Abstract
A 63-year-old woman presented to our hospital for cough, sputum, and abnormal shadows on chest X-ray. Schizophyllum commune was isolated from mucous plugs. Positive specific IgE and IgG against the fungi, elevated serum IgE, and mucous plugs with typical histologic findings of allergic bronchopulmonary mycosis (ABPM) led to the diagnosis of ABPM due to S. commune. We initially administered itraconazole unsuccessfully. Changing the antifungal agent to voriconazole resulted in improvement of the symptoms and chest imaging findings. Her ABPM has not relapsed for two years since the cessation of voriconazole, which was administered for one year.
Keywords: allergic bronchopulmonary mycosis, Schizophyllum commune, voriconazole, itraconazole
Introduction
Allergic bronchopulmonary mycosis (ABPM) is an immunologic disorder caused by a hyperimmune response to the endobronchial growth of certain fungi. ABPM due to Aspergillus sp. (ABPA) was first reported in 1952 by Hinson et al (1). Cases of ABPM due to non-Aspergillus fungi have been increasing in number. Schizophyllum commune, which is a basidiomycetes fungus found throughout Japan, was found to be the most frequent causative fungus of non-Aspergillus ABPM in Japan (2). However, definitive treatment of ABPM due to S. commune has not been established.
We experienced a case of ABPM due to S. commune in which itraconazole (ITCZ) failed but voriconazole (VRCZ) was effective. We report this case and review previous reports of ABPM due to S. commune.
Case Report
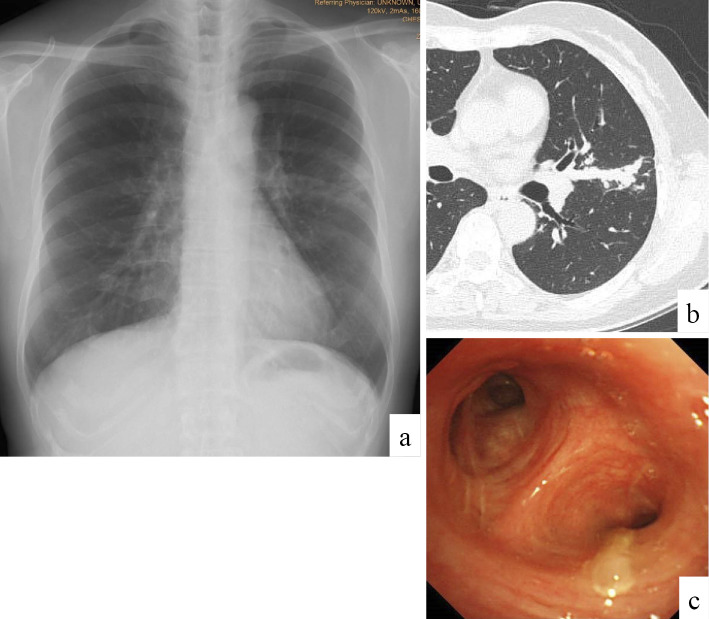
A 63-year-old woman presented to our hospital for cough, sputum, and abnormal shadows on chest X-ray. She had developed a cough since December 2012. She presented to a local physician who prescribed dextromethorphan, which was ineffective. She presented to another hospital where chest X-ray showed consolidation in the left middle lung field (Fig. 1a). Chest computed tomography (CT) showed mucoid impaction of the lingual bronchus (Fig. 1b) but did not show highly attenuated mucus, which is a CT finding highly specific for ABPM. She was therefore referred to our hospital for a further evaluation in April 2013. The patient had never smoked or been exposed to dust, nor had she ever worked in a garden or on a farm, which would suggest exposure to dense soil or water. She had never experienced periodic paroxysms of dyspnea interspersed with intervals of complete or nearly complete remission, which would suggest asthma.
Figure 1.
