Abstract
Objective
Hyponatremia is closely associated with the pathophysiology of cirrhosis. However, the association between the serum sodium level and the response to tolvaptan is unclear. This study evaluated the factors related to the tolvaptan response and the prognosis in cirrhosis patients with ascites and hyponatremia.
Methods
We retrospectively reviewed the clinical records of cirrhosis patients hospitalized for treatment with tolvaptan. The associations of patient baseline characteristics with the tolvaptan response after one week and of the characteristics after one-month tolvaptan treatment with the prognosis were analyzed.
Results
We analyzed 83 cirrhosis patients with ascites, including 34 patients with hyponatremia. The response rates to tolvaptan in patients with serum sodium <130 mEq/L, 130-135 mEq/L, and >135 mEq/L were 20%, 66%, and 58%, respectively (p=0.22). The serum sodium level was associated with the response to tolvaptan [odds ratio=1.18; 95% confidence interval (CI)=1.02-1.37; p=0.029]. In patients with hyponatremia, the serum sodium level after 1-month tolvaptan treatment was increased compared to baseline (132 mEq/L vs. 136 mEq/L, p=0.006), and an increasing serum sodium level was associated with a lower risk of mortality (hazard ratio=0.85; 95% CI=0.75-0.97; p=0.016). The survival rate was higher in patients with an increase in the serum sodium level after 1 month than in patients with a decreased serum sodium level (p=0.023).
Conclusion
Tolvaptan treatment was effective in cirrhosis patients with ascites and hyponatremia, but a low serum sodium level was associated with non-responsiveness to tolvaptan. An increased serum sodium level after one-month tolvaptan treatment may positively influence the mortality risk in cirrhosis patients with hyponatremia.
Keywords: tolvaptan, liver cirrhosis, ascites, hyponatremia
Introduction
Cirrhosis results from fibrosis of the liver. In cases of decompensation, cirrhosis patients develop ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, variceal hemorrhaging, and hepatocellular carcinoma (1). Cirrhotic ascites is treated with aldosterone antagonists and loop diuretics (2). In some cases, patients develop refractory ascites. Patients with refractory ascites are treated with several therapies, including concentrated ascites reinfusion therapy, transjugular intrahepatic portosystemic shunt, and peritoneovenous shunt (3-5). However, the efficacies of these therapies are limited; 15% of patients diagnosed with ascites die within 1 year, and 44% die within 5 years (2). Tolvaptan, an orally active non-peptide vasopressin V2 antagonist, ameliorates both hyponatremia and heart failure through the excretion of electrolyte-free water (6,7). The use of tolvaptan for fluid retention in liver cirrhosis patients has been approved in Japan. The levels of the water channel aquaporin 2 were found to be decreased after the administration of tolvaptan in liver cirrhosis patients with ascites (8). The drug's efficacy and safety for this purpose have been reported (9-11).
Hyponatremia is a frequent complication of cirrhosis caused by systemic vasodilatation and renal water retention (12). Although several studies have investigated the effect of tolvaptan in cirrhosis patients, the association between the response to tolvaptan and hyponatremia in cirrhosis patients have differed across reports (9,13). The serum sodium (Na) concentration is an important prognostic factor in cirrhosis patients. Tolvaptan improved the serum Na levels in cirrhosis patients with hyponatremia (11), and the normalization of the serum Na level after one week was associated with a favorable outcome of tolvaptan therapy (14). However, the association between the clinical data after more than one week of tolvaptan administration and the patient prognosis is still unclear.
The aim of this study was to clarify the effect of tolvaptan on cirrhosis patients with ascites and hyponatremia. We also evaluated the association between the clinical data recorded after one month of tolvaptan administration and the prognosis in this patient population.
Materials and Methods
Patients and study design
In this retrospective observational study, we reviewed the clinical records of adult cirrhosis patients with ascites who were hospitalized for treatment with tolvaptan at Fukushima Medical University Hospital from September 2013 through September 2017. They had been treated with conventional diuretics, loop diuretics, and/or anti-aldosterone agents along with a salt- and water-restricted diet. The diagnosis of cirrhosis was based on laboratory data, histological findings, and imaging tests, such as ultrasonography, computed tomography, and magnetic resonance imaging. Hyponatremia was defined as serum Na ≤135 mEq/L. Patients without hyponatremia who were treated with tolvaptan were included as the control group. The exclusion criteria were a lack of follow-up data within one week. Among the 85 patients who were initially included in this study, 2 were excluded (1 lost to follow-up within 1 week, and 1 with no data on body weight). A total of 83 patients were ultimately recruited for the analysis in this study.
