Abstract
A 75-year-old man visited our hospital complaining of a low-grade fever, dry cough, and chest abnormal shadow. Chest computed tomography revealed a nodule with a cavity in the right upper lobe. Endobronchial ultrasonography (EBUS) of the lesion suggested that the lesion was benign. Actinomyces graevenitzii was cultured from the specimen obtained by bronchoscopy using endobronchial ultrasonography with a guide sheath technique and was identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and 16S rRNA sequencing. The patient was treated with intravenous ampicillin; subsequently, his condition improved. We believe that careful observation of EBUS findings may be useful for the differential diagnosis.
Keywords: pulmonary actinomycosis, Actinomyces graevenitzii, endobronchial ultrasonography with a guide sheath (EBUS-GS), bronchoscopy
Introduction
Pulmonary actinomycosis is an indolent, slowly progressive infection caused by the anaerobic Actinomyces bacteria (1). Because the clinical and radiological findings of pulmonary actinomycosis mimic malignancy, it is frequently misdiagnosed, and adequate treatment is delayed. In addition, the identification of Actinomyces from clinical samples is difficult. Therefore, pulmonary actinomycosis is often diagnosed as a result of surgical operation. We herein report a case of pulmonary A. graevenitzii infection, which was identified by the anaerobic culture of samples obtained by bronchoscopy using endobronchial ultrasonography with a guide sheath (EBUS-GS).
Case Report
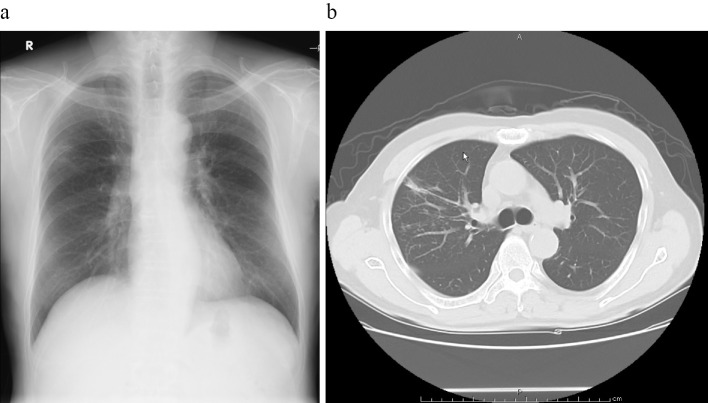
A 75-year-old man visited a regional hospital with a 10-day history of low-grade fever and dry cough. He had a smoking history of 40 cigarettes per day for 35 years. His medical history included pneumonia and Guillain-Barré syndrome, and he had periodontitis. He underwent chest radiography, which revealed infiltrate in the right upper lobe. He was referred to Miyazaki Prefectural Miyazaki Hospital for an examination under suspicion of lung cancer. His vital signs were as follows: temperature, 35.6℃; blood pressure, 117/73 mmHg; pulse, 69/min; respiratory rate, 20/min with an O2 saturation of 94% on room air. The results of a hemogram revealed a normal leucocyte count of 6,590 /μL, and the renal and liver parameters were normal. The C-reactive protein level was 4.38 mg/dL. The tumor markers CEA, CYFRA, NSE, and proGRP were negative (Table). A sputum Gram stain was negative, while sputum and blood cultures revealed no growth. Chest radiograph revealed a nodule with a cavity in the right upper lobe, while chest computed tomography (CT) demonstrated a nodule with a cavity (Fig. 1).
Table.
Laboratory Findings on Admission.
| Hematology | Biochemistry | Tumor marker | |||||||||||
| WBC | 6,590 | /mm3 | TP | 7.4 | g/dL | CEA | 1.4 | ng/dL | |||||
| Band | 0.0 | % | Alb | 3.3 | g/dL | CYFRA | 1.2 | ng/dL | |||||
| Seg | 65.0 | % | T-Bil | 0.4 | mg/dL | proGRP | 49.68 | pg/mL | |||||
| Eo | 2.0 | % | AST | 23 | U/L | NSE | 10.14 | ng/mL | |||||
| Ba | 0.0 | % | ALT | 11 | U/L | ||||||||
| Mo | 9.0 | % | LDH | 187 | U/L | Bacteriology | |||||||
| Ly | 24.0 | % | ALP | 258 | U/L | T-SPOT | (-) | ||||||
| Hb | 13.2 | g/dL | γ-GTP | 28 | U/L | ||||||||
| Ht | 39.7 | % | BUN | 18.0 | mg/dL | Serology | |||||||
| Plt | 33.4×104 | /mm3 | Cr | 0.77 | mg/dL | HBsAg | (-) | ||||||
| CRP | 4.38 | mg/dL | HCVAb | (-) | |||||||||
| Coagulation | Glu | 105 | mg/dL | ||||||||||
| PT | 11.2 | s | HbA1c | 6.3 | %(NGSP) | ||||||||
| PT-INR | 0.93 | ||||||||||||
| APTT | 41.0 | s | |||||||||||
| Fibrinogen | 850 | mg/dL | |||||||||||
Figure 1.