Chest imaging findings on admission. Chest X-ray showed band-like shadows and consolidation in the left middle lung field (a). Chest computed tomography showed mucous plugs in the lingual bronchi (b). Bronchoscopy showed a mucous plug at the lingual bronchus (c).
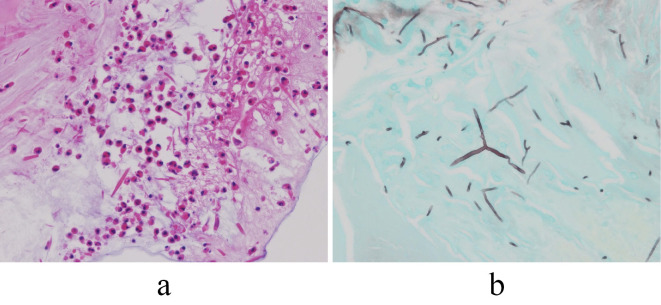
Laboratory tests did not show eosinophilia (300/mm3), but her serum IgE value was elevated to 1,363 IU/mL. Specific IgE against Aspergillus species was positive, but antibodies against Aspergillus species measured by complement fixation test were negative. Pulmonary function testing (% predicted) showed a vital capacity of 2.89 L (101.4%), forced vital capacity (FVC) of 2.93 L (102.8%), forced expiratory volume in 1 second (FEV1) of 2.52 L (137.0%), and an FEV1/FVC ratio of 86.0%, and an airway response to a beta-stimulant could not be induced. Sputum did not yield significant positive cultures, including for fungi. Lymphadenopathy and pleural effusion were not observed. We performed bronchoscopy and found mucous plugs in the lingual bronchus (Fig. 1c). Complete removal of the mucous plugs by bronchoscopy was attempted unsuccessfully. However, culture of one mucous plug that was obtained yielded a colony with a fluffy-like appearance, which was identified as S. commune at Chiba University Research Center for Pathogenic Fungi and Microbial Toxicoses. Serum values of IgE and IgG against S. commune measured at this same research center also were positive. The mucous plugs included eosinophils and Charcot-Leyden crystals, and fungal hyphae were also found in the plugs (Fig. 2). Although a clinical diagnosis of bronchial asthma was not established, we diagnosed her as having ABPM due to S. commune on the basis of histologic findings (3), culture results, and serum specific antibodies against S. commune.
Figure 2.
Histologic findings. A biopsy specimen of a mucus plug obtained via bronchoscopy showed fungal hyphae, eosinophils, and Charcot-Leyden crystals. a. Hematoxylin and Eosin staining (×200), b. Grocott stain (×200).
Despite carefully rechecking her social history, we could find no clue as to the route of infection. We started ITCZ 200 mg daily from May 2013 for 16 weeks, but her symptoms did not improve, and mucoid impaction increased in the lingual bronchus. Peripheral eosinophils increased to 500/mm3, and her serum IgE value rose to 1,439 IU/mL. We then changed the antifungal agent from ITCZ to VRCZ from August 2013, which improved her symptoms and decreased the number of mucous plugs. No mucous plugs were found on a chest CT scan obtained in August 2014 (Fig. 3), and the VRCZ was stopped. Since then, she has been followed up on an outpatient basis, and ABPM has not recurred through her most recent follow-up in November 2017. Throughout the clinical course, the level of peripheral eosinophils did not increase further, and her serum IgE value gradually decreased to 547 IU/mL at the most recent follow-up.
Figure 3.