Tolvaptan was administered at a dose of 3.75 mg, which was increased to 7.5 mg if the response to tolvaptan was insufficient on day 3 after administration. A response to tolvaptan was defined as a decrease in body weight of more than 1.5 kg on day 7 after administration (15). Laboratory data were obtained before treatment and on day 30. Patients were hospitalized for the first week of treatment with tolvaptan. After discharge, tolvaptan treatment was continued, and patients were followed at an outpatient clinic. All patients were treated with tolvaptan for more than 1 month.
We assessed laboratory data, including the total bilirubin (TB), prothrombin time-international normalized ratio (PT-INR), serum albumin (Alb), serum creatinine (Cre), blood urea nitrogen (BUN), and serum Na values, using standard methods. We also assessed the Child-Pugh score, Model for End-Stage Liver Disease (MELD) score, and MELD-Na score (12,16). The change in the score (Δ) was defined as the difference between scores obtained pretreatment and at one month after treatment. For example, ΔMELD was calculated as the (MELD score on day 30) - (MELD score before treatment).
The rates of patients with hepatocellular carcinoma (HCC) or esophageal varices were evaluated. Patients with HCC beyond the Milan criteria also evaluated (17). The degree of esophageal varices was determined using the criteria of the Japan Society for Portal Hypertension (18). The presence of varices was defined as esophageal or gastric varices greater ≥F2 or having a history of endoscopic injection sclerotherapy for varices. The prognosis was assessed by the survival time until death or liver transplantation after tolvaptan treatment.
We retrospectively reviewed patients' medical records. This study was performed according to the ethical standards of the Declaration of Helsinki at Fukushima Medical University Hospital, and the use of an opt-out consent method was approved by the ethics committee of the Fukushima Medical University School of Medicine. A website with additional information, including the opt-out consent form, was established for the study.
Statistical analysis
Clinical data are expressed as the median and 25th-75th percentile ranges. Clinical data were compared between groups by the Mann-Whitney U test. Differences in categorical variables were determined using Fisher's exact test. Multivariate logistic regression analyses were used to assess the predictors of the response to tolvaptan. Predictive factors associated with the response to tolvaptan were used for multivariate analyses. Patients who underwent abdominal paracentesis or albumin infusion within 7 days after tolvaptan administration (n = 14) were excluded when we analyzed the predictive factors associated with the tolvaptan response, as abdominal paracentesis or albumin infusion can affect the decrease in body weight. Correlations between the data were analyzed by Spearman's rank correlation test. Multivariate Cox proportional hazards regression models were used to assess the predictors of the prognosis. Factors associated with prognosis in cirrhosis patients were used for multivariate analyses. The survival rate was estimated by the Kaplan-Meier method. Differences with a value of p<0.05 were considered statistically significant. The data were analyzed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 3.2.1) (19), and a modified version of R commander (version 2.1-7).
Results
Patient baseline clinical characteristics
Among the 83 patients with cirrhotic ascites hospitalized for treatment with tolvaptan, 34 were hyponatremic, and 49 were not hyponatremic. The characteristics of the patients are shown in Table 1. The median age of the 83 patients was 67 years. All patients were classified as Child-Pugh grade B or C. Chronic hepatitis C (37%) and alcoholic liver disease (22%) were the leading causes of cirrhosis. Thirty-five patients had HCC (42%), 23 of whom had HCC beyond the Milan criteria (27%). The median follow-up period was 90 days (range: 7-836). During the follow-up period, 33 patients died, and 5 underwent liver transplantation. Among the 33 patients who died, 17 died from liver failure and 16 from HCC. The median serum Na level was 136 mEq/L. Among the 69 patients who did not undergo abdominal paracentesis or albumin infusion within 7 days after tolvaptan administration, 40 (57%) were responders to tolvaptan. The response rate to tolvaptan did not significantly differ between patients with and without hyponatremia (56% vs. 58%, p=1). We further analyzed the response rate according to the baseline serum Na value: <130 mEq/L, 130-135 mEq/L, and >135 mEq/L. The distribution of the tolvaptan response rate according to the serum Na level is shown in Fig. 1. The response rates to tolvaptan in patients whose serum Na level was <130 mEq/L, 130-135 mEq/L, and >135 mEq/L were 20%, 66%, and 58%, respectively, with no significant differences among the groups (p=0.22).
Table 1.
