Abstract
The goal of this study is to investigate the functional implications of the sexual dimorphism in transporter patterns along the proximal tubule. To do so, we have developed sex-specific computational models of solute and water transport in the proximal convoluted tubule of the rat kidney. The models account for the sex differences in expression levels of the apical and basolateral transporters, in single-nephron glomerular filtration rate, and in tubular dimensions. Model simulations predict that 70.6 and 38.7% of the filtered volume is reabsorbed by the proximal tubule of the male and female rat kidneys, respectively. The lower fractional volume reabsorption in females can be attributed to their smaller transport area and lower aquaporin-1 expression level. The latter also results in a larger contribution of the paracellular pathway to water transport. Correspondingly similar fractions (70.9 and 39.2%) of the filtered Na+ are reabsorbed by the male and female proximal tubule models, respectively. The lower fractional Na+ reabsorption in females is due primarily to their smaller transport area and lower Na+/H+ exchanger isoform 3 and claudin-2 expression levels. Notably, unlike most Na+ transporters, whose expression levels are lower in females, Na+-glucose cotransporter 2 (SGLT2) expression levels are 2.5-fold higher in females. Model simulations suggest that the higher SGLT2 expression in females may compensate for their lower tubular transport area to achieve a hyperglycemic tolerance similar to that of males.
Keywords: epithelial transport, NHE3, sex difference, SGLT2, sodium transport
INTRODUCTION
The influence of sex hormones on the development of female- and male-specific traits and on the structure and function of sex-specific organs is well understood. Recent studies have revealed key roles of sex hormones in regulating structure and/or function of nearly every tissue and organ in the mammalian body. In particular, an explosion of data has emerged concerning sex differences in kidney function (6, 7, 27, 33). A female rat is much smaller than a male, and the kidney of a female rat is about half the size of a kidney in a male (21, 27). Thus, there are undoubtedly major sex differences in renal blood flow and kidney function.
In a recent study, Veiras et al. reported sexually dimorphic patterns in transporter abundance in rodents (35). Specifically, they compared the expression, covalent modifications, and regulators of electrolyte transporters, channels, and claudins in male and female Sprague-Dawley rats (35). Their findings demonstrated that female (compared with male) rat nephrons exhibit 1) lower proximal tubule Na+-phosphate cotransporter 2 (NaPi2), aquaporin-1 (AQP1), and claudin-2 (Cldn2); 2) greater Na+/H+ exchanger isoform 3 (NHE3) phosphorylation, a marker of distribution at the base of the microvilli in an inactive domain (1); and 3) lower Na+ and HCO3− reabsorption and increased volume flow from the proximal tubule. Additionally, Sabolić et al. reported a higher Na+-glucose cotransporter 2 (SGLT2) protein abundance level in female Wistar rats compared with males (28). No correlation was found between transporter abundance and the estrus cycle stage (35).
The principal goal of this study is to assess the extent to which individual sex differences in morphology or transporter activities contribute to the observed difference in solute and water transport along the proximal convoluted tubule in male versus female rats. We focus on the proximal convoluted tubule because of the crucial role it plays in renal water and solute transport: The proximal tubule, as studied in males, is responsible for reabsorbing ~2/3 of the filtered loads of water and salt, and all the filtered glucose. To accomplish that goal, we formulate sex-specific computational models of renal epithelial transport and conduct simulations to predict solute and water transport along the proximal convoluted tubules of the male and female rat kidney under physiological, pathophysiological, and artificial conditions.
MODELING METHODOLOGY
The proximal tubule is conventionally divided into the proximal convoluted tubule and the proximal straight tubule. The present study focuses on the former. We have previously published an epithelial cell-based model of the proximal convoluted tubule of a superficial nephron in a male rat kidney (17). The model accounts for 15 solutes: Na+, K+, Cl−, HCO3−, H2CO3, CO2, NH3, NH4+, HPO42−, H2PO4−, H+, HCO2−, H2CO2, urea, and glucose. The model is formulated for steady state and predicts luminal fluid flow, hydrostatic pressure, luminal fluid solute concentrations, cytosolic solute concentrations, membrane potential, and transcellular and paracellular fluxes. A schematic diagram of a proximal tubular cell model is shown in Fig. 1. Model parameters describing tubular dimension and transport properties of the proximal tubule of the male rat kidney were taken from Layton et al. (17) and Weinstein et al. (41), with the following exceptions. In previous studies of the male rat proximal tubule (17, 41), paracellular water flux was predicted to be substantially higher than transcellular water flux. Given that AQP1-null mice retain significant water transport capability (30), the paracellular pathway is expected to play a significant role in proximal tubular water reabsorption. However, it is not clear that paracellular water flux should be substantially larger than transcellular flux. Thus, we reduced paracellular water permeability in the male rat proximal tubule model by half, to 110 cm/s. We also increase tubular diameter from 25 to 27 μm, to maintain fractional Na+ and water reabsorption at ~2/3 in males. With these parameters, water transport in the proximal tubule of the male rat kidney is predicted to be split about half-half between the transcellular and paracellular pathways; see below.
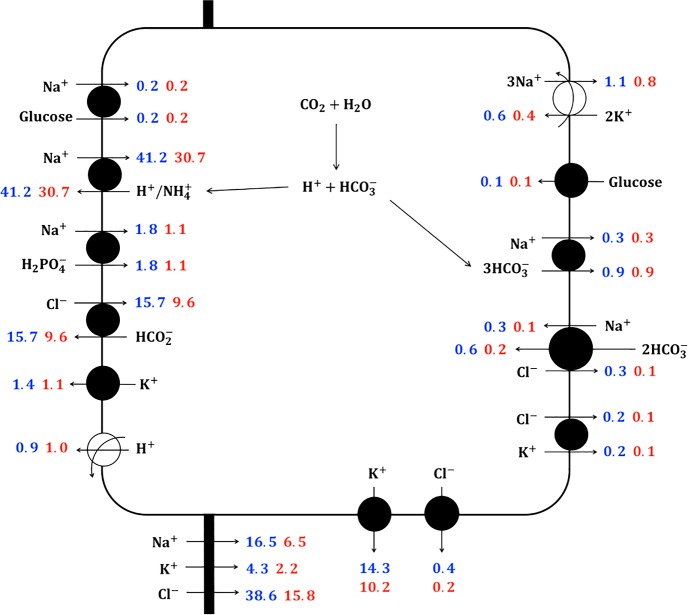
Fig. 1.
