Abstract
Women with hypertensive pregnancy complications are at greater risk of developing cardiovascular disease (CVD), metabolic diseases, stroke, and end-stage renal disease (ESRD) later in life. Pregnancy complications affect not only the mother’s long-term health but also the health of the fetus immediately after delivery and into adulthood. The health of the fetus until adulthood can be influenced by developmental programming, in which the fetus is exposed to insults that will ultimately affect the growth of the offspring and increase the offspring’s risk of developing hypertension, coronary heart disease, metabolic disease, and chronic kidney disease in adulthood. Preeclampsia, the onset of hypertension during pregnancy, is one of the major risk factors for the development of renal disease, cerebral disease, and CVD in the mother. Women with preeclampsia are at a 5–12-fold increased risk of developing ESRD, 2-fold increased risk of stroke, and 2-fold increased risk of developing CVD later in life. In this review article, we discuss 1) preeclampsia, 2) the risk of developing CVD, renal disease, or stroke later in life for women with hypertensive pregnancies, and 3) the effects of a hypertensive pregnancy on the offspring.
Keywords: cardiovascular disease, gestational hypertension, preeclampsia
PREECLAMPSIA
Preeclampsia is a hypertensive disorder that occurs during pregnancy and affects between 5 and 10% of all births in the United States each year (4, 35, 36). It is the leading cause of preterm births, morbidity, and mortality for both the mother and fetus during pregnancy (14). Preeclampsia is generally characterized as new-onset hypertension, with a blood pressure >140/90 mmHg that occurs during the 20th week of pregnancy (4, 15, 35, 36). Different from gestational hypertension, preeclampsia is a disorder that can affect many organ systems of the body and can lead to decrease in renal function, liver dysfunction, and even stroke in more severe forms of preeclampsia, such as eclampsia (4, 15, 36). Preeclampsia is commonly associated with proteinuria, severe headaches, vomiting, abdominal pain, visual disturbances, and multiorgan dysfunction (4, 15). Gestational hypertension typically is not associated with proteinuria or other organ dysfunction but can be a risk factor for the development of preeclampsia. Preeclampsia can be further broken down into two different diseases, mild and severe preeclampsia. A blood pressure of 140/90 mmHg on two different occasions after 20 wk of gestation with one of the major symptoms mentioned above is classified as mild preeclampsia (15). A blood pressure ≥160/110 mmHg with severe proteinuria (>5 g/day), severe headaches, and any of the associated side effects mentioned above is classified as severe preeclampsia. Severe preeclampsia is very dangerous and could result in eclampsia or death for both the mother and fetus (4, 15). Preeclampsia can also be categorized based on its timing, where there is early-onset preeclampsia, before 33 wk of gestation, and late-onset preeclampsia, which occurs after 33 wk of gestation (38). Early-onset preeclampsia is more closely associated with severe preeclampsia and greater risk of maternal and fetal morbidity and mortality, premature births, intrauterine growth restriction (IUGR), and small-for-gestational age (SGA) babies (38). To date, there is no cure for preeclampsia, except for the delivery of the fetus and placenta (4, 35). Although this disease ceases for most women after birth, multiple studies have shown that this event could have long-lasting negative effects on the cardiovascular, renal, metabolic, and neurological systems in both the mother and fetus later in life (1, 9, 15, 23, 25, 30, 39, 56, 63).
FACTORS THAT PLAY A ROLE IN PATHOGENESIS OF PREECLAMPSIA
It is believed that preeclampsia starts at the level of the placenta during the second trimester of pregnancy where trophoblast cells undergo apoptosis and poor invasion into the maternal decidua, leading to spiral artery remodeling (52). The inability of the trophoblast cells to invade properly causes the spiral arteries to lose vessel surface area and the ability to remodel and expand resulting in a decrease in uteroplacental blood flow compared with uncomplicated pregnancies (52). The lack of blood flow to the fetus deprives the fetus of essential nutrients and oxygen necessary for growth and development. This results in placental ischemia, which can release several factors that play a critical role in the pathogenesis of preeclampsia. These factors include and are not limited to an increase in inflammatory cytokines and cells [such as T helper 1 cells, T helper 17 cells, TNF-α, angiotensin II (ANG II) type 1 receptor agonistic autoantibodies (AT1-AAs), and natural killer cells], reactive oxygen species (such as superoxide and hydrogen peroxide), and antiangiogenic factors [such as soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin; 4, 35, 36, 52]. All these factors taken together can target endothelial cells and the release of vasoactive substances leading to endothelial dysfunction, coupled with a decrease in vasodilators [such as nitric oxide (NO)], causing an increase in vasoconstriction and hypertension.
MOTHER’S INCREASED RISK OF CARDIOVASCULAR DISEASE
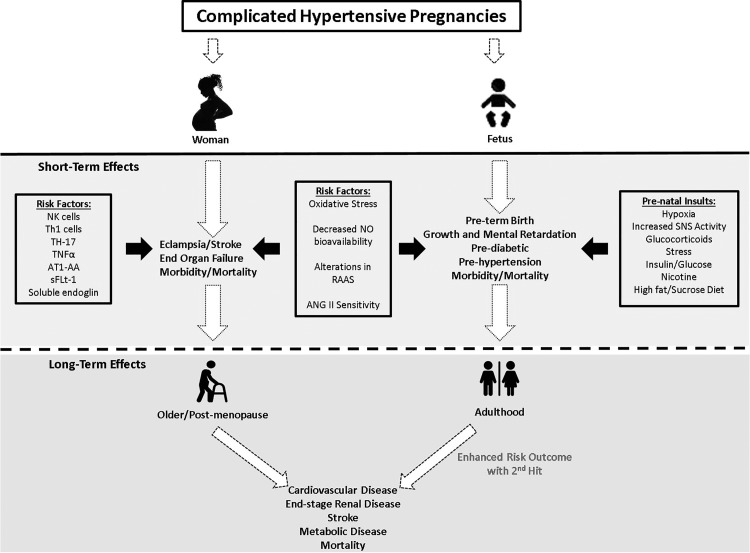
Cardiovascular disease (CVD) is the leading cause of death for women worldwide (42). The risk of CVD increases in postmenopausal women and in women with preeclampsia (1, 6, 25, 30, 39). Women with preeclampsia have a higher risk of developing myocardial infarctions and ischemic heart diseases (Fig. 1; 1, 25, 30, 39). The origins of preeclampsia are unknown; however, an interesting hypothesis suggests that preeclampsia could be an identifier of underlying cardiovascular dysfunction, allowing pregnancy to serve as a stress test that exacerbates a preexisting pathophysiology. Another thought is that preeclampsia causes lasting changes in the maternal cardiovasculature causing premature aging of the cardiovascular system leading to increased risk of CVD.
