Abstract
Objective
Clinically, patients with proton pomp inhibitor (PPI)-resistant gastroesophageal reflux disease (GERD) are very challenging to treat. The aim of this study was to determine the rates of symptom relief and adverse events among PPI-resistant GERD patients that changed their therapy from a PPI to vonoprazan.
Methods
Patients with severe gastroesophageal reflux symptoms (total GERD-Q score ≥8) without endoscopic findings of mucosal breaks who changed their medication from a PPI to vonoprazan during a 12-week period from 2015 to 2016 at 2 hospitals were selected. The primary outcome was the self-reported relief of gastroesophageal reflux symptoms. The odds ratio (OR) for the improvement of symptoms was calculated based on an exact binomial distribution using a matched-pair analysis. The secondary outcome was the GERD-Q score and adverse events.
Results
Twenty-six patients (6 men) with a mean age of 67.5 years were analyzed. After the therapy was changed from a PPI to vonoprazan, 18 patients (69.2%) reported an improvement, 6 (23.1%) reported no change, and 2 (7.7%) reported an exacerbation of symptoms. A change in therapy was significantly associated with improved self-reported symptoms (OR 9.0, p<0.001). The mean total GERD-Q score during vonoprazan treatment was significantly lower than that during PPI therapy (11.96 vs. 8.92). There were no significant differences in the incidence of adverse events between the therapies.
Conclusion
Changing the medication from a PPI to vonoprazan was significantly associated with an improvement in gastroesophageal reflux symptoms. Vonoprazan is one of the most promising treatment options for patients with PPI-resistant GERD.
Keywords: proton pump inhibitor, NERD, vonoprazan, gastroesophageal reflux symptoms, GERD-Q
Introduction
Gastroesophageal reflux disease (GERD) is a common disease encountered in daily clinical practice. The prevalence of GERD in Japan is approximately 10%, which is similar to the prevalence in Western countries (1), and is increasing (2). More than half of GERD cases in Japan show normal esophageal mucosa on endoscopy, which is known as non-erosive reflux disease (NERD) (3). Proton pump inhibitors (PPIs) are the primary treatment option for GERD and NERD and provide superior symptom relief and mucosal healing to other drugs, such as histamine H2-receptor antagonists and mucoprotective agents (4,5). As such, PPIs are recommended as first-line drugs for patients with GERD and NERD (3).
However, an important clinical issue is that a subgroup of GERD patients does not have satisfactory improvement, even after PPI therapy. In particular, NERD patients are less sensitive to PPI treatment than reflux esophagitis patients (6,7). Several medical (8,9), surgical (10,11), and endoscopic (12) approaches have been performed as treatments for NERD, but no therapy other than PPI treatment is currently recommended for NERD patients (3). Changing the type of PPI and the addition of other medications have been proposed (8,9), but the evidence for these strategies is limited. Therefore, another effective pharmacological approach is urgently required for the management of NERD.
Recently, vonoprazan, a new agent for gastric acid suppression, has been used as a treatment option for GERD in Japan (13). Vonoprazan is an active acid blocker that inhibits the proton pump activity of cytoplasmic tubulovesicles and secretory canaliculus, leading to the strong inhibition of acid production compared to conventional PPIs (14,15). Its efficacy for endoscopic erosive esophagitis was reported in a multicenter randomized controlled trial; vonoprazan showed non-inferiority to lansoprazole for mucosal healing (13). Another observational study reported that vonoprazan is effective in treating PPI-resistant reflux esophagitis (16). However, its efficacy for treating GERD symptoms, especially in NERD patients, is not well defined.
Given the strong acid-inhibition effects of vonoprazan, we hypothesized that it would improve the gastroesophageal reflux symptoms in patients with NERD. We performed a preliminary study to evaluate the effects of changing the medication from a PPI to vonoprazan. The aims of this study were to elucidate the rates of symptom relief and adverse events after changing therapy from a PPI to vonoprazan in NERD and PPI-resistant NERD patients and to identify factors that predict symptom relief among NERD patients taking vonoprazan.
Materials and Methods
Study design and setting
We performed a retrospective self-controlled study from 2015 to 2017 at the Graduate School of Medicine, the University of Tokyo, and the Japan Association for Development of Community Medicine, Nerimahikarigaoka Hospital.
We were interested in the effects of changing the therapy from a PPI to vonoprazan in patients with NERD. Because almost all patients with persistent refractory NERD had changed their therapy from a PPI to vonoprazan at our hospitals, we were unable to define a suitable control group to evaluate the effects of the therapy change. Therefore, we selected a self-controlled design to compare gastroesophageal reflux symptoms before and after the therapy change in the same patients.