Schematic diagram of the proximal convoluted cell model, showing major Na+, K+, and Cl− transport pathways. Flux values computed at the midpoint of the proximal convoluted tubule are shown in blue and red for male and female rats, respectively.
To simulate solute and water transport in the proximal tubule of a female rat, we adjusted single-nephron glomerular filtration rate (SNGFR), tubular dimensions, the activity of NHE3, NaPi, and SGLT2, membrane water permeability, and paracellular Na+ and Cl− permeabilities; see Table 1. We assume that both the male and female proximal tubule cells are similarly microvilliated (35).
Table 1.
Key parameter differences between male and female rat proximal tubule models
| Parameter | Female-Male Ratio | References |
|---|---|---|
| SNGFR | 0.8 | (3, 24, 26) |
| Proximal tubule length | 0.8 | (3, 27, 31) |
| Proximal tubule diameter | 0.8 | (3, 27, 31) |
| NHE3 activity | 0.83 | (35) |
| NaPi2 | 0.75 | (35) |
| SGLT2 | 2.5 | (28) |
| PNa (paracellular) | 0.4 | (35) |
| PCl (paracellular) | 0.4 | (35) |
| Pf (transcellular) | 0.64 | (35) |
NaPi2, Na+-phosphate cotransporter 2; NHE3, Na+/H+ exchanger isoform 3; PNa, Na+ permeability; PCl, Cl− permeability; Pf, water permeability; SGLT2, Na+-glucose cotransporter 2; SNGFR, single-nephron glomerular filtration rate.
RESULTS
Base case results.
Using base case model parameters reported by Layton et al. (17) and summarized in Table 1, the male and female rat proximal tubule models predict tubular flow, tubular fluid concentrations, cellular solute concentrations, and membrane potential. Tubular flow profiles for key solutes, volume, and pH are shown in Fig. 2. Key transcellular and paracellular solute and water fluxes obtained at the midpoint of the model proximal convoluted tubules are shown in Fig. 1.
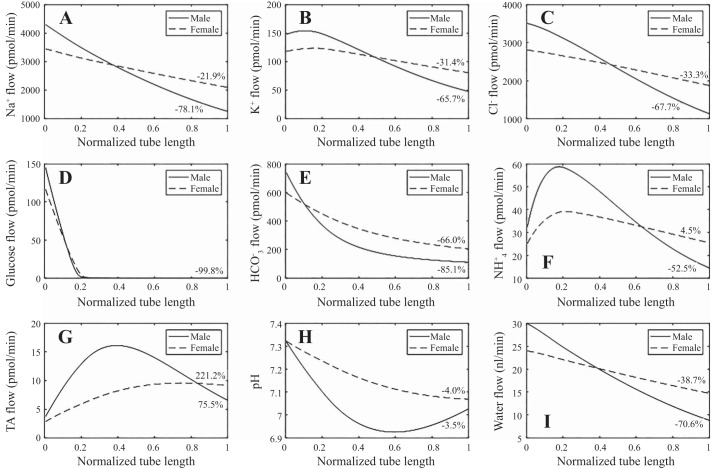
Fig. 2.
A–G: solute flow profiles along the proximal tubule of the male (solid lines) and female (dashed lines) rat kidney. H: tubular fluid pH. I: water flow profile. Substantially less Na+, K+, Cl−, and water is reabsorbed along the female rat proximal tubule compared with the male. TA, titratable acid.
Under baseline conditions, the male proximal tubule model predicts that 70.6% of filtered water is reabsorbed, assuming an SNGFR of 30 nl/min (see Fig. 2I), with 43.5 and 56.5% of the total water transport occurring transcellularly and paracellularly, respectively. In large part because of the reduced transport area and capacity (see below), the female proximal tubule model reabsorbs only 38.7% of filtered water, with 32.8 and 67.2% of the total water transport occurring transcellularly and paracellularly, respectively. A larger fraction of water goes through the paracellular pathway in females compared with males, in part because of the lower apical membrane water permeability in females, and despite the lower Cldn2 and thus paracellular Na+ and Cl− permeabilities in females (Table 1). Note also that even though SNGFR is lower in female rats compared with males (24 vs. 30 nl/min), proximal tubule outflow is predicted to be 1.67-fold higher in females (14.7 vs. 8.8 nl/min); see Fig. 2I. This prediction is consistent with the higher lithium clearance (CLi), a measure of volume flow exiting the proximal tubule, measured in female rats (35).
The model predicts that 70.9% of the filtered Na+ is reabsorbed by the proximal tubule of the male rat kidney (Fig. 2A), with 78.1 and 21.9% of the Na+ transported through the transcellular and paracellular pathways, respectively. Recall that the model assumes that NHE3 activity and paracellular Na+ permeability are 17.7 and 60%, respectively, lower in female rats compared with males (Table 1). Consequently, the model predicts that a substantially smaller fraction of 39.2% of the filtered Na+ is absorbed by the proximal tubule of the female rat kidney (Fig. 2A), with 83.9 and 16.1% of that Na+ transport going through the transcellular and paracellular pathways, respectively.
