Abstract
In and after the third trimester, the lung surface is likely to become smooth to facilitate respiratory movements. However, there are no detailed descriptions as to when and how the lung surface becomes regular. According to our observations of 33 fetuses at 9–16 weeks of gestation (crown-rump length [CRL], 39–125 mm), the lung surface, especially its lateral (costal) surface, was comparatively rough due to rapid branching and outward growing of bronchioli at the pseudoglandular phase of lung development. The pulmonary pleura was thin and, beneath the surface mesothelium, no or little mesenchymal tissue was detectable. Veins and lymphatic vessels reached the lung surface until 9 weeks and 16 weeks, respectively. In contrast, in 8 fetuses at 26–34 weeks of gestation (CRL, 210–290 mm), the lung surface was almost smooth because, instead of bronchioli, the developing alveoli faced the external surfaces of the lung. Moreover, the submesothelial tissue became thick due to large numbers of dilated veins connected to deep intersegmental veins. CD34-positive, multilayered fibrous tissue was also evident beneath the mesothelium in these stages. The submesothelial tissue was much thicker at the basal and mediastinal surfaces compared to apical and costal surfaces. Overall, rather than by a mechanical stress from the thoracic wall and diaphragm, a smooth lung surface seemed to be established largely by the thick submesothelial tissue including veins and lymphatic vessels until 26 weeks.
Keywords: Human fetus, Lung surface, Pulmonary pleura, Mesothelium, Submesothelial morphology
Introduction
It is well known that, in adults, the pulmonary pleura forms an evenly smooth lung surface. The pleura transmits the respiratory forces to the underlying lung parenchyma without frictional damage [1]. From the third fetal trimester onward, the lung surface has been shown to develop an evenly smooth morphology to adapt to respiratory movements in utero to facilitate a further development of the airway and diaphragm [2]. However, during pseudoglandular and canalicular phases in midterm fetuses, the lung surface might be shaped differently due to proliferation, branching and outward growth of the bronchiolar system. At its costal surface, the fetal lung lies adjacent to the thoracic wall, while its basal surface is located adjacent to the diaphragmatic dome and shifted cranially by the growing liver. Thus, it is likely that the shape of the lung in fetuses, at least at its costal and basal aspects, is determined by the shape of the thoracic cavity, which is firm enough to resist the pressure of the growing and expanding lungs. Hayashi et al. [3] described that in human embryos and fetuses at 6–7 weeks of gestation, a sheet of mesenchymal tissue that lines the internal aspects of the thoracic cavity gradually disappears to increase capacity of the pleural cavity. Therefore, until 8 weeks, the growing lungs seem to determine the shape of the early pleural cavity, to adjust it to the lung surface. However, there is no systematic description as to when and how the costal and basal surfaces of the fetal lung become smooth.
Here, we conducted a systematic analysis of the development of the pulmonary pleura of human fetuses at 9–16 and 26–34 weeks of gestation. Unfortunately, because of long-lasting preservation in formalin solution, the present specimens were not suitable for immunohistochemistry. However, according to Abe et al. [4] and Katori et al. [5], CD34 antibodies were likely to be useful for identification of fetal fibrous tissue configurations. Although CD34 is widely known as a marker of vascular endothelial cells and hematopoietic stem cells, it is well suitable to identify fibrocytes in specific locations such as skin and Tunica externa of lymphatics [6,7]. In addition, using elastica-Masson staining (a variation of Masson-Goldner staining), we examined whether or not the fetal pleura contains elastic fibers. According to Kinoshita et al. [8], who demonstrated initial appearance of elastic fibers in the fetal head and neck, we expected to find elastic fibers in the lung after gestational week 20. Consequently, the aim of this study was to describe the fetal pulmonary pleura in relation to the lung surface morphology.
Materials and Methods
This study was performed in accordance with the principles of the Declaration of Helsinki 1995 (as revised in 2013). For a better understanding of lobar and segmental topographical anatomy of the lung, we usually chose sagittal from 4 collections of paraffin-embedded histological sections of fetuses at midterm and late stage. We observed sections from 41 fetuses: (1) eleven Chinese specimens at 9–16 weeks (crown-rump length [CRL], 39–125 mm), (2) eight Japanese specimens at 26–34 weeks (CRL, 210–290 mm), (3) seventeen Spanish specimens at 10–15 weeks (CRL, 47–117 mm), and (4) five German specimens at 9–11 weeks (CRL, 39–64 mm). Spanish and German specimens were from serial sections, while those from China and Japan were semi-serial. The sectional plane was sagittal in 30 of 41 specimens, while the other 11 specimens were horizontally sectioned.
