Abstract
Several studies indicate an important role of gustation in intake and preference for dietary fat. The present study compared fat preference deficits produced by deletion of CD36, a putative fatty acid taste receptor, and CALHM1, an ion channel responsible for release of the ATP neurotransmitter used by taste cells. Naïve CD36 knockout (KO) mice displayed reduced preferences for soybean oil emulsions (Intralipid) at low concentrations (0.1–1%) compared with wild-type (WT) mice in 24 h/day two-bottle tests. CALHM1 KO mice displayed even greater Intralipid preference deficits compared with WT and CD36 KO mice. These findings indicate that there may be another taste receptor besides CD36 that contributes to fat detection and preference. After experience with concentrated fat (2.5–5%), CD36 KO and CALHM1 KO mice displayed normal preferences for 0.1–5% fat, although they still consumed less fat than WT mice. The experience-induced rescue of fat preferences in KO mice can be attributed to postoral fat conditioning. Short-term (3-min) two-bottle tests further documented the fat preference deficits in CALHM1 KO mice but also revealed residual preferences for concentrated fat (5–10%), which may be mediated by odor and/or texture cues.
Keywords: experience, Intralipid, postoral conditioning, soybean oil, two-bottle tests
INTRODUCTION
There is considerable interest in the role of gustation in the preference for and intake of high-fat foods. It had long been assumed that preference for fat is primarily due to textural and olfactory properties of the nutrient, but there is growing evidence that fat taste receptors contribute to this preference (1, 4). In a seminal paper, Laugerette et al. (12) confirmed an early report (7) that the CD36 fatty acid transporter is expressed in rodent taste cells and may serve as a fatty acid receptor. In particular, they reported that CD36 knockout (KO) mice missing this putative receptor, in contrast to wild-type (WT) mice, failed to prefer fatty acid (2% linoleic acid in gum suspension). We confirmed this finding and also observed that CD36 KO mice had reduced preferences for soybean oil emulsions, although, as discussed below, the findings were inconsistent (27). Additional evidence that the gustatory system has an important role in fat detection and preference comes from reports that deletion of downstream taste signaling proteins, including the transient receptor potential melastatin 5 (TRPM5) sodium channel, the CALHM1 ion channel, and the P2X2/P2X3 ATP receptor, reduce fat or fatty acid preferences (13, 25, 33, 35).
As with other taste qualities (sweet, umami, bitter) (20), there may be multiple taste receptors for fat. Cartoni et al. (5) in fact proposed that two additional fatty acid receptors, G protein-coupled receptor 40 and 120 (GPR40 and GPR120), function as taste receptors and reported fatty acid preference deficits in GPR40 KO and GPR120 KO mice. However, we found that GPR40 KO, GPR120 KO and GPR40/GPR120 double knockout (DoKO) mice did not differ from WT mice in their preference for soybean oil (Intralipid) over a wide range of concentrations (0.001–20%) (32). Ancel et al. (3) also reported normal preferences for soybean oil (0.25 or 2.5%) and rapeseed oil (0.2 or 2%) in GPR120 KO mice. In addition, they observed that GPR120 KO and WT mice did not differ in their responses to linoleic acid in short-term lick tests, long-term preference tests, or conditioned taste aversion tests. These and other findings (9) indicated that lingual GPR40 and GPR120 are not essential for the preference for fats, although they may mediate other behavioral or physiological responses to fats. The importance of GPR40 in fat taste is further questioned by inconsistent reports of its localization in rodent and human taste cells (4). In contrast to these findings, intestinal GPR40 and GPR120 receptors are clearly implicated in the postoral conditioning actions of fat (31, 32).