Fig. 1.
Hypertensive disorders of pregnancy are associated with immune, vascular, and hormonal factors that can cause detrimental long-lasting effects in both mother and baby. AT1-AA, ANG II type 1 receptor agonistic autoantibodies; NK, natural killer; RAAS, renin-angiotensin-aldosterone system; sFlt-1, soluble fms-like tyrosine kinase-1; SNS, sympathetic nervous system; Th1, T helper 1; Th17, T helper 17.
Data were collected over a 27-year period from 1968 to 1995, where over 1,400 practitioners participated in a prospective study (25) examining over 210,000 women in the United Kingdom. This study showed that women with a history of preeclampsia had an increased relative risk (RR) of 2.24 for acute myocardial infarction, RR of 1.74 for chronic ischemic heart disease, RR of 1.53 for angina pectoris, RR of 1.65 for all ischemic heart disease, and RR of 1.62 for venous thromboembolism compared with women with a normal pregnancy (25). Furthermore, a retrospective study in Iceland showed that the greater the severity of preeclampsia, the higher the RR of death for women with ischemic heart disease later in life (30). This study suggests that not only is preeclampsia a risk factor for CVD but also preeclampsia exacerbates the CVD that may occur with aging or some other risk factors associated with CVD, such as obesity, hypertension, and diabetes. Because of the time span of this study, the link between preeclampsia and severity of CVD is correlative and not causative, because other factors or events after preeclampsia may independently or in combination with preeclampsia contribute to the aggressive pathology observed in this study. These findings are alarming and of major concern in today’s society, where many women are exposed to high-fat/sugar diets, stress, and obesity and are diagnosed with diabetes, hypertension, or renal injury, which increase the risk of CVD independently without preeclampsia.
Two of the major risk factors for developing CVD are hypertension and endothelial dysfunction. An elegant Dutch study from 1993 to 1997 showed that women with preeclampsia go on to develop hypertension 7–8 yr earlier than women with a normal pregnancy, putting them at a greater risk of CVD development (26, 56). Several clinical studies examining flow-mediated dilation in women with preeclampsia versus women with a normal pregnancy showed that postpartum preeclamptic women exhibit a greater impairment of endothelial cell function up to 1 yr postpartum (1, 16). The increase in endothelial cell dysfunction was not associated with increased blood pressure or maternal risk factors, such as age, family history, body mass index, cholesterol, diabetes, smoking, and fasting glucose levels of the mother. This noncorrelation suggests that the occurrence of endothelial cell dysfunction in these women may be all that is required for the development of CVD independent of hypertension (1, 16).
MOTHER’S INCREASED END-STAGE RENAL DISEASE RISK
There is a strong link between preeclampsia and end-stage renal disease (ESRD; 23, 63). A well-designed study performed by Vikse et al. showed that women who had preeclampsia during their first pregnancy had a 4.7 RR of developing ESRD later in life (63). This study revealed that women who had recurring preeclampsia in the second pregnancy had a 6.4 RR and women who had a total of three pregnancies where two or three pregnancies resulted in preeclampsia had a 15.5 RR of developing ESRD (63). This was a landmark study in that it was the first to show the association between the risks of having multiple preeclamptic pregnancies and the development of later-life ESRD (42). Another study conducted in Norway, between 1967 and 1991, examining data from the Medical Birth Registry of Norway and the Norwegian Renal Registry, observed that women who had preeclampsia, a short gestational period, and/or low-birth weight babies have a RR >2, and >17 if all three occurrences were present during pregnancy, for their offspring to have a kidney biopsy later in life, ~16 yr after delivery (62). The occurrence of a kidney biopsy in this study was associated with renal injury. Although this study was a novel finding, the presence of a kidney biopsy does not give adequate information on the type of renal injury or the effects of preeclampsia on proteinuria, glomerular filtration rate (GFR; renal function), morphology, or fibrosis (62). However, several other studies have examined these changes in women following preeclampsia, which will be discussed below.
Both microalbuminuria and proteinuria are often present in postpartum women who had preeclampsia during pregnancy (5, 7, 9, 20, 31, 41, 43, 60). A large meta-analysis study conducted by McDonald et al., examining 273 preeclamptic patients and 333 patients with uncomplicated pregnancies, showed that 31% of women with preeclampsia have microalbuminuria, which resulted in a fourfold increased risk for proteinuria, both of which are predictive markers of renal disease (41). In addition, women with severe preeclampsia have an eightfold increased risk of having microalbuminuria compared with women with uncomplicated pregnancies (41). Microalbuminuria, which is defined as albumin-to-creatinine urine ratio ≥30 µg/mg, is a major risk factor for the development of ESRD and CVD (5). Low levels of microalbuminuria, ≥3.9 µg/mg for men and ≥7.5 µg/mg for women, are associated with a threefold increased risk of developing CVD, suggesting that just a low-grade level of albumin excretion is associated with increased risk of CVD and renal disease in nonhypertensive and nondiabetic patients (5). This new finding suggests that even a slight increase in albuminuria in women after preeclampsia is significant and may be a predictive measure for intervention or close monitoring by their provider to prevent the development of ESRD and CVD. Although more studies are needed to establish this proof of concept, studies that only show a slight change in microalbuminuria should not be discarded and counted as insignificant. It is important to also note that in the past, proteinuria was used as a diagnostic criterion for preeclampsia and that it is not surprising that women with proteinuria would have an increase in ESRD and CVD later in life.
