed that the inhibition of p-Akt in people with HCC suppresses proliferation, increases apoptosis, and induces cell-cycle arrest. According to the scientific reports published by Tan et al.39 and Li et al.,40 p-Akt participates in the invasion and metastasis-related processes of HCC by regulating matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9). Li and his colleagues41 have confirmed that the Akt-mTOR-p70S6K pathway participates in the angiogenesis of liver cancer. It has been reported that the p-p38 MAPK pathway is involved in a variety of HCC processes, including proliferation, apoptosis, cell cycle, invasion, metastasis, and angiogenesis.42, 43, 44 MLN8237 is a small-molecule inhibitor that specifically inhibits Aurora-A kinase. Phase I and II clinical trials have revealed that MLN8237 displays a satisfactory therapeutic effect and a positive safety profile.45, 46 A phase III clinical trial is currently in progress. The present study showed that MLN8237 enhances the anti-tumor activities of sorafenib through the inhibitory regulation of the downstream targets p-Akt and p-p38 MAPK. Importantly, the combined administration of the two drugs does not induce significant hepatorenal toxicity in animals, indicating that the combination therapy consisting of MLN8237 and sorafenib is safe. As the small-molecule drug is in the experimental stage, exploring the potential drug value of MLN8237 and discovering appropriate drug combinations and corresponding indications are the hotspots of current research. The synergistic use of MLN8237 and sorafenib will allow patients with HCC to receive the targeted therapy directing at multiple different tumor-specific signaling pathways, so as to improve the patient’s drug resistance and prolong survival.
In conclusion, we demonstrated that MLN8237-sorafenib combination synergistically inhibits the activation of p-Akt and p-p38 MAPK in HCC, thereby affecting a variety of the biological behaviors of HCC. Combined treatment with MLN8237 and sorafenib achieved a superior therapeutic effect compared with MLN8237 or sorafenib alone. The data presented in the current study strongly suggest that MLN8237 has great potential to treat HCC when used as a synergistic agent of sorafenib.
Materials and Methods
Cell Lines
The cell lines HepG2 and SMMC-7721 were cultured in DMEM (GIBCO-BRL) supplemented with 10% fetal bovine serum (FBS), streptomycin (100 μg/mL), and penicillin (100 U/mL). All cells were fostered at 37°C in an atmosphere containing 5% CO2. All of the cell lines were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China), where they were characterized by mycoplasma detection, DNA fingerprinting, isozyme detection, and cell vitality detection.
Cell Viability
Cell viability was assessed via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-trtrazolium bromide (MTT) assay. 5.0 × 103 cells/well were seeded in a 96-well flat-bottomed plate for 24 hr, then exposed to varying concentrations of either sorafenib or MLN8237 and cultured in normal medium. After 72 hr, the MTT solution (5 mg/mL, 20 μL) was added to each well. Following incubation for 4 hr, the media were removed and 100 μL of DMSO was added to each well. The relative number of surviving cells was assessed by measuring the optical density (OD) of cell lysates at 560 nm. All assays were performed in triplicate.
Colony Formation Assay
Clonogenic cells were seeded at a density of 500 cells per well in six-well plates and incubated for 12 days. Cells were subsequently stained with 0.5% crystal violet. The clone formation rate was determined. Independent experiments were conducted in triplicate.
Flow Cytometric Analysis of Apoptosis and Cell Cycle
Flow cytometric analyses of apoptosis and cell cycle were performed with Annexin V-FITC Apoptosis Detection Kits and propidium iodide (PI)/RNase staining buffer (BD Biosciences, USA), respectively, according to the manufacturer’s instructions.
