Abstract
The primary cilium, which protrudes from the cell surface, is associated with the pathogenesis of various diseases, including acute kidney injury (AKI). Primary cilium length dynamically changes during the progression of diseases. However, its relevance in disease and the underlying mechanism are largely unknown. In this study, we investigated the role of primary cilia in AKI induced by cisplatin, an effective anticancer drug, and the underlying mechanisms. In addition, we evaluated the usefulness of length alteration and deciliation of primary cilia into the urine for the diagnosis of AKI. Cisplatin induced shortening, elongation, and normalization of the primary cilia in kidney epithelial cells over time. During shortening, primary cilia fragments and ciliary proteins were excreted into the urine. During deciliation, cell proliferation and the expression of cyclin-dependent kinase inhibitor and proliferating cell nuclear antigen were not significantly changed. Shortening and deciliation of primary cilia were observed before significant increases in plasma creatinine and blood urea nitrogen concentration occurred. Pretreatment with Mito-Tempo, a mitochondria-targeted antioxidant, prevented cisplatin-induced primary cilium shortening and inhibited the increases in superoxide formation, lipid peroxidation, blood urea nitrogen, and tissue damage. In contrast, isocitrate dehydrogenase 2 (Idh2) gene deletion, which results in defect of the NADPH-associated mitochondrial antioxidant system, exacerbated cisplatin-induced changes in mice. Taken together, our findings demonstrate that cisplatin induces deciliation into the urine and antioxidant treatment prevents this deciliation, renal dysfunction, and tissue damage after cisplatin injection. These results suggest that cisplatin-induced AKI is associated with primary cilia and urine primary cilia proteins might be a non-invasive biomarker of kidney injury.
Keywords: Primary cilia, ROS, Deciliation, Acute kidney injury, Cisplatin, Acetylated a-tubulin, IDH2
1. Introduction
The primary cilium, a cellular organelle which is anchored on the basal body and protrudes from the cell surface, acts as a sensor and signal transducer in the cell. The length of the primary cilium is dynamically altered under physiological and pathological conditions. This alteration is strongly linked to the assembly and disassembly of the microtubule that forms the core of the primary cilium and consists of tubulins [1]. In the kidneys, primary cilia reside on the apical surface of epithelial cells, including the parietal cells of Bowman's capsule and tubular epithelial cells, and they detect changes in renal fluid flow and the composition of kidney ultrafiltrate, and activate intracellular signaling to respond to those changes [2]. Perturbations in the primary cilium structur and function, including changes in length, are not only causally but also consequentially associated with various genetic or non-genetic human diseases, including polycystic diseases of the kidney. Changes in length can occur through injury and repair. Recent studies have demonstrated that epithelial cells in the kidney can be injured by various stresses, including ischemia/reperfusion (I/R), which can lead to shortening of the primary cilium through resorption and/or disruption (deciliation) [3], [4], [5]. Such length changes are affected by multiple factors, including cell cycle, cytokines, and reactive oxygen species (ROS) [3], [4], [5], [6]. Furthermore, several studies have reported that primary cilium length influences cell susceptibility to stress [7], [8], although the exact molecular mechanisms remain to be elucidated.
Cisplatin (cis-diamminedichloridoplatinum Ⅱ) is one of the most widely used chemotherapeutic agents. Approximately one third of patients who receive cisplatin therapy develop acute kidney injury (AKI) [9], [10]. Cisplatin is mainly removed from the body through excretion via the kidneys. However, during this process, cisplatin accumulates in the kidney tubular epithelial cells and injuries the cells through various pathways [11], [12], [13], [14]. Among which oxidative stress is a major factor. Cisplatin metabolites form a complex with glutathione (GSH) the major antioxidant in the mitochondria [15], [16], which leads to depletion of GSH in the mitochondria as the cisplatin-GSH complex can easily pass through the mitochondrial membrane [4], [6]. Consequently, the mitochondrial redox balance is disrupted, resulting in oxidative damage of mitochondria and cells. Recently, we found that primary cilium length is influenced by ROS and oxidative stress [4], [6], [17]. Lavagnino et al. reported that hypoxia-inducible factor, which is linked to oxidative stress, is also associated with cilium length [18]. Furthermore, one major anti-tumor effect of cisplatin is the blockage of tubulin assembly into microtubules, resulting in cell death [19]. Recently, Wang et al. observed the shortening of primary cilia and loss of kidney tubular cells in cisplatin-injected mice, and they suggested that these changes increase kidney cell susceptibility to cisplatin. Based on these lines of evidence, we investigated why primary cilia are shortened in cisplatin-induced AKI, and whether these changes can serve as a diagnostic marker. In the present study, we found that cisplatin shortens primary cilia via deciliation rather than resorption into the cell body, and this shortening is caused by increased ROS and oxidative stress. In addition, we found that deciliated primary cilia are excreted into the urine. These findings indicate that cisplatin-nephrotoxicity is associated with primary cilia, and urine primary cilium proteins can be a useful non-invasive indicator for the diagnosis of kidney injury.
2. Materials and methods
2.1. Animal preparation
All experiments were conducted with 8-week-old male C57B/6, Idh2 gene-deleted (Idh2-/-), and wild-type (Idh2+/+) littermates [20]. C57B/6 mice were purchased from Samtaco (Seoul, Korea). The mice were allowed free access to water and standard mouse chow. The animal study was approved by the Institutional Animal Care and Use Committee of Kyungpook National University. Mice were intraperitoneally injected with either cisplatin (10 mg/kg or 20 mg/kg body weight; Sigma, St. Louis, MO, USA) or 0.9% saline (vehicle). Some mice were injected daily with (2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl) triphenylphosphonium chloride monohydrate (Mito-Tempo, a mitochondria-targeted antioxidant; 0.7 mg/kg body weight; Sigma) one week before cisplatin injection. To collect urine, mice were individually placed in metabolic cages for 6 h. At the end of experiments, kidneys were snap-frozen in liquid nitrogen or perfusion-fixed with PLP (4% paraformaldehyde, 75 mM l-lysine, 10 mM sodium periodate; Sigma) solution. Frozen tissues were stored at −80 °C until use.