Studies by Berks et al. in The Netherlands show that 14% of preeclamptic patients have elevated levels of proteinuria ~3 mo postpartum and only 2% have elevated levels ~2 yr later, which is independent of an increase in blood pressure (9). Although these rates appear low, other studies show that the presence of proteinuria after preeclampsia in patients mirrors the presence of glomerular deposits and glomerulosclerosis 18 mo postpartum (32, 57). The presence of proteinuria at 3 mo may be an implication for the nephrologist to monitor these patients, who are at greater risk of developing ESRD later in life. In fact, in a small Turkish study between 2005 and 2010 showed that of 463 preeclampsia patients, 34 women were observed to have persistent proteinuria at least 3 mo postpartum (60). Seventy-one percent of the women with persistent proteinuria postpartum had some renal biopsies taken and were diagnosed with underlying renal diseases that ranged from membrane proliferative glomerulonephritis to focal segmental glomerulosclerosis (60). The authors of this study concluded that persistent proteinuria is a major predictor of underlying renal disease after delivery and that Turkish patients with preeclampsia should be evaluated for the presence of postpartum proteinuria (60).
Limited studies have been conducted to examine the postpartum effects on blood pressure, proteinuria, and renal function after preeclampsia in African populations of women, who might have an increased risk of ESRD development after preeclampsia (31). However, 1 study by Kaze et al. examined 54 Cameroonian women with severe preeclampsia and eclampsia, which showed that 48, 32, and 2% of patients had persisting proteinuria 6 wk, 3 mo, and 6 mo postpartum, respectively (31). From this study, it was concluded that persistent proteinuria is present in Cameroonian women postpreeclampsia and that these women along with other women of African descent need to be further examined postpartum.
In a Chinese study, renal biopsies from women with preeclampsia revealed that patients displayed endotheliosis, vacuolation of podocytes, proliferation of mesangial cells, and protein casts in the tubule lumens of their kidneys (24). These patients showed glomerular lesions, which consisted of increased volume and decreased capillary lumen diameter in their kidneys (24). Note that the occlusion of the capillaries in the glomerulus causes ischemia (24). Glomerular ischemia can lead to a decrease in glomerular filtration, i.e., renal function, and increase one’s risk of developing ESRD. The authors of this study showed the presence of immunoglobulin and complement components in the renal biopsies, suggesting a role for inflammation in the kidney as a mechanism of induced renal injury from preeclamptic patients (24).
Preeclamptic women 4 mo to 20 yr postpartum have a decrease in renal function (GFR; 23, 43, 47, 54, 55, 61). A cross-sectional study of preeclamptic and normotensive women from the Netherlands showed that 20 yr after delivery, women with preeclampsia have a 30% increase in renal vascular resistance, 15% decrease in effective renal blood flow, and small decrease in creatinine clearance, without any changes in proteinuria (54). In this study, women were 55% more likely to have hypertension (54). The authors of the study concluded that preeclamptic women would benefit from constant surveillance of blood pressure and renal function. One of the major limitations of this study is that it was conducted 20 yr after, and during that 20-yr period other events could have influenced the outcomes of this study that are independent of the preeclampsia.
One marker of kidney dysfunction that has not been intensively examined is podocyturia, or podocyte injury, postpartum in preeclamptic women. Some studies show that podocyturia occurs during preeclampsia (18, 19, 34, 65). Craici et al. demonstrated that podocyturia serves as an early marker and diagnostic test for preeclampsia (19). Whether the amount of podocyturia early in pregnancy could be a predictor of the severity of preeclampsia is yet to be determined and warrants future studies. In addition, a study by Garovic et al. showed that urinary podocyte excretion was present in preeclamptic patients and was a stronger predictive factor for the diagnosis of preeclampsia than sFlt-1 and soluble endoglin, two angiogenic factors that play a major role in the pathophysiology of preeclampsia (22).
Podocytes are terminally differentiated cells located within the glomerulus that aid with the filtration of molecules, water, and small ions, but not proteins. Podocyte proteins consist of podocin, nephrin, synaptopodin, and podocalyxin, which all help to form a major part of the glomerular filtration barrier (19). When podocytes are shed and lost, they disrupt the glomerular filtration barrier and allow leakage of proteins into the urine causing proteinuria. Podocyturia can be measured by examining any of these proteins in the urine. One small study with 10 preeclamptic patients and 18 normotensive patients showed that 30% of the preeclamptic patients had podocyturia whereas none of the normotensive patients did at 5–8 wk postpartum (65). It is important to note that in this study no other factors, such as the protein-to-creatinine ratio, blood pressure, or presence of angiogenic markers (sFlt-1, placental growth factor, and endoglin), were different between the two groups of women (65). Although this was a small study, it confirmed the link between preeclampsia and podocyturia and suggested that podocyturia could be used as a biomarker for future renal disease and/or CVD later in life for preeclamptic women after delivery. This study finding was novel but needs to be reproduced and examined in a larger population with the inclusion of GFR calculations via creatinine clearance.
A more recent study examining tubular injury in preeclampsia has shown that patients with preeclampsia have elevated α1-microglobulin and complement C5b-9, which are both elevated in patients with tubular injury and cause damage to the tubules (17). Although changes were observed in these two proteins, other markers of tubular injury such as kidney injury molecule-1, retinol-binding protein, tissue inhibitor of metalloproteinase-2, and insulin-like growth factor-binding protein-7 were not different between normotensive women and preeclamptic women (17). More studies are needed to explore the relevance and the changes in expression of these markers and the long-term risk for a woman of developing hypertension/ESRD with tubular injury during pregnancy.