Patient Baseline Clinical Characteristics and the Rate of Response to Tolvaptan.
| Variables | All (n=83) |
Na ≤ 135 mEq/L (n=34) |
Na > 135 mEq/L (n=49) |
p | ||||
|---|---|---|---|---|---|---|---|---|
| Men, n (%) | 49 (59) | 26 (76) | 23 (46) | 0.012 | ||||
| Age, years | 67 (60-75) | 66 (59-72) | 69 (62-77) | 0.19 | ||||
| Etiology, n (%) | ||||||||
| Chronic hepatitis C | 31 (37) | 13 (38) | 18 (36) | 1 | ||||
| Chronic hepatitis B | 4 (4) | 0 (0) | 4 (8) | 0.14 | ||||
| Alcoholic liver disease | 19 (22) | 12 (35) | 7 (14) | 0.034 | ||||
| Nonalcoholic steatohepatitis | 9 (10) | 2 (5) | 7 (14) | 0.29 | ||||
| Autoimmune liver disease | 13 (15) | 4 (2) | 9 (18) | 0.54 | ||||
| Others | 7 (8) | 3 (8) | 4 (8) | 1 | ||||
| Child-Pugh score | 10 (9-12) | 12 (10-13) | 9 (8-10) | <0.001 | ||||
| Child-Pugh grade, B/C | 34/49 | 6/28 | 28/21 | <0.001 | ||||
| HCC, n (%) | 35 (42) | 18 (52) | 17 (34) | 0.11 | ||||
| Beyond the Milan criteria | 23 (27) | 11 (32) | 12 (24) | 0.45 | ||||
| Esophageal varices, n (%) | 48 (57) | 23 (67) | 25 (51) | 0.17 | ||||
| Dose of loop diuretics, mg | 20 (20-40) | 40 (20-40) | 20 (20-40) | 0.16 | ||||
| Dose of anti-aldosterone agent, mg | 25 (25-50) | 25 (25-50) | 25 (25-50) | 0.82 | ||||
| Albumin infusion or paracentesis, n (%) | 14 (16) | 11 (32) | 3 (6) | 0.002 | ||||
| Total bilirubin, mg/dL | 2.2 (1.2-3.9) | 3.6 (2.3-8.0) | 1.6 (1.1-2.8) | <0.001 | ||||
| Prothrombin time-INR | 1.21 (1.13-1.44) | 1.38 (1.24-1.60) | 1.15 (1.10-1.25) | <0.001 | ||||
| Serum albumin, g/dL | 2.6 (2.4-2.9) | 2.6 (2.2-2.8) | 2.7 (2.5-3.0) | 0.031 | ||||
| Serum creatinine, mg/dL | 0.87 (0.66-1.14) | 0.91 (0.69-1.18) | 0.81 (0.63-1.06) | 0.22 | ||||
| Blood urea nitrogen, mg/dL | 20.0 (14.0-27.0) | 19.0 (14.2-27.0) | 20.0 (14.0-27.0) | 0.67 | ||||
| Blood urea nitrogen/serum creatinine ratio | 21.4 (17.4-26.8) | 21.0 (18.3-24.1) | 22.1 (16.7-29.0) | 0.42 | ||||
| Serum sodium, mEq/L | 136 (134-139) | 132 (130-134) | 139 (137-140) | <0.001 | ||||
| MELD score | 11 (7-15) | 14 (10-19) | 8 (6-12) | <0.001 | ||||
| MELD-Na score | 13 (8-20) | 20 (16-24) | 9 (6-13) | <0.001 | ||||
| Change in body weight, kg | 2.1 (0.4-3.0) | 2.0 (0.8-2.4) | 2.0 (0.1-3.4) | 0.72 | ||||
| Response to tolvaptan, n (%) | 40 (57) | 13 (56) | 27 (58) | 1 |
The values presented are the median values and the 25th-75th percentile ranges.
HCC: hepatocellular carcinoma, INR: international normalized ratio, MELD: Model for End-Stage Liver Disease score
Figure 1.
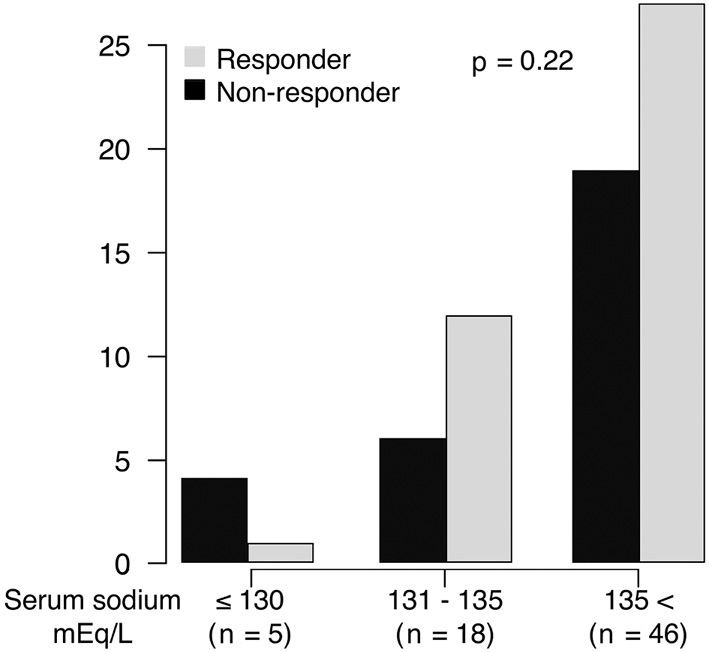
The distribution of the response to tolvaptan according to the serum sodium level. The response rates to tolvaptan in patients whose serum sodium was <130 mEq/L, 130-135mEq/L, and >135 mEq/L were 20%, 66%, and 58%, respectively (p=0.22).
