Abstract
Spinal cord injury is a significant cause of motor dysfunctions. There is no definite cure for it, and most of the therapeutic modalities are only symptomatic treatment. In this systematic review and meta-analysis, the effectiveness of stem cell therapy in the treatment of the spinal cord injuries in animal models was studied and evaluated. A systematic search through medical databases by using appropriate keywords was conducted. The relevant reports were reviewed in order to find out cases in which inclusion and exclusion criteria had been fulfilled. Finally, 89 articles have been considered, from which 28 had sufficient data for performing statistical analyses. The findings showed a significant improvement in motor functions after cell therapy. The outcome was strongly related to the number of transplanted cells, site of injury, chronicity of the injury, type of the damage, and the induction of immune-suppression. According to our data, improvements in functional recovery after stem cell therapy in the treatment of spinal cord injury in animal models was noticeable, but its outcome is strongly related to the site of injury, number of transplanted cells, and type of transplanted cells.
Keywords: Stem cell therapy, Spinal cord injuries, Meta-analysis, Contusions
Introduction
Spinal cord injury (SCI) is a catastrophic nervous system disorder. Unfortunately, it affects the younger population, and leads to persistent or long-term disabilities. Both mechanical damage or an insult induced by inflammation can result in SCI [1]. There is no recognized cure for treating SCI and most of the therapeutic modalities are focused on symptomatic relief [2,3]. Approximately 90% of patients with SCI suffer from long-term motor dysfunctions and the disease related complications. These consequences impose substantial financial and emotional burdens either directly or indirectly. Currently, in most centers, pharmacotherapy has been used for treating spinal cord injuries that has minimal impact on the functional recovery and neuropathic pain symptoms. On the other hand, putting the patient on multiple medications can lead to adverse consequences in long term [4,5].
Following a SCI, motor dysfunction will persist unless the injured region is recovered. However, neurogenesis is an uncommon phenomenon in the central nervous system (CNS) and self-healing potentials in injured cells are extremely limited. There could be an alternative way to replenish damaged cells following SCI: stem cell therapy.
Stem cells have a considerable ability to renew themselves and differentiate into any desired type of cell. Their capability to generate all kinds of cells provides an opportunity to replenish damaged and dysfunctional cells. In this regard, it seems that cell transplantation could be considered as a practical alternative for treating such injuries [6,7,8]. Actually, cell therapy can regenerate neural connections which in turn leads to improved functional recovery [9]. Many researchers are investigating the methods and procedure to improve restoring cell function. One of these attempts is differentiating mesenchymal stem cells into glial cells that play an important role in survival and function of neurons of the CNS [10,11].
Having a comprehensive knowledge of stem cell biology is crucial before any effort is taken in order to treat a SCI [12]. According to reports, stem cells have shown neuroprotective and immune-modulatory properties in the CNS of animal models. Consequently, they are presumed to be effective in treating neurodegenerative diseases such as spinal cord injuries, motor neuron diseases, Parkinson's disease, stroke, multiple sclerosis, and even peripheral diseases such as retinopathy.
Although application of stem cell therapy for treating the spinal cord injury has been widely studied [13,14,15], there are some uncertainties regarding this application. Finding the best cell source for implantation and the optimum amount of transplanted cells are two major issues. In addition, it is not entirely clear whether the immune-suppression is required or not after implantation or if it is necessary to alter implanted cells. Performing a meta-analysis may answer these questions and many more. This study is a systematic review and meta-analysis to assess the efficacy of stem cells in functional recovery in animal models of SCI.
Materials and Methods
Search strategy
Two independent reviewers performed an extended search in electronic databases of MEDLINE, EMBASE, ProQuest, BIOSIS, and Scopus in order to find papers that were published from January 2015 until the end of May 2017. This study was based on the keywords, including “spinal cord” or “injuries, spinal cord” or “cord injuries, spinal” or “cord injury, spinal” or “injury, spinal cord” or “spinal cord injury” or “myelopathy, traumatic” or “myelopathies, traumatic” or “traumatic myelopathies” or “traumatic myelopathy” or “spinal cord transection” or “cord transection, spinal” or “cord transections, spinal” or “spinal cord transections” or “transection, spinal cord” or “transections, spinal cord contusion” or “contusion, spinal cord” or “contusions, spinal cord” or “cord contusion, spinal” or “cord contusions, spinal” or “spinal cord contusions” “stem cell transplantations” or “transplantations, stem cell” or “transplantation, stem cell” “mesenchymal stromal cells” “mesenchymal stem cell” or “bone marrow stromal cells, multipotent” or “multipotent bone marrow stromal cells” or “bone marrow stromal cells” or “bone marrow stromal cell” or “Wharton jelly cells” or “cells, Wharton jelly” or “Wharton's jelly cells” or “cell, Wharton's cell, neural stem” or “pluripotent stem cells” or “iPSC” or “cells, neural stem” or “neural stem cell” or “stem cell, neural” or “stem cells, neural” embryonic stem cells. The articles found by using one of these keywords or the combination of several ones. In order to avoid missing relevant studies, reviewers chose extensive keywords. These keywords were extracted from MeSH, EMTREE, and via manual search in titles and abstracts of the articles. PubMed search included MEDLINE and archived articles in PubMed Central. For the purpose of including non-indexed reports, we also searched Google Scholar. In addition, ProQuest database was also meticulously searched for related dissertations. In cases where the article was not available online, the author(s) was (were) contacted directly. Finally, the reviewers entered the gathered studies into EndNote X7 software, and they provided a list of articles focusing on the stem cell therapies, and spinal cord injuries. We used no restriction on time for searching of studies.
