Abstract
Animal models of kidney transplantation (KTX) are widely used in studying immune response of hosts to implanted grafts. Additionally, KTX can be used in generating kidney-specific knockout animal models by transplantation of kidneys from donors with global knockout of a gene to wild-type recipients or vice versa. Dual-kidney transplantation (DKT) provides a more physiological environment for recipients than single-kidney transplantation (SKT). However, DKT in mice is rare due to technical challenges. In this study, we successfully performed DKT in mice and compared the hemodynamic response and graft function with SKT. The surgical time, complications, and survival rate of DKT were not significantly different from SKT, where survival rates were above 85%. Mice with DKT showed less injury and quicker recovery with lower plasma creatinine (Pcr) and higher glomerular filtration rate (GFR) than SKT mice (Pcr = 0.34 and 0.17 mg/dl in DKT vs. 0.50 and 0.36 mg/dl in SKT at 1 and 3 days, respectively; GFR = 215 and 131 µl/min for DKT and SKT, respectively). In addition, the DKT exhibited better renal functional reserve and long-term outcome of renal graft function than SKT based on the response to acute volume expansion. In conclusion, we have successfully generated a mouse DKT model. The hemodynamic responses of DKT better mimic physiological situations with less kidney injury and better recovery than SKT because of reduced confounding factors such as single nephron hyperfiltration. We anticipate DKT in mice will provide an additional tool for evaluation of renal significance in physiology and disease.
Keywords: dual-kidney transplantation, graft function, mouse
INTRODUCTION
Animal models of kidney transplantation (KTX) provide an essential tool in studying immune response and treatment of graft rejection (4, 7, 14). In addition, a large number of genetically modified mouse models have been generated for determination of the significance of a specific gene (8, 20). Tissue-specific gene knockout and knock-in technologies such as the Cre-loxP system are very useful to manipulate a variety of genetically modified organisms to control gene expression; however, they are difficult, time-consuming, and expensive, and there is a limitation of availability of Cre lines (5). Kidney-specific knockout/knock-in in a gene is particularly powerful in determining gene function in both healthy and diseased kidneys. Kidney transplantation can be an alternative technique in generation of kidney-specific mutant mouse by transplanting the kidneys from the donors lacking whole-body expression of a given gene to wild-type recipients or vice versa.
Traditionally, only one kidney will be transplanted, hence the name single-kidney transplantation (SKT). The increased blood volume to a single graft can cause the escalation of single nephron glomerular filtration rate (GFR) typically by ~30% following SKT, which may elevate intraglomerular pressure and induce glomerular injury (2, 3). Unaltered nephron mass supply by simultaneously transplanting two kidneys to the same recipient, called dual-kidney transplantation (DKT), would be expected to better mimic normal physiological hemodynamics in the recipient than a single kidney (11). Therefore, with the application of KTX in either the generation of a kidney-specific knockout mouse model or in the study of immune responses and treatment of graft rejection, DKT will be more promising than SKT. However, DKT in mice has not been performed because of the technical challenges. In the present study, we performed DKT in mice and compared the hemodynamics and graft function with SKT. We found that mice with DKT exhibited similar GFR to the Sham-operated mice, quicker recovery from operation, and better graft function compared with the mice with SKT.
MATERIALS AND METHODS
All procedures and experiments were approved by the Institutional Animal Care and Use Committee at the University of South Florida College of Medicine. All chemicals were purchased from Sigma (St. Louis, MO) except as otherwise indicated.
Animals.
Male C57BL/6J mice, aged 10–12 wk (23–28 g) were obtained from a vendor (Jackson Laboratory, Indianapolis, IN). After arrival, the mice were allowed to equilibrate in a temperature-controlled environment with 12:12 h light-dark cycle for 1 wk and ad libitum access to mouse chow and tap water. The animals were then randomly divided into four groups: Sham, nephrectomy, SKT, and DKT.
Kidney transplantations (SKT and DKT).
SKT was performed in five steps: donor procurement, recipient native left nephrectomy, kidney implantation, ureteral implantation, and native right kidney nephrectomy as we recently reported (16, 23, 24).
For DKT, the surgical procedures were mostly similar to SKT but different in procurement of bilateral donor kidneys and the dual anastomosis of ureteral implantation. Briefly, the donor mice were anesthetized via inhalation of isoflurane (2% in the air; flow 200 ml/min). A midline abdominal incision was utilized to fully expose the both kidneys, aorta, and inferior vena cava (IVC). The left and right ureters were isolated and cut close to the bladder. The aorta and IVC above the right renal artery and below the left renal artery were mobilized from surrounded tissue and separated. A piece of 6.0 silk suture was then tied loosely around the infrarenal aorta below the left renal artery. The aorta above the right renal artery, the distal aorta, and the IVC were cross-clamped with 5-mm microvascular clamps. The IVC above the right renal artery was transected. One milliliter of cold saline (4°C) was perfused through the aorta, followed by tightening of the ligature around the infrarenal aorta. The aorta was cut below the ligature and below the proximal clamp. The kidneys and associated vessels were removed and stored in saline at 4°C. The implantation of the kidney grafts and the recipient ureteral implantation were performed as previously described (23) with slight modification. Briefly, donor kidneys were transferred from cold saline into the recipient’s abdominal cavity. The arterial anastomosis was performed distal to the native right renal artery in an end-to-side manner between the donor and recipient aorta with 10-0 Ethilon sutures. Two stay sutures were tied at the proximal and distal apexes of the recipient’s arteriotomy with the donor’s aortic cuff. Each side of the wall of the anastomosis was sewn continuously with two to three stitches. A length of ~2–3 mm of the suture was left free at the pole of the arteriotomy. The venous anastomosis was achieved in similar fashion as the arterial anastomosis, but each side of the wall of the anastomosis was sewn continuously with six to seven stitches. The inferior and the superior clamps were released sequentially to reperfuse the transplanted kidneys.
