Abstract
Acute kidney injury (AKI) is a leading cause of morbidity and mortality. Drug-induced/toxic AKI can be caused by a number of therapeutic agents. Cisplatin is an effective chemotherapeutic agent whose administration is limited by significant nephrotoxicity. Therapies to prevent cisplatin-induced AKI are lacking. Although tumor necrosis factor-α (TNF) plays a key role in the pathogenesis of cisplatin nephrotoxicity, the innate immune signaling pathways that trigger TNF generation in this context require elucidation. In this regard, sterile injury triggers the release and activation of both isoforms of interleukin(IL)-1, IL-1α and IL-1β. In turn, stimulation of the interleukin-1 receptor (IL-1R1) by these ligands engages a proinflammatory signaling cascade that induces TNF induction. We therefore hypothesized that IL-1R1 activation exacerbates cisplatin-induced AKI by inducing TNF production, thereby augmenting inflammatory signals between kidney parenchymal cells and infiltrating myeloid cells. IL-1R1+/+ (WT) and IL-1R1−/− (KO) mice were subjected to cisplatin-induced AKI. Compared with WT mice, IL-1R1 KO mice had attenuated AKI as measured by serum creatinine and BUN, renal NGAL mRNA levels, and blinded histological analysis of kidney pathology. In the cisplatin-injured kidney, IL-1R1 KO mice had diminished levels of whole kidney TNF, and fewer Ly6G-expressing neutrophils. In addition, an unbiased machine learning analysis of intrarenal immune cells revealed a diminished number of CD11bint/CD11cint myeloid cells in IL-1R1 KO injured kidneys compared with IL-1R1 WT kidneys. Following cisplatin, IL-1R1 KO kidneys, compared with WTs, had fewer TNF-producing: macrophages, CD11bint/CD11cint cells, and neutrophils, consistent with an effect of IL-1R1 to polarize intrarenal myeloid cells toward a proinflammatory phenotype. Interruption of IL-1-dependent signaling pathways warrants further evaluation to decrease nephrotoxicity during cisplatin therapy.
Keywords: acute kidney injury, interleukin-1, myeloid
INTRODUCTION
Acute kidney injury (AKI) is one of the most common forms of organ failure as it develops in up to 5% of all hospitalized patients and 30% of critically ill patients (28). AKI leads to longer hospital stays, increased hospital costs, and increased short- and long-term mortality (9). AKI can be caused by a wide variety of stimuli, including ischemia, sepsis/infection, and drug toxicity. As the kidneys filter 25% of cardiac output and play a prominent role in drug metabolism, drug-induced nephrotoxicity complicates the administration of many pharmacological agents.
Cisplatin is a platinum-based drug that is used as a chemotherapeutic agent for the treatment of a wide variety of solid tumors. Although cisplatin is efficacious in the treatment of these tumors, its use is limited by nephrotoxicity. Nearly one-third of patients develop significant nephrotoxicity after a single dose of cisplatin, which necessitates either discontinuation of the drug or a reduced/less efficacious dose (18). Cisplatin nephrotoxicity involves proximal tubule injury, vascular damage, and renal inflammation (18). Inflammation, in particular, plays a vital role in cisplatin nephrotoxicity. Following cisplatin administration, immune cells accumulate in the kidney and proinflammatory cytokines are secreted, which together exacerbate renal injury (9, 18). Proinflammatory cytokines that play a prominent role in the pathophysiology of cisplatin nephrotoxicity include TNF (21, 22, 35) and IL-1 (5).
IL-1 isoforms, IL-1α and IL-1β, trigger activation of proinflammatory signaling pathways within cells via ligation of their cell surface receptor, IL-1R1. IL-1R1 is a member of the Toll/IL-1 receptor (TIR) family, which signals through myeloid differentiation primary response protein 88 (MyD88) to activate the NF-κB and MAPK pathways (17). Activation of these proinflammatory pathways results in the production of cytokines including TNF and chemokines, which together recruit immune cells to the site of injury. Based on the capacity of IL-1R1 activation to trigger NF-κB-dependent TNF induction, we hypothesized that genetic deletion of IL-1R1 would afford protection from cisplatin-induced AKI. We find that genetic deficiency of IL-1R1 protects from cisplatin nephrotoxicity. Moreover, through gene expression profiling of kidney lysates and fluorescent cell sorting analysis of injured kidneys, we establish that IL-1R1 activation promotes Ly6G-expressing neutrophil accumulation in the injured kidney and generation of TNF by intrarenal myeloid cells. These results indicate that IL-1 plays an important role in the pathogenesis of cisplatin nephrotoxicity and lay the groundwork for disrupting IL-1R1 signals in patients at heightened risk for cisplatin-induced AKI.
METHODS
Animals.
IL1R1−/− (IL-1R1 KO) (B6.129S7-Il1r1tm1Glm/J) mice on the C57/BL6 background were obtained from the Jackson Laboratory and backcrossed for >6 generations to the 129/SvEv strain. Littermate IL-1R1+/+ (WT) and IL-1R1 KO animals obtained by intercrossing IL1R1 heterozygotes were used for experiments. All of the animal studies were approved by the Durham Veterans Affairs Medical Center Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Female mice from 10 to 16 wk old were used for experiments.
Cisplatin-induced kidney injury.
Experimental mice were injected intraperitoneally (IP) with 30 mg/kg cisplatin (Sigma 479306) or equal volume of normal saline to induce kidney injury or serve as vehicle control animals. Mice were euthanized after 48 h for fluorescent cell sorting experiments and 72 h for all other experiments. For tissue harvest, animals were anesthetized with isoflurane (3%) via nosecone, blood was obtained via cardiac puncture, and renal tissues were sectioned and snap-frozen in liquid nitrogen or fixed in 10% formalin for histological analysis.
Assessment of renal function.
Serum creatinine and BUN levels were measured by the University of North Carolina Animal Histopathology and Laboratory Medicine Core. Urine albumin and creatinine were quantitated by ELISA (Exocell).
Histologic analyses.
After whole body perfusion with normal saline via cardiac puncture, kidney tissues were fixed with 10% neutral-buffered formalin (VWR 16004–128), embedded with paraffin, sectioned in 5-mm sections, and stained with hematoxylin and eosin (H&E) staining by the Duke Research Immunohistology Laboratory. Sections were scored by an experienced animal pathologist masked to experimental groups. Sections were graded according to a previously established scoring system (33): the percentages of tubules with cell lysis, loss of brush border, and cast formation were scored on a scale from 0 to 4 (0, no damage; 1, 25%; 2, 25–50%; 3, 50–75%; 4, >75%). Histologic scores for each kidney were obtained by adding individual component scores.