Chest X-ray and computed tomography (CT). a: The radiograph shows a nodule with a cavity in the right upper lung. b: The chest CT image shows a nodule with a cavity in the right upper lobe.
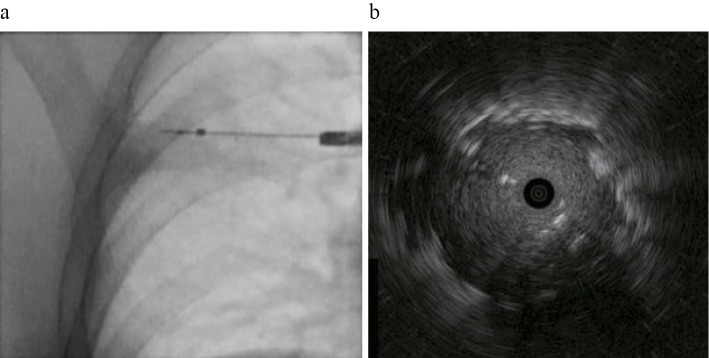
We considered lung cancer, an acid-fast bacillus infection, and pulmonary actinomycosis as the differential diagnoses based on his smoking history and the presence of periodontitis. For the diagnosis, we performed bronchoscopy using EBUS-GS, as described previously (2). After localizing the lesion using a radial probe, we obtained EBUS internal echo images (Fig. 2). The internal echo image of the lesion was homogeneous, prompting a designation of type Ib under Kurimoto's classification (2). After observing the EBUS findings, we performed bronchial brushing (BB), a transbronchial biopsy (TBB), and bronchial washing. After brushing, the brush was withdrawn, and the material's cells were transferred directly onto clean glass slides. Subsequently, we washed the brush in saline (BB washing). We then performed a TBB five times. After BB and the TBB, bronchial washings was obtained by lavage with 20 mL of normal saline. The bronchial washing was mixed with the BB washing, and 5 mL of the mixture was transferred immediately into a container for anaerobic culture.
Figure 2.
Fluoroscopy and endobronchial ultrasonography (EBUS) internal echo image. a: The probe is inserted into the lesion in the right upper lobe. b: EBUS demonstrates a hypoechoic homogeneous lesion.
Primary culturing of that mixture was performed with blood, chocolate, and Brucella agar with hemin and vitamin K1 (Brucella HK agar). Brucella HK agar was incubated at 35℃ under anaerobic conditions. After 96 hours of incubation, only molar-tooth-like colonies were observed on Brucella HK agar. The organism was a coryneform Gram-positive rod that did not produce catalase. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was performed for identification, and we identified the isolate as A. graevenitzii. For confirmation, we performed molecular identification by polymerase chain reaction (PCR) amplification and a sequencing analysis of the 16S rRNA gene using DNA extracted from the isolated organism. We performed the sequencing analysis using a GenBank BLAST search. The sequence of the 16S rRNA gene was 99.0% identical to that of the type strain of A. graevenitzii.
No other bacteria or fungi were detected in this specimen, and the results of the acid-fast staining and PCR tests of the patient's sample for Mycobacterium tuberculosis, M. avium, and M. intracellulare were negative. The histological findings of the TBB specimen and the cytological findings of BB revealed only nonspecific inflammation, and the characteristic histology of pulmonary actinomycosis, such as granuloma or sulphur granules, was not observed. In addition, no microorganisms were detected by Gram or PAS stains. These results indicated that the pulmonary lesion of our patient was caused by A. graevenitzii.