Inclusion and exclusion criteria
All controlled studies evaluated stem cell effects on functional recovery after spinal cord injuries were included. These studies were in vivo on animal models (non-human), in which compression, contusion, hemisection or transections were used for induction of spinal cord injuries. No age, sex, or phylum restrictions were applied. The minimum amount of time needed for the cell therapy to be effective for functional recovery was 3 to 4 weeks, so we considered a 4-week gap during the follow-up period as an exclusion criterion. Surveys lacking control groups (sham, saline-treated, or vehicle-treated groups) were excluded as well. Duplicate articles were removed using EndNote software (version X7, Thomson Reuters, 2014). We also excluded non-extracted data studies. Two authors independently examined the title and abstract of the articles and scrutinized the potentially eligible studies. Full-text studies were investigated, and surveys met with the selected inclusion criteria.
Data extraction
Blinded data extraction performed by researchers after removing the author, journal, and organization of the studies and recorded in a checklist. The data included animal characteristics (number, recipient species), injury model details (severity and location), cell therapy protocol (time interval between injury and treatment, delivery route, immune-suppressive agent usage and transplanted cell number). They also contained graft type (autograft, allogeneic, or xenogeneic), follow-up duration and outcome (motor function). The assessed outcome was functional recovery. Data were recorded and expressed as a mean and standard error of the mean. When the data presented in charts, data extraction method proposed by Sistrom and Mergo [16] was applied. If outcomes were reported in multiple stages of the survey, only the last published figures were included. If the same population was given multiple reports, the study with the largest sample size and the most extended follow-up period was included. Besides pooled effect size preparation from the finally selected studies according to inclusion and exclusion criteria, subgroup analyses were run for three cell types of induced pluripotent stem cell (iPSC), mesenchymal stem cell (MSC), and neural stem cell (NSC), respectively. Meanwhile, other useful data such as most popular model, the location of injuries, animal models for spinal cord injuries, route of cell injections, graft type, immuno-suppressant applications in spinal cord injuries, models of spinal cord injuries and stem cell type transplantation also extracted from the selected articles and demonstrated in Figs. 1, 2, 3, 4, 5, 6, 7.
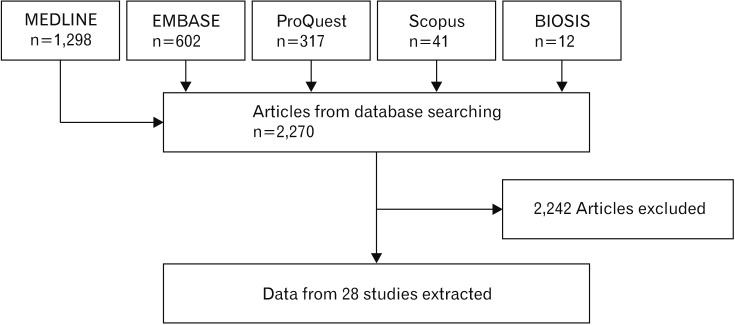
Fig. 1. Flowchart of search that was carried out through five different electronic databases including MEDLINE, EMBASE, ProQuest, BIOSIS, and Scopus. Out of 2,270 articles that were found, 28 studies were subjected to a meta-analysis that is based on inclusion and exclusion criteria.
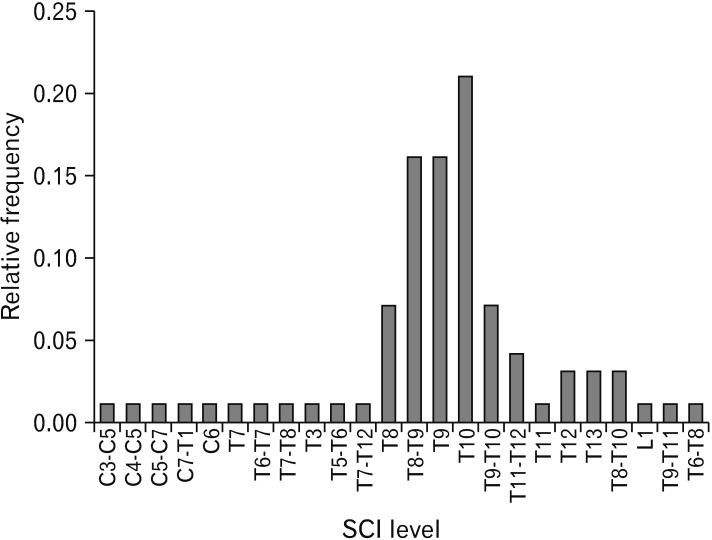
Fig. 2. Location of injuries. The site of injuries in the model of spinal cord injuries (SCIs) was primarily found to be localized at T10 level with the highest frequency.