MOTHER’S INCREASED RISK OF STROKE
Women with preeclampsia are at a fourfold increased risk of stroke later in life (15). Stroke is the leading cause of death in postmenopausal women and can occur during preeclampsia, at which point the condition is no longer termed “preeclampsia,” but “eclampsia” (15). Several studies show an increase in the risk of all stroke for women who have preeclampsia (13). According to the American Heart Association and American Stroke Association, stroke is a disease that affects the arteries leading to and within the brain, in which blood vessels become clotted or ruptured. Blood vessels that are damaged will decrease the blood flow, oxygen, and nutrient supplies to the brain. The two major classes of stroke are ischemic stroke (80% of all strokes) and hemorrhagic stroke, which are associated with an increased incidence in women with preeclampsia (13, 29, 33, 37, 51, 58, 59, 67). The Stroke Prevention in Young Women Study, in which there were 261 women with stroke and 421 women without stroke, showed that preeclampsia was associated with an increased risk of ischemic stroke with an odds ratio of 1.59 in the United States (13). In this same study, adjusted for age, race, education, and number of pregnancies, women with a history of preeclampsia had a 60% greater risk of having ischemic stroke versus women without a history of preeclampsia (13). Furthermore, in a collaborative study with 46 hospitals in central Maryland and Washington, D.C., preeclamptic women were more likely to have cerebral infarction and intracerebral hemorrhage postpartum at ~6 wk after delivery versus normotensive pregnant women (33). This was also the case for women with hypertension during pregnancy (33). The risk of stroke is increased in preeclamptic women postpartum not only in the United States but also in Sweden, Taiwan, and Scotland (51, 58, 67). Factors that might increase the risk of stroke later in life are not known and are the future of new research. Some factors that we suggest, which need to be explored, are those that involve the brain renin-angiotensin-aldosterone system (RAAS), oxidative stress, and inflammation. One plausible link from preeclampsia to cerebral damage is through microglial activation, blood-brain barrier permeability, and the loss of cerebral vascular autoregulation.
EFFECT OF HYPERTENSIVE PREGNANCY ON OFFSPRING
Multiple studies have shown that adult offspring born to mothers with a complicated pregnancy or exposed to adverse insults during pregnancy are at a greater risk of noncommunicable diseases, CVD, and hypertension later in life (2). Several other studies have shown that pregnancies affected by placental insufficiency and hypertension often create an adverse environment for the fetus during pregnancy and can affect the baby’s developmental programming. The baby’s developmental programming can be altered and reprogrammed by epigenetic changes, where there are modifications of enzymes, transcription factors, oxidative stress markers, and methylation and histone modification to DNA. These reprogramming changes can alter the baby’s sodium handing, sensitivity to ANG II, augmentation of the RAAS, nephron number, and organ development, which can all play a role in causing hypertension (48). Furthermore, fetuses born to mothers with complicated pregnancies often result in SGA babies (<2.5 kg) or large-for-gestational age babies, depending on the type of complicated pregnancy and insults (66). Both SGA and large-for-gestational age babies have an increased risk of mortality during pregnancy and their first year of life, developmental issues in childhood, and CVD in adulthood. Birth weight is a major predictor for future health outcomes in offspring (66). Complicated pregnancies, such as those involving gestational diabetes and preeclampsia, are associated with abnormal birth weight babies. Preeclampsia is a major cause of and contributor to low birth weight (70). Low birth weight is a risk factor for CVD, hypertension, type 2 diabetes, and osteoporosis (2, 3).
In the 1980s, David Barker studied the link between hypertension, CVD, and low birth weight. On the basis of the findings he derived the Barker hypothesis, often referred to as the “fetal origins of adult disease.” Barker’s studies showed that IUGR, low birth weight, and premature birth have a causal relationship to the origins of hypertension, coronary heart disease, and non-insulin-dependent diabetes in middle-aged offspring. Barker’s hypothesis and findings have been duplicated in different regions all around the world and pioneered studies in fetal programming.
Several human and animal studies have shown that environmental insults, such as nicotine, malnutrition, and hypoxia, during pregnancy can contribute to the developmental programming of CVD, hypertension, attention deficit/hyperactivity disorder, and low academic performance in childhood and adulthood (8, 11, 27, 28, 49, 53, 69). Moreover, studies have shown that children born to preeclamptic mothers have cognitive, behavioral, and mood deficits. Some of the specific outcomes that have been observed in the offspring of preeclamptic women are lower verbal reasoning and IQ scores, increased rate of depression, greater cognitive decline in old age, and reduction of cognitive and motor skill functions (21). The exact cause of these mental health issues is unknown and has been suggested to be associated with changes in size of different regions of the brain, such as the temporal lobe, amygdala, and cerebellum, determined by MRI (21).
Studies examined over 369 infants in Greece and showed that offspring from 1, 24, 48, and 72 h after delivery until 2 yr of age have high blood pressure if they were born to mothers who smoked during pregnancy (8). In this study, the number of cigarettes smoked per day was correlated with the severity of hypertension in the infant (8). Smoking nicotine during pregnancy has been recorded as being just as detrimental to the fetus as crack cocaine usage of pregnant women (53). Some of the mechanisms of how fetal insults during pregnancy program the increased risk of CVD and hypertension later in life could be due to increased glucocorticoids, oxidative stress, sympathetic nervous system activation, inflammation, endothelin, nephrogenesis, and alterations in the RAAS (2).
ALTERATIONS IN THE RAAS
Alterations in the RAAS have been shown to play a significant role in the fetal programming of CVD and hypertension in the offspring of mothers who had a complicated pregnancy or were exposed to environmental insults during pregnancy. The RAAS is important for salt and water retention, vascular tone, blood pressure regulation, and homeostasis. The pathway begins with angiotensinogen, which is cleaved by renin to produce ANG I, which is later converted to ANG II by the enzyme ANG I-converting enzyme (ACE). ANG II then binds to the ANG I receptor (AT1R) to facilitate several biological functions, such as salt and water retention, vasoconstriction, and aldosterone production. Both an increase in ANG II and activation of AT1R are often observed in hypertension. However, ANG II can bind ANG II type 2 receptor (AT2R), which has contrary biological actions that are different from AT1R activation and that include increased NO, vasodilation, and salt and water excretion.
Several human studies examining the fetuses and children of women with insults during pregnancy, such as preeclampsia and nicotine exposure, showed that insults during pregnancy result in alterations in the DNA methylation of many genes, including genes of the RAAS (10, 50). Rodents display epigenetic changes, such as DNA methylation and histone modifications, to the promoter region of the AT1R gene to influence and increase AT1R overexpression in adult offspring of rats that were calorically restricted during pregnancy (12, 68, 69). These rodents displayed an increase in blood pressure and ANG II sensitivity, suggesting that their hypertension later in life is due to the epigenetic changes to RAAS genes that occurred during fetal life (12, 68).