Participants
We selected outpatients ≥18 years of age with persistent severe gastroesophageal reflux symptoms (total GERD-Q score ≥8) without endoscopic findings of mucosal breaks on an upper gastrointestinal endoscopy examination performed before the initial PPI treatment and who changed their therapy from a PPI to vonoprazan. PPI-resistant NERD was defined as a condition in which reflux symptoms were not sufficiently mitigated (total GERD-Q score ≥8) even after the oral administration of a PPI at a standard or double dose for 8 weeks (17-20). This study was approved by the institutional review board of the University of Tokyo (No. 2058).
The evaluation of GERD symptoms and the effects of switching from a PPI to vonoprazan
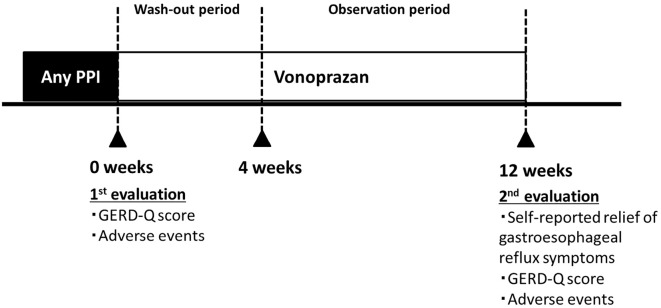
Patients had their first evaluation for gastroesophageal reflux symptoms using the GERD-Q questionnaire when they were administrated PPIs before the therapy change to vonoprazan. After obtaining consent, physicians changed the medication to vonoprazan at the same titer dose as the PPI for four weeks (wash-out period). The patients then continued to take vonoprazan for an additional eight weeks (observation period). After 12 weeks of taking vonoprazan, physicians performed interviews and administered the GERD-Q questionnaire again to assess the gastroesophageal reflux symptoms in patients (Figure).
Figure.
The evaluation of GERD symptoms and changing medication from a PPI to vonoprazan.
GERD-Q score
The GERD-Q is a self-reported questionnaire developed to diagnose GERD (21). It consists of six items rated on a 4-point scale (0 points, no symptoms; 1 point, 1 day; 2 points, 2-3 days; and 3 points, 4-7 days of symptoms for items 1, 2, 5, and 6; and 3 points, no symptoms; 2 points, 1 day; 1 point, 2-3 days; and 0 points, 4-7 days of symptoms for items 3 and 4), and patients are asked to reflect on their symptoms over the preceding week. The questionnaire has been validated in multiple languages, including Japanese (Supplementary material 1) (22-24).
Outcomes and variables
The primary outcome was the proportion of self-reported relief of gastroesophageal reflux symptoms eight weeks after the washout period (physician question: How have your gastroesophageal reflux symptoms changed? Patient response: improvement, no change, or exacerbation) (25). The secondary outcome was the total GERD-Q score (heart burn, regurgitation, pain in the upper stomach, nausea, difficulty getting a good night's sleep, and need for additional medication). We also evaluated the following adverse events as identified in an interview conducted by a physician: upper respiratory tract infection, constipation, diarrhea, nausea, liver damage, and rash.
We evaluated the known risk factors for acid reflux, such as the age, sex, body mass index (BMI), alcohol consumption, smoking, medication, Helicobacter pylori infection, and upper gastrointestinal endoscopic findings (3,26). Hiatal hernia was defined as an apparent separation of the esophagogastric junction and diaphragm impression by >2 cm, and endoscopic gastric mucosal atrophy (27) was evaluated according to the Kimura-Takemoto classification. H. pylori infection was evaluated using serological testing, a urea breath test, or a stool antigen test. Physician interviews and endoscopic findings based on the Kyoto classification of gastritis were also employed to evaluate post-eradication gastritis (28). We also assessed the medications used, including the type and duration of pretreatment PPIs (lansoprazole, omeprazole, rabeprazole, and esomeprazole); the concomitant use of other drugs [e.g. histamine 2 receptor antagonists, mucoprotective agents, low-dose aspirin, and non-steroidal anti-inflammatory drugs (NSAIDs)], anticoagulants (e.g. warfarin), and non-vitamin K antagonist oral anticoagulants (NOACs; e.g. dabigatran, rivaroxaban, edoxaban, and thienopyridine); and the adherence to the PPI and vonoprazan treatment.
Statistical analyses
We calculated the proportion of self-reported relief of gastroesophageal reflux symptoms after eight weeks (observation period). The odds ratio (OR) and 95% confidence interval (CI) for the improvement of symptoms (improvement vs. exacerbation) were calculated based on exact binomial distributions using matched-pair analyses. Because the observations before and after the therapy change for each patient were treated as matched pairs, patients with no change in symptoms did not contribute to the OR calculation (29). In addition, we also compared the GERD-Q scores, as well as the occurrence of adverse events during PPI and vonoprazan therapy, using paired t-tests and the exact matched-pair method.