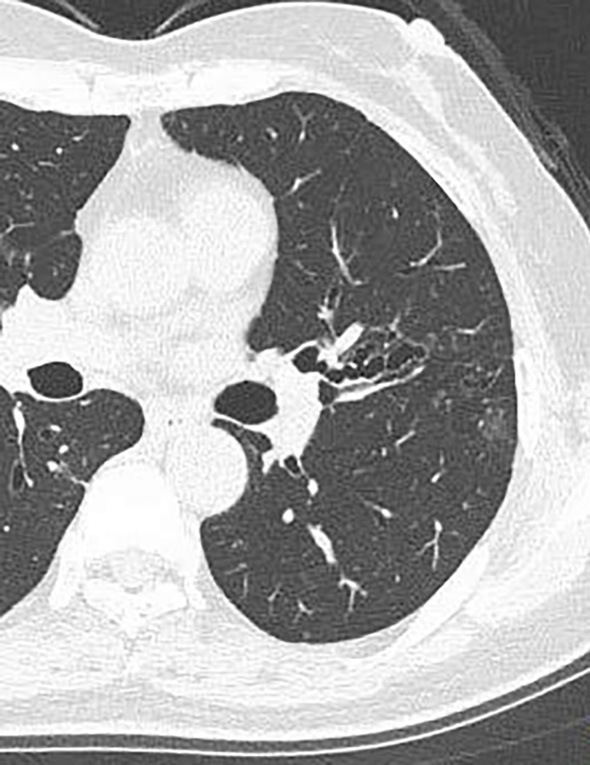
Chest computed tomography at the final follow-up examination. Chest computed tomography showed the area of central bronchiectasis that the mucous plugs had impacted.
Discussion
We herein report a case of ABPM due to S. commune in which ITCZ was ineffective. VRCZ cleared the mucous plugs, and the patient was followed up with no relapse of the ABPM for two years after one year of treatment with VRCZ.
Reports of ABPM due to S. commune have been increasing but are still relatively few, with only 25 found in the literature (4). Definitive treatment strategies have not been established, and ABPM due to S. commune has been managed with reference to the strategies of ABPA. Corticosteroid therapy is the mainstay of therapy for ABPA (5), and the guidelines recommend a combination of systemic corticosteroids and antifungal agents for ABPA (5). However, the long-term adverse effects of corticosteroid therapy may result in profound immunosuppression and debilitating metabolic abnormalities. It has been reported that pulmonary infection is not rare in ABPM patients due to the underlying impaired immune status (6). Furthermore, corticosteroid-induced immunosuppression may induce or very rarely result in progression of ABPA to invasive pulmonary aspergillosis (5). Therefore, we hesitated to start systemic corticosteroid as a first-line therapy in our patient.
Treatments of ABPM patients in previous reports include bronchial toilet, inhaled corticosteroid, systemic corticosteroid therapy, expectorant, and antifungal agents. Antifungal agents spare the effects of corticosteroids by diminishing the antigenic stimulus for bronchial inflammation, and ITCZ is administered most frequently, followed by amphotericin-B (intravenously, via inhalation, or via intrabronchial administration with a bronchoscope). There have been several reports of patients with ABPM due to S. commune successfully treated by antifungal agent alone (7,8) (Table). We therefore initially administered ITCZ alone for 16 weeks, but the serum IgE value and mucous plugs on the chest CT increased. We assumed that single ITCZ therapy was ineffective and that other treatment modalities were required. The successful treatment with VRCZ in several patients with ABPA and one with ABPM due to S. commune has been reported (9-11), and VRCZ has a low minimum inhibitory concentration (12,13) against S. commune. Therefore, we administered VRCZ alone, which decreased our patient's serum IgE value and improved the findings on chest imaging. Serum concentrations of ITCZ and VRCZ were not measured in the present case, nor was susceptibility testing of these agents performed. We were unable to clarify the exact mechanism behind the effectiveness of VRCZ in our case nor why the ITCZ was ineffective. Future studies should discuss the effectiveness of antifungals on ABPM based on the absorption, serum and tissue concentration, and susceptibility against the causative fungi.
Table.