The mice that underwent Sham laparotomy served as the control group for DKT. Mice who underwent unilateral nephrectomy of the right kidney were utilized as controls for the SKT mice.
Plasma creatinine, kidney injury molecule-1, and neutrophil gelatinase-associated lipocalin measurement.
Similar methods were used for the measurement of plasma creatinine (Pcr), kidney injury molecule-1 (KIM-1), and neutrophil gelatinase-associated lipocalin (NGAL) at 1, 3, 7, 14, and 28 days after operation as described previously (22, 26). For the Pcr concentration measurement, blood (20 µl) was collected with a heparinized micro capillary tube from the tail vein and was immediately centrifuged. Plasma (10 µl) was collected and stored in a −80°C freezer until analyzed. Pcr concentrations were measured with HPLC-mass spectrometry at O’Brien Center at the University of Alabama at Birmingham.
Plasma of 10 and 5 µl was prepared as above for KIM-1 and NGAL measurement with ELISA kits (R&D Systems, Minneapolis, MN; MKM100 and MLCN20, respectively). A dilution of 1:10 or 1:100 with diluent was performed in each sample, and the measurement followed the manufacturer’s instructions.
GFR measurement.
GFR was measured in conscious mice for all 4 groups at 7, 14, and 28 days after operation using a single bolus intravenous injection of fluorescein isothiocyanate-sinistrin (FITC-sinistrin; Fresenius Kabi Austria GmbH) as we described recently with a modification (22, 25). The FITC-sinistrin solution (0.56 mg/g body weight) was injected via retro orbital sinus of the mice under light anesthesia with isoflurane. Blood was collected (≈5 μl/each) from a small tail nick at the end of the tail into a heparinized microcapillary tube at 3, 7, 10, 15, 35, 55, 75, and 90 min after injection. The blood samples were centrifuged and plasma fractions (2 μl/each) were collected. FITC-sinistrin fluorescent intensities of the plasma samples were measured using a plate reader (Cytation5, BioTek, Winooski, VT). GFR was calculated using a two-compartment model of two-phase exponential decay (18) (GraphPad Prism, San Diego, CA) and was presented as microliters per minute (µl⋅min−1).
Renal hemodynamics and sodium excretion in response to an acute volume expansion.
Four weeks after kidney transplantation, the mice were anesthetized with ketamine (30 µg/g) and inactin (50 µg/g). One catheter was placed in the femoral artery for the measurement of mean arterial pressure (MAP), which was monitored with an AD Instruments Bridge Amp pressure transducer (AD Instruments, Sydney, Australia) and recorded on a Laboratory Chart (AD Instrument). Another catheter was placed in the femoral vein for an intravenous infusion of 2% BSA and FITC-inulin (2 mg/ml) in a 0.9% NaCl solution at a rate of 0.5 ml/hour. One or two more catheters were inserted into the ureters for the collection of urine for SKT and DKT, respectively. After surgery, urine and plasma were collected during a 30-min period after a 30-min equilibration period, followed by infusion of saline (3% body weight) in a bolus and then by the infusion of FITC-inulin at 0.5 ml/hour. Urine and plasma were collected during a 60-min period and a 60- to 90-min period after volume expansion. The urine flow rates were calculated and the Na+ excretion was measured with Easy Electrolyte analyzer (Medica Corporation, Bedford, MA). The GFR was calculated from urine inulin and plasma inulin concentration and urine flow.
Histology.
Following acute volume expansion (AVE), the mice were euthanized, and kidneys were collected for histological examination. Kidneys were removed and cut into halves by a midline incision along the longitudinal axis of the convexity. Kidney samples were fixed in 4% paraformaldehyde solution for at least 24 h and then embedded in paraffin, cut into 3-µm sections, and stained with periodic acid Schiff. Kidney injury was evaluated based on percentage of necrotic tubules as we reported (22–24, 26). Ten randomly chosen fields were captured under ×200 magnifications, and the percentage of necrotic tubules in each image was quantified. All morphometric analyses were performed by an experienced renal pathologist (L. Fu) blinded to the experimental procedures.
Immunohistochemistry.
Immunohistochemical staining of CD4 was performed on paraffin-embedded kidney sections (3 μm). Antigen retrieval was achieved using 10 mM sodium citrate buffer (pH 6.0) in a steam bath for 45 min. Antigen was blocked with 0.3% H2O2 in phosphate-buffered saline, followed by Avidin/Biotin block (Vector Laboratories, Burlingame, CA) according to the manufacturer’s instructions. Tissue sections were stained for CD4 (ab183685, Abcam, Cambridge, UK) at 1:1,000 dilution, followed by goat anti-rabbit IgG H&L (HRP) (ab97051) at 1:500 dilution. The stained tissues were then counterstained with hematoxylin to allow better visualization of tissue structure. Immunohistochemical staining for CD4+ cells was analyzed by counting the number of positive cells per high power field at ×200 magnification on 10 fields per section using the ImageJ software. All scorings were carried out by an experienced renal pathologist (L. Fu) blinded to the experimental groups.
Statistical analysis.
All data were presented as mean values ± SE. The significance of differences in mean values between and within groups were determined by analysis of variance for repeated measures and a post-hoc Fisher least-significant difference test or a paired t test where appropriate. A P value of <0.05 was considered to be statistically significant.
RESULTS
Surgical operations of SKT and DKT.
The SKTs were performed in a similar fashion as we reported previously (23, 24). For DKT, the two donor kidneys were implanted en bloc into the recipients as shown in Fig. 1. The surgical time of each step in DKT was similar to SKT except that it took ~2 min longer in the donor procurement and ureter anastomosis with DKT (n = 6; Table 1.)