Low birth weight, which is an indicator of IUGR, is associated with an increased risk of hypertension, CVD, and type 2 diabetes. Studies examining both children and teenagers between ages 4 and 16 who were born at a low birth weight showed that they have elevated aldosterone, cortisol, and blood pressure (40). These studies showed that birth weight is inversely associated with serum aldosterone levels, which may be the mechanism of hypertension via increased aldosterone (40). In this study, there was no sex difference noted. However, in another study, male adolescents born with a low birth weight to mothers with preeclampsia have increased body weight, blood pressure, circulating renin activity, and aldosterone levels compared with low-birth weight children born to normotensive women (64). This same trend was not observed in female adolescents, suggesting a sex difference at 14 yr of age (64). However, whether the sex difference continues to be present later in life is yet to be observed or tested. In rats, female offspring of mothers with placental ischemia during pregnancy displayed no changes in blood pressure during young adulthood, whereas male rats had hypertension (44). Female rats only developed hypertension after an ovariectomy, suggesting the protective role of estrogens in preventing hypertension (44). Associated with intact ovaries in female rats that were normotensive was an increase in ACE II, which generates ANG-(1-7), which acts as a negative regulator of the vasoconstrictor effects of ANG II and binds to AT2R (44). Perhaps one cause for the sex difference is increased ACE II. Male rat offspring of placental ischemic mothers have an enhanced blood pressure response to ANG II, without any changes in vessel morphology (46). On the other hand, castration abolished the increased blood pressure response to ANG II in males, suggesting a role for testosterone as a modulator of increased ANG II sensitivity in male rats born to mothers with placental ischemia (46). Ovariectomized female offspring of placental ischemic mothers demonstrated a hyperresponsiveness to ANG II (45). These data, taken together, suggest that testosterone is a positive mediator of enhanced ANG II sensitivity, in which estrogen may be protective.
In experimental studies of male offspring of mothers that were calorie restricted during pregnancy, administration of either an AT1R or renin receptor blockade inhibitor prevented the development of hypertension (27). In these studies, the male offspring were administered these drugs every day from 2 to 4 wk of age and were reexamined at 12 wk of age (27). At 12 wk of age, both aliskiren and losartan treatment decreased renal mRNA expression of angiotensinogen, whereas aliskiren alone decreased asymmetric dimethylarginine (ADMA) levels and increased AT2R and Mas protein levels in the kidneys (27). ADMA is an endogenous inhibitor of NO synthase, which is the enzyme used to make NO (60, 70). ADMA is elevated in hypertension, ESRD, and CVD (60, 70). Thus, ADMA may be an important mediator of the increased risk of CVD, hypertension, and stroke later in life for adults exposed to an adverse in utero environment during pregnancy. The increase in AT2R and Mas protein levels may be the protective mechanism in which the renin receptor blocker operates. Both activation of AT2R and activation of Mas protein increase NO production and antioxidant production and oppose the vasoconstrictor/oxidative stress response of ANG II binding to AT1R. In ovariectomized IUGR female rats, treatment for 2 wk with an ACE inhibitor (enalapril) eliminated hypertension in rats (44). In conclusion, both studies illustrate that selective blockade of the RAAS system, via renin receptor blockade, ANG II receptor blocker, and ACE inhibition, may be an effective treatment to prevent the development of hypertension in offspring that were exposed to prenatal insults in utero. However, these therapies should not be used during pregnancy because RAAS blockade causes fetal pathogenicity and interferes with renal function in the offspring.
Moreover, it is important to note that there are other mechanisms that may play a role in fetal programming of CVD and hypertension in the offspring of mothers with a complicated pregnancy. Some of those mechanisms involve augmentation and programming of the hypothalamic-pituitary-adrenal axis, the autonomic nervous system, sex hormones, renal tubular mechanisms with salt handling, endothelial mediators (such as endothelin and NO), and vascular remodeling.
Perspectives and Significance
Postpartum women with complicated hypertensive pregnancies have an increased risk of ESRD, CVD, and stroke. The same is also true for their offspring. Because of the ample evidence presented in this review article, we suggest careful monitoring of postpartum women and their offspring. We also suggest that aggressive therapy should be considered to prevent the development of these cardiovascular, renal, and cerebral complications in both women and children. Thus, there is great need for future therapies for women with hypertension during pregnancy. These therapies need to be able to prevent hypertension in the women without damage to the offspring. Furthermore, aggressive therapies for women with preeclampsia will not only help them during pregnancy but also decrease their risk of cardiorenal disease later in life.
GRANTS
This work was funded by National Institutes of Health Grants HL-105324 and HD-067541-06.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.W.C. prepared figure; M.W.C. and B.L. drafted manuscript; M.W.C. and B.L. edited and revised manuscript; M.W.C. and B.L. approved final version of manuscript.