RT-PCR analysis of mRNA expression.
mRNA was isolated with an RNeasy Mini Kit per the manufacturer’s instructions (Qiagen, Germantown, MD). For whole kidneys, a small piece of the renal pole was homogenized in Buffer RLT with 0.01% β-ME and further homogenized with Qiashredder columns (Qiagen 79654). mRNA concentration in each sample was determined by Nanodrop (ThermoFisher). cDNA was synthesized with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems 4368814) according to manufacturer’s instructions. Gene expression levels of TNFα, IL-1β, IL-1α, CXCL2, CCL2, CCL5, and NGAL were determined by RT-PCR on an ABI7900HT machine (Applied Biosystems) using TaqMan primers (Applied Biosystems; Table 1).
Table 1.
Primers used for RT-PCR analysis
| Gene | Accession No./Catalog No. |
|---|---|
| NGAL/Lcn2 | Mm01324470_m1 |
| TNFα | Mm00443259_g1 |
| IL-1α | Mm00439620_m1 |
| IL-1β | Mm00434228_m1 |
| CXCL2 | Mm00436450_m1 |
| CCL2 | Mm00441242_m1 |
| GAPDH | 4352661 |
Isolation of leukocytes from mouse kidney and fluorescent cell sorting analysis.
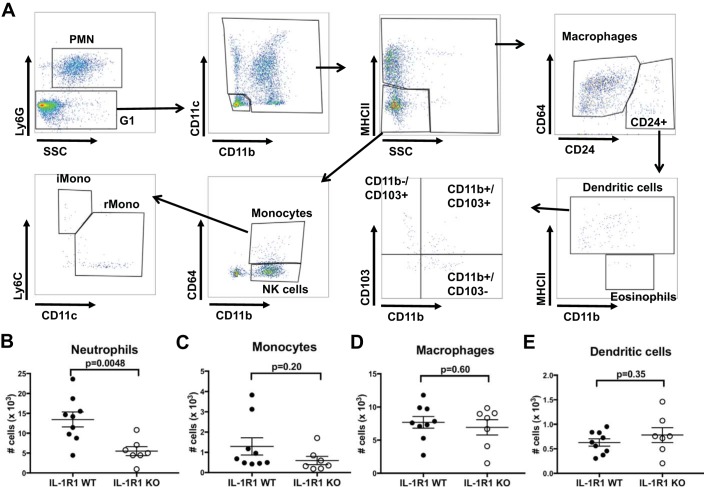
At 48 h after vehicle or cisplatin treatment, kidneys were harvested and minced, followed by incubation with 1 mg/ml collagenase type IV (GIBCO) and 10 μg/ml DNase (Sigma) for 30 min at 37°C. Cells were soft spun (10 g for 1 min), and supernatant was obtained and placed through a 70-μm cell strainer to remove large cell debris and washed with staining buffer [DPBS (Sigma), 3% heat-inactivated FBS, 2 mM EDTA]. Cells were spun down and red blood cells in pellets were lysed with ACK Lysing Buffer (GIBCO), after which single cell suspensions were washed and filtered through a 40-μm cell strainer. Cells were spun down and pellets from each kidney were resuspended in 800 μl staining buffer. For cell surface staining, cells were blocked with anti-CD16/CD32 and then stained with fluorescently-labeled antibodies (Table 2), and then Live/dead near-IR Dead Cell Stain (Invitrogen L34976). Cells were fixed with BD CytoFix. Prior to analysis, 20 μl of Count Bright absolute counting beads (Invitrogen C36950) was added to cells and samples were analyzed on an LSRII flow cytometry (BD). Total cell numbers were obtained using the enumeration formula as described in manufacturer’s instructions. The gating strategy was as described in Fig. 4.
Table 2.
Fluorescent cell sorting antibodies
| Antibody | Company | Catalog No. | Amount, µl/test |
|---|---|---|---|
| FITC anti-CD11b | Biolegend | 101206 | 0.25 |
| PerCP/Cy5.5 anti-L6G | Biolegend | 127615 | 0.25 |
| PE anti-CD11c | Biolegend | 117308 | 0.25 |
| PE-Cy7 anti-CD24 | Biolegend | 101822 | 0.125 |
| APC anti-CD64 | Biolegend | 139306 | 1 |
| BV421 anti-CD103 | Biolegend | 121422 | 1 |
| BV510 anti-Ly6C | Biolegend | 128033 | 0.5 |
| eVolve 655 anti-MHCII | eBioscience | 86–5321–42 | 0.1 |
| BV785 anti-CD45 | Biolegend | 103149 | 0.1 |
| APC anti-TNFα | eBioscience | 17–7321–82 | 0.5 |
| BV421 anti-Ly6G | BD Biosciences | 562737 | 0.5 |
| BV510 anti-CD45 | Biolegend | 103138 | 0.1 |
Fig. 4.
FACS-based enumeration of myeloid cell infiltration into kidney early in the course of cisplatin-induced AKI. At 2 days following cisplatin, fluorescent cell sorting analysis was performed on single cell suspensions from injured WT and IL-1R1 KO kidneys. A: gating strategy used to parse intrarenal myeloid cell populations after selecting for live CD45+ cells and excluding doublets. B–E: total cell numbers for PMN/neutrophils (B), monocytes (C), macrophages (D), and dendritic cells (E), respectively (n = 8 for IL-1R1 WT, n = 7 for IL-1R1 KO). Data are expressed as means ± SE. Significance determined by unpaired t-test.
T-distributed stochastic neighbor embedding (tSNE) analysis.