Eleven Chinese specimens were donated by the fetuses' families to the Department of Anatomy, Yanbian University Medical College, Yanji, China, until 2016 and the use for research was approved by the university ethics committee in Yanji (No. BS-13-35). These fetuses were obtained by induced abortion, after which the mother was orally informed by an obstetrician at the college teaching hospital of the possibility of donating the fetus for research. No attempt was made to encourage the donation actively. After the mother agreed, the fetus was assigned a specimen number and stored in 10% w/w neutral formalin solution for more than 1 month. Because of specimen number randomization, there was no possibility of contacting the family at a later date. The trunk samples were decalcified by incubating at 4℃ in a 0.5-mol/l EDTA (pH 7.5) decalcifying solution (Decalcifying Solution B, Wako, Tokyo, Japan) for 3–5 days, depending on the size of the sample. After routine procedures for paraffin embedded histology, the specimens were sectioned sagittally (5 specimens) or horizontally (6) at 20–50 micron intervals depending on sizes of the fetuses (thickness of sections, 5 µm).
Eight Japanese specimens were parts of a collection in the Department of Anatomy, Akita University, Akita, Japan. They were donated by their families to the Department during 1975–1985 and preserved in 10% w/w neutral formalin solution for more than 30 years. The available data was limited and included date of donation and gestational week, but not family name, name of obstetrician or hospital, and reason for abortion. The use for research was approved by the university ethics committee in Akita (No. 1428). After removal of lungs, heart and thymus en bloc, we prepared eight paraffin blocks. From each of the blocks, we performed semiserial sagittal sections of 5 µm at 50–200-micron interval, depending on the size of fetuses.
Most of sections from the Chinese and Japanese specimens were stained with hematoxylin and eosin (H&E), while some of them were used for immunohistochemistry as well as Elastica-Masson staining, i.e., a variation of Masson-Goldner staining [9,10]. The primary antibodies were (1) mouse monoclonal anti-human CD34 (dilution 1:100, IR632, Dako, Glostrup, Denmark); (2) monoclonal anti-human podoplanin (1:100 dilution, Nichirei D2-40, Tokyo, Japan). For the latter, we needed autoclave treatment (105℃, 10 minutes) after immersion in a ligand activator (Histofine SAB-PO Kit, Nichirei). The secondary antibody (incubation for 30 minutes, dilution 1:1,000) was horseradish peroxidase–conjugated anti-mouse IgG (Histofine Simple Stain Max-PO, Nichirei). Enzyme reaction was visualized with 3,3′-diaminobenzidine (3–5 minutes, Histofine Simple Stain DAB, Nichirei). Samples were counterstained with hematoxylin. Negative controls consisted of samples without primary antibody.
Seventeen specimens were part of the large collection at the Embryology Institute of the Universidad Complutense, Madrid, obtained from miscarriages and ectopic pregnancies at the Department of Obstetrics of the University. Most sections were stained with H&E, some with azan, orange G or silver staining. The study was approved by Universidad Complutense ethics committee (B08/374). Finally, five specimens were from the Blechschmidt collection at the Medical Museum of Georg-August-Universität Göttingen, and sectioned sagittally. Sections were stained with HE, azan or Masson trichrome staining. The use of the collection did not require a specific approval of the Institute.