In the subgroup analysis, we calculated the proportion of self-reported relief of gastroesophageal reflux symptoms and the OR for improvement of symptoms in patients with PPI-resistant NERD according to the H. pylori infection status (infected, uninfected, or eradicated).
Logistic regression analyses were performed to identify the factors associated with an improvement in gastroesophageal reflux symptoms when patients received vonoprazan. A p value <0.05 was considered statistically significant. All statistical analyses were performed using the SAS software program, ver. 9.4 (SAS Institute, Cary, USA).
Results
A total of 26 eligible patients were analyzed. The baseline patient characteristics are shown in Table 1. The mean age was 67.5 years, and 7 (26.9%) patients were men. The rate of H. pylori persistent infection was 15.4% (n=4). Prior to the medication change, PPIs of rabeprazole, lansoprazole, omeprazole, and esomeprazole were administered to 11, 6, 1, and 8 patients, respectively. The mean duration of PPI therapy was 136 weeks.
Table 1.
Baseline Patient Characteristics (n=26).
| Variable | n (%) | |
|---|---|---|
| Age, mean±SD | 67.5±12.2* | |
| Sex, male | 7 (26.9) | |
| BMI, mean±SD | 22.3±2.9* | |
| Current alcohol consumption | 6 (23.1) | |
| Current smoker | 1 (3.9) | |
| Medication | ||
| Pre-treatment PPI | ||
| Rabeprazole, 10 mg/day | 5 (19.2) | |
| Rabeprazole, 20 mg/day | 6 (23.0) | |
| Lansoprazole, 15 mg/day | 5 (19.2) | |
| Lansoprazole, 30 mg/day | 1 (3.9) | |
| Omeprazole, 10 mg/day | 1 (3.9) | |
| Omeprazole, 20 mg/day | 0 (0.0) | |
| Esomeprazole, 10 mg/day | 1 (3.9) | |
| Esomeprazole, 20 mg/day | 7 (26.9) | |
| Concomitant drug use | ||
| Histamine 2 receptor antagonists | 0 (0.0) | |
| Mucoprotective agents | 7 (26.9) | |
| Low-dose aspirin | 3 (11.5) | |
| NSAIDs | 5 (19.2) | |
| Anticoagulants | 2 (7.7) | |
| Thienopyridine | 1 (3.9) | |
| Endoscopic findings | ||
| Hiatus hernia | 7 (28.0) | |
| Endoscopic gastric mucosal atrophy | 13 (50.0) | |
| H. pylori status | ||
| Persistent infection | 4 (15.4) | |
| Eradicated | 9 (34.6) | |
| Uninfected | 13 (50.0) |
BMI: body mass index, NSAIDs: non-steroidal anti-inflammatory drugs, PPI: proton pump inhibitor, SD: standard deviation
*Mean
Of the patients who underwent vonoprazan therapy, 15 (57.7%) received 20 mg/day, and the remaining 11 (42.3%) received 10 mg/day. No significant differences in the mean adherence were found between patients who received a PPI (95.1%) and those who received vonoprazan (89.8%) (p=0.384). The responses for self-reported relief of symptoms were “improvement” in 18 patients (69.2%), “no change” in 6 (23.1%), and “exacerbation” in 2 (7.7%). Of the 18 patients with symptom improvement, 9 received vonoprazan 10 mg/day, and 9 received vonoprazan 20 mg/day. Changing therapy was significantly associated with an improvement in self-reported symptoms (OR 9.0, 95% CI 2.2-80.3, p<0.001) (Table 2). Vonoprazan significantly decreased the incidence of reflux-related symptoms, as shown by a decrease in the mean total GERD-Q score (from 11.96 to 8.92, p<0.001). In addition, the GERD-Q heartburn, regurgitation, and insomnia scores were significantly lower in patients on vonoprazan therapy than in those on PPIs (Table 3).
Table 2.
Self-reported Relief of Gastroesophageal Reflux Symptoms after Switching from a PPI to Vonoprazan.
| Outcome | Number of patients (%) | Odds ratio (95% CI) | p value | |||
|---|---|---|---|---|---|---|
| NERD (n=26) | ||||||
| Self-reported relief of symptoms | ||||||
| Improvement | 18 (69.2) | 9.0 (2.2-80.3) | <0.001 | |||
| No change | 6 (23.1) | |||||
| Exacerbation | 2 (7.7) | |||||
| PPI-resistant NERD (n=19) | ||||||
| Self-reported relief of symptoms | ||||||
| Improvement | 12 (63.2) | 6.0 (1.3-55.2) | 0.013 | |||
| No change | 5 (26.3) | |||||
| Exacerbation | 2 (10.5) |
NERD: reflux esophagitis and non-erosive reflux disease, CI: confidence interval, PPI: proton pump inhibitor
Bolding indicates statistical significance (p<0.05).