Western Blot Assay
Cell proteins were prepared using cell lysis buffer. Equal amounts of protein (50 mg) were separated by 10% SDS-PAGE and then transferred to nitrocellulose membranes (Merck Millipore) by electro-blotting. The membranes were blocked with 5% nonfat dry milk in TBST for 1 hr and then incubated with primary antibodies [anti-Aurora-A, ab13824; anti-p-Aurora-A, ab83968; anti-c-caspase3, ab2302; anti-c-caspase9, ab2324; anti-CDK4, ab137675; anti-cyclin D1, ab226977; anti-E-cadherin, ab15148; anti-N-cadherin, ab8203; anti-VEGFA, ab1316; anti-total Akt, ab8805; anti-p-Akt (S473), ab8932; anti-total p38-MAPK, ab27986; anti-p-p38-MAPK (Thr180/Tyr182), ab38238; and anti-GAPDH, ab8245] (Abcam) overnight at 4°C before subsequent incubation with the second antibody (Cell Signaling Technology) for 1 hr at 37°C. Protein binds were visualized using enhanced chemiluminescence reagent (Pierce).
Cell Migration
Cell migration was measured by transwell chamber (8-μm pore size; Corning). 48 hr after transfection, cells in serum-free media were placed into the upper chamber. Media containing 10% bovine calf serum was added into the lower chamber. Following 48-hr incubation, cells remaining in upper membrane were wiped off, while cells that migrated were fixed in methanol, stained with 0.1% crystal violet, and counted under a microscope. Three independent experiments were carried out.
Wound Healing Assay
The wound healing assay was performed to assess cell migration ability. After 24-hr starvation in serum-free medium, an artificial wound was linearly scraped on the confluent cell monolayer using a standard P-200 pipette tip. Cells that had detached from the bottom of the wells were gently aspirated. Then cells migrated into the scratch area as single cells from the confluent sides. The width of the scratch gap was monitored under an inverted microscope and photographed at 0 and 48 hr. The difference between the original width of the wound and the width after cell migration was quantified. Three replicates of each condition were used.
Immunofluorescence
Cells seeded on glass coverslips in six-well plates were fixed in 4% formaldehyde solution and permeabilized with 0.5% Triton X-100/PBS. Cells were blocked with 5% BSA-PBS for 1 hr at room temperature and incubated with primary antibody at 4°C overnight, followed by incubation with fluorescent dye-conjugated secondary antibody (Invitrogen) for 1 hr, and then stained with DAPI. Finally, images were taken under an inverted fluorescence microscope.
TUNEL Assay
Apoptosis in transplanted tumor tissues was detected using the TUNEL assay and performed according to the guidelines recommended by the TUNEL assay kit (KeyGen, Nanjing, China).
Mice Xenograft Models and Immunohistochemistry Assay
All animal experiments strictly followed the guidelines of the Institutional Review Board of Jinling Hospital. Approximately 5.0 × 106 HepG2 cells were suspended in 100 μL of PBS and injected subcutaneously into the right side of the posterior flank of female BALB/c athymic nude mice (Department of Comparative Medicine, Jinling Hospital, Nan Jing, China) at 5–6 weeks of age. Tumor volumes were examined every other day and were calculated using the equation: V = A × B2/2 (mm3), where A is the largest diameter and B is the perpendicular diameter. When the average tumor size reached approximately 50 mm3, MLN8237, sorafenib, or the combination of MLN8237 and sorafenib was administered via intraperitoneal injection at one dose of 30 mg/kg every 3 days for 4 consecutive weeks. After 4 weeks, all mice were killed, and necropsies were performed. The primary tumors were excised and analyzed by H&E staining, immunostaining of ki67, p-Akt (S473), and p-p38-MAPK (Thr180/Tyr182), and TUNEL staining.
In Vivo Tumor Metastasis Assay
All animal experiments strictly followed the guidelines of the Institutional Review Board of Jinling Hospital. For in vivo metastasis study, HepG2 cells were suspended in 20 μL of PBS and injected into the tail vein of female BALB/C athymic nude mice at 4–6 weeks of age. At the end of 2 weeks of cell injection, three mice were randomly chosen and killed to ensure the development of HCC, as confirmed by pathological analysis. Mice were then administered via intraperitoneal injection at a dose of 30 mg/kg sorafenib, 30 mg/kg MLN8237, a combination of both substances, or vehicle every 3 days for 8 consecutive weeks. Vehicle-injected normal mice were used as controls. After 8 weeks of drug treatment, mice were sacrificed for analysis (n = 5). Malignant liver and lung nodules ≥1 mm in diameter were counted by two independent investigators. Liver tissues and serum samples were collected for later analysis.