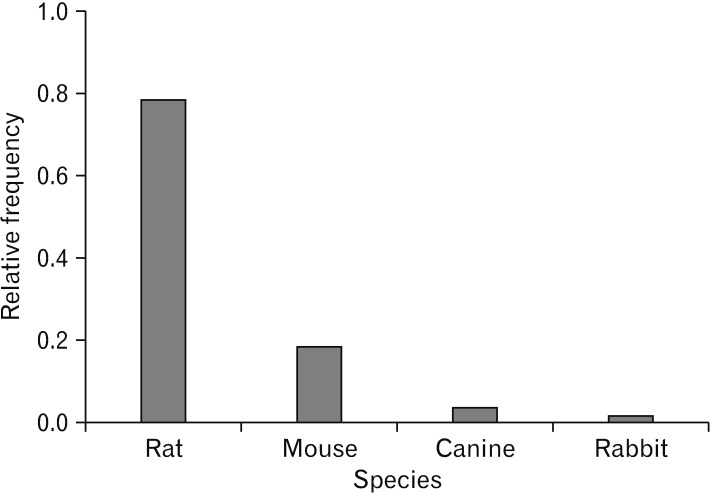
Fig. 3. Animal model species for spinal cord injuries. The data collected from 3,241 animals. As it is evident, the most frequent animal that was used was rat and the lowest was rabbit.
Fig. 4. Route of cell injections. There were six different routes of cell injections to the spinal cord. Direct injection into the spinal cord was the most frequent method that was used. ISP, intra spinal; IV, intra venous; CSF, cerebro-spinal fluid; ISP. INT, intra spinal. intrathecal.
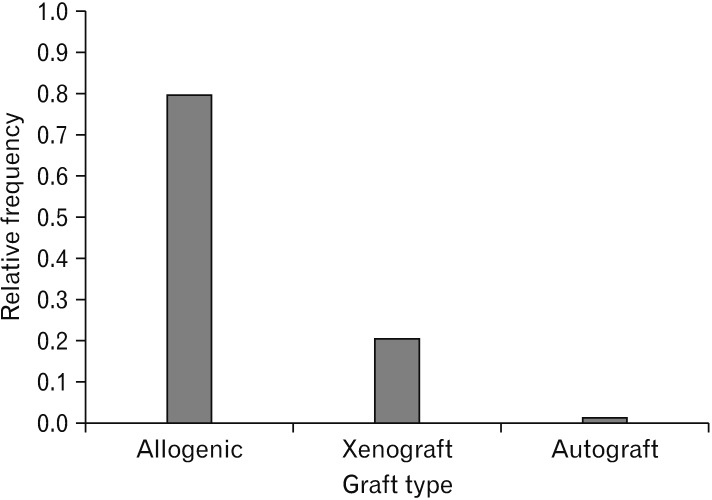
Fig. 5. Graft type. As it is obvious, allogeneic graft type of stem cells was the most common type that was performed and autograft was the least frequent graft type.
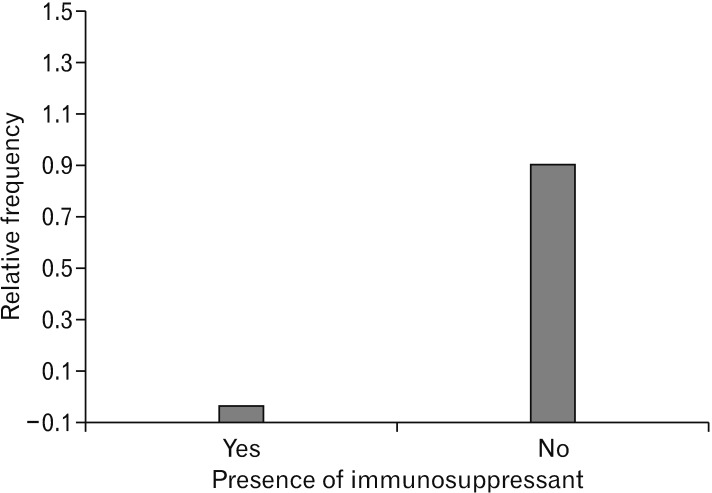
Fig. 6. Relative frequency of immuno-suppressant applications in spinal cord injuries. According to our data, using immuno-suppressant was not frequent in the cell transplantation after spinal cord injuries.
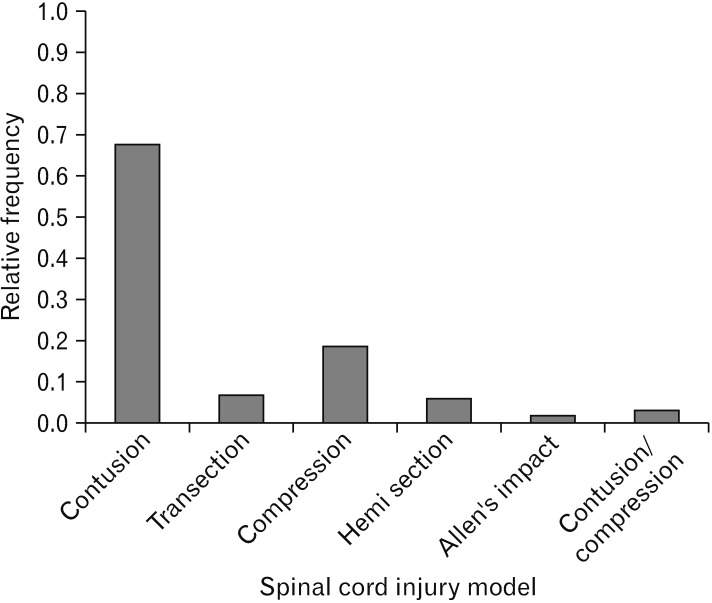
Fig. 7. Models of spinal cord injuries. In comparison with other available models, the most frequent traumatic spinal cord injury model was contusion model which was performed in 15 experiments.