REFERENCES
- 1.Agatisa PK, Ness RB, Roberts JM, Costantino JP, Kuller LH, McLaughlin MK. Impairment of endothelial function in women with a history of preeclampsia: an indicator of cardiovascular risk. Am J Physiol Heart Circ Physiol 286: H1389–H1393, 2004. doi: 10.1152/ajpheart.00298.2003. [DOI] [PubMed] [Google Scholar]
- 2.Alexander BT, Dasinger JH, Intapad S. Fetal programming and cardiovascular pathology. Compr Physiol 5: 997–1025, 2015. doi: 10.1002/cphy.c140036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander BT, Dasinger JH, Intapad S. Effect of low birth weight on women’s health. Clin Ther 36: 1913–1923, 2014. doi: 10.1016/j.clinthera.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amaral LM, Cunningham MW Jr, Cornelius DC, LaMarca B. Preeclampsia: long-term consequences for vascular health. Vasc Health Risk Manag 11: 403–415, 2015. doi: 10.2147/VHRM.S64798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnlöv J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, Benjamin EJ, D’Agostino RB, Vasan RS. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 112: 969–975, 2005. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 6.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause 13: 265–279, 2006. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 7.Bar J, Kaplan B, Wittenberg C, Erman A, Boner G, Ben-Rafael Z, Hod M. Microalbuminuria after pregnancy complicated by pre-eclampsia. Nephrol Dial Transplant 14: 1129–1132, 1999. doi: 10.1093/ndt/14.5.1129. [DOI] [PubMed] [Google Scholar]
- 8.Beratis NG, Panagoulias D, Varvarigou A. Increased blood pressure in neonates and infants whose mothers smoked during pregnancy. J Pediatr 128: 806–812, 1996. doi: 10.1016/S0022-3476(96)70333-5. [DOI] [PubMed] [Google Scholar]
- 9.Berks D, Steegers EA, Molas M, Visser W. Resolution of hypertension and proteinuria after preeclampsia. Obstet Gynecol 114: 1307–1314, 2009. doi: 10.1097/AOG.0b013e3181c14e3e. [DOI] [PubMed] [Google Scholar]
- 10.Blair JD, Yuen RK, Lim BK, McFadden DE, von Dadelszen P, Robinson WP. Widespread DNA hypomethylation at gene enhancer regions in placentas associated with early-onset pre-eclampsia. Mol Hum Reprod 19: 697–708, 2013. doi: 10.1093/molehr/gat044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blake KV, Gurrin LC, Evans SF, Beilin LJ, Landau LI, Stanley FJ, Newnham JP. Maternal cigarette smoking during pregnancy, low birth weight and subsequent blood pressure in early childhood. Early Hum Dev 57: 137–147, 2000. doi: 10.1016/S0378-3782(99)00064-X. [DOI] [PubMed] [Google Scholar]
- 12.Bogdarina I, Welham S, King PJ, Burns SP, Clark AJ. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res 100: 520–526, 2007. doi: 10.1161/01.RES.0000258855.60637.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown DW, Dueker N, Jamieson DJ, Cole JW, Wozniak MA, Stern BJ, Giles WH, Kittner SJ. Preeclampsia and the risk of ischemic stroke among young women: results from the Stroke Prevention in Young Women Study. Stroke 37: 1055–1059, 2006. doi: 10.1161/01.STR.0000206284.96739.ee. [DOI] [PubMed] [Google Scholar]
- 14.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol 28: 1–19, 2013. doi: 10.1007/s10654-013-9762-6. [DOI] [PubMed] [Google Scholar]
- 15.Bushnell C, Chireau M. Preeclampsia and stroke: risks during and after pregnancy. Stroke Res Treat 2011: 858134, 2011. doi: 10.4061/2011/858134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. JAMA 285: 1607–1612, 2001. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- 17.Codsi E, Garovic VD, Gonzalez-Suarez ML, Milic N, Borowski KS, Rose CH, Davies NP, Kashani KB, Lieske JC, White WM. Longitudinal characterization of renal proximal tubular markers in normotensive and preeclamptic pregnancies. Am J Physiol Regul Integr Comp Physiol 312: R773–R778, 2017. doi: 10.1152/ajpregu.00509.2016. [DOI] [PubMed] [Google Scholar]
- 18.Cornelis T, Odutayo A, Keunen J, Hladunewich M. The kidney in normal pregnancy and preeclampsia. Semin Nephrol 31: 4–14, 2011. doi: 10.1016/j.semnephrol.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Craici IM, Wagner SJ, Bailey KR, Fitz-Gibbon PD, Wood-Wentz CM, Turner ST, Hayman SR, White WM, Brost BC, Rose CH, Grande JP, Garovic VD. Podocyturia predates proteinuria and clinical features of preeclampsia: longitudinal prospective study. Hypertension 61: 1289–1296, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durham JH, Desnick RJ, Imbriano L, Wasserstein M, D’Agati VD, Markowitz GS. Prolonged postpartum proteinuria after early preeclampsia. Am J Kidney Dis 43: 186–191, 2004. doi: 10.1053/j.ajkd.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 21.Figueiró-Filho EA, Mak LE, Reynolds JN, Stroman PW, Smith GN, Forkert ND, Paolozza A, Rätsep MT, Croy BA. Neurological function in children born to preeclamptic and hypertensive mothers: a systematic review. Pregnancy Hypertens 10: 1–6, 2017. doi: 10.1016/j.preghy.2017.07.144. [DOI] [PubMed] [Google Scholar]
- 22.Garovic VD, Wagner SJ, Turner ST, Rosenthal DW, Watson WJ, Brost BC, Rose CH, Gavrilova L, Craigo P, Bailey KR, Achenbach J, Schiffer M, Grande JP. Urinary podocyte excretion as a marker for preeclampsia. Am J Obstet Gynecol 196: 320.e1–320.e7, 2007. doi: 10.1016/j.ajog.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Hamano T. Women with a history of preeclampsia should be monitored for the onset and progression of chronic kidney disease. Nat Clin Pract Nephrol 5: 8–9, 2009. doi: 10.1038/ncpneph0991. [DOI] [PubMed] [Google Scholar]
- 24.Han L, Yang Z, Li K, Zou J, Li H, Han J, Zhou L, Liu X, Zhang X, Zheng Y, Yu L, Li L. Antepartum or immediate postpartum renal biopsies in preeclampsia/eclampsia of pregnancy: new morphologic and clinical findings. Int J Clin Exp Pathol 7: 5129–5143, 2014. [PMC free article] [PubMed] [Google Scholar]