2.2. Measurement of functional parameters
Concentrations of blood urea nitrogen (BUN) and plasma creatinine (PCr) were measured using a Vitros 250 (Johnson & Johnson, New Brunswick, NJ, USA).
2.3. Histology
Paraffin-embedded tissues were cut into 3-μm sections using a microtome (Leica, Bensheim, Germany). To determine histological damage, kidney sections were stained with periodic acid-Schiff (PAS) according to the manufacturer's instruction. Images were captured using i-Solution software (IMT, Vancouver, Canada). Kidney tubular damage was scored as follow: 0, no damage; 1, mild damage with rounded epithelial cells and dilated tubular lumen; 2, moderate damage with flattened epithelial cells, dilated lumen, and congestion of lumen; and 3, severe damage with flat epithelial cells lacking nuclear staining and congestion of the lumen. At least 50 tubules per kidney were analyzed.
2.4. Measurement of superoxide levels in kidney tissue
Superoxide levels were measured using dihydroethidium (DHE; Sigma) as described previously [21]. Briefly, kidney lysates were transferred into wells of a 96 plate and 10 µM DHE reagent was added. The absorbance was read using an emission/excitation filter of 530 nm/620 nm at a temperature of 37 °C. Superoxide levels were expressed as a value per milligram protein of the kidney lysates.
2.5. BrdU-incorporation assay
To determine cell proliferations, 5-bromo-2′-deoxyuridine (BrdU, 50 mg/kg body weight; Sigma) incorporation assay was performed. BrdU, as a thymidine analog, is incorporated into newly synthesized DNA during the S phase of cell replication [22]. BrdU was administered to mice beginning on 1 day before cisplatin injection, every other day until sacrifice. Kidney sections were subjected to immunohistochemical staining using anti-BrdU (Serotec, Oxford, UK) antibody. The cortical and outer medullary regions were observed under a Leica microscope (DM2500, Wetzlar, Germany).
2.6. Western blot analysis
Western blot analysis was performed using anti-4-hydroxynonenal (4-HNE; Abcam, Cambridge, MA, USA), anti-acetylated-α-tubulin (ac-α-tubulin, Sigma), anti-Arl13b (Proteintech, Chicago, IL, USA), anti-proliferating cell nuclear antigen (PCNA; DAKO, Carpinteria, CA, USA), anti-p21 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; NOVUS, Littleton, CO, USA) antibodies.
2.7. Immunofluorescence
Kidney sections were deparaffinized and rehydrated, and then washed with phosphate-buffered saline (PBS) for 5 min each. The sections were incubated in PBS containing 0.1% sodium dodecyl sulfate (SDS; Sigma) for 1 min and washed in PBS for 10 min. To expose the antigen epitope, the sections were boiled in 10 mM sodium citrate buffer (pH 6.0) for 10 min, cooled for 20 min, and then washed three times with PBS for 5 min. The sections were blocked with 1% bovine serum albumin in PBS (blocking buffer) for 30 min and then incubated with anti-ac-α-tubulin, anti-AQP-1 (Alomone Labs, Jerusalem, Israel), and anti-AQP-2 (Alomone Labs) antibodies diluted in blocking buffer overnight at 4 °C. After washing, the sections were incubated with FITC-conjugated goat anti-mouse IgG (Vector Laboratories, Burlingame, CA, USA) or goat anti-rabbit IgG (Vector Laboratories) for 60 min, and then washed three times with PBS for 5 min. To stain cell nuclei, 4′-6-diamidino-2-phenylindole (DAPI; Sigma) was placed on the sections for 1 min.
To detect fragments of primary cilia in the urines, slide glasses were smeared urines, fixed, immunostained using anti-ac-α-tubulin and -Arl13b antibodies, and then observed under a Leica microscope.
2.8. Measurement of primary cilium length
Primary cilia lengths were measured as previously described [4]. Kidney sections were processed for immunofluorescence staining with anti-ac-α-tubulin antibody for primary cilia, and nuclei were counterstained with DAPI. Images were captured using a Leica microscope. Five to ten fields in the outer medulla of kidneys were randomly captured (magnification, 400 ×) and primary cilia length were measured at 1, 3, 7, or 14 days after cisplatin injection from 3 to 4 animals, using i-Solution software (IMT i-Solution Inc., Rochester, NY). For each treatment, primary cilium length was measured using more than 30 cells.
2.9. Detection of primary cilium proteins in mouse urine
Urine was collected from mice housed in a metabolic cage for 6 h. SDS sample buffer was added to the urine and the mixture was boiled for 5 min at 98 °C. Western blot analyses were conducted using anti-ac-α-tubulin antibody.
2.10. Statistics
Results were expressed as the mean ± standard error of the mean (SEM). Means were compared using Student's t-test. Each experimental group consisted of at least three mice. Differences between groups were considered statistically significant at a p value of < 0.05.