The patient was treated with 9,000 mg ampicillin/sulbactam intravenously for 1 week. After the identification of A. graevenitzii, he was treated with 6,000 mg of ampicillin intravenously for 1 week. Subsequently, he was treated with 1,500 mg of ampicillin orally for 2 months. The clinical status gradually improved. The lung nodule disappeared in a dramatic response to the antibiotics (Fig. 3). We plan to continue treatment for the next 10 months.
Figure 3.
Chest X-ray and computed tomography after treatment. a: The radiograph shows remission of the nodule in the right upper lung. b: The nodule with a cavity in the right upper lobe has disappeared.
Discussion
We encountered a case of pulmonary A. graevenitzii infection diagnosed by bronchoscopy using EBUS-GS and by molecular identification of the sample. In addition, we demonstrated that appropriate anaerobic culture of bronchial brushing samples and washing fluid were useful for the identification of the pathogen of pulmonary actinomycosis.
Pulmonary actinomycosis probably results from the aspiration of oropharyngeal or gastrointestinal secretions into the respiratory tract (3). Insufficient intraoral hygiene and associated dental disease may increase the risk (4). Pulmonary actinomycosis is rarely caused by distant hematogenous seeding (5). The patient in our case had periodontitis, so his poor oral hygiene may have been related to the pathogenesis of pulmonary actinomycosis infection.
Six species of this genus are considered pathogenic in humans: A. israeli, A. naeslundii, A. odontolyticus, A. viscosus, A. meyeri, and A. gerencseriae (6,7). A. graevenitzii was first isolated in 1997 from respiratory and bone specimens (8), while cases of pulmonary infection caused by A. graevenitzii have been reported before (9,10). However, little is known about the clinical features and pathogenesis of this bacterium. Because of its rarity, more studies on such cases are required to determine the pathogenesis of A. graevenitzii.
The diagnosis of pulmonary actinomycosis can be quite challenging. In one series, the diagnosis was suspected upon hospital admission in 7% of patients who later turned out to have the infection (11). Several reports found that 25-49% of cases of pulmonary actinomycosis were initially suspected of having lung malignancy at hospital admission (6,12,13). Even when the clinical suspicion of pulmonary actinomycosis is high, microbiological confirmation can be difficult. Medical intervention alone is sufficient for treatment; therefore, a careful diagnosis can avoid unnecessary surgery.
Bronchoscopy is a viable diagnostic method; however, the bacteriological identification of Actinomyces species from the samples obtained by bronchoscopy is reportedly very difficult. The sample should be handled anaerobically with caution, as the culture of Actinomyces species can be falsely negative if the sample is exposed to air for more than 20 minutes (6).
To diagnose pulmonary actinomycosis appropriately, it is important to consider pulmonary actinomycosis as a differential diagnosis of lung tumor-related shadow. In addition, to identify bacteria, we must ensure that we handle specimens appropriately and culture them under anaerobic conditions.
In the present case, we performed bronchoscopy using EBUS-GS. The utility of EBUS has been reported for the diagnosis of not only malignant lung diseases but also benign ones (14). Cases of actinomycosis of the lung diagnosed using EBUS have also been reported (15,16). However, in those reports, EBUS was used only to confirm the lesions. Notably, we used EBUS not only for the identification of the lesion but also to observe its internal structure. Kurimoto et al. reported that the classification of ultrasonograms suggests the pathology and histology of the lesions (2). EBUS revealed that this lesion had a homogeneous pattern without vessels or bronchioles, which is a type Ib pattern under Kurimoto's classification. Type Ib is said to primarily reflect organized pneumonia or tuberculoma. The pathological feature of pulmonary actinomycosis is chronic inflammation comprising a granulomatous change (17); therefore, the EBUS findings of our patient may reflect the pathological features, particularly the granulomatous changes of the lesion. More studies reporting EBUS findings in cases of actinomycosis are necessary. In addition, a careful analysis of the EBUS findings will encourage physicians to consider the possibility of actinomycosis as the differential diagnosis, even if it had not been considered before bronchoscopy, thereby leading physicians to perform appropriate sampling and sample processing.
To our knowledge, this is the first report describing the usefulness of EBUS internal echo imaging in diagnosing pulmonary actinomycosis caused by A. graevenitzii, which was diagnosed using a molecular analysis of the sample obtained by bronchoscopy.