Results
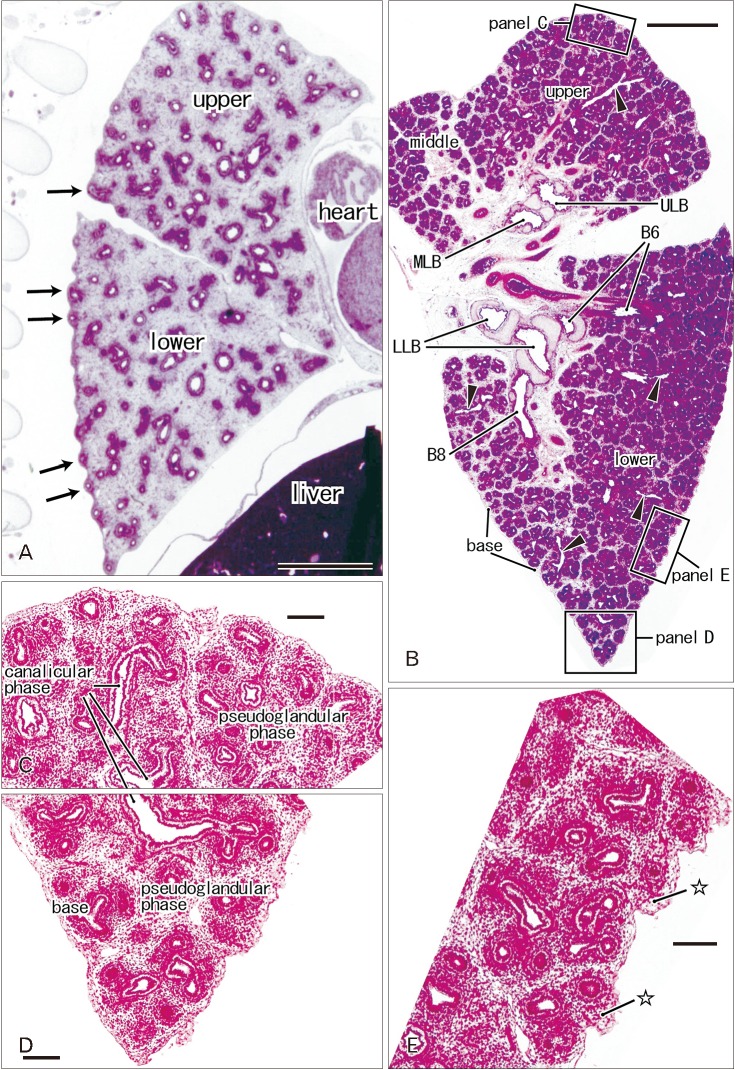
At 9–16 weeks (CRL, 39–125 mm), the pseudoglandular and canalicular phases of lung development were present in various proportions (Fig. 1). The lateral or costal surface as well as the basal surface was usually rough since the outward growing bronchioli provided abundant small protrusions at the surface (Fig. 1A, B). However, whether it was smooth or rough depended on the specimens (Fig. 1A [CRL, 39 mm]). The pulmonary pleura was thin and even difficult to identify (Table 1). Beneath the pulmonary mesothelium, no or little mesenchymal tissue was present at the superficial side of the bronchioli along the costal surface, whereas the submesothelial tissue was relatively thick at basal and medial sites (Fig. 1C–E). No intrapulmonary vein reached the pleural tissue.
Fig. 1. Rough lung surface and pulmonary pleura at the pseudoglandular and canalicular phases. H&E staining. Sagittal sections. Panel A displays the left lung from a specimen at 9 weeks (39 mm crown-rump length [CRL]), while panels B–D exhibit the right lung from a specimen at 10 weeks (56 mm CRL). Panels C–E are higher magnification view of squares in panel B, respectively. In panel A, outward growing bronchi provide small protrusions of the lateral or costal surface (arrows). A rough surface is also evident in the costal aspect in panel B, but a mesenchymal tissue (stars) is present beneath the thin pleura (E). Deep intersegmental veins do not reach the lung surface (arrowheads in B). The canalicular phase of the bronchus was mixed with the pseudoglandular phase. upper, upper lobe; middle, middle lobe; lower, lower lobe; B6 and B8, sixth and eighth segmental bronchi; LLB, lower lobar bronchus; MLB, middle lobar bronchus; ULB, upper lobar bronchus. Scale bars=1 mm (A, B), 0.1 mm (C–E).
Table 1. Studies of the pulmonary pleura and surface morphology in 33 specimens at 9–16 gestational weeks.
| Specimen (mm) | Lung surface | Pleura | Submesothelial morphology |
|---|---|---|---|
| 150429A (39) | Rough (Figs. 3A, 4A) | Very thin | Bronchi, not attaching to the pleura |
| 8-8-50 (39) | Rough (Fig.1A) | Thin | Bronchi, protruding to the lung surface |
| 17-12-48 (41) | Rough | Thin | Bronchi, protruding to the lung surface |
| 23-4-50 (45) | Rough | Thin | Bronchi, protruding to the lung surface |
| Pe 1 (47) | Rough | Very thin | Bronchi, not attaching to the pleura |
| Be 503 (48) | Rough | Very thin | Bronchi, not attaching to the pleura |
| 8-9-49 (51) | Rough | Thin | Bronchi, protruding to the lung surface |
| C56 (56) | Rough (Fig. 1B) | Thin | Thin tissue at the base and costal sides |
| B61 (61) | Rough | Thin | Bronchi, protruding to the lung surface |