3. Results
3.1. Cisplatin induces alteration of primary cilia length in the kidney tubular epithelial cells
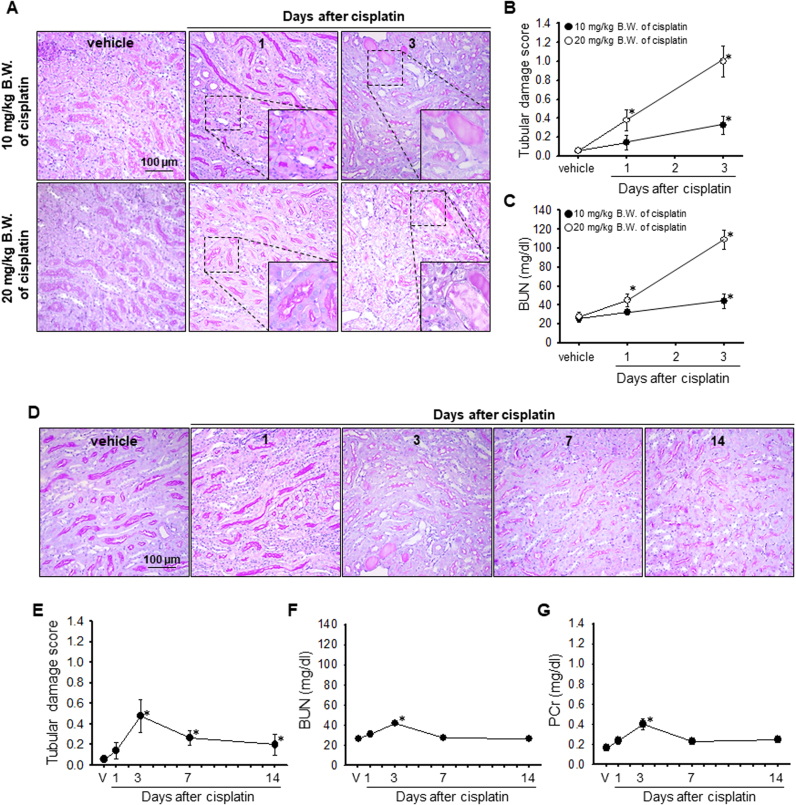
First, we determined that cisplatin (20 mg/kg B.W. for high dose or 10 mg/kg B.W. for low dose) injection induced kidney histological and functional damage, as indicated by PAS staining and BUN and PCr concentration, respectively. High dose of cisplatin resulted in significant increases BUN concentration 1 day after injection, and morphological damage 3 days after injection (Fig. 1A–C). In contrast, low dose of cisplatin did not induce significant increases in PCr and BUN after 1 day (Fig. 1C, F and G). PCr and BUN significantly increased from 3 days after cisplatin administration and then returned to normal ranges on day 7 (Fig. 1C, F and G). Consistent with the PCr and BUN level, significant tissue damage was observed 3 days after cisplatin administration (Fig. 1A, B, D and F). These data indicated that cisplatin causes kidney injury in a dose- and time-dependent manners.
Fig. 1.
Kidney function and morphology after cisplatin injection. Mice were injected with either cisplatin (20 mg/kg body weight (B.W.) for A to C and 10 mg/kg B.W. for A to G) or 0.9% saline (vehicle, V). Kidneys and blood samples were collected at the indicated times. (A and D) Kidney sections were stained with periodic acid-Schiff reagent. (B and E) Kidney tubular damage was scored as described in the Materials and methods section. (n = 3–4 per time point) (C, F, and G) Concentrations of BUN and PCr were determined at the indicated times (n = 3). Results are expressed as means ± SEs. * p < 0.05 vs. vehicle.
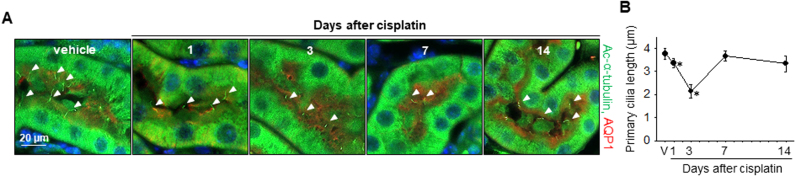
To test whether primary cilia respond to a low dose of cisplatin, we measured the lengths of primary cilia after low-dose cisplatin injection by immunostaining using acetylated-α-tubulin (ac-α-tubulin, a marker of primary cilia) antibody. Primary cilia started to shorten from day 1 after cisplatin injection until day 3 and then returned to normal length ranges at 7 days after injection (Fig. 2A and B). These results indicated that the shortening of primary cilia occurred before renal dysfunction and severe tissue damage, suggesting that primary cilia very sensitively and rapidly respond to cisplatin and that cisplatin-induced AKI is associated with the primary cilia.
Fig. 2.
Alteration of primary cilium length in the kidney tubular epithelial cells after cisplatin injection. Mice were injected with either cisplatin (10 mg/kg B.W.) or 0.9% saline (vehicle, V). Kidneys were harvested 1, 3, 7 and 14 days after cisplatin injection. (A) Paraffin-embedded kidneys were sectioned to 4 µm thickness and double-stained with anti-ac-α-tubulin antibody for primary cilia (green) and anti-AQP-1 antibody for proximal tubules (red). Nuclei were stained with DAPI (blue). Arrow heads indicate primary cilia. (B) Primary cilium length was measured as described in the Materials and methods section. Lengths of 30 primary cilia per kidney were averaged (n = 3 per time point). Results are expressed as means ± SEs. * p < 0.05 vs. vehicle.
3.2. Cisplatin induces deciliation into the urine
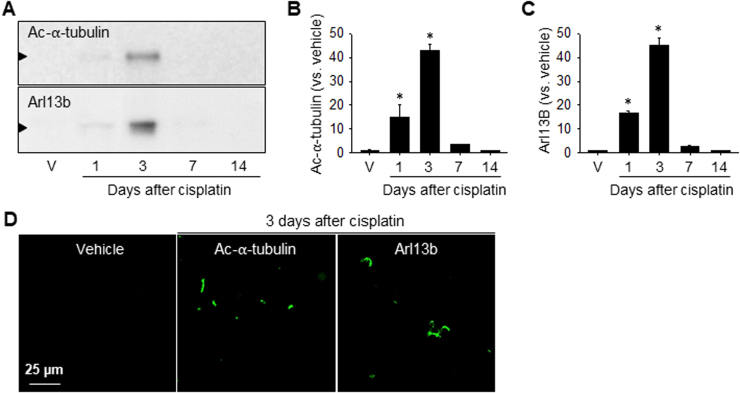
Since shortening of the primary cilia occurs via both resorption into the cell body during cell cycle entry and deciliation [23], we investigated whether cisplatin-induced shortening is associated with either deciliation or resorption by determining the levels of primary cilia proteins in the urine, cell cycle-associated protein expression, and cell proliferation. Primary cilia protrude into the tubular lumen and, if they are broken, they will be excreted into the urine through the urinary tract [24]. Therefore, to evaluate the deciliation of primary cilia, we determined the amounts of ac-α-tubulin (a marker of primary cilia) and Arl13b (a ciliary protein of the ADP-ribosylation factor family and Ras superfamily of GTPases) expression in urine samples collected from mice individually housed in metabolic cages for 6 h. Furthermore, we determined the ac-α-tubulin and Arl13B expression in the slide glasses which were smeared with urines. The urine levels of ac-α-tubulin and Arl13B increased from 1 day after low-dose cisplatin injection, peaked on day 3, and returned to normal levels thereafter (Fig. 3A-C). Many ac-α-tubulin and Arl13B-postivie signals were detected on the slide glasses smeared with the urines of cisplatin-injected mice (Fig. 3D). In the urine of non-treated mice, ac-α-tubulin and Arl13B were nearly non-detectible (Fig. 3A–D). These results indicated that cisplatin treatment results in the disruption of primary cilia and releases the breakdown products into the urine.