CAM Assay
The fertilized chicken eggs were placed in an incubator upon embryogenesis and maintained under constant humidity at 37°C. On day 8, a square window was opened in the shell after removing 2–3 mL of albumen to detach the chick chorioallantoic membrane (CAM) from the shell. Substances treated with the compounds being tested were added to the detached CAM that contained HepG2 cell-conditioned media from the groups indicated in the experiment for the capillary-like tubular formation assay. The window was sealed with parafilm and incubated for an additional 24 hr. After the second incubation, the CAM arteriosus branches in each treatment group were photographed. The assay was performed three times to ensure reproducibility.
Statistical Analysis
All statistical analysis was conducted using SPSS 17.0 statistical software, and the experimental data were presented as mean ± SD. Two group comparisons were performed with a Student’s t test. Multiple group comparisons were analyzed with one-way ANOVA. All tests performed were two-sided.
Author Contributions
K.Z. and R.W. designed the research. K.Z. and T.W. conducted the experiments, and K.Z. wrote the manuscript. H.Z., B.F., Y.Z., and K.Z. supported the experiments, contributed in reagents/materials, and provided the financial support. R.W. performed the data analysis and helped to draft the manuscript. T.W. and R.W. contributed to supervising laboratory processes. All authors approved the final version and agreed to publish the manuscript.
Conflicts of Interest
The authors have no conflicts of interest.
Acknowledgments
This project was supported by grants from the National Natural Science Foundation of China (81472266 and 81772995) and the Excellent Youth Foundation of Jiangsu Province (BK20140032).
Footnotes
Supplemental Information includes six figures and can be found with this article online at https://doi.org/10.1016/j.omtn.2018.08.014.
Supplemental Information
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Forner A., Llovet J.M., Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Maluccio M., Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J. Clin. 2012;62:394–399. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 4.Wilhelm S., Carter C., Lynch M., Lowinger T., Dumas J., Smith R.A., Schwartz B., Simantov R., Kelley S. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 2006;5:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 5.Cheng A.L., Kang Y.K., Chen Z., Tsao C.J., Qin S., Kim J.S., Luo R., Feng J., Ye S., Yang T.S. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 6.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., de Oliveira A.C., Santoro A., Raoul J.L., Forner A., SHARP Investigators Study Group Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Verslype C., van Malenstein H., Dekervel J., Windmolders P., Libbrecht L., van Eijsden R., Nevens F., van Pelt J. Resistance development after long-term sorafenib exposure in hepatocellular cancer cell lines and risk of rebound growth and epithelial to mesenchymal transition. J. Clin. Oncol. 2012;30(Suppl 4):216. [Google Scholar]