In conclusion, for the appropriate diagnosis of pulmonary actinomycosis, it is important that the samples be prepared and handled while considering the possibility of pulmonary actinomycosis. In addition, the careful observation of the EBUS findings may be useful for the differential diagnosis.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We are grateful to Kazuhiro Sakada, Issaku Yamamoto, Akira Sata (Department of Clinical Laboratory, Miyazaki prefectural Miyazaki Hospital), Nami Tsuru, and Shuji Yoshino (Miyazaki Prefectural Institute for Public Health and Environment) for their assistance in identifying A. graevenitzii.
References
- 1. Russo TA. Actinomycosis and Whipple's disease. In: Harrison's Principles of Internal Medicine. 19th ed. Kasper DL, Hauser SL, Jameson JL, et al. , Eds. McGraw-Hill, New York, 2015: 1088-1093. [Google Scholar]
- 2. Kurimoto N, Murayama M, Yoshioka S, Nishisaka T. Analysis of the internal structure of peripheral pulmonary lesions using endobronchial ultrasonography. CHEST 122: 1887-1894, 2002. [DOI] [PubMed] [Google Scholar]
- 3. Bennhoff DF. Actinomycosis: diagnostic and therapeutic considerations and a review of 32 cases. Laryngoscope 94: 1198-1217, 1984. [DOI] [PubMed] [Google Scholar]
- 4. Russo TA. Agents of actinomycosis. In: Principles and Practice of Infectious Disease. 5th ed. Mandell GL, Ed. Churchill Livingstone, New York, 1995: 2645-2654. [Google Scholar]
- 5. Apothloz C, Regamey C. Disseminated infection due to Actinomyces myeri - case report and review. Clin Infect Dis 22: 621-625, 1995. [DOI] [PubMed] [Google Scholar]
- 6. Mabeza GF, Macfarlane J. Pulmonary actinomycosis. Eur Respir J 21: 545-551, 2003. [DOI] [PubMed] [Google Scholar]
- 7. Smego RA, Foglia G. Actinomycosis. Clin Infect Dis 26: 1255-1263, 1998. [DOI] [PubMed] [Google Scholar]
- 8. Ramos CP, Falsen E, Alvarez N, Akervall E, Sjoden B, Collins MD. Actinomyces graevenitzii sp. nov., isolated from human clinical specimens. Int J Syst Bacteriol 47: 885-888, 1997. [DOI] [PubMed] [Google Scholar]
- 9. Tietz A, Aldridge KE, Figueroa JE. Disseminated coinfection with Actinomyces graevenitzii and Mycobacterium tuberculosis: case report and review of the literature. J Clin Microbiol 43: 3017-3022, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gliga S, Devaux M, Gosset Woimant M, Mompoint D, Perronne C, Dvido B. Actinomyces gravenitzii pulmonary abscess mimicking tuberculosis in a healthy young man. Can Respir J 21: 75-77, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weese WC, Smith IM. A study of 57 cases of actinomycosis over a 36-year period. Arch Intern Med 135: 1562-1568, 1975. [PubMed] [Google Scholar]
- 12. Kolditz M, Bickhardt J, Matthiessen W, Holotiuk O, Höffken G, Koschel D. Medical management of pulmonary actinomycosis: data from 49 consecutive cases. J Antimicrob Chemother 63: 839-841, 2009. [DOI] [PubMed] [Google Scholar]
- 13. Song JU, Park HY, Jeon K, Um SW, Kwon OJ. Treatment of thoracic actinomycosis: a retrospective analysis of 40 patients. Ann Thorac Med 5: 80-85, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shinagawa N, Nakano K, Asahina H, et al. Endobronchial ultrasonography with a guide sheath in the diagnosis of benign peripheral diseases. Ann Thorac Surg 93: 951-957, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Fujita Y, Iikura M, Horio Y, Ohkusu K, Kobayashi N. Pulmonary Actinomyces graevenitzii infection presenting as organizing pneumonia diagnosed by PCR analysis. J Med Microbiol 61: 1156-1158, 2012. [DOI] [PubMed] [Google Scholar]
- 16. Arimura Y, Kitamura A, Tsuchida S, et al. A case of pulmonary actinomycosis diagnosed by bronchoscopy using endobronchial ultrasonography with a guide sheath method. J Jpn Soc Respir Endosc 38: 285-290, 2016(in Japanese, Abstract in English). [Google Scholar]
- 17. Kim TS, Han J, Koh WJ, et al. Thoracic actinomycosis: CT features with histopathologic correlation. Am J Roentgenol 186: 225-231, 2006. [DOI] [PubMed] [Google Scholar]