- 25.Hannaford P, Ferry S, Hirsch S. Cardiovascular sequelae of toxemia of pregnancy. Heart 77: 154–158, 1997. doi: 10.1136/hrt.77.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heida KY, Franx A, van Rijn BB, Eijkemans MJ, Boer JM, Verschuren MW, Oudijk MA, Bots ML, van der Schouw YT. Earlier age of onset of chronic hypertension and type 2 diabetes mellitus after a hypertensive disorder of pregnancy or gestational diabetes mellitus. Hypertension 66: 1116–1122, 2015. doi: 10.1161/HYPERTENSIONAHA.115.06005. [DOI] [PubMed] [Google Scholar]
- 27.Hsu CN, Lee CT, Huang LT, Tain YL. Aliskiren in early postnatal life prevents hypertension and reduces asymmetric dimethylarginine in offspring exposed to maternal caloric restriction. J Renin Angiotensin Aldosterone Syst 16: 506–513, 2015. doi: 10.1177/1470320313514123. [DOI] [PubMed] [Google Scholar]
- 28.Jakoubek V, Bíbová J, Herget J, Hampl V. Chronic hypoxia increases fetoplacental vascular resistance and vasoconstrictor reactivity in the rat. Am J Physiol Heart Circ Physiol 294: H1638–H1644, 2008. doi: 10.1152/ajpheart.01120.2007. [DOI] [PubMed] [Google Scholar]
- 29.James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol 106: 509–516, 2005. doi: 10.1097/01.AOG.0000172428.78411.b0. [DOI] [PubMed] [Google Scholar]
- 30.Jónsdóttir LS, Arngrímsson R, Geirsson RT, Sigvaldason H, Sigfússon N. Death rates from ischemic heart disease in women with a history of hypertension in pregnancy. Acta Obstet Gynecol Scand 74: 772–776, 1995. doi: 10.3109/00016349509021195. [DOI] [PubMed] [Google Scholar]
- 31.Kaze FF, Njukeng FA, Kengne AP, Ashuntantang G, Mbu R, Halle MP, Asonganyi T. Post-partum trend in blood pressure levels, renal function and proteinuria in women with severe preeclampsia and eclampsia in sub-Saharan Africa: a 6-months cohort study. BMC Pregnancy Childbirth 14: 134, 2014. doi: 10.1186/1471-2393-14-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kincaid-Smith P. The renal lesion of preeclampsia revisited. Am J Kidney Dis 17: 144–148, 1991. doi: 10.1016/S0272-6386(12)81119-X. [DOI] [PubMed] [Google Scholar]
- 33.Kittner SJ, Stern BJ, Feeser BR, Hebel R, Nagey DA, Buchholz DW, Earley CJ, Johnson CJ, Macko RF, Sloan MA, Wityk RJ, Wozniak MA. Pregnancy and the risk of stroke. N Engl J Med 335: 768–774, 1996. doi: 10.1056/NEJM199609123351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lafayette RA, Druzin M, Sibley R, Derby G, Malik T, Huie P, Polhemus C, Deen WM, Myers BD. Nature of glomerular dysfunction in pre-eclampsia. Kidney Int 54: 1240–1249, 1998. doi: 10.1046/j.1523-1755.1998.00097.x. [DOI] [PubMed] [Google Scholar]
- 35.LaMarca B, Amaral LM, Harmon AC, Cornelius DC, Faulkner JL, Cunningham MW Jr. Placental ischemia and resultant phenotype in animal models of preeclampsia. Curr Hypertens Rep 18: 38, 2016. doi: 10.1007/s11906-016-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaMarca B, Cornelius DC, Harmon AC, Amaral LM, Cunningham MW, Faulkner JL, Wallace K. Identifying immune mechanisms mediating the hypertension during preeclampsia. Am J Physiol Regul Integr Comp Physiol 311: R1–R9, 2016. doi: 10.1152/ajpregu.00052.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanska DJ, Kryscio RJ. Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke 31: 1274–1282, 2000. doi: 10.1161/01.STR.31.6.1274. [DOI] [PubMed] [Google Scholar]
- 38.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol 209: 544.e1–544.e12, 2013. doi: 10.1016/j.ajog.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Mann JI, Doll R, Thorogood M, Vessey MP, Waters WE. Risk factors for myocardial infarction in young women. Br J Prev Soc Med 30: 94–100, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez-Aguayo A, Aglony M, Bancalari R, Avalos C, Bolte L, Garcia H, Loureiro C, Carvajal C, Campino C, Inostroza A, Fardella C. Birth weight is inversely associated with blood pressure and serum aldosterone and cortisol levels in children. Clin Endocrinol (Oxf) 76: 713–718, 2012. doi: 10.1111/j.1365-2265.2011.04308.x. [DOI] [PubMed] [Google Scholar]
- 41.McDonald SD, Han Z, Walsh MW, Gerstein HC, Devereaux PJ. Kidney disease after preeclampsia: a systematic review and meta-analysis. Am J Kidney Dis 55: 1026–1039, 2010. doi: 10.1053/j.ajkd.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 42.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Piña IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC Jr, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. Circulation 123: 1243–1262, 2011. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nisell H, Lintu H, Lunell NO, Möllerström G, Pettersson E. Blood pressure and renal function seven years after pregnancy complicated by hypertension. Br J Obstet Gynaecol 102: 876–881, 1995. doi: 10.1111/j.1471-0528.1995.tb10874.x. [DOI] [PubMed] [Google Scholar]
- 44.Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension 50: 679–685, 2007. doi: 10.1161/HYPERTENSIONAHA.107.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ojeda NB, Intapad S, Royals TP, Black JT, Dasinger JH, Tull FL, Alexander BT. Hypersensitivity to acute ANG II in female growth-restricted offspring is exacerbated by ovariectomy. Am J Physiol Regul Integr Comp Physiol 301: R1199–R1205, 2011. doi: 10.1152/ajpregu.00219.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ojeda NB, Royals TP, Black JT, Dasinger JH, Johnson JM, Alexander BT. Enhanced sensitivity to acute angiotensin II is testosterone dependent in adult male growth-restricted offspring. Am J Physiol Regul Integr Comp Physiol 298: R1421–R1427, 2010. doi: 10.1152/ajpregu.00096.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paauw ND, Luijken K, Franx A, Verhaar MC, Lely AT. Long-term renal and cardiovascular risk after preeclampsia: towards screening and prevention. Clin Sci (Lond) 130: 239–246, 2016. doi: 10.1042/CS20150567. [DOI] [PubMed] [Google Scholar]