Significant sex differences are predicted for the transport of other solutes as well: In the male and female rat proximal tubule models, 67.5 and 31.4%, respectively, of the filtered K+ is reabsorbed, and 67.7 and 33.3%, respectively, of the filtered Cl− is reabsorbed; see Fig. 2, B and C. Net decreases of 85.1 and 66.0%, respectively, are predicted for HCO3− flow for males and females (Fig. 2E). For NH4+, the male proximal tubule model predicts a 52.5% decrease in flow, whereas the female model predicts a 4.5% increase; Fig. 2F. For titratable acid (computed from H2PO4+ and HPO42−) flow, net decreases of 75.5 and 221.2% are predicted for males and females, respectively. These trends are consistent with the overall acidification of the tubular fluid (Fig. 2, G and H).
Effects of sex differences in transporter patterns.
As previously noted, the model assumes that the activities of NHE3 and NaPi, membrane water permeability, and paracellular Na+ and Cl− permeabilities are lower, whereas SGLT2 activity is higher, in the female rat proximal tubule (Table 1; 28, 35). Moreover, the female rat proximal tubule is assumed to be smaller, and SNGFR is lower. To what extent does each individual factor contribute to the sex differences in solute and water transport along the rat proximal tubule?
To answer that question, we conduct a set of simulations in which we systematically assess the extent to which each sex difference in transporter pattern, morphology, or hemodynamics contributes to the differences in water and solute (specifically, Na+) transport between males and females. To that end, we take the male rat proximal tubule model, vary each sex-specific parameter individually, and compute fractional Na+ and water reabsorption. For example, the “tubular dimensions” case consists of male rat parameters, except for tubular length and diameter, which are set to the female values. Simulation results are summarized in Fig. 3.
Fig. 3.
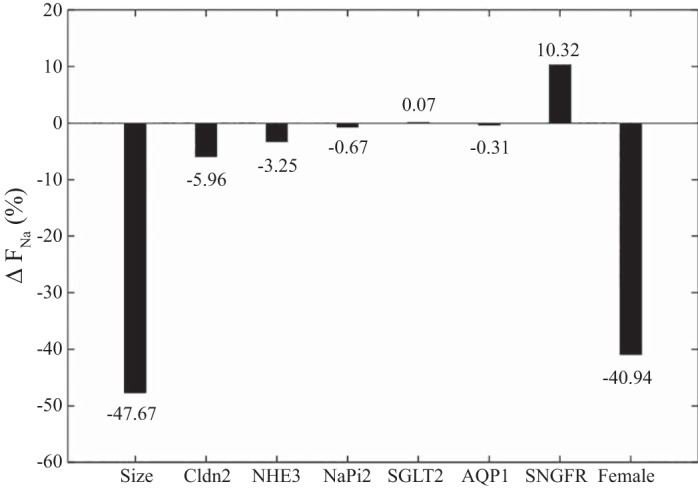
Changes in fractional Na+ reabsorption (ΔFNa) along the proximal tubule of the male rat, when individual parameters are set to female rat values. The lower single-nephron glomerular filtration rate (SNGFR) in the female increases ΔFNa because of the lower filtered load, even though absolute Na+ reabsorption decreases. AQP1, aquaporin-1; Cldn2, claudin-2; NaPi2, Na+-phosphate cotransporter 2; NHE3, Na+/H+ exchanger isoform 3; SGLT2, Na+-glucose cotransporter 2.
The model proximal tubule of the female rat is assumed to be 20% shorter than that of the male rat, with a diameter that is 20% smaller (3, 27, 31). Together, these differences imply an effective transport area that is 36% smaller in females relative to males. The models predict that if the length and tubular diameter of the male rat proximal tubule were both to be reduced by 20%, fractional Na+ reabsorption would decrease from 70.9 to 37.1%, which corresponds to a 47.7% reduction; see Fig. 3. Similar percentage decreases are predicted for fractional reabsorption of volume, K+, and Cl− (results not shown). These results suggest that the smaller tubular dimensions in female rat nephron alone more than account for its lower fractional Na+ and volume reabsorption compared with the male rat nephron, but that other factors may play a (possibly competing) role as well.
SNGFR is assumed to be 20% lower in female rats compared with male rats (3, 24, 26). When SNGFR in the male rat proximal tubule model is reduced by 20%, overall Na+ reabsorption decreases from its baseline value of 3,061 to 2,702 pmol/min, which corresponds to a 11.7% reduction. However, filtered Na+ load also decreases, by 20%. Taken together, a 20% reduction in SNGFR and filtered Na+ load yields a 10.3% increase in fractional Na+ reabsorption. Similar percentage increases are predicted for fractional reabsorption of volume, K+, and Cl− (results not shown).
The model represents sex-specific transporter patterns (28, 35). On the apical membrane, the activities of NHE3, NaPi2, and AQP1 are assumed to be 17, 25, and 36% lower, respectively, in females compared with males, whereas the activity of SGLT2 is assumed to be 2.5-fold higher in females. Taken in isolation, the 17% decrease in NHE3 activity results in a small (4%) decrease in fractional Na+ and volume reabsorption along the proximal tubule (Fig. 3). The sex differences in NaPi2, AQP1, and SGLT2 activity are predicted to have minimal impact on overall Na+ and volume reabsorption (Fig. 3). The prediction that reducing the apical membrane water permeability by 36% yields only an insignificant effect on overall water transport was unexpected. The model predicts that the lower apical water permeability yields a 11.6% decrease in transcellular water transport, but that is compensated by an increase in paracellular water transport by about the same amount. Similar results are predicted for Na+ transport. Furthermore, although the sex difference in SGLT2 activity has only minimal impact on water and Na+ transport, it does have a significant impact on glucose handling, an aspect that is discussed below.
Given the 60% lower expression level of Cldn2, which is the tight junction protein effecting paracellular NaCl reabsorption in the proximal tubule, the female rat model is assumed to have correspondingly lower paracellular Na+ and Cl− permeabilities. The models predict that, taken in isolation, those lower paracellular permeabilities would decrease overall fractional Na+, Cl−, and volume reabsorption by 6, 8, and 6%, respectively. The reduced Cldn2 expression has a disproportionally small effect on overall Na+ and Cl− transport because the decrease in paracellular transport is partially compensated by enhanced transcellular transport.