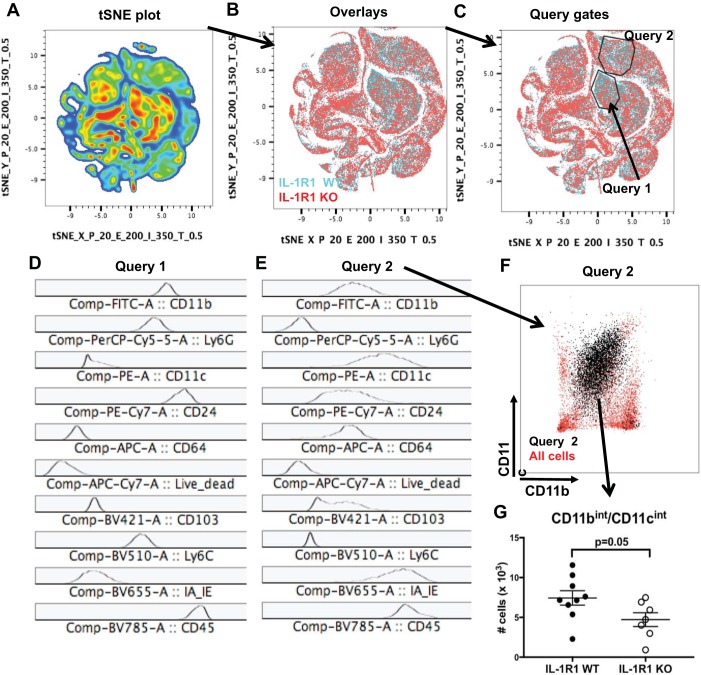
Within FlowJo analysis software (BD Biosciences), all samples from one representative experiment were concatenated and the tSNE algorithm was performed by FlowJo software using a perplexity of 20 and a learning rate of 200. tSNE plots were obtained and overlayed with individual concatenated samples from each genotype to determine whether regions contained a relative over-/underrepresentation of cells from either genotype. As shown in Fig. 5, query gates were drawn in regions with relative underrepresentation of IL-1R1 KO cells, and histograms of cell surface markers were obtained to determine characteristics of cells within region of interest.
Fig. 5.
Unbiased T-distributed stochastic neighbor embedding (tSNE) analysis reveals an underrepresentation of neutrophils and CD11bint/CD11cint myeloid cells in injured kidneys from IL-1R1 KO cohort. At 2 days after cisplatin, tSNE was conducted as detailed in methods on flow sorted samples. After exclusion of dead cells and doublets, shown is the tSNE plot of all intrarenal CD45+ cells from both IL-1R1 WT and KO mice in a representative experiment (A). B: overlay of cells based on IL-1R1 WT (blue) and IL-1R1 KO (red) genotype. C: query gates of underrepresented clusters in IL-1R1 KO cells detected by overlay with histograms of cell surface markers for cells within Query 1 (D) and Query 2 (E). F: plot of CD11c vs CD11b for cells in Query 2. G: total cell numbers for CD11bint/CD11cint cells (n = 8 for IL-1R1 WT, n = 7 for IL-1R1 KO). Data are expressed as mean ± SE.
Intracellular TNF staining.
Single cell suspensions from above were plated in 24 well culture dishes and incubated with LPS (Sigma L2630) (100 ng/ml) with 1:500 Protein Transport Inhibitor Cocktail (eBioscience) for 4 h. Then, cells were stained for cell-surface antibodies as above. Cells were fixed and permeabilized with the Foxp3/Transcription Factor Staining set (eBioscience) according to manufacturer’s instructions, after which they were blocked with 2% normal mouse serum (eBioscience 24–5544–94) and then incubated with anti-TNFα. Cells were washed and resuspended in staining buffer for analysis. Counting beads were added before analysis as above.
Statistical analysis.
The values within a group are expressed as means ± SE. For comparisons between two groups, an unpaired Student’s t-test was utilized. For comparisons between multiple treatments, statistical significance was assessed using two-way ANOVA with Sidak multiple comparisons post hoc test. All statistical analysis was performed in GraphPad Prism software (La Jolla, CA).
RESULTS
IL-1R1 activation exacerbates cisplatin-induced kidney injury.
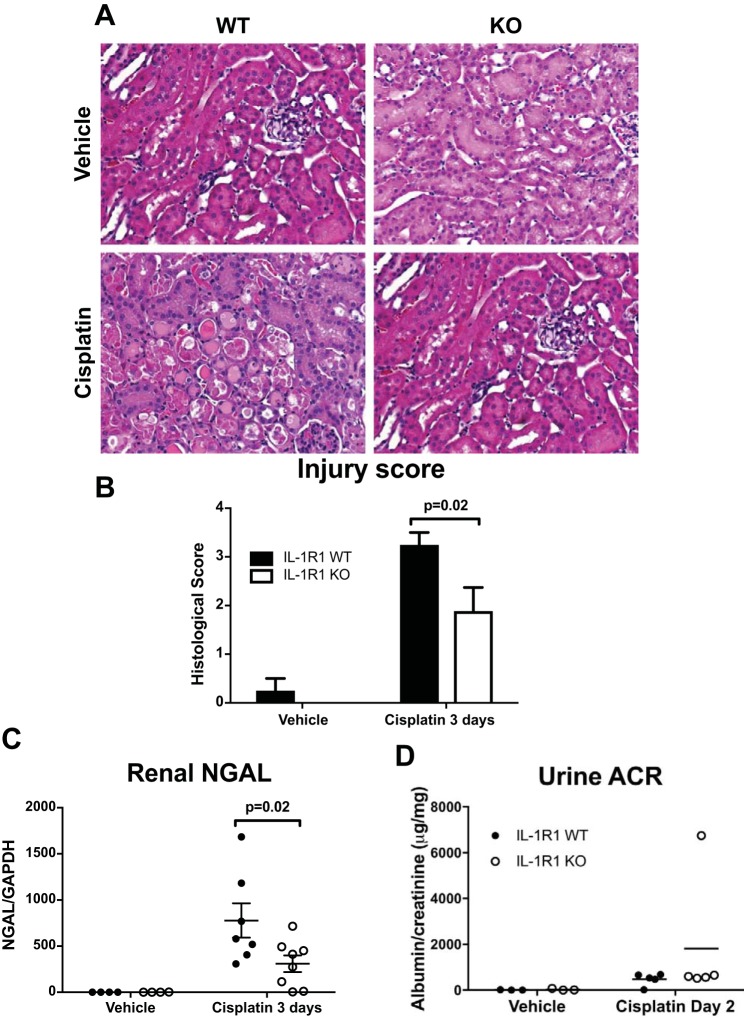
To determine whether IL-1R1 KO mice were protected from toxin/cisplatin-induced AKI, we subjected littermate IL-1R1 WT and KO mice to cisplatin-induced (30 mg/kg) AKI. At 3 days after injection, we measured survival and renal injury. Compared with IL-1R1 WT mice, IL-1R1 KO mice tended toward a survival advantage as no IL-1R1 KO mice died from cisplatin; however, this difference did not achieve statistical significance (Fig. 1A). We assayed for renal injury biomarkers and found that compared with IL-1R1 WT mice, IL-1R1 KO mice had significantly lower levels of serum creatinine (Fig. 1B) and BUN (Fig. 1C). Although not explicitly measured, these results suggest that IL-1R1 WT mice have a lower glomerular filtration rate (GFR) than IL-1R1 KO mice after cisplatin. We then assessed cisplatin-induced renal pathology in histologic tissue sections (33) and found that IL-1R1 KO mice had significantly less tubular damage than IL-1R WT controls (Fig. 2, A and B). Kidneys from cisplatin-treated IL-R1 KO animals also expressed lower mRNA levels of the renal injury marker NGAL levels (Fig. 2C). In a separate set of animals, we measured albumin excretion in the urine but did not detect high levels of albuminuria (Fig. 2D), suggesting that cisplatin does not induce substantial glomerular injury. Taken together, these results demonstrate that IL-1R1 activation exacerbates cisplatin-induced AKI.