Predictors of a response to tolvaptan
The baseline Cre and MELD-Na score were significantly lower, and the serum Na level was significantly higher in responders to tolvaptan than in non-responders (Table 2). We evaluated the predictors of a tolvaptan response after one week in a multivariate analysis (Table 3). The evaluated factors were the gender, age, Child-Pugh score, HCC beyond Milan criteria, Cre, and serum Na, which have been associated with response to tolvaptan. The serum Na level was significantly associated with a response to tolvaptan after 1 week [odds ratio (OR): 1.18, 95% confidence interval (CI): 1.02-1.37, p=0.029].
Table 2.
Differences between Responders and Non-responders to Tolvaptan.
| Variables | Responder (n=40) |
Non-responder (n=29) |
p | |||
|---|---|---|---|---|---|---|
| Men, n (%) | 21 (52) | 20 (68) | 0.21 | |||
| Age, years | 71 (60-76) | 69 (64-73) | 0.72 | |||
| Etiology, n (%) | ||||||
| Chronic hepatitis C | 19 (47) | 8 (27) | 0.13 | |||
| Alcoholic liver disease | 9 (22) | 6 (20) | 1 | |||
| Child-Pugh score | 9 (9-11) | 10 (8-12) | 0.79 | |||
| Child-Pugh grade, B/C | 10 (9-11) | 10 (8-12) | 0.79 | |||
| HCC, n (%) | 19 (47) | 11 (37) | 0.46 | |||
| Beyond the Milan criteria | 12 (30) | 8 (27) | 1 | |||
| Esophageal varices, n (%) | 24 (60) | 14 (48) | 0.46 | |||
| Dose of loop diuretics, mg | 20 (20-40) | 20 (20-40) | 0.56 | |||
| Dose of anti-aldosterone agent, mg | 25 (25-25) | 25 (0-50) | 0.064 | |||
| Total bilirubin, mg/dL | 1.8 (1.1-3.2) | 2.5 (1.1-3.6) | 0.61 | |||
| Prothrombin time-INR | 1.23 (1.12-1.38) | 1.19 (1.11-1.32) | 0.62 | |||
| Serum albumin, g/dL | 2.7 (2.4-3.0) | 2.6 (2.4-2.9) | 0.37 | |||
| Serum creatinine, mg/dL | 0.79 (0.63-0.94) | 0.92 (0.72-1.37) | 0.026 | |||
| Blood urea nitrogen, mg/dL | 17 (13-23) | 23 (17-34) | 0.030 | |||
| Blood urea nitrogen/serum creatinine ratio | 20.8 (17.3-24.8) | 23.5 (15.8-30.7) | 0.55 | |||
| Serum sodium, mEq/L | 137 (135-140) | 136 (134-137) | 0.042 | |||
| MELD score | 9 (6-13) | 11 (10-16) | 0.063 | |||
| MELD-Na score | 10 (7-16) | 16 (11-21) | 0.013 |
The values presented are the median values and the 25th-75th percentile ranges.
HCC: hepatocellular carcinoma, INR: international normalized ratio, MELD: Model for End-Stage Liver Disease score
Table 3.
Predictors of Response to Tolvaptan in Cirrhosis Patients according to a Multivariate Analysis.
| Variables | OR (95% CI) | p | ||
|---|---|---|---|---|
| Male gender | 0.74 (0.20-2.65) | 0.64 | ||
| Age, years | 1.00 (0.96-1.05) | 0.91 | ||
| Child-Pugh score | 1.16 (0.87-1.54) | 0.31 | ||
| HCC beyond Milan criteria | 1.29 (0.36-4.57) | 0.69 | ||
| Serum creatinine, mg/dL | 0.44 (0.18-1.10) | 0.079 | ||
| Serum sodium, mEq/L | 1.18 (1.02-1.37) | 0.029 |
OR: odds ratio, CI: confidence interval, HCC: hepatocellular carcinoma
Changes in clinical data from before treatment to day 30 of tolvaptan treatment
In patients with hyponatremia, the PT-INR, serum Na level, and MELD score were significantly elevated, and the Alb level and Child-Pugh score were significantly decreased. Although the MELD score was significantly elevated, the MELD-Na score did not show a significant change after treatment (Table 4). In patients without hyponatremia, no significant changes were observed after treatment.
Table 4.