In the present study, we reevaluated the primary role of CD36 as a taste receptor mediating fat preferences. In our earlier study, CD36 KO mice displayed reduced preferences for soybean oil emulsions in one test, but normal preferences in two other tests (32). The normal preferences were obtained in CD36 KO mice that had prior experience with fatty acids or soybean oil emulsions. This may be a critical factor, because in other studies we found that prior experience with nutritive fats enhanced soybean oil preferences in different inbred mouse strains (C57BL/6, 129) and KO strains (TRPM5, P2X2/P2X3) (24, 25, 33). In experiment 1 of the present study, therefore, we compared 24-h Intralipid versus water preferences of experimentally naïve and fat-experienced CD36 KO and WT mice. In experiment 2, we measured Intralipid preferences in mice missing a critical downstream taste signaling element, CALHM1, that mediates release of the ATP neurotransmitter by taste cells (36). In a prior study, we observed profound Intralipid preference deficits in P2X2/P2X3 DoKO mice missing the ATP receptor on gustatory nerves (25), which predicted that CALHM1 KO mice would also display profound deficits in fat preference. Three additional experiments were conducted to further characterize the fat preference deficits of CALHM1 KO mice. These experiments used short-term (3-min) two-bottle tests to minimize postoral influences on fat preference and also presented fat and nonfat choices as gum suspensions to minimize texture effects.
METHODS
Animals.
Naïve CD36 KO mice on a C57BL/6J background were offspring bred in the laboratory from mice obtained from Nada Abumrad (Washington University, St. Louis, MO); the WT mice were C57BL/6J mice obtained from the Jackson Laboratory (Bar Harbor, ME). Because the CD36 KO mice were interbred for multiple generations since being derived from heterozygotes, their background may not be identical to the WT stock, which is a potential weakness. The mice were 11 wk old; the CD36 KO mice weighed less than WT mice at the start of the experiment (19.1 vs. 22.2, P < 0.05). Naïve CALHM1 KO mice and CALHM1 WT littermates on a C57BL/6J background were obtained from Philippe Marambaud (Feinstein Institute for Medical Research, Manhasset, NY) (14, 37). The mice used in this study were first or second generation offspring obtained by separately interbreeding the KO and WT stock. The CALHM1 KO mice in experiment 2 weighed less than the WT mice (21.0 vs. 24.4 g, P < 0.05), but this was not the case in experiments 3–5. Approximately equal numbers of males and females of each genotype were used in the individual experiments. Samples sizes are provided in the figure legends.
The mice were singly housed in plastic tub cages kept in a room maintained at 22°C with a 12:12-h light-dark cycle and had ad libitum access to chow (LabDiet 5001; PMI Nutrition International, Brentwood, MO). Experimental protocols were approved by the Institutional Animal Care and Use Committee at Brooklyn College and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Test fluids and intake measures.
Intralipid emulsions were prepared using 20% Intralipid (Baxter, Deerfield, IL), a stable fat emulsion containing 20% soybean oil, 2.25% glycerol, and 1.2% egg yolk phospholipids. The 20% Intralipid was diluted with deionized water to produce emulsions that contained 0.1, 0.25, 0.5, 0.75, 1, and 5% soybean oil. In experiments 4 and 5, soybean oil emulsions presented as gum suspensions were used. We first prepared stable oil emulsions using food-grade soybean oil (Crisco oil; J. M. Smucker, Orrville, OH) using a sodium stearoyl lactylate emulsifier (Emplex; American Products, Kansas City, MO) and deionized water (27, 34). Soybean oil, at 0.5, 1, 2.5, 5, and 10% concentrations, was mixed with deionized water and 0.15% Emplex with an homogenizer (Ultra-Turrax T25; IKA-Works, Cincinnati, OH) and then further processed in a microfluidizer (HC-5000; Microfluidics, Newton, MA) (27). The oil emulsion was then mixed with 0.3% xanthan gum (Sigma Chemical, St. Louis, MO) using a laboratory blender. A vehicle fluid containing 0.15% Emplex, 0.3% xanthan gum, and water was prepared using the same procedure.