Fig. 1.
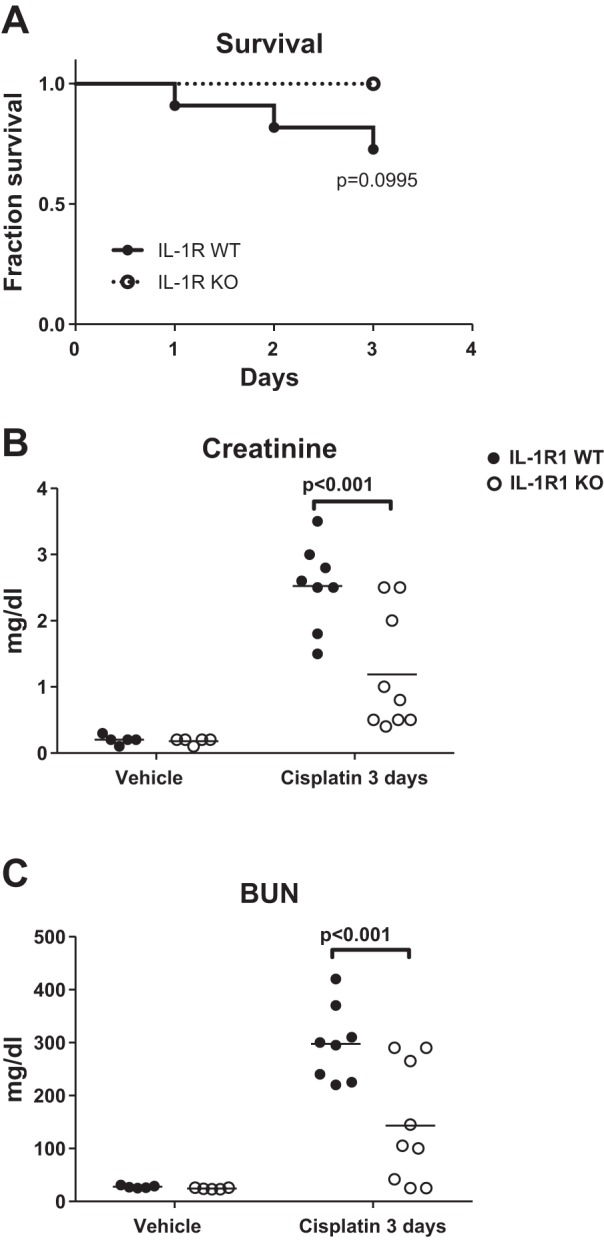
Interleukin 1 receptor (IL-1R1) activation exacerbates impairment of renal function in cisplatin nephrotoxicity. IL-1R1 WT and KO mice were injected ip with vehicle or cisplatin (30 mg/kg). At 3 days following cisplatin, survival was determined (significance determined by Mantel-Cox log-rank test) (IL-1R1 WT n = 11, IL-1R1 KO n = 9) (A); and serum levels of creatinine (B) and blood urea nitrogen (BUN; C) were measured (n = 5 for IL-1R1 WT and KO vehicle-treated mice; n = 8 for IL-1R1 WT cisplatin-treated mice, n = 9 for IL-1R1 KO cisplatin-treated mice). Data are expressed as means ± SE. Significance determined by 2-way ANOVA with Sidak posttest for multiple comparisons.
Fig. 2.
Interleukin 1 receptor (IL-1R1) ligation augments renal pathology accruing from cisplatin treatment. At 3 days following cisplatin, kidney sections were collected and stained for hematoxylin and eosin (H&E). A: representative histological kidney sections from vehicle- and cisplatin-treated IL-1R1 WT and IL-1R1 KO mice. B: sections were graded according to previously established scoring system (33) by an experienced animal pathologist masked to experimental groups. Histological scores for each kidney were obtained by adding individual component scores for IL-1R1 WT and KO kidneys (n = 4 for IL-1R1 WT and KO vehicle-treated mice; n = 8 for IL-1R1 WT cisplatin-treated mice, and n = 9 for IL-1R1 KO cisplatin-treated mice). C: at 3 days, kidney tissue was collected and renal NGAL mRNA levels were measured by RT-PCR (n = 4 for IL-1R1 WT and KO vehicle-treated mice; n = 7 for IL-1R1 WT cisplatin-treated mice, and n = 8 for IL-1R1 KO cisplatin-treated mice). D: in a separate set of animals (n = 3 in vehicle groups, 5 in cisplatin groups), albuminuria was quantitated at day 2. Shown is the ratio between urinary albumin and creatinine (ACR). Data are expressed as means ± SE. Significance determined by 2-way ANOVA with Sidak posttest for multiple comparisons.
IL-1R1 activation promotes TNF generation in the injured kidney.