| 24-4-50 (64) | Almost smooth | Very thin | Bronchi, protruding except for the base |
| 123 (73) | Rough | Thin | Bronchi, protruding except for the base |
| 124 (75) | Rough | Very thin | Bronchi, protruding except for the base |
| JR 8 (76) | Almost smooth | Very thin | Bronchi, protruding to the lung surface |
| Be 516 (82) | Rough | Very thin | Bronchi, protruding except for the base |
| SA 13 (84) | Almost smooth | Very thin | Bronchi, protruding except for the base |
| B 107 (84) | Rough | Relatively thick | Thin tissue at the base and lateral sides |
| Bu 23 (85) | Rough | Very thin | Bronchi, protruding except for the base |
| J-1 (86) | Rough | Thin | Thick tissue at the inferolateral angle |
| Cr-1 (87) | Almost smooth | Thin | Bronchi, protruding except for the base |
| Bu 18 (93) | Smooth | Relatively thick | Thick tissue at the base |
| SA 11 (96) | Almost smooth | Thin | Bronchi, protruding except for the base |
| Be1011 (97) | Rough | Relatively thick | Thick tissue at the base |
| CA 8 (101) | Almost smooth | Thin | Bronchi, protruding except for the base |
| 12J14T (107) | Rough | Thin | Bronchi, protruding except for the base |
| St 8 (105) | Almost smooth | Relatively thick | Thick tissue at the base |
| Ce 2 (115) | Almost smooth | Thin | Bronchi, protruding except for the base |
| 12J15A (115) | Rough (Fig. 3B) | Thin | Bronchi, protruding except for the base |
| B 29 (116) | Almost smooth | Thin | Bronchi, protruding except for the base |
| B 29 (117) | Almost smooth | Relatively thick | Bronchi, protruding except for the base |
| 12J15T (118) | Almost smooth | Relatively thick | Thick at the base and mediastinal sides |
| 15T (120) | Almost smooth | Relatively thick | Thick at the base and mediastinal sides |
| 154T (120) | Smooth | Relatively thick | Thick tissue at the base |
| 12J16T (125) GA1:G34 | Smooth (Fig. 4B) | Relatively thick | Thick tissue at the base |
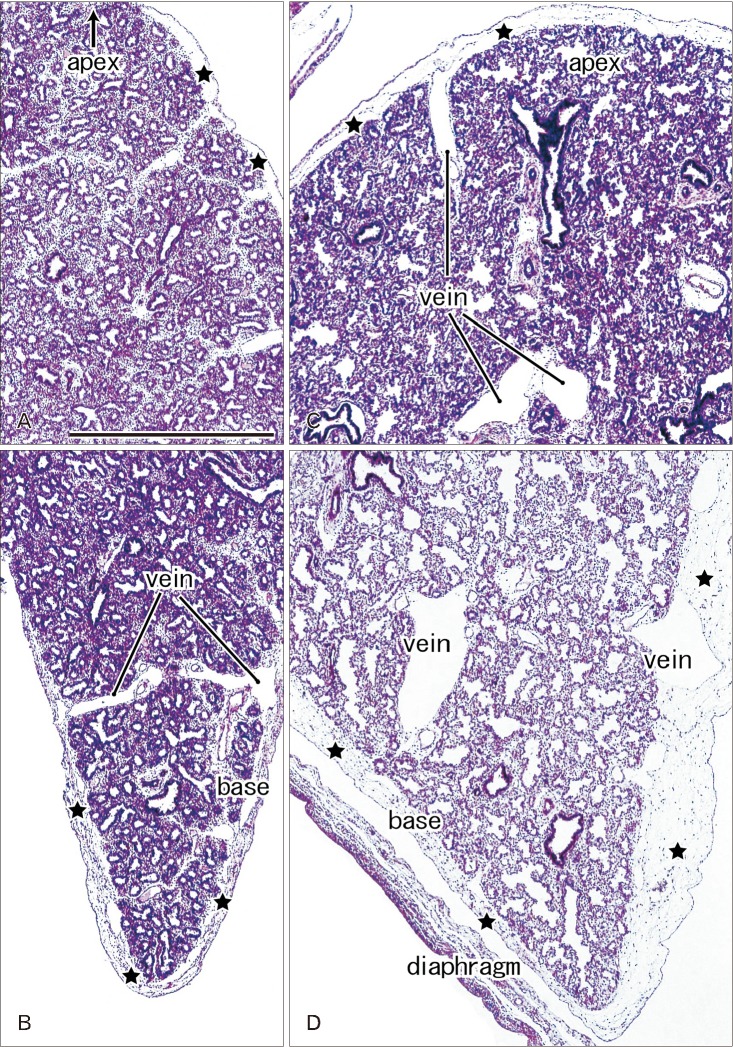
In later stages (fetuses at 26–34 weeks; CRL, 210–290 mm), the lungs were in the saccular or alveolar phases of development and carried almost smooth surfaces because, instead of outward growing bronchioli, the developing alveoli faced the lung surfaces and made them soft and flat (Fig. 2). Moreover, the prominent submesothelial tissue (lamina propria) contained numerous dilated veins connected to the deep intersegmental veins. The deep veins were also dilated. Multilayered fibers were also evident in the lamina propria. The lamina propria was usually thick at the basal and mediastinal areas (Table 1), and sometimes provided a thick fold-like protrusion at the inferolateral angle of the lower lobe.