- 8.Bolanos-Garcia V.M. Aurora kinases. Int. J. Biochem. Cell Biol. 2005;37:1572–1577. doi: 10.1016/j.biocel.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Marumoto T., Zhang D., Saya H. Aurora-A—a guardian of poles. Nat. Rev. Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 10.Wang R., Wang J.H., Chu X.Y., Geng H.C., Chen L.B. Expression of STK15 mRNA in hepatocellular carcinoma and its prognostic significance. Clin. Biochem. 2009;42:641–647. doi: 10.1016/j.clinbiochem.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Gao P., Wang R., Shen J.J., Lin F., Wang X., Dong K., Zhang H.Z. Hypoxia-inducible enhancer/alpha-fetoprotein promoter-driven RNA interference targeting STK15 suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. Cancer Sci. 2008;99:2209–2217. doi: 10.1111/j.1349-7006.2008.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang K., Chen J., Chen D., Huang J., Feng B., Han S., Chen Y., Song H., De W., Zhu Z. Aurora-A promotes chemoresistance in hepatocelluar carcinoma by targeting NF-kappaB/microRNA-21/PTEN signaling pathway. Oncotarget. 2014;5:12916–12935. doi: 10.18632/oncotarget.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui S.Y., Huang J.Y., Chen Y.T., Song H.Z., Huang G.C., De W., Wang R., Chen L.B. The role of Aurora A in hypoxia-inducible factor 1α-promoting malignant phenotypes of hepatocelluar carcinoma. Cell Cycle. 2013;12:2849–2866. doi: 10.4161/cc.25916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui S., Zhang K., Li C., Chen J., Pan Y., Feng B., Lu L., Zhu Z., Wang R., Chen L. Methylation-associated silencing of microRNA-129-3p promotes epithelial-mesenchymal transition, invasion and metastasis of hepatocelluar cancer by targeting Aurora-A. Oncotarget. 2016;7:78009–78028. doi: 10.18632/oncotarget.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manfredi M.G., Ecsedy J.A., Chakravarty A., Silverman L., Zhang M., Hoar K.M., Stroud S.G., Chen W., Shinde V., Huck J.J. Characterization of Alisertib (MLN8237), an investigational small-molecule inhibitor of aurora A kinase using novel in vivo pharmacodynamic assays. Clin. Cancer Res. 2011;17:7614–7624. doi: 10.1158/1078-0432.CCR-11-1536. [DOI] [PubMed] [Google Scholar]
- 16.Dees E.C., Cohen R.B., von Mehren M., Stinchcombe T.E., Liu H., Venkatakrishnan K., Manfredi M., Fingert H., Burris H.A., 3rd, Infante J.R. Phase I study of aurora A kinase inhibitor MLN8237 in advanced solid tumors: safety, pharmacokinetics, pharmacodynamics, and bioavailability of two oral formulations. Clin. Cancer Res. 2012;18:4775–4784. doi: 10.1158/1078-0432.CCR-12-0589. [DOI] [PubMed] [Google Scholar]
- 17.Sells T.B., Chau R., Ecsedy J.A., Gershman R.E., Hoar K., Huck J., Janowick D.A., Kadambi V.J., LeRoy P.J., Stirling M. MLN8054 and alisertib (MLN8237): discovery of selective oral Aurora A inhibitors. ACS Med. Chem. Lett. 2015;6:630–634. doi: 10.1021/ml500409n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Do T.V., Xiao F., Bickel L.E., Klein-Szanto A.J., Pathak H.B., Hua X., Howe C., O’Brien S.W., Maglaty M., Ecsedy J.A. Aurora kinase A mediates epithelial ovarian cancer cell migration and adhesion. Oncogene. 2014;33:539–549. doi: 10.1038/onc.2012.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei J.C., Meng F.D., Qu K., Wang Z.X., Wu Q.F., Zhang L.Q., Pang Q., Liu C. Sorafenib inhibits proliferation and invasion of human hepatocellular carcinoma cells via up-regulation of p53 and suppressing FoxM1. Acta Pharmacol. Sin. 2015;36:241–251. doi: 10.1038/aps.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jo H., Mondal S., Tan D., Nagata E., Takizawa S., Sharma A.K., Hou Q., Shanmugasundaram K., Prasad A., Tung J.K. Small molecule-induced cytosolic activation of protein kinase Akt rescues ischemia-elicited neuronal death. Proc. Natl. Acad. Sci. USA. 2012;109:10581–10586. doi: 10.1073/pnas.1202810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.So E.Y., Ouchi T. BRAT1 deficiency causes increased glucose metabolism and mitochondrial malfunction. BMC Cancer. 2014;14:548. doi: 10.1186/1471-2407-14-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo M., Lee S.J., Kim Y.K., Seo D.W., Baek N.I., Ryu J.H., Kang J.S., Bae G.U. Dehydrocorydaline promotes myogenic differentiation via p38 MAPK activation. Mol. Med. Rep. 2016;14:3029–3036. doi: 10.3892/mmr.2016.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z., Chen X., Fu S., Bao J., Dang Y., Huang M., Chen L., Wang Y. Dehydrocorydaline inhibits breast cancer cells proliferation by inducing apoptosis in MCF-7 cells. Am. J. Chin. Med. 2012;40:177–185. doi: 10.1142/S0192415X12500140. [DOI] [PubMed] [Google Scholar]