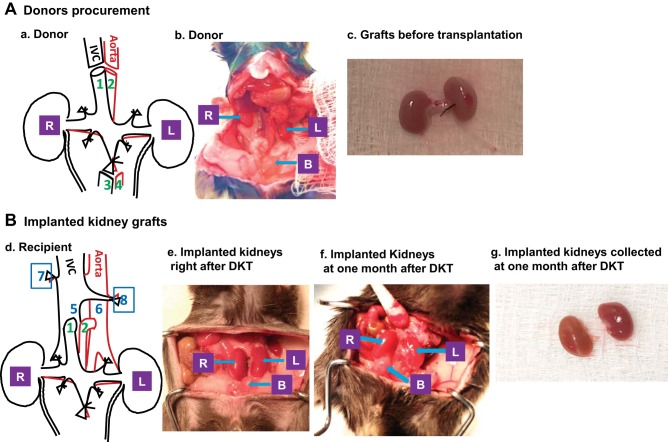
Fig. 1.
Illustration of dual-kidney transplantation (DKT). A: procurement of donor kidneys. a: schematic for donor kidneys procurement (1- suprarenal inferior vena cava; 2- suprarenal aorta; 3- inferior vena cava; 4- abdominal aorta; R- right kidney; L- left kidney). b: normal anatomical location of donor kidneys before procurement (R- right kidney; L- left kidney; B- bladder). c: two donor kidneys with ureters along with vasculatures were removed as one unit from donor. B: implantation of kidney grafts. d: schematic for DKT (1- donor suprarenal inferior vena cava will be used as renal vein for transplanted kidneys; 2- donor suprarenal aorta will be used as renal artery for transplanted kidneys; 5- recipient infrarenal inferior vena cava; 6- recipient infrarenal abdominal aorta; R and L- kidney grafts; 7- right native kidney nephrectomy; 8- left native kidney nephrectomy). e: successful DKT with good blood perfusion for both kidney grafts based on the size and color. f: anatomical location and sizes of the transplanted kidneys at 1 mo post-DKT. g: isolated transplanted kidneys at 1 mo after DKT.
Table 1.
Operation time for SKT and DKT.
| Donor Procurement | Recipient Preparation | Artery Anastomosis | Vein Anastomosis | Ureter Implantation | |
|---|---|---|---|---|---|
| SKT | 8–10 min | 7–9 min | 8–9 min | 8–9 min | 5–6 min |
| DKT | 10–12 min | 7–9 min | 8–9 min | 9–11 min | 7–9 min |
The operation in DKT took a similar time line as SKT for most steps except that the procurement of donors and ureter anastomosis in DKT took a few minutes longer (n = 6). DKT, dual-kidney transplantation; SKT, single-kidney transplantation.
Pcr, KIM-1, and NGAL concentration following KTX.
To assess the graft injury and recovery from surgeries, levels of Pcr, KIM-1, and NGAL were measured at 1, 3, 7, 14, and 28 days following transplantation. The concentrations of Pcr in mice with SKT and DKT were substantially increased at 24 h post transplantation as compared with their controls (P < 0.01, SKT vs. nephrectomy and DKT vs. Sham; n = 6; Fig. 2A). Mice with DKT demonstrated less injury and quicker recovery from surgeries than SKT mice, reflected by lower Pcr values at 1, 3, and 7 days after transplantation (Pcr = 0.34 ± 0.04, 0.17 ± 0.05 and 0.10 ± 0.03 mg/dl for DKT and Pcr = 0.50 ± 0.05, 0.36 ± 0.04 and 0.21 ± 0.04 mg/dl for SKT at 1, 3, and 7 days after surgery) (P < 0.01 SKT vs. DKT; n = 6; Fig. 2A). The Pcr concentrations for both SKT and DKT gradually returned to the basal levels after 28 days post transplantation. Plasma KIM-1 and NGAL showed similar patterns for both SKT and DKT groups (n = 6; Fig. 2, B and C).
Fig. 2.