Table 3.
GERD-Q Score during PPI and Vonoprazan Therapy.
| Outcome | PPI | Vonoprazan | Change (95% CI) | p value | ||||
|---|---|---|---|---|---|---|---|---|
| NERD (n=26) | ||||||||
| GERD-Q | Mean score±SD | Mean score±SD | ||||||
| Total | 11.96±1.89 | 8.92±2.61 | -3.04 (-4.02 to -2.05) | <0.001 | ||||
| Heartburn | 2.27±1.04 | 1.23±1.14 | -1.04 (-1.56 to -0.52) | <0.001 | ||||
| Regurgitation | 1.73±1.04 | 1.11±1.07 | -0.62 (-1.03 to -0.20) | 0.005 | ||||
| Pain | 2.58±0.50 | 2.50±0.51 | -0.08 (-0.30 to 0.15) | 0.490 | ||||
| Nausea | 2.50±0.51 | 2.77±0.43 | 0.27 (-0.00009 to 0.54) | 0.050 | ||||
| Insomnia | 2.15±1.05 | 0.92±1.09 | -1.23 (-1.76 to -0.70) | <0.001 | ||||
| Additional medication | 0.73±1.12 | 0.38±0.80 | -0.35 (-0.79 to 0.10) | 0.119 | ||||
| PPI-resistant NERD (n=19) | ||||||||
| GERD-Q | Mean score±SD | Mean score±SD | ||||||
| Total | 12.21±1.93 | 9.37±2.73 | -2.84 (-4.13 to -1.55) | <0.001 | ||||
| Heartburn | 2.42±0.90 | 1.37±1.11 | -1.05 (-1.66 to -0.44) | 0.002 | ||||
| Regurgitation | 1.84±0.96 | 1.32±1.11 | -0.53 (-0.93 to -0.12) | 0.014 | ||||
| Pain | 2.58±0.51 | 2.47±0.51 | -0.11 (-0.38 to 0.17) | 0.429 | ||||
| Nausea | 2.53±0.51 | 2.68±0.48 | 0.16 (-0.18 to 0.49) | 0.331 | ||||
| Insomnia | 2.11±1.05 | 1.05±1.13 | -1.05 (-1.70 to -0.40) | 0.003 | ||||
| Additional medication | 0.74±1.10 | 0.47±0.90 | -0.26 (-0.79 to 0.27) | 0.310 | ||||
NERD: non-erosive reflux disease, CI: confidence interval, PPI: proton pump inhibitor, SD: standard deviation
Bolding indicates statistical significance (p<0.05).
Among these NERD patients, 19 received standard or double PPI doses and were therefore categorized as having PPI-resistant NERD. In the PPI-resistant NERD patients, the responses for self-reported symptom relief were “improvement” in 12 patients (63.2%), “no change” in 5 (26.3%), and “exacerbation” in 2 (10.5%). Changing therapy was significantly associated with an improvement in self-reported symptoms (OR 6.0, 95% CI 1.3-55.2, p=0.013) (Table 2) and a decrease in the mean total GERD-Q score (from 12.21 to 9.37, p<0.001) (Table 3).
The therapeutic responses according to the H. pylori infection status (infected, uninfected and eradicated) are shown in Supplementary material 2 and 3. Treatment with vonoprazan was significantly associated with an improvement in self-reported symptoms and in the mean total GERD-Q score, regardless of the H. pylori status.
The results of univariate analyses of predictive factors leading to an improvement in reflux symptoms are shown in Table 4. No significant associations were found between symptom relief and these factors, including the BMI, smoking, endoscopic findings, and H. pylori status.
Table 4.
