Abstract
We herein describe a case of pulmonary tumor thrombotic microangiopathy (PTTM) with rapidly progressing colon cancer. A 61-year-old man who had been receiving treatment for type 2 diabetes mellitus for 3 years was hospitalized due to critical hypoxemia. Computed tomography, which had not shown any abnormalities 3 months previously, revealed a tumor in the ascending colon, multiple nodules in the liver, and the absence of any lung abnormalities. On day 3 of hospitalization, a sudden onset of severe dyspnea and tachycardia occurred, followed by death. Autopsy revealed microscopic metastatic tumor emboli in multiple pulmonary vessels with fibrin thrombus and intimal proliferation, which led to a diagnosis of PTTM.
Keywords: pulmonary tumor thrombotic microangiopathy, dyspnea, colon cancer, signet-ring cell carcinoma, type 2 diabetes, autopsy
Introduction
Pulmonary tumor thrombotic microangiopathy (PTTM) is characterized by rapidly progressing respiratory failure (1). In the past, the concept of PTTM has been described as a condition that tends to be detected on autopsy because of the limitations of imaging techniques (2). We experienced one case of PTTM, which progressed rapidly within 3 days after the detection of the primary lesion in the colon of a patient with uncontrolled type 2 diabetes mellitus. In this case, PTTM manifested as a sudden onset of severe dyspnea and was proven by autopsy as to be the cause of the patient's death. In this report, we present and discuss the finding with consideration of the related literature.
Case Report
A 61-year-old Japanese man with type 2 diabetes mellitus was referred to our hospital from a nearby clinic due to an elevated blood glucose level. Upon assessment, his glycated hemoglobin (HbA1c) level was markedly elevated at 11.3%; thus, he was hospitalized to investigate the cause of his uncontrolled diabetes. The patient was hypertensive and his relevant history included cerebral infarction 3 years previously. His medications included vildagliptin, glimepiride, valsartan, amlodipine, bezafibrate, and clopidogrel. He worked as a welder in a construction company and was not a smoker. His family history was unremarkable. A physical examination on admission revealed a conscious, coherent patient with the following findings: body mass index, 25.9; blood pressure, 128/81 mmHg; sinus rhythm at a rate of 65 beats per minute; and no fever or respiratory distress. The patient's peripheral oxygen saturation (SpO2) was 99% on room air. The palpebral conjunctivae were pink and the bulbar conjunctivae were anicteric. Electrocardiography (ECG) demonstrated a sinus rhythm at a rate of 62 beats per minute. The other organ systems were unremarkable.
Additional examinations revealed a normal complete blood count, hypertriglyceridemia, and slightly elevated levels of liver transaminases, γ-glutamyltransferase, and uric acid. The patient's carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9) levels were within the normal range. The patient's urine C-peptide immunoreactivity, which indicates insulin secretory ability, was high. A fecal occult blood test was positive (Table 1). On plain computed tomography (CT) of the chest and abdomen, severe fatty liver and vascular calcification were observed, but no other space-occupying lesions were seen in the abdomen or chest.
Table 1.
Laboratory Data on First Admission.
| CBC | ||||||
| WBC | 7,200 | /mm3 | Na | 137 | mmol/L | |
| RBC | 538×104 | /mm3 | K | 4.3 | mmol/L | |
| Hb | 16.3 | g/dL | Cl | 104 | mmol/L | |
| Ht | 47.70 | % | e-GFR | 66.6 | mL/min/1.73mm2 | |
| Plt | 30.7×104 | /mm3 | Glu | 218 | mg/dL | |
| Biochemistry | HbA1c (NGSP) | 10.6 | % | |||
| Alb | 4.3 | g/dL | Insulin | 17.1 | μIU/mL | |
| CRP | 0.35 | mg/dL | sCPR | 2.65 | ng/mL | |
| CK | 47 | U/L | uCPR | 182 | μg/day | |
| AST | 82 | U/L | CEA | 2.6 | ng/mL | |
| ALT | 86 | U/L | CA19-9 | <0.4 | U/mL | |
| LDH | 223 | U/L | Urinalysis | |||
| ALP | 195 | U/L | Protein | (-) | ||
| γ-GTP | 147 | U/L | Glucose | (4+) | ||
| Amy | 76 | U/L | Ketone body | (-) | ||
| Cre | 0.9 | mg/dL | Microalbuminurea | 32.9 | mg/L | |
| UA | 7.9 | mg/dL | Glucagon load test | |||
| BUN | 14 | mg/dL | Pre-load | 3.04 | ng/mL | |
| T-chol | 216 | mg/dL | Post-load | 5.28 | ng/mL | |
| TG | 265 | mg/dL | Fecal occult blood 1 | (+) | ||
| HDL | 34 | mg/dL | Fecal occult blood 2 | (+) | ||
| LDL | 138 | mg/dL | ||||
During this hospitalization, insulin therapy (Insulin Lispro; Eli Lilly Japan, Kobe, Japan) was started immediately. On day 9, an optimal pre-prandial blood glucose level was achieved. Thereafter, on day 20, insulin therapy was changed to treatment with oral hypoglycemic agents, including sitagliptin, glimepiride, miglitol, and metformin. He was advised to undergo a further examination to determine the cause of the positive fecal occult blood test, but he refused. He was therefore discharged on day 27 after the adjustment of his diabetes medications.