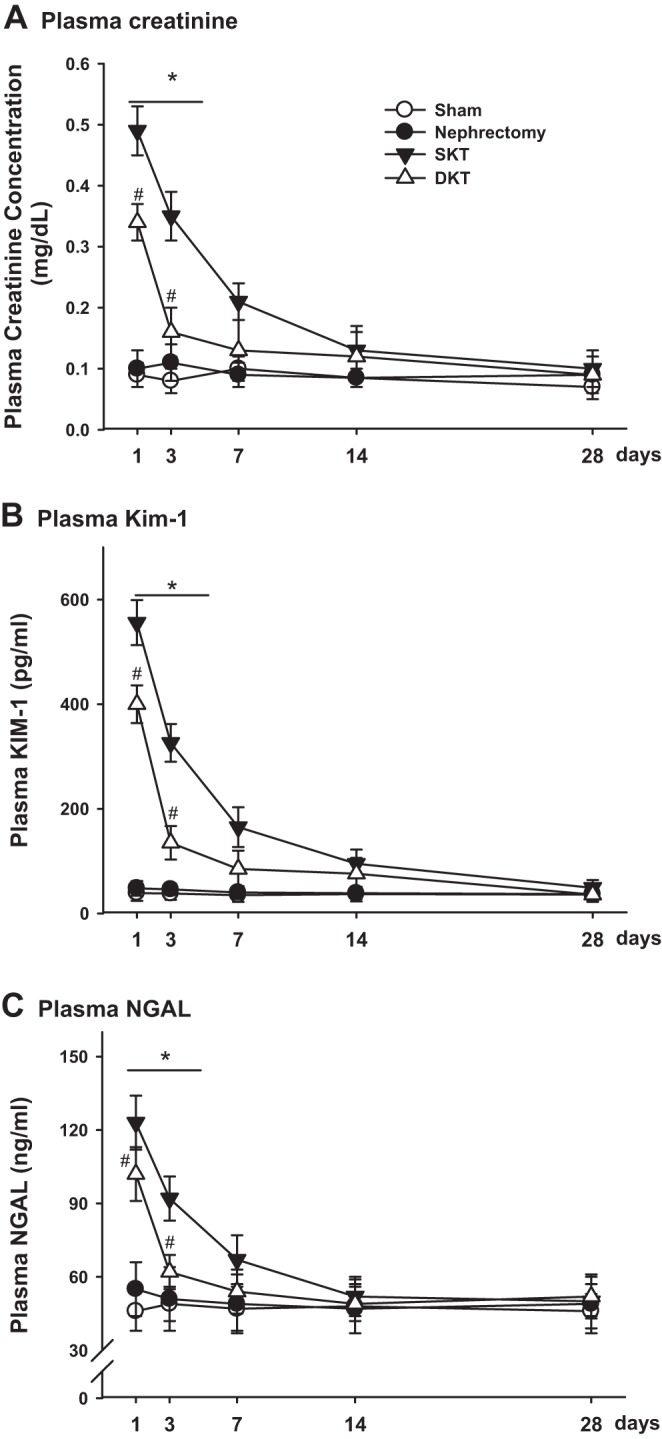
Injury marker measurement over time. Plasma creatinine (A), plasma kidney injury molecule-1 (KIM-1) (B), and plasma neutrophil gelatinase-associated lipocalin (NGAL) (C) were measured and compared at 1, 3, 7, 14, and 28 days after transplantation among different groups. (*P < 0.01 vs. other groups; #P < 0.05 vs. Sham; n = 6). DKT, dual-kidney transplantation; SKT, single-kidney transplantation.
GFR in conscious mice.
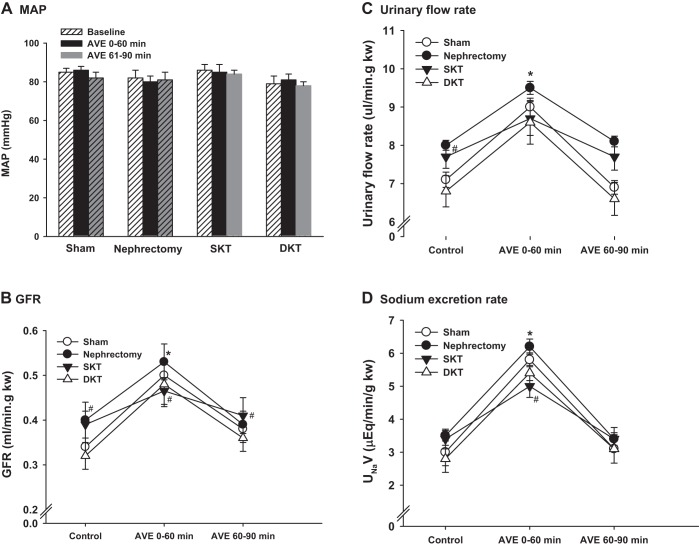
GFR was measured in conscious mice at 7, 14, and 28 days after transplantation to evaluate the restoration of graft function. Sham and nephrectomy groups showed consistent GFR during the observation period of 4 wk, which were 263.4 ± 5.2 µl/min and 206.8 ± 4.6 µl/min, respectively. At 7 days after transplantation, GFR in mice with DKT (215.6 ± 8.4 µl/min) and SKT (131.4 ± 8.6 µl/min) were ~20% and 30% lower than their respective controls, Sham operated and nephrectomized mice (P < 0.01 SKT vs. nephrectomy; P < 0.01 DKT vs. Sham; n = 6; Fig. 3A). At 14 days after operation, the GFR in DKT mice had returned to a level comparable to Sham-operated mice, which was 242.3 ± 4.8 µl/min. However, the GFR in SKT mice was still ~17% lower than nephrectomized mice, indicating a slower recovery of SKT than DKT mice from operation (P < 0.01 SKT vs. DKT; n = 6; Fig. 3A). At 4 wk after transplantation, the GFR for both SKT and DKT was almost back to baseline. The GFR⋅g−1⋅kw−1 (kidney weight) was ~20% higher in SKT than in DKT, indicating hyperfiltration in the SKT mice (P < 0.01 nephrectomy and SKT vs. Sham and DKT; n = 6; Fig. 3B).
Fig. 3.