Changes in Clinical Data from before Treatment to Day 30 of Tolvaptan Treatment.
| Na ≤ 135 mEq/L | ||||||
|---|---|---|---|---|---|---|
| Variables | Before treatment | Day 30 | p | |||
| Total bilirubin, mg/dL | 3.6 (2.3-8.0) | 3.3 (2.2-13.4) | 0.85 | |||
| Prothrombin time-INR | 1.38 (1.24-1.60) | 1.40 (1.22-1.52) | <0.001 | |||
| Serum albumin, g/dL | 2.6 (2.2-2.8) | 2.4 (2.0-2.8) | 0.048 | |||
| Serum creatinine, mg/dL | 0.91 (0.69-1.18) | 0.97 (0.77-1.13) | 0.15 | |||
| Blood urea nitrogen, mg/dL | 19.0 (14.2-27.0) | 20.0 (14.0-25.0) | 0.55 | |||
| Blood urea nitrogen/ | ||||||
| Serum creatinine ratio | 21. 0 (18.3-24.1) | 19.4 (16.0-24.6) | 0.60 | |||
| Serum sodium, mEq/L | 132 (130-134) | 136 (133-139) | 0.006 | |||
| ΔSerum sodium, mEq/L | 3 (1-7) | |||||
| MELD score | 14 (10-19) | 15 (11-19) | 0.008 | |||
| ΔMELD | 1 (0-3) | |||||
| MELD-Na score | 20 (16-24) | 18 (13-22) | 0.32 | |||
| ΔMELD-Na | -1 (-3-1) | |||||
| Child-Pugh score | 12 (10-13) | 12 (10-13) | 0.033 | |||
| ΔChild-Pugh score | 0 (-1-0) | |||||
| Na>135 mEq/L | ||||||
| Variables | Before treatment | Day 30 | p | |||
| Total bilirubin, mg/dL | 1.6 (1.1-2.8) | 1.4 (0.9-2.2) | 0.36 | |||
| Prothrombin time-INR | 1.15 (1.10-1.25) | 1.15 (1.09-1.38) | 0.73 | |||
| Serum albumin, g/dL | 2.7 (2.5-3.0) | 2.8 (2.4-3.2) | 0.58 | |||
| Serum creatinine, mg/dL | 0.81 (0.63-1.06) | 0.90 (0.72-1.09) | 0.37 | |||
| Blood urea nitrogen, mg/dL | 20.0 (14.0-27.0) | 20.0 (14.0-28.0) | 0.87 | |||
| Blood urea nitrogen/Serum creatinine ratio | 22.1 (16.7-29.0) | 22.6 (16.8-28.6) | 0.36 | |||
| Serum sodium, mEq/L | 139 (137-140) | 139 (135-141) | 0.11 | |||
| ΔSerum sodium, mEq/L | -1 (-4-1) | |||||
| MELD score | 8 (6-12) | 9 (6-11) | 0.54 | |||
| ΔMELD | 0 (-1-2) | |||||
| MELD-Na score | 9 (6-13) | 10 (7-15) | 0.21 | |||
| ΔMELD-Na | 0 (-2-3) | |||||
| Child-Pugh score | 9 (8-10) | 9 (8-10) | 0.46 | |||
| ΔChild-Pugh score | 0 (0-0) | |||||
The values presented are the median values and the 25th-75th percentile ranges.
INR: international normalized ratio, MELD: Model for End-Stage Liver Disease score, Δ: the difference in scores between pretreatment and at 30 days after treatment
We next evaluated the correlations between the change in the serum Na level and laboratory data before treatment. The ΔNa was significantly correlated with the baseline serum Na level (r=-0.53, p<0.001) but did not correlate with the Child-Pugh score, BUN, Cre, or BUN/Cre ratio (Table 5).
Table 5.
Correlations between the Change in the Serum Sodium Level and the Pretreatment Laboratory Data.
| r | p | |||
|---|---|---|---|---|
| Serum sodium, mEq/L | -0.53 | <0.001 | ||
| Child-Pugh score | 0.22 | 0.072 | ||
| Blood urea nitrogen, mg/dL | 0.12 | 0.33 | ||
| Serum creatinine, mg/dL | 0.13 | 0.26 | ||
| Blood urea nitrogen/serum creatinine ratio | 0.19 | 0.41 |
Predictors of mortality in cirrhosis patients with hyponatremia treated with tolvaptan at day 30
Among the 83 patients, the survival rate was significantly lower in patients with hyponatremia at baseline than in those without hyponatremia (log-rank test: p<0.001). We evaluated the association between the clinical data after one month and the time of mortality in patients with hyponatremia (Table 6). The evaluated factors were the gender, age, Child-Pugh score, HCC beyond Milan criteria, Cre, and ΔNa. In the multivariate analysis, the Child-Pugh score [hazard ratio (HR): 1.66, 95% CI: 1.09-2.55, p=0.018], Cre (HR: 19.36, 95% CI: 3.22-116.0, p=0.001), and ΔNa (HR: 0.85, 95% CI: 0.75-0.97, p=0.016) at day 30 of tolvaptan treatment were significantly associated with mortality. For patients with hyponatremia, the survival rate was significantly higher in the ΔNa >0 group than in the ΔNa ≤0 group (p=0.023, Fig. 2).