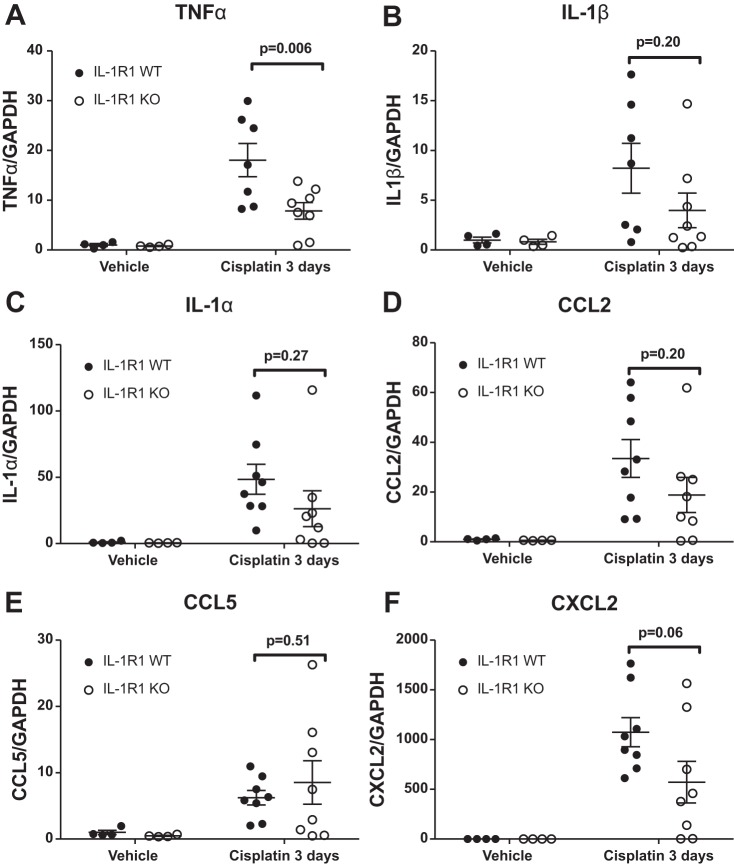
As IL-1R1 activation exacerbated cisplatin-induced renal injury (Figs. 1 and 2), we next sought to determine mechanisms of IL-1R1-mediated renal injury. We first examined the inflammatory milieu in the kidney by profiling the whole kidney tissue mRNA expression of cytokines and chemokines relevant to the pathogenesis of AKI. Compared with IL-1R1 WT mice, we found that IL-1R1 KO mice had significantly lower levels of TNF (Fig. 3A). Levels of IL-1α, IL-1β, CCL2, and CCL5 were similar between IL-1R1 WT and KO mice (Fig. 3, B–E). We detected a marked trend toward decreased expression of the neutrophil-recruiting chemokine CXCL2 (25) in IL-1R1 KO kidneys compared with IL-1R1 WTs (Fig. 3F), but this difference did not reach statistical significance. Thus IL-1R1 activation promotes intrarenal TNF generation and the CXCL2-mediated recruitment of neutrophils, which together promote further renal injury.
Fig. 3.
Interleukin 1 receptor (IL-1R1) signals drive TNFα and CXCL2 generation in the injured kidney. At 3 days following cisplatin, kidney tissue was obtained from WT and IL-1R1 KO mice and RT-PCR was performed for TNFα (A), IL-1β (B), IL-1α (C), CCL2 (D), CCL5 (E), and CXCL2 (F) (n = 4 for IL-1R1 WT and KO vehicle-treated mice; n = 7–8 for IL-1R1 WT cisplatin-treated mice, and n = 8 for IL-1R1 KO cisplatin-treated mice). Data expressed as means ± SE. Significance determined by 2-way ANOVA with Sidak posttest for multiple comparisons.
IL-1R1 activation facilitates neutrophil accumulation in the injured kidney following cisplatin.
We next sought to determine whether IL-1R1 stimulation modulates leukocyte recruitment into the kidney during cisplatin-mediated injury. We injected mice with cisplatin and measured leukocyte recruitment at 2 days when AKI is still developing. As IL-1 is a classical myeloid cell cytokine, we used fluorescent cell sorting with an established gating strategy to parse intrarenal myeloid cell populations (31) (Fig. 4A). Compared with IL-1R1 WT mice, IL-1R1 KO mice had similar numbers of monocytes (CD11b+/CD64+/MHCII−/Ly6G−), macrophages (CD64+/CD24−/MHCII+/Ly6G−), and dendritic cells (CD11c+/CD24hi/MHCII+/Ly6G−) (Fig. 4, C–E), but significantly fewer proportions and numbers of neutrophils (Ly6G+/SSChi) (Fig. 4B). These data indicate that IL-1R1 activation promotes the intrarenal accumulation of neutrophils, possibly via the induction of the neutrophil-recruiting chemokine CXCL2 (Fig. 3F).
Unbiased machine-learning algorithm demonstrates increased recruitment of neutrophils and macrophage-like myeloid cells in IL-1R1-expressing mice.
As an unbiased fluorescent cell sorting analysis approach to identify divergent hematopoietic cell populations in injured IL-1R1 WT and KO kidneys, we employed T-distributed stochastic neighbor embedding (t-SNE). t-SNE is a machine learning algorithm that can reduce multiparameter, high-dimensional flow cytometry into two-dimensional clusters (30). After excluding dead cells and doublets and selecting for CD45+ hematopoietic cells that have infiltrated the kidney, Fig. 5A shows a two-dimensional tSNE plot of all cells from all samples of a representative experiment. We next overlayed the tSNE graph with color coding based on whether cells were derived from IL-1R1 WT (blue) or KO mice (red) (Fig. 5B). Query gates were drawn around regions with a visual underrepresentation of IL-1R1 KO cells (Fig. 5C). Figure 5D shows histograms of cell surface markers for cells within Query 1. These cells are CD11b+/CD24hi/Ly6G+/Ly6C+/MHCII− (Fig. 5D), which is validated by the increased numbers of neutrophils we found in our targeted gating approach (Fig. 4B). Figure 5E demonstrates that cells in Query 2 are CD11b+/CD11c+/CD24mid/CD64+/Ly6C−/Ly6G−/MHCII+. To further evaluate this myeloid population, we plotted cells from Query gate 2 based on CD11b and CD11c expression (Fig. 5F). These cells correspond to a macrophage-like CD11bint/CD11cint population (12). We enumerated these cells in the injured kidney and found that IL-1R1 WT mice showed increased numbers of CD11bint/CD11cint cells compared with IL-1R1 KO mice (Fig. 5G). Thus, in addition to promoting neutrophil infiltration (Fig. 4B and Fig. 5D), IL-1R1 activation leads to enhanced accumulation of CD11bint/CD11cint cells in the injured kidneys (12).
IL-1R1 activation promotes TNF generation by intrarenal myeloid cells following cisplatin.