- 48.Paauw ND, van Rijn BB, Lely AT, Joles JA. Pregnancy as a critical window for blood pressure regulation in mother and child: programming and reprogramming. Acta Physiol (Oxf) 219: 241–259, 2017. doi: 10.1111/apha.12702. [DOI] [PubMed] [Google Scholar]
- 49.Piper BJ, Corbett SM. Executive function profile in the offspring of women that smoked during pregnancy. Nicotine Tob Res 14: 191–199, 2012. doi: 10.1093/ntr/ntr181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richmond RC, Simpkin AJ, Woodward G, Gaunt TR, Lyttleton O, McArdle WL, Ring SM, Smith AD, Timpson NJ, Tilling K, Davey Smith G, Relton CL. Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Hum Mol Genet 24: 2201–2217, 2015. doi: 10.1093/hmg/ddu739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ros HS, Lichtenstein P, Bellocco R, Petersson G, Cnattingius S. Pulmonary embolism and stroke in relation to pregnancy: how can high-risk women be identified? Am J Obstet Gynecol 186: 198–203, 2002. doi: 10.1067/mob.2002.119177. [DOI] [PubMed] [Google Scholar]
- 52.Shah DA, Khalil RA. Bioactive factors in uteroplacental and systemic circulation link placental ischemia to generalized vascular dysfunction in hypertensive pregnancy and preeclampsia. Biochem Pharmacol 95: 211–226, 2015. doi: 10.1016/j.bcp.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther 285: 931–945, 1998. [PubMed] [Google Scholar]
- 54.Spaan JJ, Ekhart T, Spaanderman ME, Peeters LL. Remote hemodynamics and renal function in formerly preeclamptic women. Obstet Gynecol 113: 853–859, 2009. doi: 10.1097/AOG.0b013e31819caf0f. [DOI] [PubMed] [Google Scholar]
- 55.Spaanderman ME, Van Beek E, Ekhart TH, Van Eyck J, Cheriex EC, De Leeuw PW, Peeters LL. Changes in hemodynamic parameters and volume homeostasis with the menstrual cycle among women with a history of preeclampsia. Am J Obstet Gynecol 182: 1127–1134, 2000. doi: 10.1067/mob.2000.105342. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki H, Watanabe Y, Arima H, Kobayashi K, Ohno Y, Kanno Y. Short- and long-term prognosis of blood pressure and kidney disease in women with a past history of preeclampsia. Clin Exp Nephrol 12: 102–109, 2008. doi: 10.1007/s10157-007-0018-1. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki S, Gejyo F, Ogino S, Maruyama Y, Ueno M, Nishi S, Kimura H, Arakawa M. Postpartum renal lesions in women with pre-eclampsia. Nephrol Dial Transplant 12: 2488–2493, 1997. doi: 10.1093/ndt/12.12.2488. [DOI] [PubMed] [Google Scholar]
- 58.Tang CH, Wu CS, Lee TH, Hung ST, Yang CY, Lee CH, Chu PH. Preeclampsia-eclampsia and the risk of stroke among peripartum in Taiwan. Stroke 40: 1162–1168, 2009. doi: 10.1161/STROKEAHA.108.540880. [DOI] [PubMed] [Google Scholar]
- 59.Tate J, Bushnell C. Pregnancy and stroke risk in women. Womens Health (Lond) 7: 363–374, 2011. doi: 10.2217/WHE.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Unverdi S, Ceri M, Unverdi H, Yilmaz R, Akcay A, Duranay M. Postpartum persistent proteinuria after preeclampsia: a single-center experience. Wien Klin Wochenschr 125: 91–95, 2013. doi: 10.1007/s00508-013-0320-8. [DOI] [PubMed] [Google Scholar]
- 61.van Beek E, Ekhart TH, Schiffers PM, van Eyck J, Peeters LL, de Leeuw PW. Persistent abnormalities in plasma volume and renal hemodynamics in patients with a history of preeclampsia. Am J Obstet Gynecol 179: 690–696, 1998. doi: 10.1016/S0002-9378(98)70066-3. [DOI] [PubMed] [Google Scholar]
- 62.Vikse BE, Irgens LM, Bostad L, Iversen BM. Adverse perinatal outcome and later kidney biopsy in the mother. J Am Soc Nephrol 17: 837–845, 2006. doi: 10.1681/ASN.2005050492. [DOI] [PubMed] [Google Scholar]
- 63.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med 359: 800–809, 2008. doi: 10.1056/NEJMoa0706790. [DOI] [PubMed] [Google Scholar]
- 64.Washburn LK, Brosnihan KB, Chappell MC, Diz DI, Gwathmey TM, Nixon PA, Russell GB, Snively BM, O’Shea TM. The renin-angiotensin-aldosterone system in adolescent offspring born prematurely to mothers with preeclampsia. J Renin Angiotensin Aldosterone Syst 16: 529–538, 2015. doi: 10.1177/1470320314526940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White WM, Garrett AT, Craici IM, Wagner SJ, Fitz-Gibbon PD, Butters KA, Brost BC, Rose CH, Grande JP, Garovic VD. Persistent urinary podocyte loss following preeclampsia may reflect subclinical renal injury. PLoS One 9: e92693, 2014. doi: 10.1371/journal.pone.0092693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilcox AJ. On the importance–and the unimportance–of birthweight. Int J Epidemiol 30: 1233–1241, 2001. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- 67.Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, Smith WC. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ 326: 845, 2003. doi: 10.1136/bmj.326.7394.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu L, Shi A, Zhu D, Bo L, Zhong Y, Wang J, Xu Z, Mao C. High sucrose intake during gestation increases angiotensin II type 1 receptor-mediated vascular contractility associated with epigenetic alterations in aged offspring rats. Peptides 86: 133–144, 2016. doi: 10.1016/j.peptides.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 69.Xiao D, Xu Z, Huang X, Longo LD, Yang S, Zhang L. Prenatal gender-related nicotine exposure increases blood pressure response to angiotensin II in adult offspring. Hypertension 51: 1239–1247, 2008. doi: 10.1161/HYPERTENSIONAHA.107.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiong X, Demianczuk NN, Saunders LD, Wang FL, Fraser WD. Impact of preeclampsia and gestational hypertension on birth weight by gestational age. Am J Epidemiol 155: 203–209, 2002. doi: 10.1093/aje/155.3.203. [DOI] [PubMed] [Google Scholar]