Fig. 3.
Excretion of primary cilia into the urine after cisplatin injection. Mice were injected with either cisplatin (10 mg/kg B.W.) or 0.9% saline (vehicle, V). Urine samples were collected at the indicated times after housing the mice in individual metabolic cages for 6 h. (A) Ac-α-tubulin and Arl13b expression in the samples as determined by western blot analysis. (B and C) Band densities were measured using ImageJ. (D) Urine smeared slide glasses were immunostained with anti-ac-α-tubulin (green) and Arl13b (green) antibodies. Results are expressed as means ± SEs. * p < 0.05 vs. vehicle.
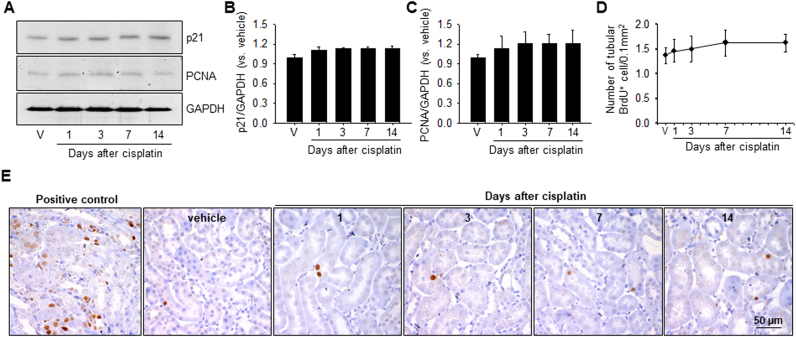
To test whether the shortening of primary cilia is also associated with resorption due to cell cycle entry, we assessed the expression of p21, a cyclin-dependent kinase inhibitor, and proliferating cell nuclear antigen (PCNA), a marker protein of cell proliferation in kidney tissues. In addition, we assessed cell proliferation by BrdU incorporation assay. BrdU is incorporated into DNA during the S phase of the cell cycle [25]. After cisplatin injection, the protein levels of p21 and PCNA slightly, albeit not statistically significantly, increased on 1 day, without further increases at 3, 7, and 14 days (Fig. 4A-C). BrdU-positive cells were rarely observed in the kidneys of both cisplatin- and vehicle-injected mice, and there were no significant differences between these two groups (Fig. 4D and E). These results indicated that cisplatin-induced shortening of the primary cilia may not be due to cell cycle entry and cell proliferation.
Fig. 4.
Expression of p21 and PCNA and cell proliferation in the kidney after cisplatin injection. Mice were injected with either cisplatin (10 mg/kg B.W.) or 0.9% saline (vehicle, V). Some mice were administered with BrdU intraperitoneally every other day, beginning on 1 day before cisplatin injection, until the end of the experiment. Kidneys were harvested 1, 3, 7 and 14 days after cisplatin injection. (A) Expression of p21 and PCNA as evaluated by western blotting analysis using anti-p21 and anti-PCNA antibodies. GAPDH was used as loading control. (B and C) Band densities were measured using ImageJ. (D and E) Kidney sections were immunohistochemically stained using anti-BrdU antibody (brown). A mouse kidney Section 24 h after 30 min of ischemia was included as a control. Hematoxylin was used to stain the nuclei (blue). (D) BrdU-positive (BrdU+) cells were counted in the outer medulla. Results are expressed as means ± SEs (n = 3). * p < 0.05 vs. vehicle.
3.3. Mitochondrial antioxidant treatment prevents cisplatin-induced deciliation, whereas mitochondrial antioxidant enzyme defect exacerbates deciliation
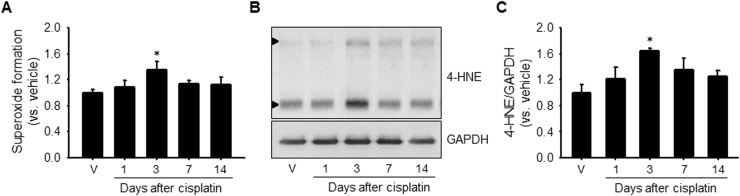
Since mitochondrial oxidative stress is one of the major causes of cisplatin-induced AKI, we investigated whether the shortening of primary cilia and cisplatin-induced AKI are associated with ROS production and oxidative stress. Cisplatin increased superoxide formation in mouse kidneys; this increase was observed from 1 day after cisplatin injection, reached statistical significance at day 3, and then returned to normal levels over time (Fig. 5A). The expression pattern of 4-hydroxynoneal (4-HNE), a marker of lipid peroxidation, in the kidney was similar to that of superoxide formation (Fig. 5B and C). These data indicated that cisplatin induces an increase in ROS and consequently, oxidative stress.
Fig. 5.
Superoxide formation and lipid peroxidation in the kidney after cisplatin injection. Mice were injected with either cisplatin (10 mg/kg B.W.) or 0.9% saline (vehicle). Kidneys were harvested 1, 3, 7 and 14 days after cisplatin injection. (A) Superoxide formation was measured as described in the Materials and methods section. (B) Level of lipid peroxidation was determined by western blot analysis using anti-4-HNE antibody. 4-HNE-positive signals are indicated by arrow head. GAPDH was used as a loading control. (C) Band densities were measured using the NIH Image J program. Results are expressed as means ± SEs (n = 3–4). * p < 0.05 vs. respective vehicle.