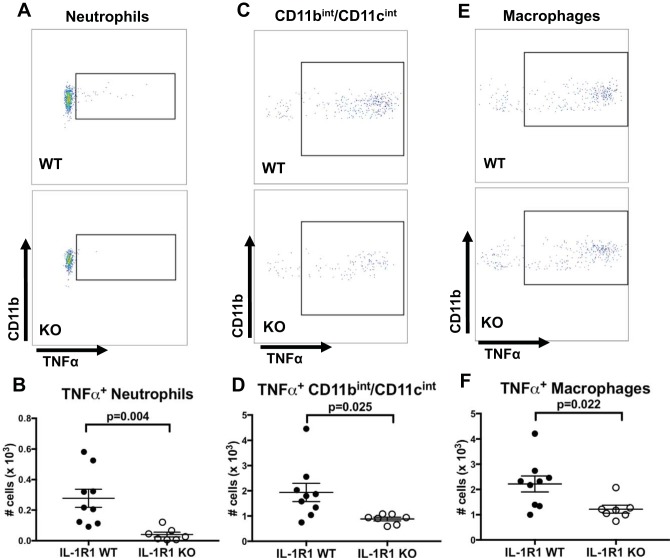
Since we found increased TNF generation within the kidney following cisplatin (Fig. 3A), and increased renal accumulation of myeloid cells (Fig. 4B and Fig. 5, D–G), we posited that IL-1R1 activation on intrarenal myeloid cells would promote their generation of TNF. To test this hypothesis, we performed intracellular cytokine staining for TNF on myeloid cells isolated from cisplatin-injured kidneys. We found increased total numbers of TNF-producing neutrophils (Fig. 6, A and B) and CD11bint/CD11cint cells (Fig. 6, C and D). Interestingly, although total numbers of CD64+ macrophages were not different between IL-1R1 WT and KO mice following cisplatin (Fig. 4D), we found increased total numbers of TNF-expressing macrophages (Fig. 6, E and F). Taken together, these results indicate that IL-1R1 activation on intra-renal myeloid cells stimulates their production of TNF, a key driver of cisplatin nephrotoxicity (21).
Fig. 6.
IL-1R1 stimulation promotes TNF generation from intrarenal myeloid cells. At 2 days following cisplatin, single cell suspensions from IL-1R1 WT and KO kidneys were stimulated ex vivo for 4 h with LPS after which cell surface staining and intracellular staining for TNF were performed. A–F: representative pseudocolor plots and total numbers of TNF+ cells for neutrophils (A and B), CD11bint/CD11cint cells (C and D), and macrophages (E and F), respectively (n = 8 for IL-1R1 WT, n = 7 for IL-1R1 KO). Data are expressed as means ± SE. Significance determined by unpaired t-test.
DISCUSSION
Cisplatin is an efficacious chemotherapeutic agent; however, nephrotoxicity continues to be a major hindrance to its administration. Inflammatory responses are central to the renal injury that complicates cisplatin therapy. Among the inflammatory mediators detected in the injured kidney, the macrophage cytokine TNF plays a critical role in the pathogenesis of cisplatin nephrotoxicity (21), but the upstream signals that trigger TNF generation in this context warrant investigation. As IL-1R1 activation stimulates NF-κB-dependent Tnfa mRNA transcription, we hypothesized that genetic deficiency of IL-1R1 would be protective in cisplatin-induced AKI. We find that mice genetically deficient in IL-1R1 have attenuated cisplatin nephrotoxicity as demonstrated by serum and renal tissue biomarkers and grading of renal pathology. Compared with WT controls, IL-1R1 KO mice also have lower levels of TNF expression, fewer neutrophils, and fewer TNF-producing myeloid cells in the injured kidney. Thus IL-1R1 activation following cisplatin administration promotes a proinflammatory renal microenvironment, marked by the induction of TNF in intrarenal myeloid cells, to exacerbate kidney injury.
As both sterile and infectious insults converge to activate IL-1 via the NRLP3 inflammasome complex, therapies targeted at blocking the biological effects of IL-1 during inflammation have received intense scrutiny. Accordingly, IL-1 blockade ameliorates a range of both acute and chronic inflammatory conditions (4, 13, 23). Blockade of the IL-1 pathway, whether through genetic deletion or pharmacological agents has also been explored in the setting of kidney disease. Genetic deficiency of IL-1R1 hastens recovery following ischemic AKI (7), limits angiotensin II-induced blood pressure elevation (34), and limits renal injury and fibrosis following unilateral ureteral obstruction (10, 36). Despite previous reports that myeloid cell-dependent immune responses have widely divergent actions in ischemia- and cisplatin-induced AKI (16, 27), our findings in the current study demonstrate that IL-1R1 genetic deficiency affords protection following cisplatin-induced AKI symmetric to that seen in ischemic AKI (7). Nevertheless, compared with genetic deletion, pharmacological blockade of IL-1’s actions has yielded inconsistent results in reducing renal injury. Whereas IL-1R1 receptor antagonism improves renal function following endotoxemia (11) and mediates protection from ischemic-AKI in rats (24), IL-1R1 blockade in mice fails to attenuate ischemic- or cisplatin-induced AKI (5, 7). The reasons for the discrepant effects of IL-1R1 receptor antagonism compared with genetic deficiency are unclear. With receptor antagonism a low level of IL-1R1 signaling may persist to drive inflammation whereas genetic deficiency completely abolishes IL-1R1 signaling. Indeed, signaling through IL-1R1 can occur in cells expressing less than 10 receptors (26). Alternatively, receptor antagonists may not reach the site of action to antagonize IL-1’s activity. The current studies do not discriminate between these possibilities.
Cisplatin nephrotoxicity has been associated with increased production of proinflammatory cytokines (5, 14, 20, 21, 35). We find that cisplatin administration increases levels of several inflammatory cytokines and chemokines in the kidney; but among these only the induction of TNF required IL-1R1 activation. In contrast, we find that, compared with WT controls, IL-1R1 KO kidneys have blunted induction of TNF following cisplatin. One function of IL-1 is to amplify the innate immune response through NF-κB activation, which then induces a network of inflammatory cytokines and chemokines, one of which is TNF (3). TNF has been strongly implicated in the pathogenesis of cisplatin nephrotoxicity (21, 22, 35), prompting investigation into the specific cellular sources of TNF responsible for exacerbating cisplatin nephrotoxicity. Two separate groups have demonstrated that renal tubule-derived TNF is important in cisplatin-induced AKI (32, 35). Liu et al. (14) have also shown that T cell-deficient mice produce less TNF post-cisplatin. Our results would indicate IL-1R1 activation also triggers release of TNF from intrarenal myeloid cells, which contributes to cisplatin nephrotoxicity.
In our immune cell profiling of cisplatin-injured kidneys, we find that IL-1R1 activation promotes neutrophil accumulation. These data are consistent with previous reports that IL-1R1 signaling promotes neutrophil accumulation in injured tissues, likely through prosurvival effects in neutrophils and the upregulation of neutrophil-recruiting chemokines (1, 6, 8, 19, 29). Regarding chemokine induction, IL-1R1 activation has stimulated expression of the neutrophil-recruiting chemokine CXCL2 in diverse contexts (1, 19, 29), and we detected a strong trend toward decreased expression of CXCL2 in injured IL-1R1 KO kidneys compared with WT controls. Like macrophages, neutrophils can produce TNF but also release reactive oxygen species that contribute to AKI accruing from cisplatin (18) and other injurious stimuli (2, 15). Nevertheless, in a model of ischemic renal injury, IL-1 did not foster oxidative injury (6).