Fig. 2. Lung surface and pulmonary pleura at the saccular phase in late stage fetuses. H&E staining. Sagittal sections. Panels A and B display the left lung from a specimen at 26 weeks (215 mm crown-rump length [CRL]), while panels C and D exhibit the right lung from a specimen at 31 weeks (255 mm CRL). The subpleural tissue (stars) is thicker in the basal area than in the upper area near the apex of the lung and it contains dilated superficial veins those connected with deep, intersegmental veins. All panels were prepared at the same magnification. Scale bar=1 mm (A).
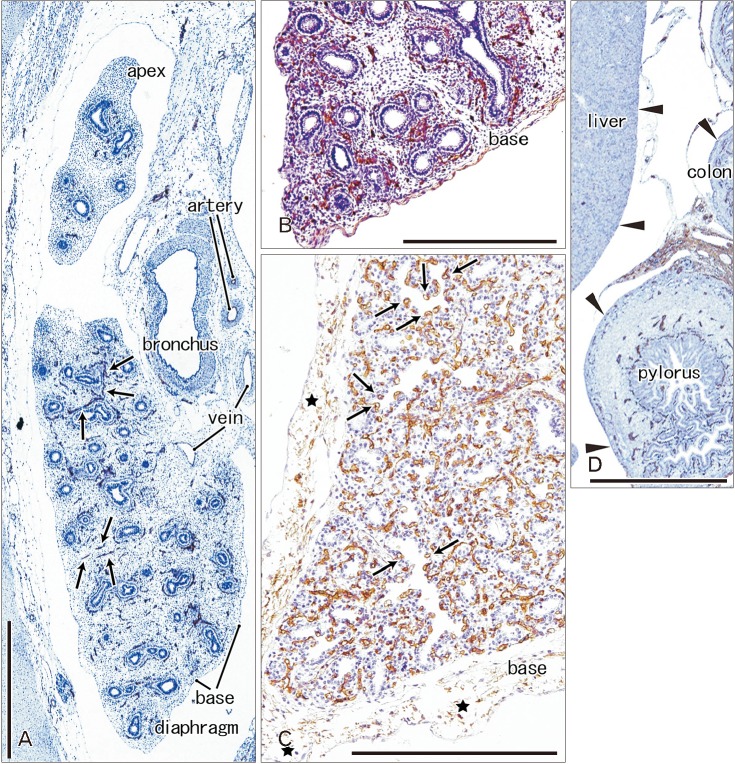
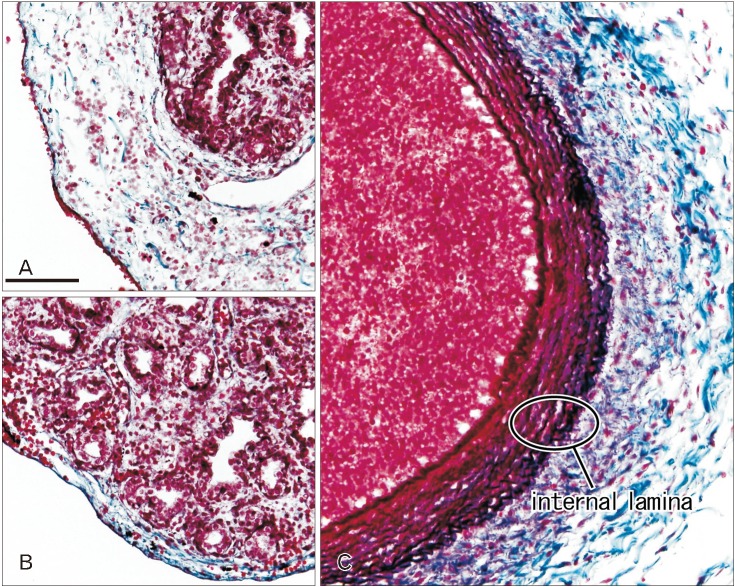
Immunoreactivity for CD34 first appeared in developing vessels between bronchioli (Fig. 3A) and extended to the submesothelial tissue (Fig. 3B, C). Capillaries adjacent to the developing alveoli, characteristic of the canalicular and saccular phases, also expressed CD34 in the late stage fetuses (Fig. 3C). Actually, the subpleural tissue was still thin at 15 weeks (Fig. 3B), but serosal surfaces of the liver and abdominal gastrointestinal tract carried no or little CD34-positive fibers or tissues (Fig. 3D). The pulmonary pleura was weakly positive or negative for CD34: this was same as in the peritoneum. D2-40–positive lymphatic vessels were identified at and near the lung surface even in the smallest specimen (CRL 39 mm) in this study (Fig. 4A). At 16 weeks, the lymphatic vessels were thick and increased in number in the intersegmental fibrous tissue as well as in the submesothelial tissue (Fig. 4B). D2-40 reactivity was not seen in the larger specimens possibly due to long preservation in formalin solution. Finally, we found elastic fibers in larger specimens especially in the internal lamina of any thick arteries (Fig. 5), but the lung contained no or only few elastic fibers. The pleura was stained darkly gray with the elastic fiber staining (Fig. 5A, B), but according to the morphology, the positive structure was different from wavy elastic fibers seen in the arterial wall.