At two weeks after discharge, he started to lose his appetite. A further blood examination revealed an HbA1c level of 7.4%. Two weeks later, he started experience dyspnea, for which he was subsequently readmitted to our hospital. On admission, he was found to have an intact consciousness, and a physical examination revealed the following findings: low blood pressure (73/45 mmHg); heart rate, 105 beats per minute; tachypnea (respiratory rate 28 breaths per minute), and a low SpO2 on room air (88%). The palpebral conjunctivae were pale, but jaundice was not observed. Lung auscultation was normal. A cardiac examination revealed no murmur or gallop. The patient's abdomen was flat. The other organ systems were unremarkable.
As shown in Table 2, the laboratory data on readmission revealed marked leukocytosis, with normal differential, red blood cell, and platelet counts. Marked liver and kidney dysfunction were observed. HbA1c level had decreased significantly to 6.4%. The CEA level was elevated, but the CA 19-9 level remained within the normal range. The levels of fibrinogen degradation products and D-dimer were elevated. Hypoxemia was confirmed on an arterial blood gas analysis. ECG demonstrated sinus tachycardia at a rate of 120 beats per minute, without findings of right atrial or right ventricular overload, such as P-wave abnormalities. Echocardiography showed a normokinetic left ventricle and the normal chamber sizes of the right atrium and ventricle. Moreover, mild tricuspid regurgitation (up to 1.8 m/s) was observed; however, the right ventricular systolic pressure could not be estimated due to poor imaging of the inferior vena cava.
Table 2.
Laboratory Data on Second Admission.
| CBC | Na | 129 | mmol/L | |||
| WBC | 29,200 | /mm3 | K | 5.0 | mmol/L | |
| RBC | 485×104 | /mm3 | Cl | 94 | mmol/L | |
| Hb | 14.3 | g/dL | T-Bil | 1.4 | mg/dL | |
| Ht | 41.5 | % | Glu | 312 | mg/dL | |
| Plt | 23.7×104 | /mm3 | HbA1c (NGSP) | 6.4 | % | |
| Biochemistry | CEA | 9.5 | ng/mL | |||
| Fibrinogen | 241 | mg/dL | CA19-9 | <0.4 | U/mL | |
| Fibrinolysis | 10.4 | sec | AFP | 2.2 | ng/mL | |
| FDP | 102.1 | µg/mL | PIVKA-II | 142 | mAU/mL | |
| D-dimer | 51.1 | µg/mL | Arterial Blood Gas (room air) | |||
| TP | 6.4 | g/dL | pH | 7.452 | ||
| Alb | 2.6 | g/dL | PCO2 | 22.4 | mmHg | |