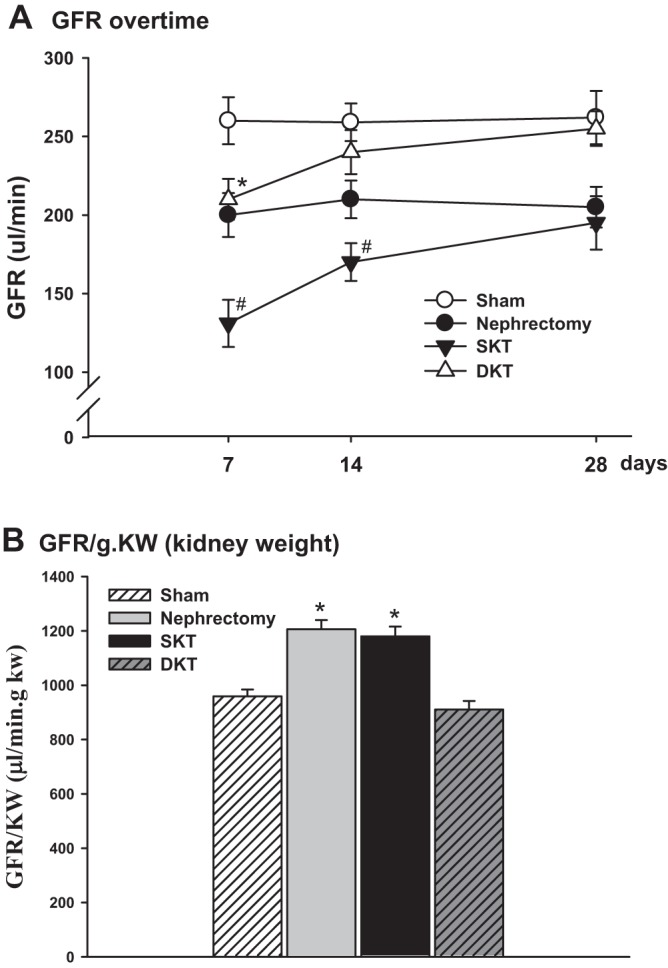
Glomerular filtration rate (GFR) in conscious mice. A: GFR was measured in conscious mice at 7, 14, and 28 days following transplantation (*P < 0.01 vs. Sham; #P < 0.01 vs. other groups; n = 6). B: GFR values were normalized by kidney weight. GFR⋅g−1⋅kw−1 (kidney weight) was ~20% higher in nephrectomy and SKT than in Sham and DKT. (*P < 0.01 vs. Sham and DKT; n = 6). DKT, dual-kidney transplantation; SKT, single-kidney transplantation.
Renal hemodynamics and sodium excretion in response to AVE.
To evaluate the renal hemodynamics and sodium excretion, we measured kidney clearance function in response to an AVE by intravenous infusion of saline in all groups of mice at 4 wk after transplantation.
The MAPs were constant in all groups of mice before and during AVE. There were no significant differences in MAP among the four groups (n = 4; Fig. 4A). The basal GFR values were similar between SKT and nephrectomy groups and between DKT and Sham groups. The GFRs normalized by kidney weight (ml⋅min−1⋅g−1⋅kw−1) in DKT and Sham-operated mice at baseline were ~27% lower than that in SKT and nephrectomized groups (P < 0.01 Sham and DKT vs. nephrectomy and SKT; n = 4; Fig. 4B). GFR (ml⋅min−1⋅g−1⋅kw−1) rose by ~57%, 50%, 33%, and 54% during 60 min after AVE for Sham, nephrectomy, SKT, and DKT, respectively (P < 0.01; 0–60 min vs. basal and 60–90 min; n = 4; Fig. 4B). The mice with SKT exhibited blunted effect in the increase of GFR and natriuretic in response to the acute saline load. The comparison of urinary flow rate and sodium excretion rate among the groups followed a pattern similar to that of GFR (n = 4; Fig. 4, C and D).
Fig. 4.
Kidney clearance function measurement. Mean arterial pressure (MAP) (A), glomerular filtration rate (GFR) (B), urinary flow rate (C), and sodium excretion rate (D) were measured in mice of the four groups in response to a bolus infusion of saline of 3% body weight at 28 days after transplantation. Kidney clearance function was assessed during 0 to 60 min and 60 to 90 min after volume expansion. (*P < 0.01 vs. basal, #P < 0.05 vs. DKT and Sham; n = 4). AVE, acute volume expansion; DKT, dual-kidney transplantation; SKT, single-kidney transplantation.
Histology.
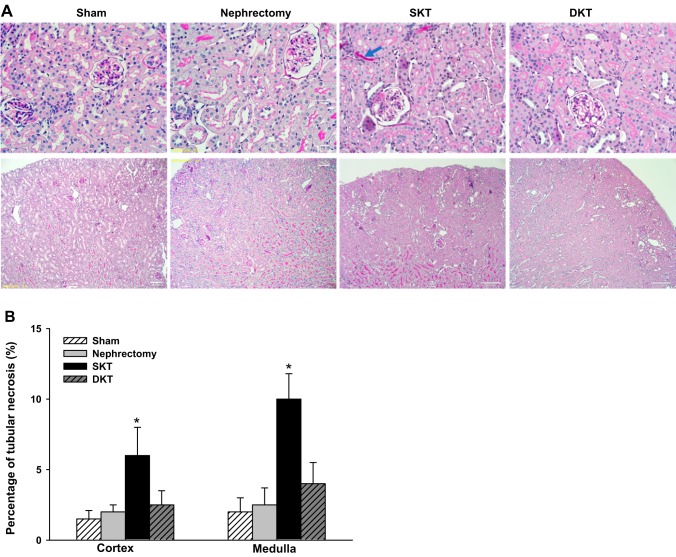
Histology by periodic acid Schiff staining demonstrated preserved architecture in all the groups (n = 4; Fig. 5A). Focal minor tubular injuries were only observed in the SKT group but not in DKT, nephrectomy, or Sham groups (P < 0.01; SKT vs. other groups; n = 6; Fig. 5B).
Fig. 5.
Histology. A: representative periodic acid Schiff-stained histology images. Preserved architecture was observed in all the groups. Focal minor tubular injuries (blue arrow) were only observed in the SKT group. B: kidney injury scores. Based on the percentage of necrotic tubules, the SKT group exhibited the very mild tubular injury. There was no structural abnormality detected in Sham, nephrectomy, and DKT groups (*P < 0.01 vs. other groups; n = 6). DKT, dual-kidney transplantation; SKT, single-kidney transplantation.
Immunohistochemistry.