Fig. 3. CD34 immunohistochemistry of the lung and pleura. Sagittal sections. (A) The left lung from a specimen at 9 weeks (pseudoglandular phase; 39 mm crown-rump length [CRL]). (B) The left lung from a specimen at 15 weeks (a mixture of the pseudoglandular and canalicular phases; 115 mm CRL). (C) The left lung from a specimen at 30 weeks (saccular phase; 250 mm CRL). Panels B and D are photos of the same section and panel D displays the liver, pylorus and transverse colon. CD34 immunoreactivity is restricted in the developing vessels (arrows in A). At 15 weeks, the subpleural tissue is thin at the lung base (B), while no or little serosal tissue is seen along the liver, pylorus and colon (arrowheads in D). At 30 weeks, the subpleural tissue contains multilayered fibers expressing CD34 weakly (C). Capillaries adjacent to alveoli express CD34 strongly (arrows in C). Scale bars=1 mm.
Fig. 4. D2-40 immunohistochemistry of lymphatic vessels at the lung surface. (A) The right lung base from a specimen at 9 weeks (the same specimen as shown in Fig. 3A; 39 mm crown-rump length [CRL]). (B) The right lung base from a specimen at 16 weeks (a mixture of the pseudoglandular and canalicular phases; 125 mm CRL). D2-40–positive lymphatic vessels reach the lung surface until 9 weeks (arrows in A) and they are thick and abundant at 16 weeks (arrows in B). Bronchial epithelia also express reactivity of D2-40. Veins reach the lung surface at 16 weeks. Two panels were prepared at the same magnification. Scale bar=1 mm (A).
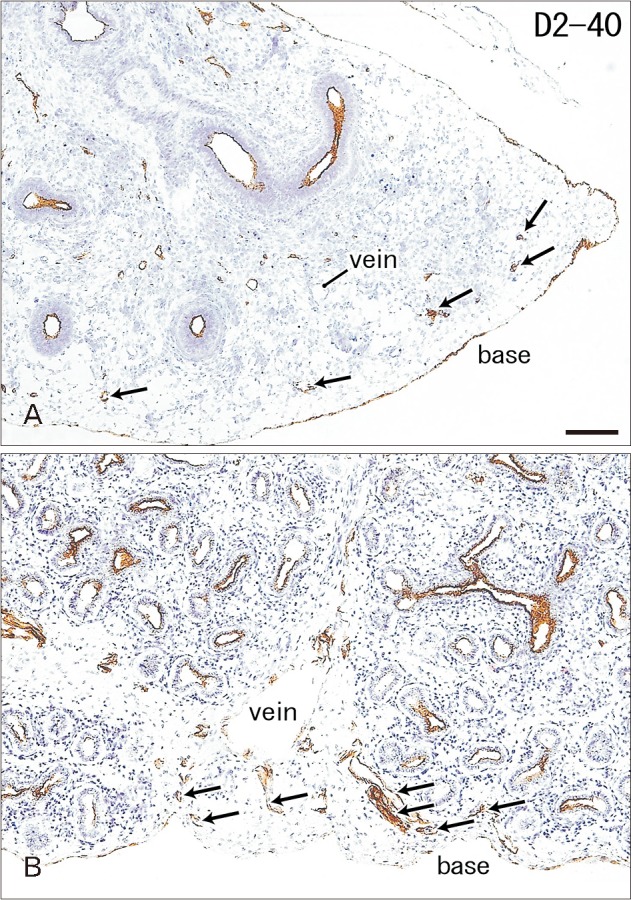
Fig. 5. Elastica Masson staining of the lung in late stage fetuses. Sagittal sections. Panels A and B display the left lung from a specimen at 26 weeks (215 mm crown-rump length [CRL]) and the right lung from a specimen at 31 weeks (255 mm CRL), respectively. Panel C exhibits a thick branch of the pulmonary artery from a specimen at 31 weeks (255 mm CRL). The pleura and subpleural tissue are stained dark gray (A, B), but they are different from wavy elastic fibers seen in the internal lamina of the arterial wall (C). All panels were prepared at the same magnification. Scale bar=0.1 mm (A).