Statistical analysis
Data were summarized and entered in the RevMan statistical software according to the corresponding mean and standard deviation. In cases in which standard errors were available, standard deviations calculated according to the study sample size. For each comparison, we calculated a standardized mean difference (SMD) with a confidence interval of 95% (95% CI), and then the pooled effect size was presented. Chi-square test was used for assessing of heterogeneity. A P-value of 0.1 or less considered as the existence of heterogeneity. The fixed effect model used for homogenous studies, and if the positive heterogeneity held, subgroup analysis was performed to determine its source. Subgroup analyses were carried out based on animal sex, injury model (contusion, compression, hemisection, transection), location (cervical, thoracic, lumbar), and severity (moderate, severe), an intervention phase (acute, sub-acute, chronic), delivery, graft type (allogeneic, xenogeneic), stem cell type, number of transplanted cells, and co-treatment use (antibiotic and/or immune-suppressive agents).
Results
Search in electronic sources yielded 1,435 nonduplicated studies. By screening through their titles and abstracts, we found 129 relevant articles, from which 59 articles met the inclusion criteria. Only 28 articles reported sufficient data to perform a statistical test, including subgroup evaluation (Fig. 1) [2,3,4,5,6,7,14,15,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Besides effect size preparation from these 28 studies, other useful data such as the most popular model, the location of injuries and types of stem cell also extracted from the articles and they are shown in Figs. 1, 2 3 4 5 6 7 8. The data collected from 3,241 animals. Contusion model was used mostly for injury induction and it was performed in 15 experiments. Besides pooled effect size preparation in 28 studies in accordance with inclusion and exclusion criteria (Fig. 9), subgroup analyses were run for three cell types of iPSC (Fig. 10), MSCs (Fig. 11), and NSCs (Fig. 12), respectively. The effect size in each forest plot presented under both fixed and random effect models. However, due to the heterogeneity of studies, as reported by I2, the random effect model is more appropriate for the analysis of data in each forest plot. The estimated effect size for the different type of injury models is shown in Fig. 6. Experiment-induced injuries were severe in half and moderate in the other half. The mean time interval between injury and treatment was 9.1±12.6 days (ranged from 1 to 63 days). In 19 experiments, transplantation was performed right after injury induction (acute phase), in six procedures were 3–10 days apart (sub-acute phase), and in 3 this gap was equal to or more than 2 weeks (chronic phase). The number of transplanted cells ranged from 1×105 to 4×107 cells per kilogram of the animal's body weight. Mesenchymal stem cells were used in 12 studies. Meanwhile, neural stem cells and induced pluripotent stem cells were applied in eleven and five studies, respectively. The effects of each administered cell type subgroup on motor functions have been demonstrated in Figs. 3, 4, and 5, respectively. The most prominent effect belonged to neural stem cells (fixed effect model, 5.84; 95% CI, 5.24–6.40) followed by induced pluripotent stem cells (fixed effect model, 1.53; 95% CI, 1.14–1.92) and mesenchymal stem cells (fixed effect model, 1.51; 95% CI, 1.33–1.70). Findings showed better functional recovery where more than 3×106 cell dose/kg was transplanted (SMD, 4.78; 95% CI, 4.43–4.95) compared to lower doses injection (SMD, 1.94; 95% CI, 1.67–2.12). Motor function recovery improved to a greater extent when cells were transplanted in acute (SMD, 4.52; 95% CI, 4.36–4.64) or sub-acute (SMD, 3.38; 95% CI, 3.08–4.67) phases compared to the chronic phase (SMD, 1.804; 95% CI, 1.07–2.60; P=0.01). Immuno-suppressive administration led to lower efficacies (SMD, 1.87; 95% CI, 1.57–2.28). Motor function recovery found to be lower when the cells were transplanted in the cervical injuries (SMD, 2.54; 95% CI, 2.16–3.04) compared to thoracic injuries (SMD, 4.55; 95% CI, 4.28–4.75).
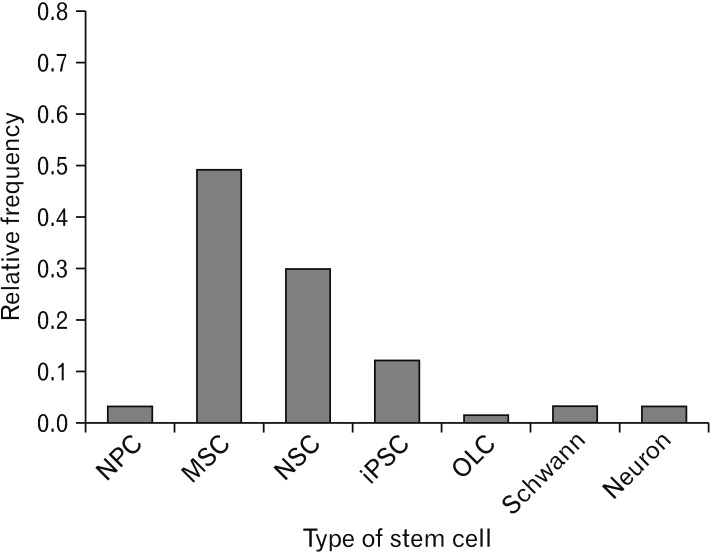
Fig. 8. Types of stem cell using for transplantation. Mesenchymal stem cells were mostly used in comparison with other transplanted cell types. NPC, neuroprogenitor cell; MSCs, mesenchymal stem cell; NSC, neural stem cell; iPSC, induced pluripotent stem cells; OLC, oligodendrocyte cells.