Table 6.
Predictors of Mortality in Cirrhosis Patients with Hyponatremia Treated with Tolvaptan at Day 30.
| Variables | HR (95% CI) | p | ||
|---|---|---|---|---|
| Male gender | 6.35 (1.08-37.24) | 0.040 | ||
| Age, years | 0.95 (0.90-1.01) | 0.13 | ||
| HCC beyond Milan criteria | 1.21 (0.29-5.03) | 0.78 | ||
| Child-Pugh score | 1.66 (1.09-2.55) | 0.018 | ||
| Serum creatinine, mg/dL | 19.36 (3.22-116.0) | 0.001 | ||
| ΔSerum sodium, mEq/L | 0.85 (0.75-0.97) | 0.016 |
HR: hazard ratio, CI: confidence interval, HCC: hepatocellular carcinoma, Δ: the difference in scores between pretreatment and at 30 days after treatment
Figure 2.

Kaplan-Meier plots for the survival in patients with hyponatremia. The survival rate was significantly higher in the ΔNa >0 group than in the ΔNa ≤0 group (p=0.023).
Discussion
The present study demonstrated that the response to tolvaptan did not significantly differ between cirrhosis patients with and without hyponatremia, but a low serum Na level was significantly associated with a poor response to tolvaptan in a multivariate analysis. The ΔNa after one-month tolvaptan treatment was significantly associated with the prognosis in cirrhosis patients with ascites and hyponatremia. Furthermore, an increase in the serum Na level after one-month tolvaptan treatment was a predictor of a good prognosis.
There was no significant differences in the response rate between patients with and without hyponatremia, but a significant association was noted between a low serum Na level and the response. Based on these results, we speculate that the response to tolvaptan, as indicated by weight reduction, varies according to the degree of hyponatremia. The change in the serum Na level is known to differ between patients with mild and severe hyponatremia (11). The serum Na level was significantly increased in patients with hyponatremia but not in patients without hyponatremia in our study. Tolvaptan has been shown to improve hyponatremia in patients with cirrhosis (11). However, the efficacy of tolvaptan in patients with cirrhosis and severe hyponatremia appears to be limited (20). The improvement of hyponatremia by tolvaptan is induced via the excretion of free water (7). Weight reduction by tolvaptan is also induced via the excretion of free water (13). Although there were no significant differences in the response rate between patients with different Na levels, the response rate of patients whose serum Na was <130 mEq/L was only 20% in this study. Thus, the effect of weight reduction by tolvaptan may vary according to patients' degree of hyponatremia.
Various results concerning the association between the serum Na level and tolvaptan-induced weight loss in cirrhosis patients have been reported. Kawaratani et al. reported that the serum Na level was significantly lower in non-responders to tolvaptan than in responders (13). Other reports did not find significant differences in the serum Na level between responders to tolvaptan and non-responders (9,21-23). Multiple factors affect the response to tolvaptan, such as the renal function, Child-Pugh score, and presence of advanced HCC (9,21,24). These factors are associated with each other and differ in every reported cohort. Therefore, the interaction of these factors may have caused the differences in results concerning the serum Na level. Indeed, in the present study, the serum Na level was significantly associated with the response to tolvaptan in a multivariate analysis that included the Child-Pugh score, renal function, and advanced HCC. Because these factors are closely associated in the clinical setting, differences in the response rate to tolvaptan between patients with and without hyponatremia may appear small. Consistent with the above-mentioned reports suggesting that the effect of tolvaptan varies according to the degree of hyponatremia, our results suggested that the effect of tolvaptan may decrease as hyponatremia progresses. Given that the response rate of patients with a low serum Na level (<130 mEq/L) was only 20% in this study, starting treatment with tolvaptan before hyponatremia progresses may result in better outcomes.