| CRP | 15.15 | mg/dL | PO2 | 62.0 | mmHg | |
| CK | 42 | U/L | HCO3- | 15.3 | mmol/L | |
| AST | 121 | U/L | BE | -6.6 | mmol/L | |
| ALT | 77 | U/L | sO2 | 92.3 | % | |
| LDH | 685 | U/L | Arterial Blood Gas (O2 9L/min 2days after) | |||
| ALP | 731 | U/L | pH | 7.428 | ||
| γ-GTP | 791 | U/L | PCO2 | 18.9 | mmHg | |
| Cre | 1.6 | mg/dL | PO2 | 63.9 | mmHg | |
| UA | 14.4 | mg/dL | HCO3- | 12.2 | mmol/L | |
| BUN | 66 | mg/dL | BE | -9.4 | mmol/L | |
| NH3 | 115 | μg/dL | sO2 | 92.9 | % | |
| TG | 294 | mg/dL | ||||
| HDL | 34 | mg/dL | ||||
| LDL | 138 | mg/dL | ||||
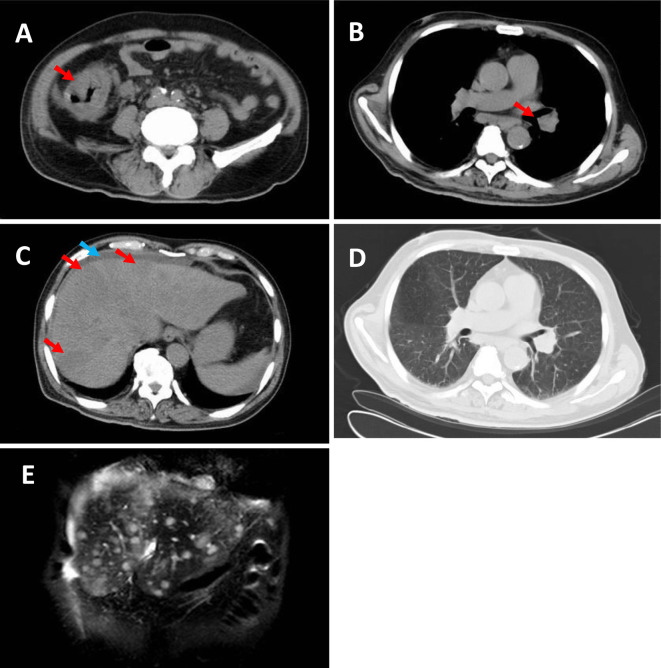
Repeat CT revealed thickening of the wall of the ascending colon and multiple lymphadenopathies in the supraclavicular, mediastinal, left hilar, paraaortic, and ileocolic areas (Fig. 1A and B). Fatty liver was not recognized in this CT scan, but there were new findings of multiple liver nodules and ascites (Fig. 1C). However this CT scan had no abnormal findings that could explain the patient's respiratory distress (Fig. 1D). Magnetic resonance imaging of the liver on the same day showed nodules that were hypointense on T2W1, and hyperintense on T1W1 and diffusion-weighted imaging (Fig. 1E). These data suggested liver metastases or abscess.
Figure 1.
CT during the second hospitalization (A-D). Significant wall thickening in the ascending colon (arrow) (A), swollen lymph nodes in the hilar region of the left lung (arrow) (B), multiple hypodense nodules in the liver (red arrow) and ascites (blue arrow) (C) were observed. There were no abnormal findings in the lungs (D). MRI T2WI during the second hospitalization showed multiple nodules in the liver (E).
On day 1, dopamine infusion (3 μg/kg/min) was started for blood pressure support, with a target systolic blood pressure of >100 mmHg; the maximum blood pressure achieved was 123/89 mmHg. Oxygen (6-8 L/min by reservoir mask) was given to improve his respiratory condition. Because the presence of multiple liver abscesses was one of the differential diagnoses, he was treated for severe infection using meropenem and immunoglobulin preparation. On day 3, he developed supraventricular tachycardia at a rate of 192 beats per minute and severe hypoxemia. Despite intensive care, the patient died a few hours later. Autopsy was performed on the same day.