Factors Associated with an Improvement in the Gastroesophageal Reflux Symptoms after Changing Therapy (n=26).
| Variable | Improved/Not improved | OR (95% CI) | p value | |||
|---|---|---|---|---|---|---|
| Age, years | 68.6/65.0* | 1.03 (0.96-1.10) | 0.481 | |||
| Sex, male | 5/2 | 1.15 (0.17-7.74) | 0.883 | |||
| BMI, kg/m2 | 22.4/22.0* | 1.04 (0.78-1.40) | 0.787 | |||
| Current alcohol consumption | 5/1 | 2.69 (0.26-27.8) | 0.406 | |||
| Current smoker | 0/1 | NA | NA | |||
| Pre-treatment PPI | ||||||
| Rabeprazole | 8/3 | 1.33 (0.24-7.35) | 0.741 | |||
| Lansoprazole | 4/2 | 0.86 (0.12-6.01) | 0.877 | |||
| Omeprazole | 1/0 | NA | NA | |||
| Esomeprazole | 5/3 | 0.64 (0.11-3.74) | 0.621 | |||
| Concomitant drug use | ||||||
| Histamine 2 receptor antagonists | 0/0 | NA | NA | |||
| Mucoprotective agents | 5/2 | 1.15 (0.17-7.74) | 0.883 | |||
| Low-dose aspirin | 2/1 | 0.88 (0.07-11.3) | 0.919 | |||
| NSAIDs | 2/3 | 0.21 (0.03-1.62) | 0.134 | |||
| Anticoagulants | 2/0 | NA | NA | |||
| Thienopyridine | 1/0 | NA | NA | |||
| Vonoprazan adherence (%) | 88.6/93.4* | 0.97 (0.89-1.07) | 0.540 | |||
| Endoscopic findings | ||||||
| Hiatus hernia | 5/2 | 1.25 (0.19-8.44) | 0.819 | |||
| Endoscopic gastric mucosal atrophy | 8/5 | 0.48 (0.09-2.65) | 0.399 | |||
| H. pylori status | ||||||
| Uninfected or eradicated | 10/3 | 1.00 | ||||
| Persistent infected | 8/5 | 0.48 (0.09-2.65) | 0.399 |
BMI: body mass index, CI: confidence interval, NA: not applicable, NSAIDs: non-steroidal anti-inflammatory drugs, OR: odds ratio, PPI: proton pump inhibitor
*Mean
Adverse events during PPI and vonoprazan therapy are shown in Table 5. No significant differences in the rate of adverse events were found between treatment periods. Only one case had a new adverse event (rash) during vonoprazan therapy. Other adverse events, such as upper respiratory tract infection and diarrhea, were observed in the same patients during both PPI and vonoprazan therapy.
Table 5.
Adverse Events during PPI and Vonoprazan Therapy (n=26).
| Number of patients with AE | ||||||||
|---|---|---|---|---|---|---|---|---|
| AE | Both PPI and vono | PPI only | Vono only | p value | ||||
| Upper respiratory tract infection | 4 | 1 | 0 | 1.000 | ||||
| Constipation | 0 | 1 | 0 | 1.000 | ||||
| Diarrhea | 2 | 1 | 0 | 1.000 | ||||
| Nausea | 0 | 1 | 0 | 1.000 | ||||
| Rash | 1 | 3 | 1 | 0.250 | ||||
AE: adverse event, NA: not applicable, PPI: proton pump inhibitor, vono: vonoprazan
Discussion
We found that changing therapy from a PPI to vonoprazan was associated with an improvement in gastroesophageal reflux symptoms in patients with NERD and PPI-resistant NERD. No significant differences were found in the rate of adverse events between patients receiving PPIs and those receiving vonoprazan therapy.
Vonoprazan is the only potassium-competitive acid blocker available for clinical use. It was developed to overcome the shortcomings of PPIs, such as the short half-life, insufficient acid suppression, slow onset of action, and metabolic variation (30). Vonoprazan causes effective acid suppression by strongly inhibiting the H-K ATPase activity of parietal cells; this has led to superior clinical results against various acid-related diseases (31-33). Regarding GERD, a previous study investigated the efficacy of vonoprazan on esophageal mucosal healing (13). Ashida et al. reported that 99% of patients with erosive esophagitis had mucosal healing after 8 weeks of vonoprazan therapy (13). This trial showed no inferiority over lansoprazole in mucosal healing, and some subgroup analyses even showed that vonoprazan was superior to lansoprazole. However, clinical data indicating that vonoprazan leads to an improvement in NERD symptoms are very limited.
In this self-controlled study, we found that switching from a PPI to vonoprazan for 12 weeks significantly improved gastroesophageal reflux symptoms in patients with NERD, including PPI-resistant NERD. Our results validate the efficacy of vonoprazan for treating GERD not only in erosive esophagitis but also in NERD patients. Our results are consistent with those of a recent retrospective observational study that reported an improvement in GERD symptoms in NERD patients treated with vonoprazan for 4 weeks (34).