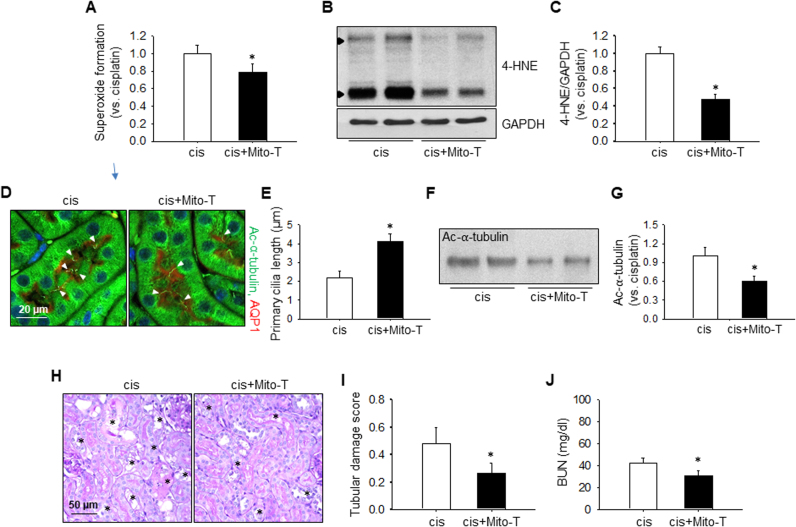
Next, we investigated whether cisplatin-induced AKI and shortening of primary cilia can be prevented by pretreatment with Mito-Tempo, a mitochondria-targeted antioxidant. Mito-Tempo treatment significantly reduced cisplatin-induced superoxide formation and 4-HNE expression when compared with vehicle (Fig. 6A–C). Furthermore, Mito-Tempo treatment prevented the shortening and deciliation of primary cilia in the kidney tubular cells and attenuated kidney dysfunction and tissue damage (Fig. 6D–J).
Fig. 6.
Effect of Mito-Tempo, a mitochondria-targeted antioxidant, on cisplatin-induced changes. Mice were injected with either cisplatin (10 mg/kg B.W.) or 0.9% saline (vehicle, V). Some mice were injected with Mito-Tempo (0.7 mg/kg B.W.) daily from 1 week before cisplatin injection until the end of the experiment. Urine was collected 3 days after cisplatin injection for 6 h using individual metabolic cages. Kidneys were harvested 3 days after cisplatin injection. (A) Superoxide formation as measured by dihydroethidium assay. (B) Expression level of 4-HNE as detected by western blot analysis. 4-HNE- positive signals are indicated by arrow head. GAPDH was used as a loading control. (C) Band densities were measured using ImageJ. (D) Paraffin-embedded kidneys were sectioned to 4 µm thickness and double-stained with anti-ac-α-tubulin antibody for primary cilia (green) and anti-AQP-1 antibody for proximal tubules (red). Nuclei were stained with DAPI (blue). The arrow head indicates a primary cilium. (E) Primary cilium length. Lengths of 30 primary cilia per kidney were averaged (n = 3). (F) Ac-α-tubulin protein levels in urine samples as determined by western blot analysis. (G) Band densities were measured using ImageJ. (H) Kidney sections were stained with periodic acid-Schiff reagent. The asterisk indicates injured tubule. (I) Kidney tubular damage was scored as described in the Materials and methods section (n = 3). (J) BUN concentration was measured 3 days after cisplatin injection. Results are expressed as means ± SEs (n = 3–4). * p < 0.05 vs. cisplatin.
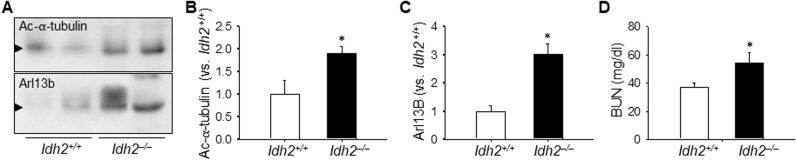
Finally, to define the role of the mitochondrial antioxidant system defect, we investigated cisplatin-induced deciliation in Idh2–/– mice. IDH2 is a critical enzyme in the NADPH-associated mitochondrial antioxidant system and its defect impairs this system [15]. Cisplatin-induced deciliation as well as kidney structural and functional damages were greater in Idh2–/– mice than in wild-type littermates (Fig. 7A–E). These results indicated that cisplatin-induced shortening of primary cilia is due to oxidative stress and cisplatin-induced nephrotoxicity is associated with the disruption of primary cilia.
Fig. 7.
Deciliation of primary cilia in Idh2 gene deleted mice after cisplatin injection. Idh2‒/‒ mice and wild-type (Idh2+/+) littermates were intraperitoneally injected with either cisplatin (10 mg/kg B.W.) or 0.9% saline (vehicle, V). (A and C) Acetylated α-tubulin level in the urine as examined by western blot analysis using anti-ac-α-tubulin antibody. (B and D) Band densities were measured using ImageJ. (E) BUN concentration was measured 3 days after cisplatin injection. Results are expressed as means ± SEs (n = 4). * p < 0.05 vs. Idh2+/+.
4. Discussion
In this study, we found that cisplatin induces the deciliation of primary cilia of kidney epithelial cells into the urine, along with shortening of the cilia on those cells, and that mitochondrial antioxidant treatment can prevent this deciliation as well as kidney injury. In contrast, defect of the mitochondrial antioxidant system exacerbates deciliation and kidney dysfunction. These findings demonstrate for the first time that cisplatin induces the deciliation of primary cilia into the urine via oxidative stress, and the increase in primary cilia proteins in the urine indicates kidney injury.