The serum Na level is associated with the prognosis of liver cirrhosis. The MELD-Na score is an important predictive model of the prognosis for cirrhosis patients (12). A low serum Na level is associated with a poor survival rate in cirrhosis patients (25). Furthermore, the survival rate was significantly lower in patients with hyponatremia at baseline than in those without hyponatremia in this study. Although tolvaptan improved hyponatremia and ascites, two meta-analyses that reviewed trials of vaptans concluded that vaptans did not affect mortality (26,27). However, 1 meta-analysis included only 1 trial of tolvaptan among 12 trials, while the other included 3 trials of tolvaptan among 16 trials. Therefore, the effect of tolvaptan on mortality has not yet been fully elucidated. Kogiso et al. reported that the normalization of hyponatremia was associated with a favorable outcome in cirrhosis patients treated with tolvaptan (14). They speculated that serum Na correction may be associated with an improved survival through a reduction in NO production and complications (14). In the present study, the serum Na level was significantly elevated after one-month treatment with tolvaptan, and a lower serum Na level was associated with an increased risk of mortality. Furthermore, elevation of the serum Na level after treatment with tolvaptan was a predictor of a good prognosis. An increased serum Na level may positively influence the mortality risk in cirrhosis patients with hyponatremia. Changes in the serum Na level were correlated with the baseline serum Na level but not correlated with the Child-Pugh score, BUN, Cre, or BUN/Cre ratio in this study. Even if patients had a high Child-Pugh score or a low renal function, their serum Na level had the potential to increase after one-month treatment with tolvaptan.
The pathways of hyponatremia development and water retention in cirrhosis patients may overlap. Hyponatremia results from either water retention or a loss of electrolytes. In patients with cirrhosis, systemic vasodilation and arterial underfilling are the main mechanisms leading to hyponatremia and portal hypertension (28). Subsequently, the increase in circulating vasodilators plays an important role in the pathogenesis of splanchnic vasodilation (29). In contrast, antidiuretic hormone (ADH) regulates serum osmolality. ADH is released when hypovolemia occurs, binding to the V2 receptor and increasing water reabsorption via aquaporin-2 (30). Therefore, another major factor responsible for hyponatremia is the increased production of ADH due to non-osmotic hypersecretion related to circulatory dysfunction in advanced cirrhosis (31). Consistent with our present results, these reports suggest that the serum Na level is associated with ascites in cirrhosis patients.
Several limitations associated with the present study warrant mention. First, this was a single-center, retrospective study. A multicenter, prospective study is needed to confirm these findings. Second, the number of patients included in this analysis was small. While future studies in a larger number of patients and adding other predictive factors are desirable, our results suggest that the serum Na level is closely associated with the effect of tolvaptan in cirrhosis patients with ascites and hyponatremia. Third, we did not analyze other predictive factors of the response to tolvaptan, such as the urinary sodium excretion and urine Na/K ratio (22,23,32). We also failed to analyze the association between the response to tolvaptan and the urinary electrolyte excretion, as there were few patients in whom urinary electrolytes had been measured. In addition, we did not analyze the symptom reduction by tolvaptan. Hiramine et al. found that two indicators-symptom reduction and body weight loss-were sufficient to evaluate the response to tolvaptan (15). The association between the serum Na level and the symptom reduction by tolvaptan is still unclear.
Conclusion
In conclusion, tolvaptan treatment was effective in cirrhosis patients with ascites and hyponatremia, but a low serum Na level was a predictor of a poor response to tolvaptan. The serum Na level after one month of tolvaptan treatment was associated with the prognosis in cirrhosis patients with ascites and hyponatremia. Therefore, treatment with tolvaptan before hyponatremia progresses may lead to better outcomes in cirrhosis patients with ascites.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Ge PS, Runyon BA. Treatment of patients with cirrhosis. N Engl J Med 375: 767-777, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Planas R, Montoliu S, Balleste B, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol 4: 1385-1394, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Takaki A, Maeshima Y, Yagi T, et al. Peritoneovenous shunting for refractory ascites results in worsening of nephrotic syndrome. Hepatol Res 42: 1048-1053, 2012. [DOI] [PubMed] [Google Scholar]
- 4. Taki Y, Kanazawa H, Narahara Y, et al. Predictive factors for improvement of ascites after transjugular intrahepatic portosystemic shunt in patients with refractory ascites. Hepatol Res 44: 871-877, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Kozaki K, Iinuma M, Takagi T, et al. Cell-free and concentrated ascites reinfusion therapy for decompensated liver cirrhosis. Ther Apher Dial 20: 376-382, 2016. [DOI] [PubMed] [Google Scholar]