Several factors may explain the effectiveness of vonoprazan. First, it has a strong acid-inhibition effect. A pharmacological study on healthy adults showed that patients treated with vonoprazan had a significantly longer pH 4 holding time than those treated with conventional PPIs (35). In addition, once-daily vonoprazan led to sustained acid suppression, which resulted in an excellent nighttime pH 4 holding time ratio. This might reduce nocturnal acid breakthrough, which is often associated with refractory symptoms of GERD (3). Second, due to the open-label non-randomized design of our study, the placebo effect may have been associated with improved clinical outcomes.
Previous observational studies have evaluated the pathogenesis of PPI-resistant NERD and reported that 10-30% of PPI-resistant NERD patients had acid-related reflux (17,20). In the present study, we showed that vonoprazan improved symptoms even in PPI-resistant NERD patients. However, two patients experienced an exacerbation of symptoms after vonoprazan treatment, although this may have been due to other causal factors, such as non-acid reflux and esophageal motility disorder (17,20).
The results of our study suggest a new treatment option for patients with NERD. We found that vonoprazan improved gastroesophageal reflux symptoms in 69.2% of NERD and 63.2% of PPI-resistant NERD patients. This rate is higher than that achieved by studies that escalated the dose of PPI (36), co-administered a prokinetic drug (37,38), and treated patients with Japanese herbal medicine (39). In our study, after 12 weeks of vonoprazan and PPI therapy, there were no marked differences in the rate of adverse events reported by patients, suggesting the safety of vonoprazan therapy. However, short-term (four weeks) vonoprazan therapy in a recent trial failed to improve GERD symptoms in NERD patients (40). Thus, a large-scale prospective study is required to confirm the effects of long-term vonoprazan treatment for PPI-resistant NERD.
Our study had several strengths. First, this is the first study to show the efficacy of vonoprazan for both NERD and PPI-resistant NERD. Second, we performed upper endoscopy to exclude non-GERD gastrointestinal diseases, such as peptic ulcers and malignancies. Studies that are based only on a questionnaire assessment do not exclude these diseases. Third, using a self-controlled design that included patient data from different time periods, study participants acted as their own controls; as such, the confounding effects of risk factors that are generally time-invariant were theoretically minimized. Therefore, the estimated effects of changing therapy were less likely to be biased by time-invariant factors, such as sex, the BMI, genetic factors, and the medical history, although our sample size was relatively small (41).
However, our study also had several limitations. First, we used a retrospective, open-label, non-randomized, controlled design, which has several biases. Second, because the sample size was small, the statistical power might have been insufficient to adjust for time-varying confounders, such as transient concomitant medication use and transient physical activity. Third, CYP2C19 genotyping and pH monitoring were not performed in our patients.
In conclusion, switching from a PPI to vonoprazan was significantly associated with an improvement in gastroesophageal reflux symptoms in patients with NERD and PPI-resistant NERD without an increase in adverse events. Therefore, vonoprazan represents a promising treatment option for patients with NERD and PPI-resistant NERD.
The institutional review board of the University of Tokyo approved this study.
Author's disclosure of potential Conflicts of Interest (COI).
Mitsuhiro Fujishiro: Research funding, Hoya and Pentax.
Financial Support
This work was supported by grants from the KAKENHI Grant-in-Aid for Scientific Research, 17K15928 (R.N).
Supplementary Material
The GERD-Q questionnaire. Respondents were asked to enter their frequency scores after reflecting on their symptoms from the previous week.
Self-reported relief of acid reflux symptoms after therapy change from a PPI to vonoprazan according to H.pylori infection status
GERD-Q score during PPI and vonoprazan therapy according to H. pylori infection status.