Sex difference in SGLT2 activity and glucose handling.
As noted above, the expression level of SGLT2 has been shown to be 2.5-fold higher in female rats compared with male rats (28). Our simulation suggests that the higher SGLT2 activity in females has minimal impact on overall Na+ transport (Fig. 3). However, what is the impact on glucose transport? Under baseline conditions (in which blood glucose concentration is taken to be 5 mM), essentially all filtered glucose is reabsorbed by the proximal convoluted tubule in both sexes. The question is, how do males and females respond to elevated blood glucose levels? At what point would glucosuria occur in each sex? What if the SGLT2 expression level in females were the same as in males (instead of 2.5-fold higher)? Recall that filtered glucose load is lower in females (because of the 20% lower SNGFR), but so is its transport area (36% lower, see above). If SGLT2 expression level were the same in males and females, would glucosuria occur sooner (or later) in females?
To answer these questions, we conducted simulations in which plasma glucose concentration was elevated from baseline (5 mM) to 10, 15, 20, 25, and 30 mM. These simulations were conducted for the 1) male proximal tubule model, 2) “baseline female” proximal tubule model with elevated SGLT2 activity (i.e., parameters as shown in Table 1), and 3) female proximal tubule model with parameters shown in Table 1, except with SGLT2 activity set to the male level (which we refer to as “low-SGLT2 female”). Key results for the baseline male and female models (but not low-SGLT2 female) are summarized in Fig. 4.
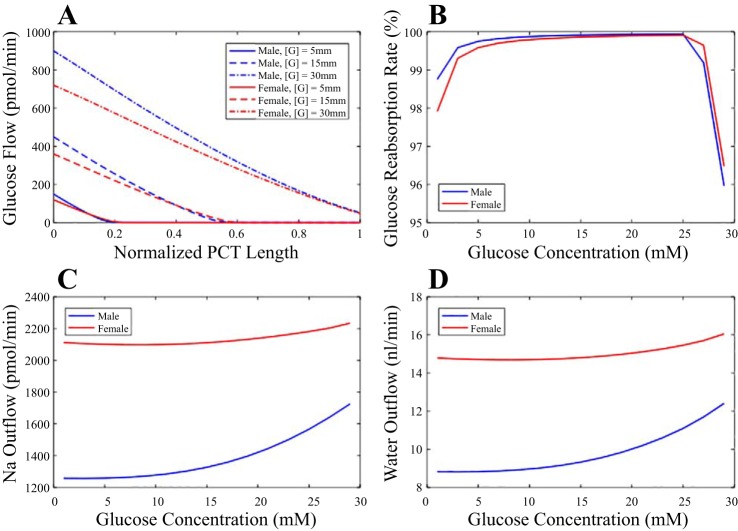
Fig. 4.
Glucose transport along the proximal tubule of the male (blue) and female (red) rat kidney at differing hyperglycemic levels ([G]). A: tubular glucose flow at selected [G]. B: fractional glucose reabsorption as a function of [G]. Glucosuria occurs at similar hyperglycemic level for male and female rats. C: proximal tubular Na+ outflow as a function of [G]. D: proximal tubular water outflow as a function of [G]. As [G] increases, fractional reabsorption of both Na+ and water decreases. PCT, proximal convoluted tubule.
Under euglycemic conditions, all three models predict that essentially all filtered glucose is reabsorbed by the proximal convoluted tubule. Nonetheless, a subtle yet significant difference distinguishes low-SGLT2 female from the other two cases. In both the male and baseline female models, essentially all filtered glucose is reabsorbed along the initial ~20% of the proximal convoluted tubule. In contrast, for the low-SGLT2 female model, a significant amount of glucose remains for the initial 36% of the nephron segment.
We hypothesize that without elevated SGLT2 expression, glucosuria would occur in a female rat at a significantly lower hyperglycemic level. To determine the critical hyperglycemic levels among the three cases, we consider the predicted proximal tubule glucose outflow. Recall that the model represents only the proximal convoluted tubule. The S3 segment that follows expresses SGLT1 and reabsorbs glucose as well. Thus, we assume that the glucosuria occurs when >10% of the filtered glucose exits the model proximal convoluted tubule. With this definition, the model predicts a similar critical hyperglycemic level of 32 mM for the male and baseline female proximal tubule but a significantly lower critical hyperglycemia level of 16 mM for the low-SGLT2 female proximal tubule. In all three cases, as plasma glucose concentration increases, the higher filtrate osmolality impedes the reabsorption of water and consequently other solutes such as Na+, K+, Cl−, etc. Specifically, hyperglycemia reduces fractional volume and Na+ reabsorption, with the effect significantly more prominent in the male model. The model predicts that as plasma glucose concentration increases from baseline (5 mM) to 30 mM, fractional volume and Na+ reabsorption in the male proximal tubule model decrease from 70.6 and 70.9% (baseline) to 57.4 and 59.1%, respectively. Those changes are attenuated in females, whose proximal tubule reabsorbs much less water under baseline conditions. The baseline female model predicts that the same increase in plasma glucose results in a reduction in fractional volume and Na+ reabsorption from 38.7 and 39.2% (baseline) to 32.3 and 34.9%, respectively. The corresponding changes for low-SGLT2 female are fractional volume reabsorption from 38.6% (baseline) to 21.4% and fractional Na+ reabsorption from 39.1% (baseline) to 28.0%.
Taken together, these results suggest that in large part because of its smaller transport area, a substantially elevated SGLT2 expression level is required in the female rat proximal tubule for it to achieve a tolerance for hyperglycemia similar to that of males. Furthermore, the effects of hyperglycemia on proximal tubular water and Na+ transport are attenuated in females, a result that may blunt diuretic and natriuretic effects of hyperglycemia in females.