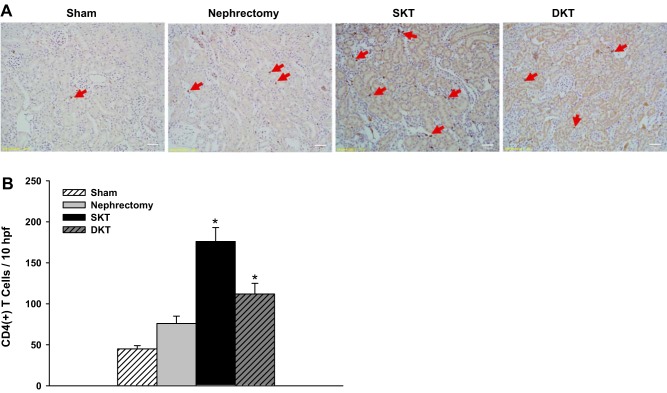
The infiltration of CD4+ T cells in kidney tissue was examined by immunohistochemical assay. The CD4+ T cells were found primarily in the interstitium. The SKT group showed the most significant increase in CD4+ T cells followed by DKT, nephrectomy, and Sham groups (176 ± 17, 112 ± 13, 76 ± 9, and 45 ± 4 CD4+ T cells/10 high power field, respectively) (P < 0.01 vs. other groups; n = 6; Fig. 6).
Fig. 6.
Immunohistochemistry analysis. A: immunohistochemical staining of CD4. Red arrow indicates the positive cells. B: average number of CD4+ T cells per 10 fields was analyzed.(*P < 0.01 vs. other groups; n = 6). DKT, dual-kidney transplantation; hpf, high power field; SKT, single-kidney transplantation.
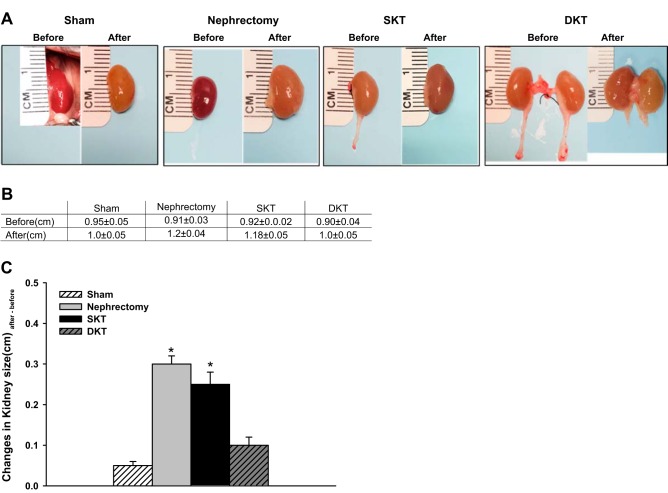
Body weight, kidney weight, kidney size, and survival rate.
Four weeks after transplantation, the kidney size and weight in the nephrectomy and SKT groups were significantly increased. However, the kidneys with Sham and DKT did not change evidently (n = 6; Fig. 7). The ratios of single kidney weight over body weight in nephrectomy and SKT were ~30% and 35% higher than these in Sham and DKT groups (n = 6; Table 2). The survival rate was >90% for all the groups of mice.
Fig. 7.
Kidney size comparison. A: respective kidney sizes measured before operation (Before) and 28 days after operation (After) for Sham, nephrectomy, SKT, and DKT groups. B: summary of the kidney sizes. C: changes of the kidney sizes 28 days after operation. (*P < 0.01 vs. other groups; n = 6). DKT, dual-kidney transplantation; SKT, single-kidney transplantation.
Table 2.
Body weight and kidney weight
| Sham | Nephrectomy | SKT | DKT | |
|---|---|---|---|---|
| BW, g | 23.8 ± 0.3 | 25.4 ± 1.0 | 28.2 ± 0.5 | 25.9 ± 0.3 |
| KW, g | L: 0.1240 ± 0.05 R: 0.1192 ± 0.04 | 0.1731 ± 0.05 | 0.1903 ± 0.04 | L: 0.1440 ± 0.06 R: 0.1506 ± 0.07 |
An apparent increase of the kidney weight was observed in SKT group. The ratios of single kidney weigh over body weight in nephrectomy and SKT group were ~30% and 35% higher than these in Sham and DKT groups (n = 6). BW, body weight; DKT, dual-kidney transplantation; KW, kidney weight; L, left kidney; R, right kidney; SKT, single-kidney transplantation.
DISCUSSION
In the present study, we successfully performed DKT in mice and compared the hemodynamic response and graft function with SKT mice. The mice with DKT showed ~40% less kidney injury and better recovery after surgery than SKT according to Pcr and GFR levels. The improved renal functional reserve was demonstrated in mice with DKT versus SKT as assessed by the response to an acute salt loading at 4 wk after transplantation. Histological examination also exhibited less kidney injury in DKT than SKT.
The genetically modified mouse model is a powerful tool in determining gene function in health and diseases, particularly tissue-specific gene manipulated mouse models. Gene knockout and knock-in technologies such as the Cre-loxP system are very useful in tissue-specific knockout animal model generation; however, it is relatively difficult, time-consuming, expensive, and limited by the availability of Cre lines (5). Kidney transplantation can be an alternative strategy in generation of kidney-specific gene-modified mouse by implantation of the kidney from the donor lacking whole-body expression of a target gene to wild type or vice versa. In addition, as mouse can offer unmatched advantages of genetic manipulability and extensive treatment options, mouse kidney transplantation has been extensively used in the study of mechanisms underlying antibody-mediated rejection. Traditionally, only one kidney will be transplanted to the host animal, which subsequently induces hyperfiltration of a single nephron. DKT would be expected to provide more physiological situations in hemodynamics in recipients than a single kidney (11). Although DKT has been performed in large animals and human beings (12, 13, 15), DKT in mice is lacking due to technical challenging. In this study, we successfully performed DKT in mice and compared the hemodynamics, surgery complication, and renal functional reserve to mice with SKT.