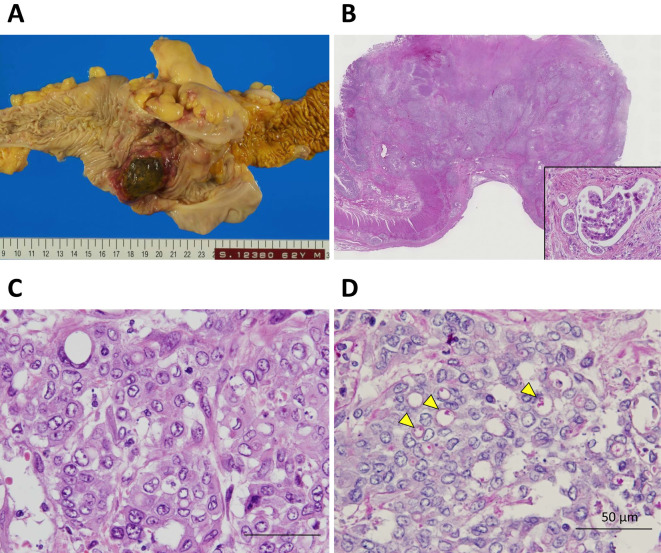
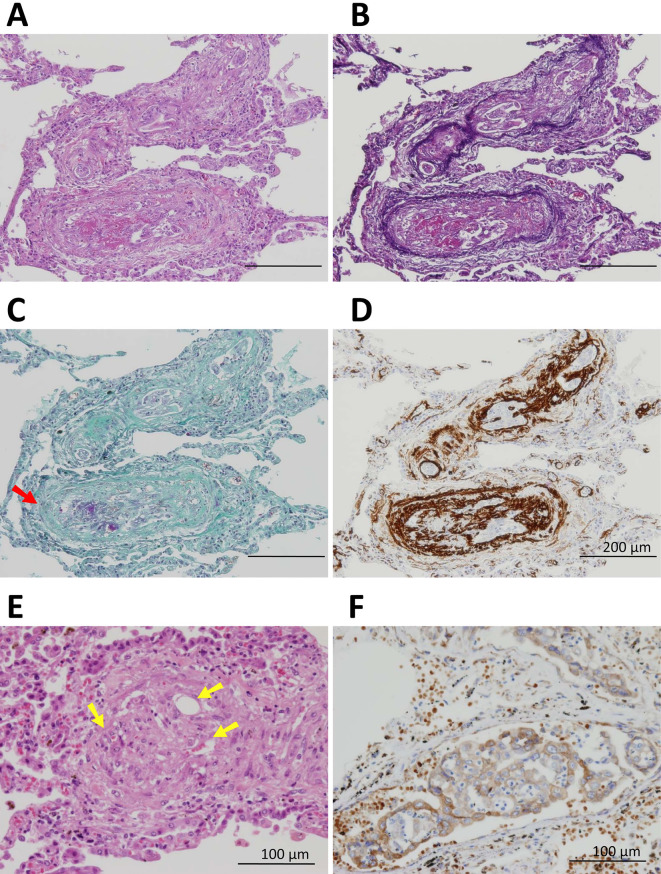
The histological findings of all layers of the ascending colon revealed poorly differentiated adenocarcinoma, with non-solid and signet-ring cell types, mucinous substrate in the cytoplasm, and multiple lymphovascular invasion (Fig. 2). Consistent with the macroscopic and microscopic observations, poorly differentiated adenocarcinoma of colon origin was found to have metastasized to the portal vein and formed a mass with liver necrosis and multiple lymph nodes. Although no solid lung mass was observed macroscopically, the histologic findings revealed tumor cells inside multiple pulmonary vessels and outside the vessels in the form of a small nodule. These tumor cells were similar to the poorly differentiated adenocarcinoma lesion in the colon and liver. Furthermore, in the pulmonary vessels, an abundance of fibrin thrombi were detected by Fraser-Lendrum staining, and intimal proliferation was detected by immunohistochemical staining for alpha smooth muscle actin. Although there were no tumor cells in some of the pulmonary vessels, obliteration due to intimal proliferation and recanalization were observed. A diagnosis of PTTM was confirmed based on the histological findings and the immunohistochemical expression of vascular endothelial growth factor (VEGF) in the cytoplasm of the tumor cells (Fig. 3).
Figure 2.
The macroscopic and microscopic findings of ascending colon cancer. Macroscopically, type I tumor invasion was seen in all layers of the colon wall (A). Microscopically, multiple lymphovascular invasion (inset) (B) and poorly differentiated adenocarcinoma of the non-solid with signet-cell types on Hematoxylin and Eosin staining (C) and periodic acid-Schiff staining (D) were observed; arrowheads indicate the cytoplasmic substrate.
Figure 3.