- 6. Vaduganathan M, Marti CN, Mentz RJ, et al. Serum osmolality and postdischarge outcomes after hospitalization for heart failure. Am J Cardiol 117: 1144-1150, 2016. [DOI] [PubMed] [Google Scholar]
- 7. Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 355: 2099-2112, 2006. [DOI] [PubMed] [Google Scholar]
- 8. Nakanishi H, Kurosaki M, Hosokawa T, et al. Urinary excretion of the water channel aquaporin 2 correlated with the pharmacological effect of tolvaptan in cirrhotic patients with ascites. J Gastroenterol 51: 620-627, 2016. [DOI] [PubMed] [Google Scholar]
- 9. Chishina H, Hagiwara S, Nishida N, et al. Clinical factors predicting the effect of tolvaptan for refractory ascites in patients with decompensated liver cirrhosis. Dig Dis 34: 659-664, 2016. [DOI] [PubMed] [Google Scholar]
- 10. Sakaida I, Terai S, Kurosaki M, et al. Effectiveness and safety of tolvaptan in liver cirrhosis patients with edema: interim results of post-marketing surveillance of tolvaptan in liver cirrhosis (START study). Hepatol Res 47: 1137-1146, 2016. [DOI] [PubMed] [Google Scholar]
- 11. Cardenas A, Gines P, Marotta P, et al. Tolvaptan, an oral vasopressin antagonist, in the treatment of hyponatremia in cirrhosis. J Hepatol 56: 571-578, 2012. [DOI] [PubMed] [Google Scholar]
- 12. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 359: 1018-1026, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawaratani H, Fukui H, Moriya K, et al. Predictive parameter of tolvaptan effectiveness in cirrhotic ascites. Hepatol Res 47: 854-861, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Kogiso T, Kobayashi M, Yamamoto K, et al. The outcome of cirrhotic patients with ascites is improved by the normalization of the serum sodium level by tolvaptan. Intern Med 56: 2993-3001, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hiramine Y, Uojima H, Nakanishi H, et al. Response criteria of tolvaptan for the treatment of hepatic edema. J Gastroenterol 53: 258-268, 2018. [DOI] [PubMed] [Google Scholar]
- 16. Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 124: 91-96, 2003. [DOI] [PubMed] [Google Scholar]
- 17. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334: 693-699, 1996. [DOI] [PubMed] [Google Scholar]
- 18. Idezuki Y. General rules for recording endoscopic findings of esophagogastric varices (1991). Japanese Society for Portal Hypertension. World J Surg 19: 420-422; discussion 423, 1995. [DOI] [PubMed] [Google Scholar]
- 19. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR' for medical statistics. Bone Marrow Transplant 48: 452-458, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pose E, Sola E, Piano S, et al. Limited efficacy of tolvaptan in patients with cirrhosis and severe hyponatremia: real-life experience. Am J Med 130: 372-375, 2016. [DOI] [PubMed] [Google Scholar]
- 21. Yamada T, Ohki T, Hayata Y, et al. Potential effectiveness of tolvaptan to improve ascites unresponsive to standard diuretics and overall survival in patients with decompensated liver cirrhosis. Clin Drug Investig 36: 829-835, 2016. [DOI] [PubMed] [Google Scholar]
- 22. Kogiso T, Yamamoto K, Kobayashi M, et al. Response to tolvaptan and its effect on prognosis in cirrhotic patients with ascites. Hepatol Res 47: 835-844, 2017. [DOI] [PubMed] [Google Scholar]
- 23. Komiyama Y, Kurosaki M, Nakanishi H, et al. Prediction of diuretic response to tolvaptan by a simple, readily available spot urine Na/K ratio. PLoS One 12: e0174649, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miyazaki M, Yada M, Tanaka K, et al. Efficacy of tolvaptan for the patients with advanced hepatocellular carcinoma. World J Gastroenterol 23: 5379-5385, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Umemura T, Shibata S, Sekiguchi T, et al. Serum sodium concentration is associated with increased risk of mortality in patients with compensated liver cirrhosis. Hepatol Res 45: 739-744, 2015. [DOI] [PubMed] [Google Scholar]
- 26. Yan L, Xie F, Lu J, et al. The treatment of vasopressin V2-receptor antagonists in cirrhosis patients with ascites: a meta-analysis of randomized controlled trials. BMC Gastroenterol 15: 65, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dahl E, Gluud LL, Kimer N, Krag A. Meta-analysis: the safety and efficacy of vaptans (tolvaptan, satavaptan and lixivaptan) in cirrhosis with ascites or hyponatraemia. Aliment Pharmacol Ther 36: 619-626, 2012. [DOI] [PubMed] [Google Scholar]
- 28. John S, Thuluvath PJ. Hyponatremia in cirrhosis: pathophysiology and management. World J Gastroenterol 21: 3197-3205, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gines P, Fernandez-Esparrach G, Arroyo V, Rodes J. Pathogenesis of ascites in cirrhosis. Semin Liver Dis 17: 175-189, 1997. [DOI] [PubMed] [Google Scholar]
- 30. Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci U S A 92: 1013-1017, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gines P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology 48: 1002-1010, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Uojima H, Kinbara T, Hidaka H, et al. Close correlation between urinary sodium excretion and response to tolvaptan in liver cirrhosis patients with ascites. Hepatol Res 47: E14-E21, 2017. [DOI] [PubMed] [Google Scholar]