References
- 1. Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 54: 710-717, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fujiwara Y, Arakawa T. Epidemiology and clinical characteristics of GERD in the Japanese population. J Gastroenterol 44: 518-534, 2009. [DOI] [PubMed] [Google Scholar]
- 3. Iwakiri K, Kinoshita Y, Habu Y, et al. . Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J Gastroenterol 51: 751-767, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Habu Y, Maeda K, Kusuda T, et al. . “Proton-pump inhibitor-first” strategy versus “step-up” strategy for the acute treatment of reflux esophagitis: a cost-effectiveness analysis in Japan. J Gastroenterol 40: 1029-1035, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Sigterman KE, van Pinxteren B, Bonis PA, Lau J, Numans ME. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev 5: CD002095, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tew S, Jamieson GG, Pilowsky I, Myers J. The illness behavior of patients with gastroesophageal reflux disease with and without endoscopic esophagitis. Dis Esophagus 10: 9-15, 1997. [DOI] [PubMed] [Google Scholar]
- 7. Richter JE, Kovacs TO, Greski-Rose PA, Huang section sign B, Fisher R. Lansoprazole in the treatment of heartburn in patients without erosive oesophagitis. Aliment Pharmacol Ther 13: 795-804, 1999. [DOI] [PubMed] [Google Scholar]
- 8. Futagami S, Iwakiri K, Shindo T, et al. . The prokinetic effect of mosapride citrate combined with omeprazole therapy improves clinical symptoms and gastric emptying in PPI-resistant NERD patients with delayed gastric emptying. J Gastroenterol 45: 413-421, 2010. [DOI] [PubMed] [Google Scholar]
- 9. Miyamoto M, Manabe N, Haruma K. Efficacy of the addition of prokinetics for proton pump inhibitor (PPI) resistant non-erosive reflux disease (NERD) patients: significance of frequency scale for the symptom of GERD (FSSG) on decision of treatment strategy. Intern Med 49: 1469-1476, 2010. [DOI] [PubMed] [Google Scholar]
- 10. Anvari M, Allen C, Marshall J, et al. . A randomized controlled trial of laparoscopic nissen fundoplication versus proton pump inhibitors for treatment of patients with chronic gastroesophageal reflux disease: one-year follow-up. Surg Innov 13: 238-249, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Gillies RS, Stratford JM, Booth MI, Dehn TC. Does laparoscopic antireflux surgery improve quality of life in patients whose gastro-oesophageal reflux disease is well controlled with medical therapy? Eur J Gastroenterol Hepatol 20: 430-435, 2008. [DOI] [PubMed] [Google Scholar]
- 12. Tokudome K, Funaki Y, Sasaki M, et al. . Efficacy of endoluminal gastroplication in Japanese patients with proton pump inhibitor-resistant, non-erosive esophagitis. World J Gastroenterol 18: 5940-5947, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ashida K, Sakurai Y, Hori T, et al. . Randomised clinical trial: Vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther 43: 240-251, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Otake K, Sakurai Y, Nishida H, et al. . Characteristics of the novel potassium-competitive acid blocker vonoprazan fumarate (TAK-438). Adv Ther 33: 1140-1157, 2016. [DOI] [PubMed] [Google Scholar]
- 15. Kagami T, Sahara S, Ichikawa H, et al. . Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther 43: 1048-1059, 2016. [DOI] [PubMed] [Google Scholar]
- 16. Hoshino S, Kawami N, Takenouchi N, et al. . Efficacy of vonoprazan for proton pump inhibitor-resistant reflux esophagitis. Digestion 95: 156-161, 2017. [DOI] [PubMed] [Google Scholar]
- 17. Kawamura O, Hosaka H, Shimoyama Y, et al. . Evaluation of proton pump inhibitor-resistant nonerosive reflux disease by esophageal manometry and 24-hour esophageal impedance and pH monitoring. Digestion 91: 19-25, 2015. [DOI] [PubMed] [Google Scholar]
- 18. Tamura Y, Funaki Y, Izawa S, et al. . Pathophysiology of functional heartburn based on Rome III criteria in Japanese patients. World J Gastroenterol 21: 5009-5016, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zerbib F, Duriez A, Roman S, Capdepont M, Mion F. Determinants of gastro-oesophageal reflux perception in patients with persistent symptoms despite proton pump inhibitors. Gut 57: 156-160, 2008. [DOI] [PubMed] [Google Scholar]
- 20. Kawami N, Takenouchi N, Umezawa M, et al. . Pathogenesis of double-dose proton pump inhibitor-resistant non-erosive reflux disease, and mechanism of reflux symptoms and gastric acid secretion-suppressive effect in the presence or absence of Helicobacter pylori infection. Digestion 95: 140-145, 2017. [DOI] [PubMed] [Google Scholar]
- 21. Jones R, Junghard O, Dent J, et al. . Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther 30: 1030-1038, 2009. [DOI] [PubMed] [Google Scholar]
- 22. Jonasson C, Wernersson B, Hoff DA, Hatlebakk JG. Validation of the GerdQ questionnaire for the diagnosis of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 37: 564-572, 2013. [DOI] [PubMed] [Google Scholar]