Primary cilium length is tightly controlled, and length alteration contributes to the onset and progression of various diseases. In the kidneys, primary cilium length is dynamically altered under the influence of not only physiological factors, such as renal flow and ultrafiltrate composition, but also pathological factors, including ROS and cytokines [4], [5], [7], [8], [17], [26], [27]. Recent evidence demonstrated that perturbation of primary cilium length is associated with a number of renal diseases, including AKI [3], [5], [26]. However, the causal relationship of primary cilium length and AKI had not been established. Using a cisplatin-induced AKI mouse model and in-vitro experiments, Wang et al. found that cisplatin induces shortening and loss of primary cilia in kidney epithelial cells, and this increased kidney susceptibility to cisplatin [7]. The authors suggested that this shortening may be caused by resorption or disassembly of primary cilia via an imbalance of cilium length regulatory proteins or functional loss of cilium proteins [7]. In this present study, we detected expression of primary ciliary proteins and various size of fragments of primary cilia in the urine, even before severe renal injury appeared after cisplatin injection, and the urine primary cilium proteins gradually increased overtime in positive correlation with kidney injury. This result indicates that cisplatin causes disruption, disassembly, or deciliaiton of primary cilia, and this shortening or deciliation may enhance kidney susceptibility to cisplatin.
Shortening of primary cilia occurs via two distinct mechanisms; resorption and deciliation, or disassembly [23]. Resorption of primary cilia is a normal process that occurs during progression from the G0/G1 phase to the S to G2 phases of the cell cycle [23], [28]. When a cell divides, the primary cilium is retracted into the cell body to precede mitosis. In contrast, deciliation is the disruption of cilia in response to environmental stresses [23], [28]. To define the mechanisms of shortening of primary cilia, we investigated changes in p21 and PCNA expression in the kidneys after cisplatin injection. The levels of p21 and PCNA expression were slightly increased 1 day after low-dose cisplatin injection; however, there were no further increases at later time points, when significant kidney dysfunction and damage were observed. This result indicates that the cell cycle change is not critical for cisplatin-induced shortening. In support of this, BrdU-incorporated cells were barely observed in the kidney tubular epithelial cells after cisplatin injection. This indicates that the shortening of primary cilia after cisplatin is not due to reabsorption for cell proliferation. Based on these findings, we speculate that the cilia shortening seen in the kidney tubular cells after cisplatin injection may be mainly due to deciliation, or disruption, rather than cell cycle- and cell proliferation-related resorption.
Although the exact molecular mechanisms of deciliation are currently unknown, accumulating evidences demonstrates that primary cilium shortening is affected by various intra- and extracellular factors, including MAPK, p53 and ROS [7], [29], [30]. Furthermore, Wang et al. reported that the shortening of primary cilia after cisplatin treatment is due to ERK activation, which subsequently suppresses cilium maintenance proteins, such as Polaris, whereas ERK inhibitor prevents this shortening [7]. However, the role of ERK activation in determining cilium length is controversial. In a previous study, we found that ERK inhibitor shortened the primary cilia of Madin-Darby canine kidney (MDCK) cells [4]. Abdual-Majeed et al. reported that MAPK inhibition resulted in the shortening of primary cilia length [31]. Furthermore, we previously found that kidneys recovering from I/R injury highly express the active form of ERK and have longer primary cilia when compared with normal kidneys [4], [5], [6]. In the present study, we found that cisplatin activates ERK before renal dysfunction and severe kidney damage occur (data not shown). Based on our and the above observations, despite the different experimental settings and the fact that the role of ERK in primary ciliogenesis is not clear, we speculate that ERK plays a critical role in the primary cilium length alterations in AKI. However, the exact role of ERK requires further study.
ROS production/oxidative stress is a major cause of cisplatin-induced AKI. Recently, we found that a high dose of H2O2 induced disruption, or deciliation, of primary cilia of cultured kidney tubular epithelial cells into the cultured medium, and this disruption was prevented by antioxidant treatment [4]. In addition, in an I/R-induced AKI mouse model, shortening of primary cilia was attenuated by antioxidant treatment [4]. Therefore, we investigated whether cisplatin-induced deciliation is associated with ROS and oxidative stress. In the present study, cisplatin increased mitochondrial ROS and oxidative stress, and mitochondrial antioxidant treatment reduced cisplatin-induced deciliation along with a reduction in kidney dysfunction and structural damage. To further define the role of mitochondrial ROS, we used Idh2 KO mice. IDH2 deletion impairs the mitochondrial NADPH-GSH-associated antioxidant system and thus increases susceptibility to cisplatin [15]. In the present study, defect of the mitochondrial antioxidant system exacerbated the deciliation of primary cilia at 1 day after injection, when severe histological and functional damages were not yet observed. These results indicate that cisplatin-induced deciliation is mediated by increased ROS production and oxidative stress. Accordingly, it has been reported that reactive carbonyl compounds, products of oxidative damage, induce the loss of primary cilia in human kidney proximal tubular cells without primary cilium resorption [32], [33].
Dynamic changes in the microtubular core of primary cilia are linked to primary ciliogenesis and are regulated by translational modifications, including acetylation, tyrosination, glutamylation, and glycation of tubulins [34], [35], [36], [37]. One of anti-tumor effect of cisplatin is a blockage of tubulin assembly into microtubule [19], [38]. Recently, Tang et al. reported that cisplatin increased histone deacetylase 6 (HDAC6) expression and activity and blockages of HDAC6 by HDAC inhibitor or HDAC6 siRNA treatment protected against cisplatin-induced AKI [39]. Kratzer et el. reported that ROS depolymerize and disorganize microtubules and disrupts the cytoskeleton in lung cells [40]. Therefore, we speculate that cisplatin-induced deciliation may be associated with an caused by increased ROS and oxidative stress.