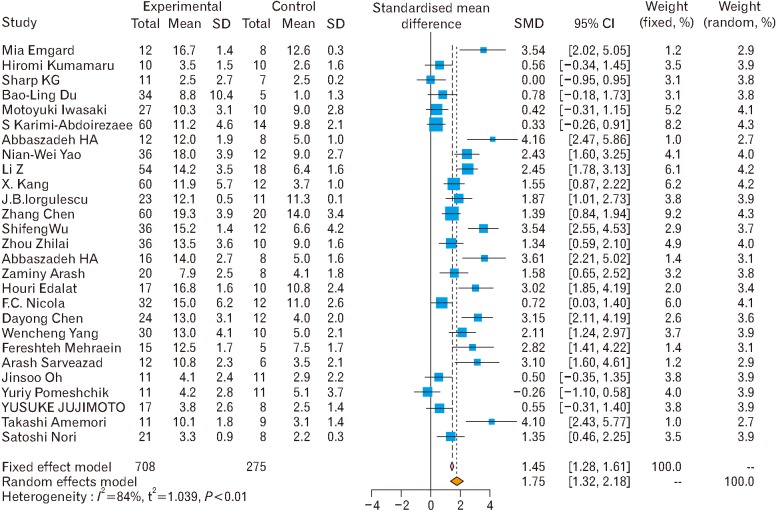
Fig. 9. Forest plot of stem cell therapy on motor functions without considering the type of the stem cell [2,4,5,6,7,14,15,17,18,19,20,21,22,23,24,25,26,27,28,29,31,32,33,34,35,36,38]. The overall effect size of stem cell therapy on motor functions under both fixed (odds ratio [OR], 1.86; 95% confidence interval [CI], 1.7–2.02) and random (OR, 4.36; 95% CI, 3.20–5.51) effect model was shown.
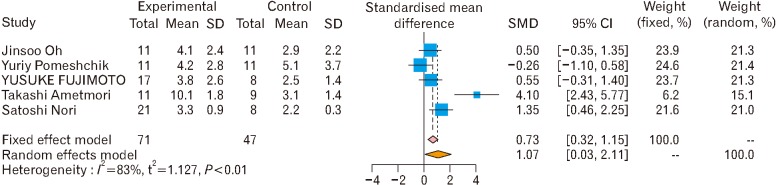
Fig. 10. Subgroup analysis of induced pluripotent stem cells (iPSC) effects on motor function [6,7,18,31,32]. The pooled effect size of induced pluripotent stem cell therapy on motor functions shows the effectiveness of iPSC therapy on motor functions under both fixed and random effect models. For each comparison, we calculated a standardized mean difference (SMD) with a confidence interval of 95% (95% CI), and then the pooled effect size was presented. Chi-square test was used for assessing of heterogeneity. A P-value of 0.1 or less considered as the existence of heterogeneity.
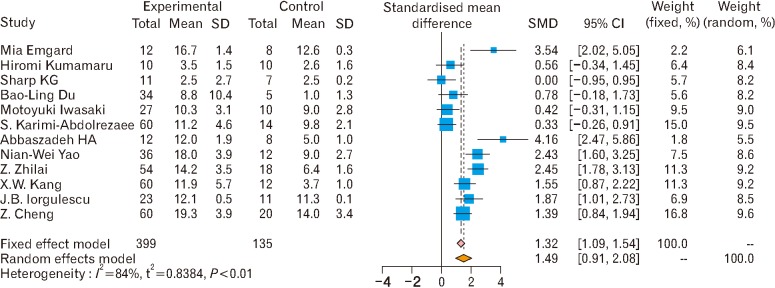
Fig. 11. Subgroup analysis of mesenchymal stem cell effects on motor function [2,4,5,14,17,19,20,22,23,24,35,36]. The pooled effect size of mesenchymal stem cell therapy on motor functions shows the effectiveness of mesenchymal stem cell therapy on motor functions under both fixed and random effect models. SMD, standardized mean difference; CI, confidence interval.
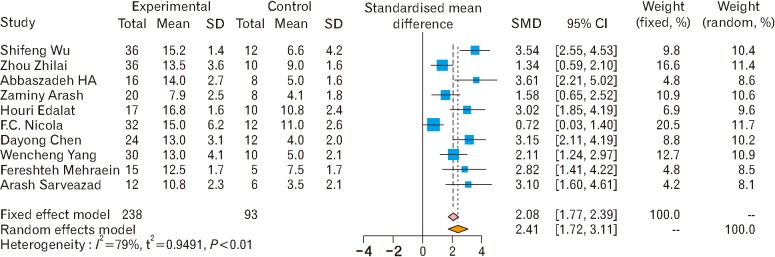
Fig. 12. Subgroup analysis of neural stem cells effects on motor function [3,14,15,25,26,27,28,29,34,36]. The pooled effect size of neural stem cell therapy on motor functions shows the effectiveness of neural stem cell therapy on motor functions under both fixed and random effect model. SMD, standardized mean difference; CI, confidence interval.
Discussion
In this study, we presented a precise systematic analysis of the recent reports on animal studies and described treatments based on stem cell therapies in the SCI by using standard methods. In this study, the quality, design and methodological characteristics of the published articles were scrutinized.