In 24-h home cage tests, the oil emulsion and water or vehicle were presented in two 50-ml plastic tubes with stainless steel sipper spouts. The tubes were placed on the tub cage tops to the right of the feeder and were fixed in place with clips. Fluid intakes were measured to the nearest 0.1 g with an electronic balance interfaced to a laptop computer. Fluid spillage was estimated by recording the change in weights of two drinking tubes placed on an empty cage.
Brief access two-bottle lick tests were conducted in plastic test cages as previously described (41). Sipper spouts were attached to 50-ml glass tubes that were mounted on motorized bottle holders. The bottle holders positioned the spouts 1 mm in front of the cage at the start of a session and retracted them 3 min after the animal had emitted 10 licks. Licking behavior was monitored with electronic lickometers interfaced to a microcomputer.
Experiment 1: 24-h Intralipid preferences in CD36 KO and WT mice.
The mice were adapted to their cages with two water bottles for 1 wk. They were then given a series of two-bottle Intralipid emulsion vs. water tests at the 0.1–5% fat concentrations (test 1). They were next given water only for 4 days followed by a second series of Intralipid emulsion versus water tests at the same concentrations (test 2). In these and all subsequent 24-h tests in this study, each concentration was presented for 2 days in an ascending order and the left-right position of the fluids were alternated daily.
Experiment 2: 24-h Intralipid preferences in CALHM1 KO and WT mice.
The CALHM1 KO and WT mice were given 24-h Intralipid emulsion versus water tests using procedures identical to those of the first experiment.
Experiment 3: short-term Intralipid preferences in CALHM1 KO and WT mice.
Intralipid preferences observed in 24-h tests, particularly at high concentrations, may reflect learned responses based on the postoral actions of the nutrient that condition significant flavor preferences (2, 27). To minimize postoral effects, previous studies have used brief access (e.g., 5-min) two-bottle tests to evaluate fat preference (15). Consistent with this approach, we found that intragastric infusions of Intralipid did not influence licking behavior until 12 min into the session (40). Experiment 3 was conducted with 3-min lick tests with 1–5% Intralipid emulsions to determine if CALHM1 KO mice display fat preferences when postoral effects are minimized. Naïve CALHM1 KO and WT mice were housed as in prior experiments. The animals were familiarized with the lick test cages and bottle retractors by housing them overnight in the cages with ad libitum chow and access to two water bottles for 30 min every hour. The mice were then returned to their home cages. Next, they were water deprived overnight and allowed to drink from two water bottles in the test cages for 5 min. On the following 2 days the mice were given two-bottle access to 1% Intralipid emulsion versus water for 3 min. One hour after the session, a second 3-min two-bottle test was conducted with the left-right positions of the Intralipid and water bottles reversed. On the first two water-restriction days the mice were given 1-h access to water in the home cage after lick training to maintain hydration. On the third day and thereafter, they were given ad libitum access to water but restricted chow rations (1–3 g) so as to maintain them at 90% of their ad libitum body weight. While food restricted, they were tested for 3 days with 1% Intralipid emulsion versus water, followed by 2 days with 2.5% and then 5% Intralipid emulsion versus water.