Pulmonary tumor thrombotic microangiopathy secondary to metastatic poorly differentiated adenocarcinoma of colon origin. Poorly differentiated adenocarcinoma cells similar to colon cancer were seen in multiple pulmonary vessels on Hematoxylin and Eosin staining (A) and Elastica-HE staining (B). A fibrin thrombus (arrow) was detected on Fraser-Lendrum staining (C) and intimal proliferation was detected by immunohistochemical staining for alpha smooth muscle actin (D). In some pulmonary vessels, recanalization (yellow arrows) was observed (E). The expression of vascular endothelial growth factor (VEGF) was detected in the cytoplasm of tumor cells (F).
Discussion
In 1990, Herbay et al. (1) proposed PTTM as a special subtype of pulmonary artery tumor embolism wherein a tumor embolus is exclusively formed in the pulmonary microarteries, leading to pulmonary hypertension that causes acute respiratory failure. The clinical features of PTTM include progressive dyspnea and cough, enhanced coagulation and fibrinolysis, and a significant drop in the pulmonary alveolar diffusing capacity on a blood gas analysis. The histological characteristics of PTTM include fibrocellular intimal thickening of pulmonary endoplasmic microarteries, tumor embolus, and thrombus organization and recanalization. In this case, almost all symptoms of PTTM-with the exception of cough-were observed, and all of the characteristic histological findings of PTTM were seen.
PTTM is commonly diagnosed on autopsy. In some cases, however, the presence of PTTM is proven by a lung perfusion scan (3,4) or transbronchial biopsy (5). In one case in which a lung abnormality was observed on a CT scan, lung biopsy with video-assisted thoracoscopic surgery revealed findings that were compatible with PTTM (6). In addition, Swan-Ganz catheterization for percutaneous thrombectomy was reported to have provided a definite diagnosis of PTTM based on cytology (7). In cases without any chest CT or ECG abnormalities-such as the present case-PTTM cannot be excluded in the differential diagnoses. Furthermore, in a previous case report, a lung perfusion scan showed no abnormal findings and the findings of diffuse intravascular tumor emboli were only detected on autopsy (8). Thus, the use of multiple imaging modalities might be able to detect PTTM. In this case, however, the patient's sudden and rapid clinical deterioration precluded the diagnosis of PTTM.
Previous reports have stated that PTTM progresses very rapidly. In this case, PTTM, as well as the primary lesion and its metastases, rapidly progressed, leading to the patient's death. We considered that the hyperinsulinemic state during the first hospitalization was the cause of the rapid development of the malignant tumor in this patient. The activation of the PI3 kinase/Akt pathway during hyperinsulinemia has been reported to activate the mitogenic signaling of tumor cells (9). In addition, activation of insulin-like growth factor-1 during hyperinsulinemia was shown to reduce apoptosis and stimulate cellular proliferation in tumors (10). In cancer cells, insulin resistance was reported to upregulate the isoform A of insulin receptor (IR-A) (11), and subsequently insulin-like growth factor-2/ IR-A signaling, thereby promoting cancer cell growth (12). Considering this relationship between diabetes mellitus and cancer, tumors are expected to progress more rapidly in patients with diabetes mellitus in comparison to non-diabetic individuals. Previously, poor glycemic control in patients with Type 2 diabetes mellitus was reported to be associated with a clinically aggressive course of colorectal cancer (13). In this case, the underlying uncontrolled diabetes mellitus with hyperinsulinemia might have enhanced the rapid progression of the primary and metastatic lesions, and resulted in PTTM. In fact, in a Japanese epidemiological study, diabetes mellitus was reported to be a risk factor for several cancers, including liver, pancreas, and colon cancers (14). We therefore emphasize the importance of screening for liver, pancreatic, and colon cancers in patients with diabetes mellitus.
In the present case, signet-ring cells were histologically detected on autopsy. Primary signet-ring cell carcinoma (SRCC) of the colon and rectum is reported to be a rare variant of colorectal adenocarcinoma (15,16) with an aggressive clinical course and a poor prognosis (17). In a previous autopsy study, the histologic subtype was found to strongly influence the pattern of metastasis in patients with colorectal cancer (18). In that study, the incidence of primary cancer was only 1.2% for SRCC, while the incidence of adenocarcinoma and mucinous adenocarcinoma was 84.9% and 13.9%, respectively. However, metastatic disease was present in 28.9% of patients and was found in 61.2%, 27.6%, and 33.9% of patients with SRCC, adenocarcinoma and mucinous adenocarcinoma, respectively. Thus, colon SRCC seems to be a rare subtype that can easily metastasize. In this case, the SRCC histologic subtype of the primary tumor was therefore considered to be a major factor that predisposed the patient to the development of PTTM.