Treatment Duration and Effects of Antifungals in Previous Reports of ABPM/MIB Caused by Schizophyllum commune.
| Age/Sex | Antifungals | Duration | Effect | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| 54/F | ITCZ | 10 months | Ineffective | 18 | ||||
| 72/F | ITCZ and inhalation of amphotericin-B | 1 year | Effective | 19 | ||||
| 44/F | Inhalation of amphotericin-B plus ICS plus oral PSL | Unknown | Effective | 20 | ||||
| 51/F | Intravenous amphotericin-B | 1 month | Effective | 21 | ||||
| 51/F | ITCZ | 4 months | Effective | 22 | ||||
| 54/F | ITCZ | 3 months | Effective | 23 | ||||
| 75/F | ITCZ | 4 months | Effective | 24 | ||||
| 55/M | Oral PSL plus ITCZ | 134 days | Effective | 25 | ||||
| 64/F | ITCZ plus oral PSL | 3 months | Effective | 26 | ||||
| 63/F | Inhalation and intrabronchial administration of amphotericin-B | 2 months | Effective | 27 | ||||
| 53/F | Fluconazole Inhalation and intrabronchial administration of amphotericin-B |
Unknown Unknown |
Ineffective Effective |
27 | ||||
| 82/F | Oral PSL plus ITCZ plus inhalation of ICS | Unknown | Effective | 27 | ||||
| 53/F | ITCZ plus ICS and SM combination | Unknown | Effective | 28 | ||||
| 70/F | ITCZ | 3 months | Effective | 29 | ||||
| 61/F | Oral ITCZ and PSL | Unknown | Effective | 30 | ||||
| 59/F | VRCZ plus ICS | Unknown | Effective | 31 |
ABPM: allergic bronchopulmonary mycosis, MIB: mucoid impaction of the bronchi, S. commune: Schizophyllum commune, ITCZ: itraconazole, VRCZ: voriconazole, SM: salmeterol, PSL: prednisolone, ICS: inhaled corticosteroid
Although reports of VRCZ use for ABPM due to S. commune are limited, the present report suggests that the administration of VRCZ may be effective for patients with ABPM due to S. commune when ITCZ is ineffective.
The recommended duration of ITCZ therapy for ABPA is 16 weeks (14), but the adequate treatment period of ITCZ and other antifungal agents for ABPM due to S. commune remains unclear. Treatment periods with antifungal agents differ among reports (Table). Most patients who received antifungals are treated for three to four months. We previously reported a patient with ABPM due to S. commune who has not relapsed for seven years after the three-month administration of ITCZ (8). However, we also experienced a patient who relapsed after ITCZ therapy for four months (7). There is one report of a patient who relapsed 4 months after 10-month treatment with ITCZ (15), but ITCZ and inhalation of amphotericin-B administered for 1 year improved another patient's condition (16). Only one case of ABPM due to S. commune treated with VRCZ has been reported, and the recommended treatment period of VRCZ is unclear. However, by referring to these experiences and previous reports, we administered VRCZ for one year, and our patient has not relapsed in the two years since the cessation of the VRCZ. Further accumulation of cases is needed to clarify the appropriate length of treatment.
Our report has several limitations. First, our and other previous reports include limited information on the patients' long-term prognosis. Most reports of patients with ABPM due to S. commune have focused on a short clinical course after the initiation of treatment. Long-term problems of ABPA include irreversible pulmonary and bronchial destruction. These same problems may be present in ABPM due to S. commune, and thus treatment strategies need to be discussed from a long-term perspective. Second, we did not conduct a sensitivity test of VRCZ against S. commune, which is suggested when using antifungal agents (17).
In conclusion, we suggest that VRCZ may be an effective treatment option for ABPM due to S. commune, but further studies are needed to confirm the benefits of VRCZ for this condition.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Katsuhiko Kamei at Chiba University Research Center for Pathogenic Fungi and Microbial Toxicoses for the identification of S. commune and measurement of serum-specific IgE and IgG against S. commune.