Responses to variations in SNGFR.
Hyperfiltration is a precursor to glomerular injury and is also found in diabetes. To investigate the impacts of variations in SNGFR in tubular transport in the two sexes, we conducted simulations in which SNGFR was varied by ±10, ±20, and ±30%. Plasma composition was unchanged. The resulting fluid, Na+, and glucose flows at the proximal tubule outlet are shown in Fig. 5.
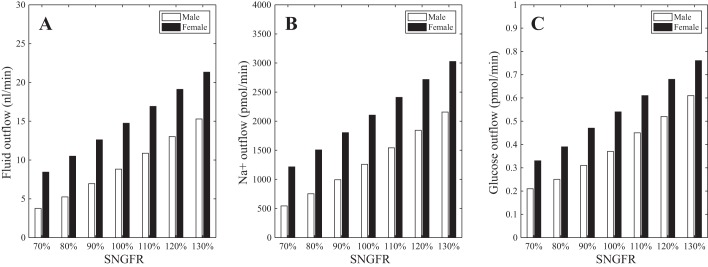
Fig. 5.
Effects of variations in single-nephron glomerular filtration rate (SNGFR) on proximal tubular transport. As SNGFR is varied from 30% below baseline to 30% above, fluid flow (A), Na+ flow (B), and glucose flow (C) at the proximal tubule outlet increase. Male and female rat proximal tubule models exhibit similar tolerance to elevated glucose load due to hyperfiltration.
As SNGFR varied from 30% below baseline to 30% above, fractional Na+ reabsorption decreased from 82 to 62% in males (baseline 71%) and from 50 to 33% in females (baseline 39%). Similar trends were predicted for fluid transport (Fig. 5). Thus, both models exhibit some degree of glomerulotubular balance, but that balance is imperfect. Glucose reabsorption remained at nearly 100%, even when SNGFR was elevated to 30% above baseline. Thus, these results suggest that females, with their elevated SGLT2 expression level, and males are similarly adept at handling enhanced glucose load and avoiding glucosuria due to hyperfiltration.
DISCUSSION
Major sex differences have been reported in renal structure and functions under various physiological, pharmacological, and toxicological conditions, based on clinical observations in humans and on studies in experimental animals in vivo and in models in vitro (27). It is generally believed that the sex hormone-regulated expression and action of transporters in the apical and basolateral membrane of nephron epithelial cells play a key role in the sexual differences in renal functions, but the extent of their contributions has not been fully understood. This line of inquiry is particularly timely, given the recent burst of data quantifying sexual dimorphism in transporter patterns in rodents (4, 28, 33, 35). Thus, the principal goal of this study is to assess the effects of the known sex differences in renal hemodynamics and structure in the rat on functions of the proximal convoluted tubule under physiological and hyperglycemic conditions. Computational model simulations suggest that fractional volume reabsorption is lower in the female rat because of their smaller transport area and lower AQP1 expression level. The latter is predicted to result in a larger contribution of the paracellular pathway to water transport. Similarly, fractional Na+ reabsorption is also lower in the female rat, primarily because of their smaller transport area and lower NHE3 and Cldn2 expression levels. The higher SGLT2 expression in females is predicted to compensate for their lower transport area to achieve a hyperglycemic tolerance similar to that of males.
The simulation results indicating lower fractional volume reabsorption are consistent with physiological measurements collected in direct comparison of salt and volume handling in females and males at baseline (35): First, NHE3, a central mediator of proximal tubule Na+ reabsorption and H+ secretion driving HCO3− reabsorption, is more highly phosphorylated and localized to the base of the microvilli, both consistent with less NHE activity (1, 10). Second, when females and males were stressed with an isotonic fluid load, females excreted a larger percentage of the load earlier: 30% versus 15% by 3 h. Third, the rate of bicarbonate reabsorption along the proximal tubule, an indicator of NHE activity measured by in vivo stationary microperfusion, was 35% lower in females than males. Since the proximal tubule is a leaky epithelium, this finding predicts similarly lower fluid reabsorption in this region. Fourth, endogenous CLi was employed as a noninvasive measure of volume flow from the proximal tubule. This method is based on data indicating that low (endogenous) levels of lithium are handled like sodium along the proximal tubule, but not farther along the nephron; thus, CLi can indirectly estimate Na+ and volume handling by the proximal tubule (20, 34). Urine collected overnight in a metabolic cage and a blood sample collected in the morning were used in the following formula: urine volume × urine [Li+]/plasma [Li+]. CLi was measured to be twice as high in females versus males [consistent with higher CLi, plasma [Li+] was lower in females than in males (35)]. Taken together, these four lines of evidence support lower fractional reabsorption along the proximal tubule.
The methodology employed in this study is computational modeling (11). Since Weinstein’s first computational model of epithelial transport in the kidney (40), a series of epithelial models have been published, focusing on different tubular segments or populations of nephrons and on various aspects of renal functions (4a, 12–16, 18, 36–38). However, perhaps in part because of the historic lack of data and adequate understanding of the transport properties of the female rat nephron, all published renal epithelial transport computational models are based on the male. The present study is the first to address, computationally, the other half of the rat population and analyzes solute and water epithelial transport in a female rat nephron segment. With the increasing understanding of the sex differences in kidney structures and functions in humans and in experimental animals, we should no longer assume that results obtained in one sex, be they measurements or model predictions, can always be generalized to the other sex. Consequently, the computational model of renal epithelial transport in a female rat developed here is requisite.
Computational models provide a systemic approach for investigating system perturbations, such as those induced by drug administration or genetic alterations. In the present study, we conduct model simulations to assess the impacts of individual sex differences in renal hemodynamics, structure, and transport properties. We consider each parameter and ask, what if this were the only difference between the male and female rat kidney? How would their solute and water transport be different then? Model simulations have identified tubular dimensions, which scale with transport area, as the most important factor in determining the sex difference between proximal tubule functions (Fig. 3), followed by SNGFR. The sexually dimorphic transporter expressions have relatively minor impact on baseline water and Na+ transport, especially NaPi2, SGLT2, and AQP1 (Fig. 3).