Discussion
In the present study, we demonstrated that smooth lung surface was established until 26 weeks of human fetal development. Instead of the outward growing bronchioli at the pseudo-glandular and canalicular phases, the smooth surface was characterized by increasing numbers of alveoli facing the lung surface. Subsequent development of the submesothelial tissue appeared to make the smooth surface complete. Thickening of the submesothelial tissue seemed to depend on the development of superficial veins that were connected with deep intersegmental veins. Lymphatic vessels developed much earlier than superficial veins, but their volumes seemed to be limited in the submesothelial tissue. The submesothelial tissue is known as a major lymphatic drainage route from the deep, subsegmental parenchyma to the hilar nodes, as a morphological basis for early metastasis of lung cancer [11,12].
Actually, the lamina propria of the pleura was often thick at the basal and mediastinal surfaces. However, rather than a possible mechanical stress from the thoracic wall and diaphragm during respiratory movement in utero, the submesothelial tissue including vessels seemed to much contribute to provide the smooth lung surface. Even in full-term babies, the number of alveoli is only 8% of that in adults [13]. Therefore, it is unlikely that the fetal lung occupies the full size of the potential pleural space. A thick fold of the submesothelial tissue at the inferolateral angle of the lower lobe may occupy an excess space of the pleural cavity.
Matrices in the fetal pulmonary interstitium have been of major interest for embryologists with a focus on epithelial-mesenchymal interactions and epithelio-mesenchymal transitions [14,15,16,17]. Some components of the extracellular matrix (ECM), such as tenascin-C and others, are likely to mechanistically support branching and growth of bronchioli [17]. Likewise, the pulmonary pleura and submesothelial tissue are likely to contain ECM components against the mechanical contact with the thoracic wall. We were not able to perform immunohistochemistry of ECM components because of a limitation of materials, but using immunohistochemistry of CD34, we demonstrated a fiber configuration of the tissues. CD34-positive fibrous tissues are evident in and along viscera but not in and around skeletal muscles: this fact makes a clear site-dependent difference in distribution [4,5]. Submesothelial fibrous components were fewer in and along the liver and abdominal gastrointestinal tract than the lung in a same section. The thin submesothelial tissue in the abdomen might be a result of no or few veins and lymphatics contained [18,19]. The subpleural fibers might conduct mechanical stress from the thoracic wall to the lung ECM with or without modification.
Acknowledgements
We are grateful to Mr. H.G. Sydow for his assistance with the Blechschmidt collection, Göttingen. This study was supported in part by a Grant-in-Aid for Scientific Research (JSPS KAKENHI No. 16K08435) from the Ministry of Education, Culture, Sports, Science and Technology in Japan.
References
- 1.Finley DJ, Rusch VW. Anatomy of the pleura. Thorac Surg Clin. 2011;21:157–163. doi: 10.1016/j.thorsurg.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 2.O'Rahilly R, Müller F. Human embryology and teratology. 2nd ed. New York: Wiley-Liss; 1996. pp. 265–271. [Google Scholar]
- 3.Hayashi S, Fukuzawa Y, Rodríguez-Vázquez JF, Cho BH, Verdugo-López S, Murakami G, Nakano T. Pleuroperitoneal canal closure and the fetal adrenal gland. Anat Rec (Hoboken) 2011;294:633–644. doi: 10.1002/ar.21351. [DOI] [PubMed] [Google Scholar]
- 4.Abe S, Suzuki M, Cho KH, Murakami G, Cho BH, Ide Y. CD34-positive developing vessels and other structures in human fetuses: an immunohistochemical study. Surg Radiol Anat. 2011;33:919–927. doi: 10.1007/s00276-011-0854-2. [DOI] [PubMed] [Google Scholar]
- 5.Katori Y, Kiyokawa H, Kawase T, Murakami G, Cho BH. CD34-positive primitive vessels and other structures in human fetuses: an immunohistochemical study. Acta Otolaryngol. 2011;131:1086–1090. doi: 10.3109/00016489.2011.590152. [DOI] [PubMed] [Google Scholar]