New machine learning approaches to analysis of flow cytometry data afford an opportunity to identify subpopulations of hematopoietic cells that feature prominently in tissue lesions without a preconceived and potentially misdirected focus on specific cell types (34). Employing this type of unbiased analysis, we find that IL-1R1 activation also stimulates accumulation of CD11bint/CD11cint myeloid cells in the injured kidney. These cells are the most abundant myeloid population in the kidney, and they were demonstrated to resemble macrophages with both pro- and anti-inflammatory properties (12). After isolating these cells in vivo from cisplatin-injured kidneys and stimulating them for 4 h ex vivo, we find that a large proportion of these cells produce TNF via an IL-1R1-dependent mechanism. It is likely that the type and/or timing of the stimulus determines whether the pro- or anti-inflammatory properties of these cells predominate.
A recent flow strategy for identifying tissue macrophages involves extracting MHCII-expressing cells displaying CD64 but not CD24 (31). Following cisplatin, this myeloid subpopulation accumulated to a similar extent in the WT and IL-1R1 KO kidneys, but fewer of these cells expressed TNF in the IL-1R1-deficient cohort. Thus IL-1R1 activation within intrarenal macrophages promotes a proinflammatory polarization as reflected by their capacity to generate TNF. Taken together, these results indicate that IL-1R1 activation within multiple myeloid cell populations facilitates a proinflammatory phenotype that augments TNF-dependent nephrotoxicity.
In conclusion, cisplatin-induced AKI complicates chemotherapy for a broad range of solid tumors. We find that IL-1R1 genetic deficiency confers protection from cisplatin nephrotoxicity through salutary effects on multiple subpopulations of intrarenal myeloid cells. These studies should facilitate the identification of cell-specific strategies for disrupting IL-1-dependent nephrotoxicity while preserving the chemotherapeutic efficacy of platinum-based regimens.
GRANTS
This work was supported by National Institute of Health (NIH) Grants DK-087893 and HL-128355; Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Grant BX000893; and Duke O’Brien Center for Kidney Research (National Institute of Diabetes and Digestive and Kidney Diseases Grant P30-DK-096493) to S. Crowley. This work was also supported by NIH Grant 5T32-GM-008600 to the Duke Department of Anesthesiology (J. R. Privratsky as fellow); and International Anesthesia Research Society Mentored Research Award and Duke Department of Anesthesiology Dream Innovation Grant to J. R. Privratsky.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.R.P., J.Z., M.D.G., and S.D.C. conceived and designed research; J.R.P., J.Z., X.L., N.R., Q.W., and Y.-R.Y. performed experiments; J.R.P., J.Z., X.L., N.R., Q.W., Y.-R.Y., and S.D.C. analyzed data; J.R.P., J.Z., X.L., Q.W., and S.D.C. interpreted results of experiments; J.R.P. and J.Z. prepared figures; J.R.P. drafted manuscript; J.R.P. and S.D.C. edited and revised manuscript; J.R.P., J.Z., X.L., N.R., Q.W., Y.-R.Y., M.D.G., and S.D.C. approved final version of manuscript.
REFERENCES
- 1.Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S, LeibundGut-Landmann S. IL-1 coordinates the neutrophil response to C. albicans in the oral mucosa. PLoS Pathog 12: e1005882, 2016. doi: 10.1371/journal.ppat.1005882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basile DP, Leonard EC, Beal AG, Schleuter D, Friedrich J. Persistent oxidative stress following renal ischemia-reperfusion injury increases ANG II hemodynamic and fibrotic activity. Am J Physiol Renal Physiol 302: F1494–F1502, 2012. doi: 10.1152/ajprenal.00691.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood 87: 2095–2147, 1996. [PubMed] [Google Scholar]
- 4.Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 11: 633–652, 2012. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, Oh D-JJ, Lu L, Klein CL, Dinarello CA, Edelstein CL. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther 322: 8–15, 2007. doi: 10.1124/jpet.107.119792. [DOI] [PubMed] [Google Scholar]
- 6.Guidot DM, Linas SL, Repine MJ, Shanley PF, Fisher HS, Repine JE. Interleukin-1 treatment increases neutrophils but not antioxidant enzyme activity or resistance to ischemia-reperfusion injury in rat kidneys. Inflammation 18: 537–545, 1994. doi: 10.1007/BF01560700. [DOI] [PubMed] [Google Scholar]
- 7.Haq M, Norman J, Saba SR, Ramirez G, Rabb H. Role of IL-1 in renal ischemic reperfusion injury. J Am Soc Nephrol 9: 614–619, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Hsu L-C, Enzler T, Seita J, Timmer AM, Lee C-Y, Lai T-Y, Yu G-Y, Lai L-C, Temkin V, Sinzig U, Aung T, Nizet V, Weissman IL, Karin M. IL-1β-driven neutrophilia preserves antibacterial defense in the absence of the kinase IKKβ. Nat Immunol 12: 144–150, 2011. doi: 10.1038/ni.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol 11: 88–101, 2015. doi: 10.1038/nrneph.2014.180. [DOI] [PubMed] [Google Scholar]
- 10.Jones LK, O’Sullivan KM, Semple T, Kuligowski MP, Fukami K, Ma FY, Nikolic-Paterson DJ, Holdsworth SR, Kitching AR. IL-1RI deficiency ameliorates early experimental renal interstitial fibrosis. Nephrol Dial Transplant 24: 3024–3032, 2009. doi: 10.1093/ndt/gfp214. [DOI] [PubMed] [Google Scholar]
- 11.Kadova Z, Dolezelova E, Cermanova J, Hroch M, Laho T, Muchova L, Staud F, Vitek L, Mokry J, Chladek J, Havlinova Z, Holecek M, Micuda S. IL-1 receptor blockade alleviates endotoxin-mediated impairment of renal drug excretory functions in rats. Am J Physiol Renal Physiol 308: F388–F399, 2015. doi: 10.1152/ajprenal.00266.2014. [DOI] [PubMed] [Google Scholar]