References
- 1. Hinson KF, Moon AJ, Plummer NS. Broncho-pulmonary aspergillosis; a review and a report of eight new cases. Thorax 7: 317-333, 1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chowdhary A, Agarwal K, Kathuria S, Gaur SN, Randhawa HS, Meis JF. Allergic bronchopulmonary mycosis due to fungi other than Aspergillus: a global overview. Crit Rev Microbiol 40: 30-48, 2014. [DOI] [PubMed] [Google Scholar]
- 3. Bosken CH, Myers JL, Greenberger Katzenstein AL. Pathologic features of allergic bronchopulmonary aspergillosis. Am J Surg Pathol 12: 216-222, 1988. [DOI] [PubMed] [Google Scholar]
- 4. Ishiguro T, Takayanagi N, Kagiyama N, Shimizu Y, Yanagisawa T, Sugita Y. Clinical characteristics of biopsy-proven allergic bronchopulmonary mycosis: varieties in causative fungi and laboratory findings. Intern Med 53: 1407-1411, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Walsh TJ, Anaissie EJ, Denning DW, et al. ; Infectious Diseases Society of America.. Treatment of aspergillosis; clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 46: 327-360, 2008. [DOI] [PubMed] [Google Scholar]
- 6. Ishiguro T, Takayanagi N, Baba Y, Takaku Y, Kagiyama N, Sugita Y. Pulmonary nontuberculous mycobacteriosis and chronic lower respiratory tract infections in patients with allergic bronchopulmonary mycosis without cystic fibrosis. Intern Med 55: 1067-1070, 2016. [DOI] [PubMed] [Google Scholar]
- 7. Ishiguro T, Takayanagi N, Harasawa K, et al. Mucoid impaction of the bronchi caused by Schizophyllum commune which developed after discontinuation of itraconazole administration. Nihon Kokyuki Gakkai Zasshi 47: 296-303, 2009(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 8. Ishiguro T, Takayanagi N, Tokunaga D, et al. Pulmonary Schizophyllum Commune infection developing mucoid impaction of the bronchi. Yale J Biol Med 80: 105-111, 2007. [PMC free article] [PubMed] [Google Scholar]
- 9. Erwin GE, Fitzgerald JE. Case report: allergic bronchopulmonary aspergillosis and allergic fungal sinusitis successfully treated with voriconazole. J Asthma 44: 891-895, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Glackin L, Leen G, Elnavir B, Greally P. Voriconazole in the treatment of allergic bronchopulmonary aspergillosis in cystic fibrosis. Ir Med J 102: 29, 2009. [PubMed] [Google Scholar]
- 11. Shen Q, Yao YK, Yang Q, Zhou JY. Schizophyllum commune-induced pulmonary mycosis. Chin Med J 129: 2141-2142, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. González GM, Sutton DA, Thompson E, Tijerina R, Rinaldi MG. In vitro activities of approved and investigational antifungal agents against 44 clinical isolated of basidiomycetous fungi. Antimicrob Agents Chemother 45: 633-635, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chowdhary A, Kathuria S, Singh PK, et al. Molecular characterization and in vitro antifungal susceptibility profile of Schizophyllum commune, an emerging basidiomycete in bronchopulmonary mycoses. Antimicrob Agents Chemother 57: 2845-2848, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guidelines Committee for Deep-seated Mycoses.. Guidelines for management of deep-seated mycoses 2014. Kyowa-Kikaku, Tokyo, 2014(in Japanese). [Google Scholar]
- 15. Kamei K, Unno H, Nagao K, Kuriyama T, Nishimura K, Miyaji M. Allergic bronchopulmonary mycosis caused by the basidiomycetous fungus Schizophyllum commune. Clin Infect Dis 18: 305-309, 1994. [DOI] [PubMed] [Google Scholar]