To avoid extra trauma and reduce operative time, we implanted both donor kidneys en bloc into the recipients following a similar procedure as that performed for SKT, as we reported previously (21, 23, 24). Compared with SKT, additional dissection was needed during the donors’ procurement, as we used the IVC and the aorta artery above the right renal artery as the renal vein and artery for the DKT, respectively, for the dual transplanted kidneys. The anastomosis of the vessels was similar to SKT except that dual anastomosis of ureters was required with DKT. The surgical times for DKT were ~4 min longer than those of SKT; however, the surgical complications associated with DKT were comparable to SKT with only one graft failure caused by graft thrombosis in the seven DKTs. Although extra work as mentioned above was needed and the anastomosis of the vessels was more challenging due to the limited working space with DKT, with practice, we were able to successfully accomplish the surgery with a survival rate above 85%, which was not different from SKT.
Even though the surgical time was a little bit longer (~4 min), the mice with DKT exhibited significantly less kidney injury than SKT, reflected by ~40% lower Pcr values 24 h after operation and quicker recovery from operation than SKT mice. Following SKT, the size of the transplanted kidney rapidly increases because of increased single nephron GFR because of the tremendously reduced total nephron numbers (9, 10, 17), which may elevate intraglomerular pressure and accentuate glomerular injury in the transplanted grafts (2, 19). In our study, hypertrophy was noticed in the grafts with SKT. The size and weight of the kidney in SKT group were increased ~30% 1 mo after operation. However, the kidneys with DKT were not different from Sham-operated controls. GFR normalized by kidney weight was ~20% higher in mice in SKT compared with DKT, indicating hyperfiltration in SKT mice. There were no obvious structural abnormalities in the DKT mice, and only focal minor tubular injuries were observed in the SKT group with less than 10% necrotic tubules. The immunohistochemical staining of CD4 showed more significant increase in CD4+ T cells in the SKT group than DKT group, suggesting more inflammation in SKT than DKT mouse, which may further amplify necroinflammation in SKT group. The hyperfiltration in SKT mice may be one of the reasons that mice with DKT revealed less renal injury at 24 h after operation and showed much quicker recovery from the operation compared with SKT.
To determine the long-term graft function, we measured the kidney clearance function in response to the AVE by measurement of GFR, sodium excretion, and urine flow rate at 1 mo after surgery. The transplanted grafts with DKT exhibited a similar hemodynamic response and sodium excretory ability as the Sham-operated mice, demonstrating an intact reservation of graft function and a normal physiological environment in the recipient. However, the SKT mice exhibited glomerular hyperfiltration at baseline and a blunted rise in GFR and natriuretic response to an acute salt load, indicating the partial loss of renal functional reservation. Our results agree with the findings in human transplantation that sufficient nephron mass from DKT compared with SKT prevented long-term deterioration in kidney functions (1, 6). These findings demonstrated that applying DKT to the study of immune response and the generation of kidney specific knockout mice will avoid the confounding factors caused by hyperfiltration and provide more physiological environment than SKT for the subsequent research.
In conclusion, we have successfully generated a mouse model of DKT. The hemodynamic responses following DKT better mimic physiological situations with less kidney injury and better recovery after surgery. We believe that DKT will provide a new tool and meaningful approach to the evaluation of renal significance in physiology and disease.
GRANTS
This work was supported by NIH Grants DK099276 (R. Liu) and DK098582 (R. Liu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.W. and J.W. conceived and designed research; L.W. performed experiments; L.W., X.W., S.J., J.B., and L.F. analyzed data; L.W., X.W., J.B., L.F., J.Z., and R.L. interpreted results of experiments; L.W. and L.F. prepared figures; L.W. drafted manuscript; L.W., X.W., S.J., J.W., J.B., L.F., J.Z., and R.L. edited and revised manuscript; R.L. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of X. Wang: Shandong Medical Imaging Research Institute, Shandong Provincial Key Laboratory of Diagnosis and Treatment of Cardio-Cerebral Vascular Disease, Shandong University, Jinan, Shandong, China.