It is noteworthy that almost all observed sex differences in the rat proximal convoluted tubule have the effect of lowering transport in the female: reduced tubular transport area, lower SNGFR, and lower transporter protein expression (Table 1). All have this effect, except for the abundance of mSGLT2 protein, which is 2.5-fold higher in the female (28). Whereas ovariectomy of adult females did not affect SGLT2 abundance or localization, castration of adult males increased the abundance of rat SGLT2 (rSGLT2) protein, an effect prevented by testosterone and enhanced by estradiol treatment (28). These findings implicate a negative influence of androgens on SGLT2 in males. Interestingly, Sabolić et al. also reported that the sex-dependent pattern in mice reversed: there was more abundant SGLT2 in males (28).
Under baseline conditions, the higher SGLT2 expression level and activity in females have little impact on net proximal tubule Na+ and glucose reabsorption: With or without the enhanced SGLT2 expression, the female rat proximal convoluted tubule reabsorbs all filtered glucose and about the same amount of filtered Na+. A major difference in glucose reabsorption is revealed under hyperglycemic conditions. Our model simulations predict that without elevated SGLT2 expression, glucosuria would occur in a female rat at a significantly lower plasma glucose level, at 16 mM, compared with 32 mM in a male rat (Fig. 4). Thus, model results suggest that to maintain a glucose transport capacity similar to that of the male rat (in the sense that glucosuria occurs at similarly elevated plasma glucose levels), SGLT2 expression level must be enhanced in the female rat, to compensate for the smaller tubular transport area.
Note also that the smaller tubular transport area in the female proximal convoluted tubule affects the transport capacity of all solute and water in this region: Under baseline conditions, only 39.2 and 38.7% of the filtered Na+ and water, respectively, is reabsorbed in females compared with 70.9 and 70.6% in males. Results are qualitatively similar for other solutes such as K+ and Cl−. As discussed above, transport capacity for glucose is maintained via the enhanced SGLT2 activity. In contrast, the abundance of some Na+ transporter proteins is actually lower in the female rat (Table 1). This points to a different strategy in females for maintaining Na+ and volume balance, one that involves a substantially larger contribution by the distal nephron segments. Indeed, Veiras et al. (35) have found higher Na+-K+-Cl− cotransporter 2 and Na+-K+-ATPase protein densities along the medullary nephron segments of the female rat compared with males and higher Na+-Cl− cotransporter, Cldn7, and epithelial Na+ channel abundance in the distal nephron and collecting duct. Although the functional implications of the sex-specific Na+- and water-handling strategies have yet to be fully comprehended, female rats exhibit more efficient excretion of a saline load (29). Additionally, the higher proximal tubular outflow in the female rat likely implies significant sex differences in the tubuloglomerular feedback system. However, whether diuretics that target distal and collecting duct transporters are more efficacious in females remains unclear (4).
Perspectives.
The differences in salt handling in the male and female rat kidney almost surely have major implications in their blood pressure regulation. Essential for the kidney’s long-term control of blood pressure is the pressure-natriuresis mechanism, whereby increases in renal perfusion pressure lead to increases in Na+ excretion, which in turn lower salt and water retention and reduce effective circulating volume. Females tend to exhibit a leftward shift in the pressure-natriuresis relation relative to males, such that females excrete the same amount of Na+ as males at a lower arterial pressure under physiological conditions (7, 9). Pressure-natriuresis responses encompass multiple levels of Na+ transporter regulation (22), including NHE3, and are substantially modulated by the renin-angiotensin-aldosterone system (RAAS; 23). Important sex-based differences have long been reported and analyzed in the RAAS (19, 32).
It should be clear by now that major sex differences exist in essentially every aspect of blood pressure regulation: renal tubular transport, renal salt handling, pressure-natriuresis response, RAAS, etc. These differences may explain the sex differences in blood pressure and the prevalence of hypertension found in many mammalian and avian species (29): In humans (42) and in genetic models of hypertension such as spontaneously hypertensive rats and Dahl salt-sensitive rats (25), males develop an earlier and more severe hypertension than females. Despite these well-known differences, hypertensive men and women typically receive the same therapeutic treatments, with less effective outcomes in women (5). Thus, a key message of this study is that there is a critical need to develop sex-specific antihypertensive treatments.
Model limitations and future extensions.
The present models are based on the rat. Even in rodents, significant differences have been reported in proximal tubule function between the rat and the mouse (35). It goes without saying that significant differences exist between the rat kidney and the human kidney, in terms of anatomy and hemodynamics. Consequently, although results obtained using a rat kidney model may shed insights into human kidney function, those results do not always or entirely translate. In terms of sexual dimorphism, the male rat and its kidney are about twice the size of a female rat and its kidney, whereas men and women, and their organs, are much more similar in size. A key result of this study is that the size differences between male and female rats account for much of their observed differences in proximal tubular salt and water transport. The fact that men and women do not exhibit comparable size differences may, taken in isolation, suggest that the sexual dimorphism in renal tubular transport may be significantly attenuated in humans compared with rats.
The present analysis represents only the proximal convoluted tubule of a superficial nephron. Thus, although it accounts for a major fraction of salt and water transport, it does not predict urinary outputs. To accomplish that, the model must be extended to include the proximal straight tubule, the loop of Henle and distal tubular segments, as well as corresponding segments of the juxtamedullary nephrons, as given by Layton et al. (16) and Weinstein (39). SNGFR is assumed known a priori in the present model; that is, the model does not represent autoregulation, unlike in Refs. 31a and 31b. The function implications of the sex differences in autoregulatory mechanisms such as tubuloglomerular feedback (2), given the differences in renal epithelial transport, would make a worthwhile modeling study.