Interestingly, we observed primary cilium shortening before the development of severe kidney dysfunction and structural damage. This finding suggests that this shortening may precede severe injury. In a previous I/R-induced AKI animal model study, we found that perturbation of the microtubule acetylation and deacetylation balance occurs before renal functional and morphological impairments [41]. Wang et al. reported that the shortening and loss of primary cilia increase cell susceptibility to cisplatin via exacerbation of cisplatin-induced caspase activation, and that primary cilium length is inversely correlated with caspase activity [7]. Patel et al. also reported that the loss of cilia sensitizes to I/R-induced AKI, and cilium-defect in mice accelerated ischemic AKI-induced PKD development [42]. However, some studies have demonstrated that primary cilium elongation increases cell susceptibility to inflammatory responses, because primary cilia contain various cytokine receptors [43]. Therefore, although we cannot assert whether shortening and deciliation of primary cilia is harmful or beneficial in cisplatin nephrotoxicity and the exact molecular mechanisms remain to be clarified, our data and those of others clearly that primary cilium length changes are tightly associated with the development of cisplatin-related nephrotoxicity.
Early and accurate diagnosis is critical for rapid and appropriate intervention for improving the outcome of AKI. In present study, we found that urine cilium proteins are increased under mild kidney injury, which does not induce a significant increase in BUN levels, and that the amount of urine primary cilium protein is correlated with the extent of kidney injury. This indicates that the determination of primary cilia in the urine may be a good non-invasive approach to detecting epithelial cell injury. Future studies on how ROS regulate primary cilia and on the detection of primary cilium proteins in patients suffering from kidney injury will be valuable for understanding the role of primary cilia in kidney diseases and the development of useful, non-invasive diagnostic tools.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Minister of Health & Welfare, Republic of Korea (grant number : HI18C1116).
Acknowledgments
Disclosures
The authors declare no conflicts of interest.
References
- 1.Hilton L.K., Gunawardane K., Kim J.W., Schwarz M.C., Quarmby L.M. The kinases LF4 and CNK2 control ciliary length by feedback regulation of assembly and disassembly rates. Curr. Biol.: CB. 2013;23:2208–2214. doi: 10.1016/j.cub.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 2.Singla V., Reiter J.F. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 3.Verghese E., Weidenfeld R., Bertram J.F., Ricardo S.D., Deane J.A. Renal cilia display length alterations following tubular injury and are present early in epithelial repair. Nephrol. Dial., Transplant.: Off. Publ. Eur. Dial. Transplant. Assoc. - Eur. Ren. Assoc. 2008;23:834–841. doi: 10.1093/ndt/gfm743. [DOI] [PubMed] [Google Scholar]
- 4.Kim J.I., Kim J., Jang H.S., Noh M.R., Lipschutz J.H., Park K.M. Reduction of oxidative stress during recovery accelerates normalization of primary cilia length that is altered after ischemic injury in murine kidneys. Am. J. Physiol. Ren. Physiol. 2013;304:F1283–F1294. doi: 10.1152/ajprenal.00427.2012. [DOI] [PubMed] [Google Scholar]
- 5.Jang H.S., Han S.J., Kim J.I., Lee S., Lipschutz J.H., Park K.M. Activation of ERK accelerates repair of renal tubular epithelial cells, whereas it inhibits progression of fibrosis following ischemia/reperfusion injury. Biochim. Et. Biophys. Acta. 2013;1832:1998–2008. doi: 10.1016/j.bbadis.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Han S.J., Jang H.S., Seu S.Y., Cho H.J., Hwang Y.J., Kim J.I., Park K.M. Hepatic ischemia/reperfusion injury disrupts the homeostasis of kidney primary cilia via oxidative stress. Biochim. Et. Biophys. Acta. 2017;1863:1817–1828. doi: 10.1016/j.bbadis.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Wang S., Wei Q., Dong G., Dong Z. ERK-mediated suppression of cilia in cisplatin-induced tubular cell apoptosis and acute kidney injury. Biochim. Et. Biophys. Acta. 2013;1832:1582–1590. doi: 10.1016/j.bbadis.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel V., Li L., Cobo-Stark P., Shao X., Somlo S., Lin F., Igarashi P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum. Mol. Genet. 2008;17:1578–1590. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanigan M.H., Devarajan P. Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther. 2003;1:47–61. [PMC free article] [PubMed] [Google Scholar]
- 10.Arany I., Safirstein R.L. Cisplatin nephrotoxicity. Semin. Nephrol. 2003;23:460–464. doi: 10.1016/s0270-9295(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 11.Yonezawa A. Platinum agent-induced nephrotoxicity via organic cation transport system. Yakugaku zasshi: J. Pharm. Soc. Jpn. 2012;132:1281–1285. doi: 10.1248/yakushi.12-00211. [DOI] [PubMed] [Google Scholar]
- 12.Ishida S., Lee J., Thiele D.J., Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl. Acad. Sci. USA. 2002;99:14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D., Lippard S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 14.Pabla N., Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 15.Kong M.J., Han S.J., Kim J.I., Park J.W., Park K.M. Mitochondrial NADP(+)-dependent isocitrate dehydrogenase deficiency increases cisplatin-induced oxidative damage in the kidney tubule cells. Cell death Dis. 2018;9:488. doi: 10.1038/s41419-018-0537-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasherman Y., Sturup S., Gibson D. Is glutathione the major cellular target of cisplatin? A study of the interactions of cisplatin with cancer cell extracts. J. Med. Chem. 2009;52:4319–4328. doi: 10.1021/jm900138u. [DOI] [PubMed] [Google Scholar]