- 23. Jonasson C, Moum B, Bang C, Andersen KR, Hatlebakk JG. Randomised clinical trial: a comparison between a GerdQ-based algorithm and an endoscopy-based approach for the diagnosis and initial treatment of GERD. Aliment Pharmacol Ther 35: 1290-1300, 2012. [DOI] [PubMed] [Google Scholar]
- 24. Wang K, Duan LP, Ge Y, Xu ZJ, Xia ZW. Diagnostic values of GerdQ, 24 hours ambulatory oesophageal pH and impedance-pH monitoring in Barrett's esophagus, reflux esophagitis and non-erosive reflux disease. Zhonghua Yi Xue Za Zhi 91: 1228-1232, 2011(in Chinese, Abstract in English). [PubMed] [Google Scholar]
- 25. Tran T, Lowry AM, El-Serag HB. Meta-analysis: the efficacy of over-the-counter gastro-oesophageal reflux disease therapies. Aliment Pharmacol Ther 25: 143-153, 2007. [DOI] [PubMed] [Google Scholar]
- 26. Kohata Y, Fujiwara Y, Watanabe T, et al. . Long-term benefits of smoking cessation on gastroesophageal reflux disease and health-related quality of life. PLoS One 11: e0147860, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 3: 87-97, 1969. [Google Scholar]
- 28. Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Koike K. Association between gastric cancer and the Kyoto classification of gastritis. J Gastroenterol Hepatol 32: 1581-1586, 2017. [DOI] [PubMed] [Google Scholar]
- 29. Greenland S. Applications of stratified analysis methods. In: Modern Epidemiology. 3rd ed. Greenland S, Rothman KJ, Lash TL, Eds. Lippincott Williams and Wilkins, Philadelphia, PA, 2008: 283-302. [Google Scholar]
- 30. Sakurai Y, Nishimura A, Kennedy G, et al. . Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (vonoprazan) doses in healthy male Japanese/non-Japanese subjects. Clin Transl Gastroenterol 6: e94, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 65: 1439-1446, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shichijo S, Hirata Y, Niikura R, et al. . Vonoprazan versus conventional proton pump inhibitor-based triple therapy as first-line treatment against Helicobacter pylori: a multicenter retrospective study in clinical practice. J Dig Dis 17: 670-675, 2016. [DOI] [PubMed] [Google Scholar]
- 33. Miwa H, Uedo N, Watari J, et al. . Randomised clinical trial: Efficacy and safety of vonoprazan vs. lansoprazole in patients with gastric or duodenal ulcers-results from two phase 3, non-inferiority randomised controlled trials. Aliment Pharmacol Ther 45: 240-252, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Asaoka D, Nagahara A, Hojo M, et al. . Efficacy of a potassium-competitive acid blocker for improving symptoms in patients with reflux esophagitis, non-erosive reflux disease, and functional dyspepsia. Biomed Rep 6: 175-180, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sakurai Y, Mori Y, Okamoto H, et al. . Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects: a randomised open-label cross-over study. Aliment Pharmacol Ther 42: 719-730, 2015. [DOI] [PubMed] [Google Scholar]
- 36. Furuta T, Shimatani T, Sugimoto M, et al. . Acid-Related Symptom Research Group (2011) Investigation of pretreatment prediction of proton pump inhibitor (PPI)-resistant patients with gastroesophageal reflux disease and the dose escalation challenge of PPIs-TORNADO study: a multicenter prospective study by the Acid-Related Symptom Research Group in Japan. J Gastroenterol 46: 1273-1283, 2011. [DOI] [PubMed] [Google Scholar]
- 37. Madan K, Ahuja V, Kashyap PC, Sharma MP. Comparison of efficacy of pantoprazole alone versus pantoprazole plus mosapride in therapy of gastroesophageal reflux disease: a randomized trial. Dis Esophagus 17: 274-278, 2004. [DOI] [PubMed] [Google Scholar]
- 38. Miwa H, Inoue K, Ashida K, et al. . Randomised clinical trial: efficacy of the addition of a prokinetic, mosapride citrate, to omeprazole in the treatment of patients with non-erosive reflux disease-a double-blind, placebo-controlled study. Aliment Pharmacol Ther 33: 323-332, 2011. [DOI] [PubMed] [Google Scholar]
- 39. Tominaga K, Iwakiri R, Fujimoto K, et al. . Rikkunshito improves symptoms in PPI-refractory GERD patients: a prospective, randomized, multicenter trial in Japan. J Gastroenterol 47: 284-292, 2012. [DOI] [PubMed] [Google Scholar]
- 40. Kinoshita Y, Sakurai Y, Shiino M, et al. . Evaluation of the efficacy and safety of vonoprazan in patients with nonerosive gastroesophageal reflux disease: a phase III, randomized, double-blind, placebo-controlled, multicenter study. Curr Ther Res Clin Exp 81-82: 1-7, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hallas J, Pottegard A. Use of self-controlled designs in pharmacoepidemiology. J Intern Med 275: 581-589, 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The GERD-Q questionnaire. Respondents were asked to enter their frequency scores after reflecting on their symptoms from the previous week.
Self-reported relief of acid reflux symptoms after therapy change from a PPI to vonoprazan according to H.pylori infection status
GERD-Q score during PPI and vonoprazan therapy according to H. pylori infection status.