- 24.Pang R., Tse E., Poon R.T. Molecular pathways in hepatocellular carcinoma. Cancer Lett. 2006;240:157–169. doi: 10.1016/j.canlet.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 25.Ringel M.D., Hayre N., Saito J., Saunier B., Schuppert F., Burch H., Bernet V., Burman K.D., Kohn L.D., Saji M. Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res. 2001;61:6105–6111. [PubMed] [Google Scholar]
- 26.Page C., Huang M., Jin X., Cho K., Lilja J., Reynolds R.K., Lin J. Elevated phosphorylation of AKT and Stat3 in prostate, breast, and cervical cancer cells. Int. J. Oncol. 2000;17:23–28. doi: 10.3892/ijo.17.1.23. [DOI] [PubMed] [Google Scholar]
- 27.Yap T.A., Yan L., Patnaik A., Fearen I., Olmos D., Papadopoulos K., Baird R.D., Delgado L., Taylor A., Lupinacci L. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J. Clin. Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 28.Mack P.C., Farneth N., Mahaffey C., Lara P., Gandara D.R. Impact of AKT inhibitor MK-2206 on erlotinib resistance in non-small cell lung cancer (NSCLC) J. Clin. Oncol. 2011;29(Suppl 15):7573. [Google Scholar]
- 29.Pandey V., Bhaskara V.K., Babu P.P. Implications of mitogen-activated protein kinase signaling in glioma. J. Neurosci. Res. 2016;94:114–127. doi: 10.1002/jnr.23687. [DOI] [PubMed] [Google Scholar]
- 30.Lei Y.Y., Wang W.J., Mei J.H., Wang C.L. Mitogen-activated protein kinase signal transduction in solid tumors. Asian Pac. J. Cancer Prev. 2014;15:8539–8548. doi: 10.7314/apjcp.2014.15.20.8539. [DOI] [PubMed] [Google Scholar]
- 31.Lali F.V., Hunt A.E., Turner S.J., Foxwell B.M. The pyridinyl imidazole inhibitor SB203580 blocks phosphoinositide-dependent protein kinase activity, protein kinase B phosphorylation, and retinoblastoma hyperphosphorylation in interleukin-2-stimulated T cells independently of p38 mitogen-activated protein kinase. J. Biol. Chem. 2000;275:7395–7402. doi: 10.1074/jbc.275.10.7395. [DOI] [PubMed] [Google Scholar]
- 32.Birkenkamp K.U., Tuyt L.M., Lummen C., Wierenga A.T., Kruijer W., Vellenga E. The p38 MAP kinase inhibitor SB203580 enhances nuclear factor-kappa B transcriptional activity by a non-specific effect upon the ERK pathway. Br. J. Pharmacol. 2000;131:99–107. doi: 10.1038/sj.bjp.0703534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H., Chen G.G., Zhang Z., Chun S., Leung B.C., Lai P.B. Induction of autophagy in hepatocellular carcinoma cells by SB203580 requires activation of AMPK and DAPK but not p38 MAPK. Apoptosis. 2012;17:325–334. doi: 10.1007/s10495-011-0685-y. [DOI] [PubMed] [Google Scholar]
- 34.Tei H., Miyake H., Fujisawa M. Enhanced sensitivity to sorafenib by inhibition of Akt1 expression in human renal cell carcinoma ACHN cells both in vitro and in vivo. Hum. Cell. 2015;28:114–121. doi: 10.1007/s13577-015-0112-8. [DOI] [PubMed] [Google Scholar]