- 6.Wilting J, Papoutsi M, Christ B, Nicolaides KH, von Kaisenberg CS, Borges J, Stark GB, Alitalo K, Tomarev SI, Niemeyer C, Rössler J. The transcription factor Prox1 is a marker for lymphatic endothelial cells in normal and diseased human tissues. FASEB J. 2002;16:1271–1273. doi: 10.1096/fj.01-1010fje. [DOI] [PubMed] [Google Scholar]
- 7.Hasselhof V, Sperling A, Buttler K, Ströbel P, Becker J, Aung T, Felmerer G, Wilting J. Morphological and molecular characterization of human dermal lymphatic collectors. PLoS One. 2016;11:e0164964. doi: 10.1371/journal.pone.0164964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinoshita H, Umezawa T, Omine Y, Kasahara M, Rodríguez-Vázquez JF, Murakami G, Abe S. Distribution of elastic fibers in the head and neck: a histological study using late-stage human fetuses. Anat Cell Biol. 2013;46:39–48. doi: 10.5115/acb.2013.46.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motohashi O, Suzuki M, Shida N, Umezawa K, Ohtoh T, Sakurai Y, Yoshimoto T. Subarachnoid haemorrhage induced proliferation of leptomeningeal cells and deposition of extracellular matrices in the arachnoid granulations and subarachnoid space. Immunhistochemicalstudy. Acta Neurochir (Wien) 1995;136:88–91. doi: 10.1007/BF01411441. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi T, Kumasaka T, Mitani K, Yao T, Suda K, Seyama K. Loss of heterozygosity on tuberous sclerosis complex genes in multifocal micronodular pneumocyte hyperplasia. Mod Pathol. 2010;23:1251–1260. doi: 10.1038/modpathol.2010.114. [DOI] [PubMed] [Google Scholar]
- 11.Riquet M. Anatomic basis of lymphatic spread from carcinoma of the lung to the mediastinum: surgical and prognostic implications. Surg Radiol Anat. 1993;15:271–277. doi: 10.1007/BF01627878. [DOI] [PubMed] [Google Scholar]
- 12.Topol M, Masłoń A. The problem of direct lymph drainage of the bronchopulmonary segments into the mediastinal and hilar lymph nodes. Clin Anat. 2009;22:509–516. doi: 10.1002/ca.20790. [DOI] [PubMed] [Google Scholar]
- 13.Skandalakis JE, Gray SW, Symbas PN. The trachea and lungs. In: Skandalakis JE, Gray SW, editors. Embryology for Surgeons. 2nd ed. Baltimore, MD: Williams & Wilkins; 1972. pp. 414–450. [Google Scholar]
- 14.Virtanen I, Laitinen A, Tani T, Pääkkö P, Laitinen LA, Burgeson RE, Lehto VP. Differential expression of laminins and their integrin receptors in developing and adult human lung. Am J Respir Cell Mol Biol. 1996;15:184–196. doi: 10.1165/ajrcmb.15.2.8703474. [DOI] [PubMed] [Google Scholar]
- 15.Arai H, Hirano H, Mushiake S, Nakayama M, Takada G, Sekiguchi K. Loss of EDB+ fibronectin isoform is associated with differentiation of alveolar epithelial cells in human fetal lung. Am J Pathol. 1997;151:403–412. [PMC free article] [PubMed] [Google Scholar]
- 16.Wright C, Strauss S, Toole K, Burt AD, Robson SC. Composition of the pulmonary interstitium during normal development of the human fetus. Pediatr Dev Pathol. 1999;2:424–431. doi: 10.1007/s100249900145. [DOI] [PubMed] [Google Scholar]
- 17.Lambropoulou M, Limberis V, Koutlaki N, Simopoulou M, Ntanovasilis D, Vandoros GP, Tatsidou P, Kekou I, Koutsikogianni I, Papadopoulos N. Differential expression of tenascin-C in the developing human lung: an immunohistochemical study. Clin Exp Med. 2009;9:333–338. doi: 10.1007/s10238-009-0057-x. [DOI] [PubMed] [Google Scholar]
- 18.Jin ZW, Nakamura T, Yu HC, Kimura W, Murakami G, Cho BH. Fetal anatomy of peripheral lymphatic vessels: a D2-40 immunohistochemical study using an 18-week human fetus (CRL 155 mm) J Anat. 2010;216:671–682. doi: 10.1111/j.1469-7580.2010.01229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Han EH, Jin ZW, Lee HK, Fujimiya M, Murakami G, Cho BH. Fetal topographical anatomy of the upper abdominal lymphatics: its specific features in comparison with other abdominopelvic regions. Anat Rec (Hoboken) 2012;295:91–104. doi: 10.1002/ar.21527. [DOI] [PubMed] [Google Scholar]