The Basso, Beattie and Bresnahan locomotor rating scale test applied in 54% of the animals. Improvement in motor outcome appeared to be under influence of several factors related to the treatment and the lesion. It is also dependent to the biological characteristics of the stem cells. For instance, neural stem cells led to a significantly more prominent response. It seems this type of stem cell could be considered as the primary source of cells for stem cell therapy in the future. Furthermore, the time of administration was also a crucial factor. The cells with more neuroprotective features seem more efficient when applied earlier and those with the neuroregenerative effect could be more efficient when they apply later. Motor function recovery improved to a greater extent when cells were transplanted in acute phase as compared to the chronic phase. Regarding the application of immune-suppressant in the spinal cord injury, we found that there are few reports focusing on the role of immunomodulation in stem cell therapy. In a systematic review about stem cell therapy in focal cerebral ischemia, prescription of cyclosporine was correlated with increased efficacy [37,38]. This suggests that the use of immune-suppressant for increasing the survival of transplanted cells has some degrees of value and worsts a thorough investigation. In this point, more data is required for judging about the effects of immunosuppression.
In general, allogeneic stem cell increased motor outcome after spinal cord injuries by approximately 32%. In studies related to motor outcomes, a considerably more significant effect was seen when cells were delivered for thoracic injuries, rather than for cervical insults; suggesting that either local changes may mediate the impacts of stem cells or local implantation makes an extra injury that conceals the advantage provided by the stem cells. Some univariate analyses, multivariate meta-regression or stepwise partitioning of heterogeneity must be provided which cast light on this matter.
We believe that stem cell therapy may still be the best treatment option for other diseases such as multiple sclerosis as the recent meta-analysis showed that the regulatory T cell number does not explain any differences between multiple sclerosis patients and controls [39].
There are many other proposed therapies for spinal cord injuries [36,40,41]. In this study, we did not run metaanalyses related to combination of stem cell therapy and other drugs or non-drug therapies. Such analyses are feasible when stem cell therapy is investigated more and when we — hopefully — have a better understanding about all aspects of spinal cord injuries.
Although, based on overall size effects, stem cell therapy is an effective treatment method for SCI, the degree of this success is dependent on factors such as the number and type of the transplanted cells and the phase of the injury (acute or chronic). Transplantation of autograft cells is more effective than other types of the cell preparations. Moreover, compression injuries were better resolved than a contusion and hemisectioned injuries. The race of the animal has a minor effect.
Acknowledgements
We are grateful for the support provided by Hearing Disorders Research Center and thanks for the Clinical Research Development Unit (CRDU) Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- 1.Kim Y, Kim J, Ahn M, Shin T. Lithium ameliorates rat spinal cord injury by suppressing glycogen synthase kinase-3beta and activating heme oxygenase-1. Anat Cell Biol. 2017;50:207–213. doi: 10.5115/acb.2017.50.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emgård M, Piao J, Aineskog H, Liu J, Calzarossa C, Odeberg J, Holmberg L, Samuelsson EB, Bezubik B, Vincent PH, Falci SP, Seiger Å, Åkesson E, Sundström E. Neuroprotective effects of human spinal cord-derived neural precursor cells after transplantation to the injured spinal cord. Exp Neurol. 2014;253:138–145. doi: 10.1016/j.expneurol.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Wu S, Cui G, Shao H, Du Z, Ng JC, Peng C. The cotransplantation of olfactory ensheathing cells with bone marrow mesenchymal stem cells exerts antiapoptotic effects in adult rats after spinal cord injury. Stem Cells Int. 2015;2015:516215. doi: 10.1155/2015/516215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumamaru H, Saiwai H, Kubota K, Kobayakawa K, Yokota K, Ohkawa Y, Shiba K, Iwamoto Y, Okada S. Therapeutic activities of engrafted neural stem/precursor cells are not dormant in the chronically injured spinal cord. Stem Cells. 2013;31:1535–1547. doi: 10.1002/stem.1404. [DOI] [PubMed] [Google Scholar]