GRANTS
This research was supported by the National Institutes of Health: National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-106102 to A. T. Layton and Grant R01-DK-083785 to A. A. McDonough, and by funding from the Canada 150 Research Chair program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Q.L. and A.T.L. conceived and designed research; Q.L. performed experiments; Q.L., A.A.M., and A.T.L. analyzed data; Q.L., A.A.M., and A.T.L. interpreted results of experiments; Q.L. prepared figures; Q.L., A.A.M., H.E.L., and A.T.L. edited and revised manuscript; Q.L., A.A.M., H.E.L., and A.T.L. approved final version of manuscript; A.T.L. drafted manuscript.
REFERENCES
- 1.Brasen JC, Burford JL, McDonough AA, Holstein-Rathlou NH, Peti-Peterdi J. Local pH domains regulate NHE3-mediated Na+ reabsorption in the renal proximal tubule. Am J Physiol Renal Physiol 307: F1249–F1262, 2014. doi: 10.1152/ajprenal.00174.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown RD, Hilliard LM, Head GA, Jones ES, Widdop RE, Denton KM. Sex differences in the pressor and tubuloglomerular feedback response to angiotensin II. Hypertension 59: 129–135, 2012. doi: 10.1161/HYPERTENSIONAHA.111.178715. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Sullivan JC, Edwards A, Layton AT. Sex-specific computational models of the spontaneously hypertensive rat kidneys: factors affecting nitric oxide bioavailability. Am J Physiol Renal Physiol 313: F174–F183, 2017. doi: 10.1152/ajprenal.00482.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colafella KM, Denton KM. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol 14: 185–201, 2018. doi: 10.1038/nrneph.2017.189. [DOI] [PubMed] [Google Scholar]
- 4a.Edwards A, Castrop H, Laghmani K, Vallon V, Layton AT. Effects of NKCC2 isoform regulation on NaCl transport in thick ascending limb and macula densa: a modeling study. Am J Physiol Renal Physiol 307: F137–F146, 2014. doi: 10.1152/ajprenal.00158.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu Q, Burt VL, Paulose-Ram R, Dillon CF. Gender differences in hypertension treatment, drug utilization patterns, and blood pressure control among US adults with hypertension: data from the National Health and Nutrition Examination Survey 1999–2004. Am J Hypertens 21: 789–798, 2008. doi: 10.1038/ajh.2008.185. [DOI] [PubMed] [Google Scholar]
- 6.Hatano R, Onoe K, Obara M, Matsubara M, Kanai Y, Muto S, Asano S. Sex hormones induce a gender-related difference in renal expression of a novel prostaglandin transporter, OAT-PG, influencing basal PGE2 concentration. Am J Physiol Renal Physiol 302: F342–F349, 2012. doi: 10.1152/ajprenal.00366.2011. [DOI] [PubMed] [Google Scholar]
- 7.Hilliard LM, Nematbakhsh M, Kett MM, Teichman E, Sampson AK, Widdop RE, Evans RG, Denton KM. Gender differences in pressure-natriuresis and renal autoregulation: role of the Angiotensin type 2 receptor. Hypertension 57: 275–282, 2011. doi: 10.1161/HYPERTENSIONAHA.110.166827. [DOI] [PubMed] [Google Scholar]
- 9.Khraibi AA, Liang M, Berndt TJ. Role of gender on renal interstitial hydrostatic pressure and sodium excretion in rats. Am J Hypertens 14: 893–896, 2001. doi: 10.1016/S0895-7061(01)02164-1. [DOI] [PubMed] [Google Scholar]
- 10.Kocinsky HS, Girardi AC, Biemesderfer D, Nguyen T, Mentone S, Orlowski J, Aronson PS. Use of phospho-specific antibodies to determine the phosphorylation of endogenous Na+/H+ exchanger NHE3 at PKA consensus sites. Am J Physiol Renal Physiol 289: F249–F258, 2005. doi: 10.1152/ajprenal.00082.2004. [DOI] [PubMed] [Google Scholar]
- 11.Layton AT. A new microscope for the kidney: mathematics. Am J Physiol Renal Physiol 312: F671–F672, 2017. doi: 10.1152/ajprenal.00648.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Layton AT, Edwards A, Vallon V. Renal potassium handling in rats with subtotal nephrectomy: modeling and analysis. Am J Physiol Renal Physiol 314: F643–F657, 2017. doi: 10.1152/ajprenal.00460.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Layton AT, Laghmani K, Vallon V, Edwards A. Solute transport and oxygen consumption along the nephrons: effects of Na+ transport inhibitors. Am J Physiol Renal Physiol 311: F1217–F1229, 2016. doi: 10.1152/ajprenal.00294.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Layton AT, Moore LC, Layton HE. Multistable dynamics mediated by tubuloglomerular feedback in a model of coupled nephrons. Bull Math Biol 71: 515–555, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Layton AT, Vallon V. SGLT2 inhibition in a kidney with reduced nephron number: modeling and analysis of solute transport and metabolism. Am J Physiol Renal Physiol 314: F969–F984, 2018. doi: 10.1152/ajprenal.00551.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Layton AT, Edwards A, Vallon V. Adaptive changes in GFR, tubular morphology, and transport in subtotal nephrectomized kidneys: modeling and analysis. Am J Physiol Renal Physiol 313: F199–F209, 2017. doi: 10.1152/ajprenal.00018.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Layton AT, Vallon V, Edwards A. A computational model for simulating solute transport and oxygen consumption along the nephrons. Am J Physiol Renal Physiol 311: F1378–F1390, 2016. doi: 10.1152/ajprenal.00293.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Layton AT, Vallon V, Edwards A. Modeling oxygen consumption in the proximal tubule: effects of NHE and SGLT2 inhibition. Am J Physiol Renal Physiol 308: F1343–F1357, 2015. doi: 10.1152/ajprenal.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Layton AT, Vallon V, Edwards A. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am J Physiol Renal Physiol 310: F1269–F1283, 2016. doi: 10.1152/ajprenal.00543.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leete JG, Gurley S, Layton AT. Modeling sex differences in the renin angiotensin system and the efficacy of antihypertensive therapies. Comput Chem Eng 112: 253–264, 2018. doi: 10.1016/j.compchemeng.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leyssac PP, Christensen P. A comparison between endogenous and exogenous lithium clearance in the anaesthetized rat. Acta Physiol Scand 151: 173–179, 1994. doi: 10.1111/j.1748-1716.1994.tb09735.x. [DOI] [PubMed] [Google Scholar]