- 12.Kawakami T, Lichtnekert J, Thompson LJ, Karna P, Bouabe H, Hohl TM, Heinecke JW, Ziegler SF, Nelson PJ, Duffield JS. Resident renal mononuclear phagocytes comprise five discrete populations with distinct phenotypes and functions. J Immunol 191: 3358–3372, 2013. doi: 10.4049/jimmunol.1300342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, Gitton X, Widmer A, Patel N, Hawkins PN; Canakinumab in CAPS Study Group . Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med 360: 2416–2425, 2009. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 14.Liu M, Chien CC, Burne-Taney M, Molls RR, Racusen LC, Colvin RB, Rabb H. A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J Am Soc Nephrol 17: 765–774, 2006. doi: 10.1681/ASN.2005010102. [DOI] [PubMed] [Google Scholar]
- 15.Liu ZZ, Schmerbach K, Lu Y, Perlewitz A, Nikitina T, Cantow K, Seeliger E, Persson PB, Patzak A, Liu R, Sendeski MM. Iodinated contrast media cause direct tubular cell damage, leading to oxidative stress, low nitric oxide, and impairment of tubuloglomerular feedback. Am J Physiol Renal Physiol 306: F864–F872, 2014. doi: 10.1152/ajprenal.00302.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu L, Faubel S, He Z, Andres Hernando A, Jani A, Kedl R, Edelstein CL. Depletion of macrophages and dendritic cells in ischemic acute kidney injury. Am J Nephrol 35: 181–190, 2012. doi: 10.1159/000335582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev 226: 10–18, 2008. doi: 10.1111/j.1600-065X.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- 18.Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res Int 2014: 967826, 2014. doi: 10.1155/2014/967826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulsson JM, Moshfegh A, Dadfar E, Held C, Jacobson SH, Lundahl J. In-vivo extravasation induces the expression of interleukin 1 receptor type 1 in human neutrophils. Clin Exp Immunol 168: 105–112, 2012. doi: 10.1111/j.1365-2249.2011.04548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramesh G, Kimball SR, Jefferson LS, Reeves WB. Endotoxin and cisplatin synergistically stimulate TNF-alpha production by renal epithelial cells. Am J Physiol Renal Physiol 292: F812–F819, 2007. doi: 10.1152/ajprenal.00277.2006. [DOI] [PubMed] [Google Scholar]
- 21.Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110: 835–842, 2002. doi: 10.1172/JCI200215606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramesh G, Reeves WB. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol 285: F610–F618, 2003. doi: 10.1152/ajprenal.00101.2003. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group . Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med 377: 1119–1131, 2017. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 24.Rusai K, Huang H, Sayed N, Strobl M, Roos M, Schmaderer C, Heemann U, Lutz J. Administration of interleukin-1 receptor antagonist ameliorates renal ischemia-reperfusion injury. Transpl Int 21: 572–580, 2008. doi: 10.1111/j.1432-2277.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 25.Schiwon M, Weisheit C, Franken L, Gutweiler S, Dixit A, Meyer-Schwesinger C, Pohl J-MM, Maurice NJ, Thiebes S, Lorenz K, Quast T, Fuhrmann M, Baumgarten G, Lohse MJ, Opdenakker G, Bernhagen J, Bucala R, Panzer U, Kolanus W, Gröne H-JJ, Garbi N, Kastenmüller W, Knolle PA, Kurts C, Engel DR. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell 156: 456–468, 2014. doi: 10.1016/j.cell.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stylianou E, O’Neill LA, Rawlinson L, Edbrooke MR, Woo P, Saklatvala J. Interleukin 1 induces NF-kappa B through its type I but not its type II receptor in lymphocytes. J Biol Chem 267: 15836–15841, 1992. [PubMed] [Google Scholar]
- 27.Tadagavadi RK, Reeves WB. Renal dendritic cells ameliorate nephrotoxic acute kidney injury. J Am Soc Nephrol 21: 53–63, 2010. doi: 10.1681/ASN.2009040407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med 334: 1448–1460, 1996. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 29.Uribe-Herranz M, Lian L-H, Hooper KM, Milora KA, Jensen LE. IL-1R1 signaling facilitates Munro’s microabscess formation in psoriasiform imiquimod-induced skin inflammation. J Invest Dermatol 133: 1541–1549, 2013. doi: 10.1038/jid.2012.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Maaten LJP, Hinton GE. Visualizing high-dimensional data using t-SNE. J Mach Learn Res 9: 2579–2605, 2008. [Google Scholar]
- 31.Yu Y-RA, O’Koren EG, Hotten DF, Kan MJ, Kopin D, Nelson ER, Que L, Gunn MD. A protocol for the comprehensive flow cytometric analysis of immune cells in normal and inflamed murine non-lymphoid tissues. PLoS One 11: e0150606, 2016. doi: 10.1371/journal.pone.0150606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang B, Ramesh G, Norbury CC, Reeves WB. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-alpha produced by renal parenchymal cells. Kidney Int 72: 37–44, 2007. doi: 10.1038/sj.ki.5002242. [DOI] [PubMed] [Google Scholar]
- 33.Zhang D, Liu Y, Wei Q, Huo Y, Liu K, Liu F, Dong Z. Tubular p53 regulates multiple genes to mediate AKI. J Am Soc Nephrol 25: 2278–2289, 2014. doi: 10.1681/ASN.2013080902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Rudemiller NP, Patel MB, Karlovich NS, Wu M, McDonough AA, Griffiths R, Sparks MA, Jeffs AD, Crowley SD. Interleukin-1 receptor activation potentiates salt reabsorption in angiotensin ii-induced hypertension via the NKCC2 co-transporter in the nephron. Cell Metab 23: 360–368, 2016. doi: 10.1016/j.cmet.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Rudemiller NP, Patel MB, Wei Q, Karlovich NS, Jeffs AD, Wu M, Sparks MA, Privratsky JR, Herrera M, Gurley SB, Nedospasov SA, Crowley SD. Competing actions of type 1 angiotensin II receptors expressed on T lymphocytes and kidney epithelium during cisplatin-induced AKI. J Am Soc Nephrol 27: 2257–2264, 2016. doi: 10.1681/ASN.2015060683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang JD, Patel MB, Griffiths R, Dolber PC, Ruiz P, Sparks MA, Stegbauer J, Jin H, Gomez JA, Buckley AF, Lefler WS, Chen D, Crowley SD. Type 1 angiotensin receptors on macrophages ameliorate IL-1 receptor-mediated kidney fibrosis. J Clin Invest 124: 2198–2203, 2014. doi: 10.1172/JCI61368. [DOI] [PMC free article] [PubMed] [Google Scholar]