- 5.Sharp KG, Yee KM, Steward O. A re-assessment of long distance growth and connectivity of neural stem cells after severe spinal cord injury. Exp Neurol. 2014;257:186–204. doi: 10.1016/j.expneurol.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh J, Lee KI, Kim HT, You Y, Yoon DH, Song KY, Cheong E, Ha Y, Hwang DY. Human-induced pluripotent stem cells generated from intervertebral disc cells improve neurologic functions in spinal cord injury. Stem Cell Res Ther. 2015;6:125. doi: 10.1186/s13287-015-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pomeshchik Y, Puttonen KA, Kidin I, Ruponen M, Lehtonen S, Malm T, Åkesson E, Hovatta O, Koistinaho J. Transplanted human induced pluripotent stem cell-derived neural progenitor cells do not promote functional recovery of pharmacologically immunosuppressed mice with contusion spinal cord injury. Cell Transplant. 2015;24:1799–1812. doi: 10.3727/096368914X684079. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen HX, Beck KD, Anderson AJ. Quantitative assessment of immune cells in the injured spinal cord tissue by flow cytometry: a novel use for a cell purification method. J Vis Exp. 2011;(50):2698. doi: 10.3791/2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei GJ, An G, Shi ZW, Wang KF, Guan Y, Wang YS, Han B, Yu EM, Li PF, Dong DM, Wang LP, Teng ZW, Zhao DL. Suppression of microRNA-383 enhances therapeutic potential of human bone-marrow-derived mesenchymal stem cells in treating spinal cord injury via GDNF. Cell Physiol Biochem. 2017;41:1435–1444. doi: 10.1159/000468057. [DOI] [PubMed] [Google Scholar]
- 10.Abbaszadeh HA, Tiraihi T, Delshad A, Saghedizadeh M, Taheri T, Kazemi H, Hassoun HK. Differentiation of neurosphere-derived rat neural stem cells into oligodendrocyte-like cells by repressing PDGF-alpha and Olig2 with triiodothyronine. Tissue Cell. 2014;46:462–469. doi: 10.1016/j.tice.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Abbaszadeh HA, Tiraihi T, Delshad AR, Saghedi Zadeh, M, Taheri T. Bone marrow stromal cell transdifferentiation into oligodendrocyte-like cells using triiodothyronine as a inducer with expression of platelet-derived growth factor alpha as a maturity marker. Iran Biomed J. 2013;17:62–70. doi: 10.6091/ibj.11162.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Zhao Y, Cheng S, Han S, Shu M, Chen B, Chen X, Tang F, Wang N, Tu Y, Wang B, Xiao Z, Zhang S, Dai J. Cetuximab modified collagen scaffold directs neurogenesis of injury-activated endogenous neural stem cells for acute spinal cord injury repair. Biomaterials. 2017;137:73–86. doi: 10.1016/j.biomaterials.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 13.Morita T, Sasaki M, Kataoka-Sasaki Y, Nakazaki M, Nagahama H, Oka S, Oshigiri T, Takebayashi T, Yamashita T, Kocsis JD, Honmou O. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a model of chronic spinal cord injury. Neuroscience. 2016;335:221–231. doi: 10.1016/j.neuroscience.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 14.Zhilai Z, Biling M, Sujun Q, Chao D, Benchao S, Shuai H, Shun Y, Hui Z. Preconditioning in lowered oxygen enhances the therapeutic potential of human umbilical mesenchymal stem cells in a rat model of spinal cord injury. Brain Res. 2016;1642:426–435. doi: 10.1016/j.brainres.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Yang W, Yang Y, Yang JY, Liang M, Song J. Treatment with bone marrow mesenchymal stem cells combined with plumbagin alleviates spinal cord injury by affecting oxidative stress, inflammation, apoptotis and the activation of the Nrf2 pathway. Int J Mol Med. 2016;37:1075–1082. doi: 10.3892/ijmm.2016.2498. [DOI] [PubMed] [Google Scholar]
- 16.Sistrom CL, Mergo PJ. A simple method for obtaining original data from published graphs and plots. AJR Am J Roentgenol. 2000;174:1241–1244. doi: 10.2214/ajr.174.5.1741241. [DOI] [PubMed] [Google Scholar]
- 17.Iwasaki M, Wilcox JT, Nishimura Y, Zweckberger K, Suzuki H, Wang J, Liu Y, Karadimas SK, Fehlings MG. Synergistic effects of self-assembling peptide and neural stem/progenitor cells to promote tissue repair and forelimb functional recovery in cervical spinal cord injury. Biomaterials. 2014;35:2617–2629. doi: 10.1016/j.biomaterials.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Fujimoto Y, Abematsu M, Falk A, Tsujimura K, Sanosaka T, Juliandi B, Semi K, Namihira M, Komiya S, Smith A, Nakashima K. Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell-derived long-term self-renewing neuroepithelial-like stem cells. Stem Cells. 2012;30:1163–1173. doi: 10.1002/stem.1083. [DOI] [PubMed] [Google Scholar]
- 19.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657–1676. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao NW, Lu Y, Shi LQ, Xu F, Cai XH. Neuroprotective effect of combining tanshinone IIA with low-dose methylprednisolone following acute spinal cord injury in rats. Exp Ther Med. 2017;13:2193–2202. doi: 10.3892/etm.2017.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Zhao W, Liu W, Zhou Y, Jia J, Yang L. Transplantation of placenta-derived mesenchymal stem cell-induced neural stem cells to treat spinal cord injury. Neural Regen Res. 2014;9:2197–2204. doi: 10.4103/1673-5374.147953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang XW, Hu JL, Wang SK, Wang J. Effectiveness of muscle basal lamina carrying neural stem cells and olfactory ensheathing cells in spinal cord repair. Genet Mol Res. 2015;14:13437–13455. doi: 10.4238/2015.October.28.5. [DOI] [PubMed] [Google Scholar]
- 23.Iorgulescu JB, Patel SP, Louro J, Andrade CM, Sanchez AR, Pearse DD. Acute putrescine supplementation with Schwann cell implantation improves sensory and serotonergic axon growth and functional recovery in spinal cord injured rats. Neural Plast. 2015;2015:186385. doi: 10.1155/2015/186385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng Z, Bosco DB, Sun L, Chen X, Xu Y, Tai W, Didier R, Li J, Fan J, He X, Ren Y. Neural stem cell-conditioned medium suppresses inflammation and promotes spinal cord injury recovery. Cell Transplant. 2017;26:469–482. doi: 10.3727/096368916X693473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaminy A, Shokrgozar MA, Sadeghi Y, Norouzian M, Heidari MH, Piryaei A. Transplantation of schwann cells differentiated from adipose stem cells improves functional recovery in rat spinal cord injury. Arch Iran Med. 2013;16:533–541. [PubMed] [Google Scholar]