- 16. Tomita K, Hashizume I, Kasamatsu N, et al. Allergic bronchopulmonary mycosis caused by Schizophyllum commune. Nihon Kyobu Shikkan Gakkai Zasshi 34: 804-809, 1996(in Japanese). [PubMed] [Google Scholar]
- 17. Chowdhary A, Kathuria S, Agarwal K, Meis JF. Reply to “implications of high antifungal susceptibility on Schizophyllum commune-associated allergy in clinical practice”. Antimicrob Agents Chemother 57: 5784, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamei K, Unno H, Nagao K, et al. Allergic bronchopulmonary mycosis caused by the basidiomycetous fungus Shizophyllum commune. Clin Infect Dis 18: 305-309, 1994. [DOI] [PubMed] [Google Scholar]
- 19. Tomita K, Hashizume I, Kasamatsu N, et al. Allergic bronchopulmonary mycosis caused by Schizophyllum commune. Nihon Kyobu Shikkan Gakkai Zasshi 34: 804-809, 1996(in Japanese). [PubMed] [Google Scholar]
- 20. Yamashina S. ABPM due to Shizophyllum commune. Jpn J Antibiotics 50: 51-54, 1997(in Japanese). [PubMed] [Google Scholar]
- 21. Yamazaki Y, Sakashita H, Tanaka T, Kamei K, Nishimura K, Yoshizawa Y. Mucoid impaction caused by monokaryotic mycelium of Shizophyllum commune in association with bronchiectasis. Intern Med 39: 160-162, 2000. [DOI] [PubMed] [Google Scholar]
- 22. Ito Y, Sasaki S, Watanabe S, et al. A case of mucoid impaction of bronchi (MIB) due to Shizophyllum commune. Nihon Kokyuki Gakkai Zasshi 39: 266-270, 2001(in Japanese). [PubMed] [Google Scholar]
- 23. Ishiguro T, Takayanagi N, Tokunaga D, et al. Pulmonary Shizophyllum commune infection developing mucoid impaction of the bronchi. Yale J Biol Med 80: 105-111, 2007. [PMC free article] [PubMed] [Google Scholar]
- 24. Ishiguro T, Takayanagi N, Harasawa K, et al. Mucoid impaction of the bronchi caused by Shizophyllum commune which developed after discontinuation of Itraconazole administration. Nihon Kokyuki Gakkai Zasshi 47: 296-303, 2009(in Japanese). [PubMed] [Google Scholar]
- 25. Amemiya Y, Shirai R, Tokimatsu I, et al. Allergic bronchopulmonary mycosis induced by Schizophyllum commune: case report and review of the literature. Nihon Kokyuki Gakkai Zasshi 47: 692-697, 2009(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 26. Uruga H, Imafuku A, Hanada S, et al. A case of allergic bronchopulmonary mycosis caused by Shizophyllum commune presenting with hyperattenuated mucoid impaction. Nihon Kokyuki Gakkai Zasshi 48: 749-754, 2010(in Japanese). [PubMed] [Google Scholar]
- 27. Masunaga A, Morimoto K, Ando T, Ikushima S, Takemura T, Oritsu M. Nihon Kokyuki Gakkai Zasshi 48: 912-917, 2010(in Japanese). [PubMed] [Google Scholar]
- 28. Ishiguro T, Takayanagi N, Saito A, et al. Allergic bronchopulmonary mycosis due to Shizophyllum commune and Aspergillus fumigatus. Nihon Kokyuki Gakkai Zasshi 49: 612-618, 2011(in Japanese). [PubMed] [Google Scholar]
- 29. Ogawa H, Fujimura M, Takeuchi Y, Makimura K, Satoh K. The definitive diagnostic process and successful treatment for ABPM caused by Shizophyllum commune: a report of two cases. Allergol Intern 61: 163-169, 2012. [DOI] [PubMed] [Google Scholar]
- 30. Seki M, Ohno H, Hotoh K, et al. Allergic bronchopulmonary mycosis due to co-infection with Aspergillus fumigatus and Shizophyllum commune. IDCases 1: 5-8, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shen Q, Yao YK, Yang Q, Zhou JY. Schizophyllum commune-induced Pulmonary Mycosis. Chin Med J 129: 2141-2142, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]