- 17.Han S.J., Jang H.S., Kim J.I., Lipschutz J.H., Park K.M. Unilateral nephrectomy elongates primary cilia in the remaining kidney via reactive oxygen species. Sci. Rep. 2016;6:22281. doi: 10.1038/srep22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavagnino M., Oslapas A.N., Gardner K.L., Arnoczky S.P. Hypoxia inhibits primary cilia formation and reduces cell-mediated contraction in stress-deprived rat tail tendon fascicles. Muscles, Liga. Tendons J. 2016;6:193–197. doi: 10.11138/mltj/2016.6.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tulub A.A., Stefanov V.E. Cisplatin stops tubulin assembly into microtubules. A new insight into the mechanism of antitumor activity of platinum complexes. Int. J. Biol. Macromol. 2001;28:191–198. doi: 10.1016/s0141-8130(00)00159-8. [DOI] [PubMed] [Google Scholar]
- 20.Kim S., Kim S.Y., Ku H.J., Jeon Y.H., Lee H.W., Lee J., Kwon T.K., Park K.M., Park J.W. Suppression of tumorigenesis in mitochondrial NADP(+)-dependent isocitrate dehydrogenase knock-out mice. Biochim. Et. Biophys. Acta. 2014;1842:135–143. doi: 10.1016/j.bbadis.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Jang H.S., Kim J.I., Kim J., Na Y.K., Park J.W., Park K.M. Bone marrow derived cells and reactive oxygen species in hypertrophy of contralateral kidney of transient unilateral renal ischemia-induced mouse. Free Radic. Res. 2012;46:903–911. doi: 10.3109/10715762.2012.686664. [DOI] [PubMed] [Google Scholar]
- 22.Takaya K., Koya D., Isono M., Sugimoto T., Sugaya T., Kashiwagi A., Haneda M. Involvement of ERK pathway in albumin-induced MCP-1 expression in mouse proximal tubular cells. Am. J. Physiol. Ren. Physiol. 2003;284:F1037–F1045. doi: 10.1152/ajprenal.00230.2002. [DOI] [PubMed] [Google Scholar]
- 23.Quarmby L.M., Parker J.D. Cilia and the cell cycle? J. Cell Biol. 2005;169:707–710. doi: 10.1083/jcb.200503053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larkins C.E., Aviles G.D., East M.P., Kahn R.A., Caspary T. Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol. Biol. Cell. 2011;22:4694–4703. doi: 10.1091/mbc.E10-12-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gratzner H.G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: a new reagent for detection of DNA replication. Science. 1982;218:474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- 26.Verghese E., Ricardo S.D., Weidenfeld R., Zhuang J., Hill P.A., Langham R.G., Deane J.A. Renal primary cilia lengthen after acute tubular necrosis. J. Am. Soc. Nephrol. 2009;20:2147–2153. doi: 10.1681/ASN.2008101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L., Weidenfeld R., Verghese E., Ricardo S.D., Deane J.A. Alterations in renal cilium length during transient complete ureteral obstruction in the mouse. J. Anat. 2008;213:79–85. doi: 10.1111/j.1469-7580.2008.00918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quarmby L.M. Cellular deflagellation. Int. Rev. Cytol. 2004;233:47–91. doi: 10.1016/S0074-7696(04)33002-0. [DOI] [PubMed] [Google Scholar]
- 29.Wang S., Livingston M.J., Su Y., Dong Z. Reciprocal regulation of cilia and autophagy via the MTOR and proteasome pathways. Autophagy. 2015;11:607–616. doi: 10.1080/15548627.2015.1023983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S., Dong Z. Primary cilia and kidney injury: current research status and future perspectives. Am. J. Physiol. Ren. Physiol. 2013;305:F1085–F1098. doi: 10.1152/ajprenal.00399.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdul-Majeed S., Moloney B.C., Nauli S.M. Mechanisms regulating cilia growth and cilia function in endothelial cells. Cell. Mol. Life Sci.: CMLS. 2012;69:165–173. doi: 10.1007/s00018-011-0744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Ribera L., Slattery C., Mc Morrow T., Marcos R., Pastor S. Reactive carbonyl compounds impair wound healing by vimentin collapse and loss of the primary cilium. Food Chem. Toxicol.: Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017;108:128–138. doi: 10.1016/j.fct.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 33.Park K.M. Can tissue cilia lengths and urine cilia proteins be markers of kidney diseases? Chonnam Med. J. 2018;54:83–89. doi: 10.4068/cmj.2018.54.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seeley E.S., Nachury M.V. The perennial organelle: assembly and disassembly of the primary cilium. J. Cell Sci. 2010;123:511–518. doi: 10.1242/jcs.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma N., Kosan Z.A., Stallworth J.E., Berbari N.F., Yoder B.K. Soluble levels of cytosolic tubulin regulate ciliary length control. Mol. Biol. Cell. 2011;22:806–816. doi: 10.1091/mbc.E10-03-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song Y., Brady S.T. Post-translational modifications of tubulin: pathways to functional diversity of microtubules. Trends Cell Biol. 2015;25:125–136. doi: 10.1016/j.tcb.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Z., Schaedel L., Portran D., Aguilar A., Gaillard J., Marinkovich M.P., Théry M., Nachury M.V. Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science. 2017;356:328–332. doi: 10.1126/science.aai8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boekelheide K., Arcila M.E., Eveleth J. Cis-Diamminedichloroplatinum (II) (cisplatin) alters microtubule assembly dynamics. Toxicol. Appl. Pharmacol. 1992;116:146–151. doi: 10.1016/0041-008x(92)90156-m. [DOI] [PubMed] [Google Scholar]
- 39.Tang J., Shi Y., Liu N., Xu L., Zang X., Li P., Zhang J., Zheng X., Qiu A., Zhuang S. Blockade of histone deacetylase 6 protects against cisplatin-induced acute kidney injury. Clin. Sci. 2018;132:339–359. doi: 10.1042/CS20171417. [DOI] [PubMed] [Google Scholar]
- 40.Kratzer E., Tian Y., Sarich N., Wu T., Meliton A., Leff A., Birukova A.A. Oxidative stress contributes to lung injury and barrier dysfunction via microtubule destabilization. Am. J. Respir. Cell Mol. Biol. 2012;47:688–697. doi: 10.1165/rcmb.2012-0161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han S.J., Kim J.H., Kim J.I., Park K.M. Inhibition of microtubule dynamics impedes repair of kidney ischemia/reperfusion injury and increases fibrosis. Sci. Rep. 2016;6:27775. doi: 10.1038/srep27775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel V., Chowdhury R., Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr. Opin. Nephrol. Hypertens. 2009;18:99–106. doi: 10.1097/MNH.0b013e3283262ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu D., Shi S., Wang H., Liao K. Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes. J. Cell Sci. 2009;122:2760–2768. doi: 10.1242/jcs.046276. [DOI] [PubMed] [Google Scholar]