- 21.MacKay L, MacKay E. Factors which determine renal weight. III. Sex. Am J Physiol 83: 196–201, 1927. doi: 10.1152/ajplegacy.1927.83.1.196. [DOI] [Google Scholar]
- 22.McDonough AA, Nguyen MT. Maintaining balance under pressure: integrated regulation of renal transporters during hypertension. Hypertension 66: 450–455, 2015. doi: 10.1161/HYPERTENSIONAHA.115.04593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirabito KM, Hilliard LM, Head GA, Widdop RE, Denton KM. Pressor responsiveness to angiotensin II in female mice is enhanced with age: role of the angiotensin type 2 receptor. Biol Sex Differ 5: 13, 2014. doi: 10.1186/s13293-014-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munger K, Baylis C. Sex differences in renal hemodynamics in rats. Am J Physiol Renal Fluid Electrolyte Physiol 254: F223–F231, 1988. doi: 10.1152/ajprenal.1988.254.2.F223. [DOI] [PubMed] [Google Scholar]
- 25.Ouchi Y, Share L, Crofton JT, Iitake K, Brooks DP. Sex difference in the development of deoxycorticosterone-salt hypertension in the rat. Hypertension 9: 172–177, 1987. doi: 10.1161/01.HYP.9.2.172. [DOI] [PubMed] [Google Scholar]
- 26.Remuzzi A, Puntorieri S, Mazzoleni A, Remuzzi G. Sex related differences in glomerular ultrafiltration and proteinuria in Munich-Wistar rats. Kidney Int 34: 481–486, 1988. doi: 10.1038/ki.1988.206. [DOI] [PubMed] [Google Scholar]
- 27.Sabolić I, Asif AR, Budach WE, Wanke C, Bahn A, Burckhardt G. Gender differences in kidney function. Pflügers Arch 455: 397–429, 2007. doi: 10.1007/s00424-007-0308-1. [DOI] [PubMed] [Google Scholar]
- 28.Sabolić I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, Ljubojevic M, Brzica H, Sebastiani A, Thal SC, Sauvant C, Kipp H, Vallon V, Koepsell H. Expression of Na+-d-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiol Cell Physiol 302: C1174–C1188, 2012. doi: 10.1152/ajpcell.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ 3: 7, 2012. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnermann J, Chou CL, Ma T, Traynor T, Knepper MA, Verkman AS. Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc Natl Acad Sci USA 95: 9660–9664, 1998. doi: 10.1073/pnas.95.16.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silbiger SR, Neugarten J. The impact of gender on the progression of chronic renal disease. Am J Kidney Dis 25: 515–533, 1995. doi: 10.1016/0272-6386(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 31a.Sgouralis I, Layton AT. Theoretical assessment of renal autoregulatory mechanisms. Am J Physiol Renal Physiol 306: F1357–F1371, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31b.Sgouralis I, Layton AT. Autoregulation and conduction of vasomotor responses in a mathematical model of the rat afferent arteriole. Am J Physiol Renal Physiol 303: F229–F239, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan JC. Sex and the renin-angiotensin system: inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol 294: R1220–R1226, 2008. doi: 10.1152/ajpregu.00864.2007. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan JC, Gillis EE. Sex and gender differences in hypertensive kidney injury. Am J Physiol Renal Physiol 313: F1009–F1017, 2017. doi: 10.1152/ajprenal.00206.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomsen K, Shirley DG. The validity of lithium clearance as an index of sodium and water delivery from the proximal tubules. Nephron 77: 125–138, 1997. doi: 10.1159/000190264. [DOI] [PubMed] [Google Scholar]
- 35.Veiras LC, Girardi AC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu AS, McDonough AA. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol 28: 3504–3517, 2017. doi: 10.1681/ASN.2017030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinstein AM. A mathematical model of the rat proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 250: F860–F873, 1986. doi: 10.1152/ajprenal.1986.250.5.F860. [DOI] [PubMed] [Google Scholar]
- 37.Weinstein AM. A mathematical model of rat ascending Henle limb. III. Tubular function. Am J Physiol Renal Physiol 298: F543–F556, 2010. doi: 10.1152/ajprenal.00232.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinstein AM. A mathematical model of rat proximal tubule and loop of Henle. Am J Physiol Renal Physiol 308: F1076–F1097, 2015. doi: 10.1152/ajprenal.00504.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinstein AM. A mathematical model of the rat nephron: glucose transport. Am J Physiol Renal Physiol 308: F1098–F1118, 2015. doi: 10.1152/ajprenal.00505.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinstein AM. Nonequilibrium thermodynamic model of the rat proximal tubule epithelium. Biophys J 44: 153–170, 1983. doi: 10.1016/S0006-3495(83)84287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weinstein AM, Weinbaum S, Duan Y, Du Z, Yan Q, Wang T. Flow-dependent transport in a mathematical model of rat proximal tubule. Am J Physiol Renal Physiol 292: F1164–F1181, 2007. doi: 10.1152/ajprenal.00392.2006. [DOI] [PubMed] [Google Scholar]
- 42.Wiinberg N, Høegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens 8: 978–986, 1995. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]