- 35.Dong J., Zhai B., Sun W., Hu F., Cheng H., Xu J. Activation of phosphatidylinositol 3-kinase/AKT/snail signaling pathway contributes to epithelial-mesenchymal transition-induced multi-drug resistance to sorafenib in hepatocellular carcinoma cells. PLoS ONE. 2017;12:e0185088. doi: 10.1371/journal.pone.0185088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roth G.S., Macek Jilkova Z., Zeybek Kuyucu A., Kurma K., Ahmad Pour S.T., Abbadessa G., Yu Y., Busser B., Marche P.N., Leroy V., Decaens T. Efficacy of AKT inhibitor ARQ 092 compared with sorafenib in a cirrhotic rat model with hepatocellular carcinoma. Mol. Cancer Ther. 2017;16:2157–2165. doi: 10.1158/1535-7163.MCT-16-0602-T. [DOI] [PubMed] [Google Scholar]
- 37.Mao J., Yang H., Cui T., Pan P., Kabir N., Chen D., Ma J., Chen X., Chen Y., Yang Y. Combined treatment with sorafenib and silibinin synergistically targets both HCC cells and cancer stem cells by enhanced inhibition of the phosphorylation of STAT3/ERK/AKT. Eur. J. Pharmacol. 2018;832:39–49. doi: 10.1016/j.ejphar.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 38.Wilson J.M., Kunnimalaiyaan S., Gamblin T.C., Kunnimalaiyaan M. MK2206 inhibits hepatocellular carcinoma cellular proliferation via induction of apoptosis and cell cycle arrest. J. Surg. Res. 2014;191:280–285. doi: 10.1016/j.jss.2014.05.083. [DOI] [PubMed] [Google Scholar]
- 39.Tan W., Zhu S., Cao J., Zhang L., Li W., Liu K., Zhong J., Shang C., Chen Y. Inhibition of MMP-2 expression enhances the antitumor effect of sorafenib in hepatocellular carcinoma by suppressing the PI3K/AKT/mTOR pathway. Oncol. Res. 2017;25:1543–1553. doi: 10.3727/096504017X14886444100783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X., Yang Z., Song W., Zhou L., Li Q., Tao K., Zhou J., Wang X., Zheng Z., You N. Overexpression of Bmi-1 contributes to the invasion and metastasis of hepatocellular carcinoma by increasing the expression of matrix metalloproteinase (MMP)-2, MMP-9 and vascular endothelial growth factor via the PTEN/PI3K/Akt pathway. Int. J. Oncol. 2013;43:793–802. doi: 10.3892/ijo.2013.1992. [DOI] [PubMed] [Google Scholar]
- 41.Li W., Tan D., Zhang Z., Liang J.J., Brown R.E. Activation of Akt-mTOR-p70S6K pathway in angiogenesis in hepatocellular carcinoma. Oncol. Rep. 2008;20:713–719. [PubMed] [Google Scholar]
- 42.Chiu C.C., Chen J.Y., Lin K.L., Huang C.J., Lee J.C., Chen B.H., Chen W.Y., Lo Y.H., Chen Y.L., Tseng C.H. p38 MAPK and NF-kappaB pathways are involved in naphtho[1,2-b] furan-4,5-dione induced anti-proliferation and apoptosis of human hepatoma cells. Cancer Lett. 2010;295:92–99. doi: 10.1016/j.canlet.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Liu W., Ning R., Chen R.N., Huang X.F., Dai Q.S., Hu J.H., Wang Y.W., Wu L.L., Xiong J., Hu G. Aspafilioside B induces G2/M cell cycle arrest and apoptosis by up-regulating H-Ras and N-Ras via ERK and p38 MAPK signaling pathways in human hepatoma HepG2 cells. Mol. Carcinog. 2016;55:440–457. doi: 10.1002/mc.22293. [DOI] [PubMed] [Google Scholar]
- 44.Wu R., Duan L., Cui F., Cao J., Xiang Y., Tang Y., Zhou L. S100A9 promotes human hepatocellular carcinoma cell growth and invasion through RAGE-mediated ERK1/2 and p38 MAPK pathways. Exp. Cell Res. 2015;334:228–238. doi: 10.1016/j.yexcr.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Friedberg J.W., Mahadevan D., Cebula E., Persky D., Lossos I., Agarwal A.B., Jung J., Burack R., Zhou X., Leonard E.J. Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas. J. Clin. Oncol. 2014;32:44–50. doi: 10.1200/JCO.2012.46.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dickson M.A., Mahoney M.R., Tap W.D., D’Angelo S.P., Keohan M.L., Van Tine B.A., Agulnik M., Horvath L.E., Nair J.S., Schwartz G.K. Phase II study of MLN8237 (Alisertib) in advanced/metastatic sarcoma. Ann. Oncol. 2016;27:1855–1860. doi: 10.1093/annonc/mdw281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.