Options such as molecular-targeted therapy with bevacizumab, are now available and have been shown to be effective for treating PTTM from colon cancer (19). Because the time from the onset of symptoms to death is short, an early diagnosis of PTTM is essential for enabling intervention. Correspondingly, early cancer detection in diabetic patients is important for preventing metastasis and the possible development of PTTM.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Von Herbay A, Illes A, Waldherr R, et al. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer 66: 587-592, 1990. [DOI] [PubMed] [Google Scholar]
- 2. Gavin MC, Morse D, Partridge AH, Levy BD, Loscalzo J. Clinical problem-solving. Breathless. N Engl J Med 366: 75-81, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Roberts KE, Hamele-Bene D, Saqi A, Stein CA, Cole RP. Pulmonary tumor embolism : a review of the literature. Am J Med 115: 228-232, 2003. [DOI] [PubMed] [Google Scholar]
- 4. Crane R, Rudd TG, Dail D. Tumor microembolism: pulmonary perfusion pattern. J Nucl Med 25: 877-880, 1984. [PubMed] [Google Scholar]
- 5. Noguchi S, Imanaga T, Shimizu M, Nakano T, Miyazaki N. A case of pulmonary tumor thrombotic microangiopathy diagnosed by transbronchial lung biopsy. Nihon Kokyuki Gakkai Zasshi 46: 493-496, 2008(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 6. Kayatani H, Matsuo K, Ueda Y, et al. Pulmonary tumor thrombotic microangiopathy diagnosed antemortem and treated with combination chemotherapy. Intern Med 51: 2767-2770, 2012. [DOI] [PubMed] [Google Scholar]
- 7. Keenan NG, Nicholson AG, Oldershaw PJ. Fatal acute pulmonary hypertension caused by pulmonary tumour thrombotic microangiopathy. Int J Cardiol 124: e11-e13, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Nakamura H, Adachi H, Sudoh A, et al. Subacute cor Pulmonale due to tumor embolism. Intern Med 43: 420-422, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2: 489-501, 2002. [DOI] [PubMed] [Google Scholar]
- 10. Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrin Met 17: 328-336, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Algire C, Amrein L, Bazile M, David S, Zakikhani M, Pollak M. Diet and tumour LKB1 expression interact to determine sensitivity to anti-neoplastic effects of metformin in vivo. Oncogene 30: 1174-1182, 2011. [DOI] [PubMed] [Google Scholar]
- 12. Garofalo C, Manara MC, Nicoletti G, et al. Efficacy of and resistance to anti-IGF-1R therapies in Ewing's sarcoma is dependent on insulin receptor signaling. Oncogene 30: 2730-2740, 2011. [DOI] [PubMed] [Google Scholar]
- 13. Siddiqui AA, Spechler SJ, Huerta S, Dredar S, Little BB, Cryer C. Elevated HbA1c is an independent predictor of aggressive clinical behavior in patients with colorectal cancer: a case-control study. Digest Dis Sci 53: 2486-2494, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med 166: 1871-1877, 2006. [DOI] [PubMed] [Google Scholar]
- 15. Almagro UA. Primary signet-ring carcinoma of the colon. Cancer 52: 1453-1457, 1983. [DOI] [PubMed] [Google Scholar]
- 16. Giacchero A, Aste H, Baracchini P, et al. Primary signet-ring carcinoma of the large bowel. Report of nine cases. Cancer 56: 2723-2726, 1985. [DOI] [PubMed] [Google Scholar]
- 17. Sim HL, Tan KY, Poon PL, Cheng A. Primary rectal signet-ring cell carcinoma with peritoneal dissemination and gastric secondaries. World J Gastroenterol 14: 2118-2120, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hugen N, van de Velde JH, de Wilt JH, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol 25: 651-657, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higo K, Kubota K, Takeda A, Higashi M, Ohishi M. Successful antemortem diagnosis and treatment of pulmonary tumor thrombotic microangiopathy. Intern Med 53: 2595-2599, 2014. [DOI] [PubMed] [Google Scholar]