- 26.Edalat H, Hajebrahimi Z, Pirhajati V, Movahedin M, Tavallaei M, Soroush MR, Mowla SJ. Transplanting p75-suppressed bone marrow stromal cells promotes functional behavior in a rat model of spinal cord injury. Iran Biomed J. 2013;17:140–145. doi: 10.6091/ibj.1193.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen D, Zeng W, Fu Y, Gao M, Lv G. Bone marrow mesenchymal stem cells combined with minocycline improve spinal cord injury in a rat model. Int J Clin Exp Pathol. 2015;8:11957–11969. [PMC free article] [PubMed] [Google Scholar]
- 28.Mehraein F, Golipoor Z. Transplant of very small embryoniclike stem cells to spinal cord injury in a rat model promotes movement recovery. Exp Clin Transplant. 2015;13:256–261. [PubMed] [Google Scholar]
- 29.Sarveazad A, Bakhtiari M, Babahajian A, Janzade A, Fallah A, Moradi F, Soleimani M, Younesi M, Goudarzi F, Joghataei MT. Comparison of human adipose-derived stem cells and chondroitinase ABC transplantation on locomotor recovery in the contusion model of spinal cord injury in rats. Iran J Basic Med Sci. 2014;17:685–693. [PMC free article] [PubMed] [Google Scholar]
- 30.Neirinckx V, Agirman G, Coste C, Marquet A, Dion V, Rogister B, Franzen R, Wislet S. Adult bone marrow mesenchymal and neural crest stem cells are chemoattractive and accelerate motor recovery in a mouse model of spinal cord injury. Stem Cell Res Ther. 2015;6:211. doi: 10.1186/s13287-015-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amemori T, Ruzicka J, Romanyuk N, Jhanwar-Uniyal M, Sykova E, Jendelova P. Comparison of intraspinal and intrathecal implantation of induced pluripotent stem cell-derived neural precursors for the treatment of spinal cord injury in rats. Stem Cell Res Ther. 2015;6:257. doi: 10.1186/s13287-015-0255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nori S, Okada Y, Nishimura S, Sasaki T, Itakura G, Kobayashi Y, Renault-Mihara F, Shimizu A, Koya I, Yoshida R, Kudoh J, Koike M, Uchiyama Y, Ikeda E, Toyama Y, Nakamura M, Okano H. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelialmesenchymal transition. Stem Cell Reports. 2015;4:360–373. doi: 10.1016/j.stemcr.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaminy A, Ali Shokrgozar M, Sadeghi Y, Noroozian M, Hassan Heidari M, Piryaei A. Mesenchymal stem cells as an alternative for Schwann cells in rat spinal cord injury. Iran Biomed J. 2013;17:113–122. doi: 10.6091/ibj.1121.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicola FC, Rodrigues LP, Crestani T, Quintiliano K, Sanches EF, Willborn S, Aristimunha D, Boisserand L, Pranke P, Netto CA. Human dental pulp stem cells transplantation combined with treadmill training in rats after traumatic spinal cord injury. Braz J Med Biol Res. 2016;49:e5319. doi: 10.1590/1414-431X20165319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du BL, Zeng X, Ma YH, Lai BQ, Wang JM, Ling EA, Wu JL, Zeng YS. Graft of the gelatin sponge scaffold containing genetically-modified neural stem cells promotes cell differentiation, axon regeneration, and functional recovery in rat with spinal cord transection. J Biomed Mater Res A. 2015;103:1533–1545. doi: 10.1002/jbm.a.35290. [DOI] [PubMed] [Google Scholar]
- 36.Abbaszadeh HA, Tiraihi T, Noori-Zadeh A, Delshad AR, Sadeghizade M, Taheri T. Human ciliary neurotrophic factor-overexpressing stable bone marrow stromal cells in the treatment of a rat model of traumatic spinal cord injury. Cytotherapy. 2015;17:912–921. doi: 10.1016/j.jcyt.2015.03.689. [DOI] [PubMed] [Google Scholar]
- 37.Piltti KM, Funes GM, Avakian SN, Salibian AA, Huang KI, Carta K, Kamei N, Flanagan LA, Monuki ES, Uchida N, Cummings BJ, Anderson AJ. Increasing human neural stem cell transplantation dose alters oligodendroglial and neuronal differentiation after spinal cord injury. Stem Cell Reports. 2017;8:1534–1548. doi: 10.1016/j.stemcr.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abbaszadeh HA, Tiraihi T, Sadeghi Y, Delshad AR, Sadeghizadeh M, Taheri T, Noori-Zadeh A. Decrease in cavity size and oligodendrocyte cell death using neurosphere-derived oligodendrocyte-like cells in spinal cord contusion model. Iran Biomed J. 2018;22:246–257. doi: 10.22034/ibj.22.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noori-Zadeh A, Mesbah-Namin SA, Bistoon-Beigloo S, Bakhtiyari S, Abbaszadeh HA, Darabi S, Rajabibazl M, Abdanipour A. Regulatory T cell number in multiple sclerosis patients: a meta-analysis. Mult Scler Relat Disord. 2016;5:73–76. doi: 10.1016/j.msard.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Abdanipour A, Schluesener HJ, Tiraihi T, Noori-Zadeh A. Systemic administration of valproic acid stimulates overexpression of microtubule-associated protein 2 in the spinal cord injury model to promote neurite outgrowth. Neurol Res. 2015;37:223–228. doi: 10.1179/1743132814Y.0000000438. [DOI] [PubMed] [Google Scholar]
- 41.Shams Nooraei M, Noori-Zadeh A, Darabi S, Rajaei F, Golmohammadi Z, Abbaszadeh HA. Low level of autophagy-related gene 10 (ATG10) expression in the 6-hydroxydopamine rat model of Parkinson's disease. Iran Biomed J. 2018;22:15–21. doi: 10.22034/ibj.22.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]