REFERENCES
- 1.Andrés A, Morales JM, Herrero JC, Praga M, Morales E, Hernández E, Ortuño T, Rodício JL, Martínez MA, Usera G, Díaz R, Polo G, Aguirre F, Leiva O. Double versus single renal allografts from aged donors. Transplantation 69: 2060–2066, 2000. doi: 10.1097/00007890-200005270-00015. [DOI] [PubMed] [Google Scholar]
- 2.Brenner BM. Hemodynamically mediated glomerular injury and the progressive nature of kidney disease. Kidney Int 23: 647–655, 1983. doi: 10.1038/ki.1983.72. [DOI] [PubMed] [Google Scholar]
- 3.Brenner BM, Cohen RA, Milford EL. In renal transplantation, one size may not fit all. J Am Soc Nephrol 3: 162–169, 1992. [Erratum in J Am Soc Nephrol 3: 1038, 1992] [DOI] [PubMed] [Google Scholar]
- 4.Franceschini N, Cheng O, Zhang X, Ruiz P, Mannon RB. Inhibition of prolyl-4-hydroxylase ameliorates chronic rejection of mouse kidney allografts. Am J Transplant 3: 396–402, 2003. doi: 10.1034/j.1600-6143.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 5.Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer 7: 645–658, 2007. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- 6.Gill J, Cho YW, Danovitch GM, Wilkinson A, Lipshutz G, Pham PT, Gill JS, Shah T, Bunnapradist S. Outcomes of dual adult kidney transplants in the United States: an analysis of the OPTN/UNOS database. Transplantation 85: 62–68, 2008. doi: 10.1097/01.tp.0000296855.44445.af. [DOI] [PubMed] [Google Scholar]
- 7.Gueler F, Rong S, Gwinner W, Mengel M, Bröcker V, Schön S, Greten TF, Hawlisch H, Polakowski T, Schnatbaum K, Menne J, Haller H, Shushakova N. Complement 5a receptor inhibition improves renal allograft survival. J Am Soc Nephrol 19: 2302–2312, 2008. doi: 10.1681/ASN.2007111267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen K, Khanna C. Spontaneous and genetically engineered animal models; use in preclinical cancer drug development. Eur J Cancer 40: 858–880, 2004. doi: 10.1016/j.ejca.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 9.Hartman ME, Bonfilio AC. Effect of nephrectomy on the minute renal circulation. Am J Physiol 196: 1119–1121, 1959. doi: 10.1152/ajplegacy.1959.196.5.1119. [DOI] [PubMed] [Google Scholar]
- 10.Katz AI, Epstein FH. Relation of glomerular filtration rate and sodium reabsorption to kidney size in compensatory renal hypertrophy. Yale J Biol Med 40: 222–230, 1967. [PMC free article] [PubMed] [Google Scholar]
- 11.Khalil MAM, Tan J, Khan TFT, Khalil MAU, Azmat R. Dual kidney transplantation: a review of past and prospect for future. Int Sch Res Notices 2017: 2693681, 2017. doi: 10.1155/2017/2693681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu AD, Carter JT, Weinstein RJ, Prapong W, Salvatierra O, Dafoe DC, Alfrey EJ. Excellent outcome in recipients of dual kidney transplants: a report of the first 50 dual kidney transplants at Stanford University. Arch Surg 134: 971–975, 1999. doi: 10.1001/archsurg.134.9.971. [DOI] [PubMed] [Google Scholar]
- 13.Mackenzie HS, Azuma H, Rennke HG, Tilney NL, Brenner BM. Renal mass as a determinant of late allograft outcome: insights from experimental studies in rats. Kidney Int Suppl 52: S38–S42, 1995. [PubMed] [Google Scholar]
- 14.Mannon RB, Kopp JB, Ruiz P, Griffiths R, Bustos M, Platt JL, Klotman PE, Coffman TM. Chronic rejection of mouse kidney allografts. Kidney Int 55: 1935–1944, 1999. doi: 10.1046/j.1523-1755.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- 15.Perico N, Ruggenenti P, Scalamogna M, Locatelli G, Remuzzi G. One or two marginal organs for kidney transplantation? Transplant Proc 34: 3091–3096, 2002. doi: 10.1016/S0041-1345(02)03624-2. [DOI] [PubMed] [Google Scholar]
- 16.Rong S, Lewis AG, Kunter U, Haller H, Gueler F. A knotless technique for kidney transplantation in the mouse. J Transplant 2012: 127215, 2012. doi: 10.1155/2012/127215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rous SN, Wakim KG. Kidney function before, during and after compensatory hypertrophy. J Urol 98: 30–35, 1967. doi: 10.1016/S0022-5347(17)62817-9. [DOI] [PubMed] [Google Scholar]
- 18.Sturgeon C, Sam AD II, Law WR. Rapid determination of glomerular filtration rate by single-bolus inulin: a comparison of estimation analyses. J Appl Physiol (1985) 84: 2154–2162, 1998. doi: 10.1152/jappl.1998.84.6.2154. [DOI] [PubMed] [Google Scholar]
- 19.Terasaki PI, Koyama H, Cecka JM, Gjertson DW. The hyperfiltration hypothesis in human renal transplantation. Transplantation 57: 1450–1454, 1994. doi: 10.1097/00007890-199405000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Vandamme TF. Use of rodents as models of human diseases. J Pharm Bioallied Sci 6: 2–9, 2014. doi: 10.4103/0975-7406.124301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Wei J, Jiang S, Li HH, Fu L, Zhang J, Liu R. Effects of different storage solutions on renal ischemia tolerance after kidney transplantation in mice. Am J Physiol Renal Physiol 314: F381–F387, 2018. doi: 10.1152/ajprenal.00475.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Song J, Buggs J, Wei J, Wang S, Zhang J, Zhang G, Lu Y, Yip KP, Liu R. A new mouse model of hemorrhagic shock-induced acute kidney injury. Am J Physiol Renal Physiol 312: F134–F142, 2017. doi: 10.1152/ajprenal.00347.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Song J, Wang S, Buggs J, Chen R, Zhang J, Wang L, Rong S, Li W, Wei J, Liu R. Cross-sex transplantation alters gene expression and enhances inflammatory response in the transplanted kidneys. Am J Physiol Renal Physiol 313: F326–F338, 2017. doi: 10.1152/ajprenal.00039.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Wang X, Qu HY, Jiang S, Zhang J, Fu L, Buggs J, Pang B, Wei J, Liu R. Role of kidneys in sex differences in angiotensin II-induced hypertension. Hypertension 70: 1219–1227, 2017. doi: 10.1161/HYPERTENSIONAHA.117.10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Chandrashekar K, Wang L, Lai EY, Wei J, Zhang G, Wang S, Zhang J, Juncos LA, Liu R. Inhibition of nitric oxide synthase 1 induces salt-sensitive hypertension in nitric oxide synthase 1α knockout and wild-type mice. Hypertension 67: 792–799, 2016. doi: 10.1161/HYPERTENSIONAHA.115.07032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei J, Song J, Jiang S, Zhang G, Wheeler D, Zhang J, Wang S, Lai EY, Wang L, Buggs J, Liu R. Role of intratubular pressure during the ischemic phase in acute kidney injury. Am J Physiol Renal Physiol 312: F1158–F1165, 2017. doi: 10.1152/ajprenal.00527.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]