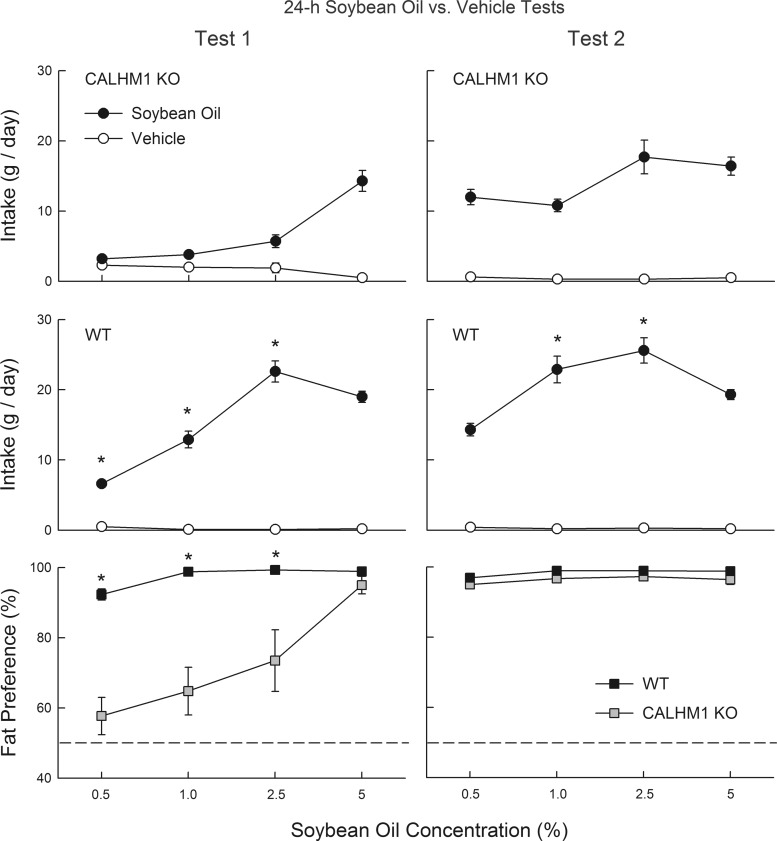
Experiment 4: 24-h soybean oil preferences in CALHM1 KO and WT mice.
The texture of liquid or solid fat is a prominent orosensory feature of this nutrient. To minimize texture effects, many prior studies offered animals the choice of fat suspended in xanthan gum vs. a xanthan gum vehicle (12, 17). Experiments 4 and 5 were conducted with two-bottle tests with soybean oil in gum versus gum only vehicle to determine if CALHM1 KO mice display fat preferences when texture effects are minimized. Because Intralipid contains added glycerol and phospholipids, which may influence its flavor, the fat emulsions were prepared using food-grade soybean oil and Emplex emulsifier; the emulsifier was included in the xanthan gum vehicle as well. In this experiment, the CALHM1 KO and WT mice were given 24-h tests as in experiment 2 except with gum vehicle and soybean oil emulsions at concentrations of 0.5, 1, 2.5, and 5% oil.
Experiment 5: short-term soybean oil preferences in CALHM1 KO and WT mice.
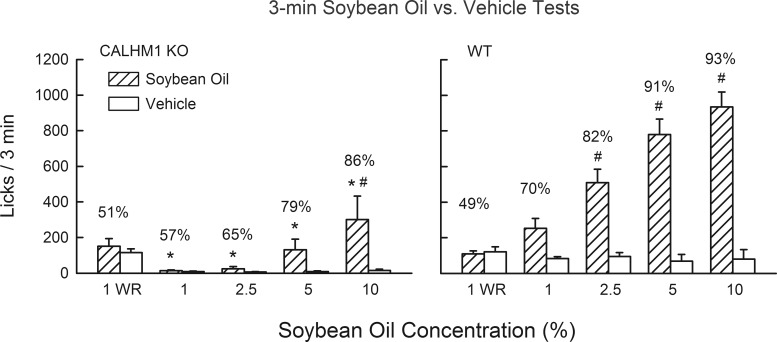
To minimize postoral influences on the preference for soybean oil/gum emulsions, 3-min two-bottle tests were conducted with vehicle and soybean oil emulsion at concentrations of 1, 2.5, 5, and 10% using the procedures of experiment 3.
Data analysis.
Fluid intakes in the 24-h tests were averaged over the 2 days at each concentration. Fat preferences were expressed as percent intakes (e.g., Intralipid intake/total intake × 100). Group differences in fat intakes and preferences were evaluated using separate mixed model ANOVAs with group (genotype) and concentration as between-group and within-group factors, respectively. Significant interaction effects were evaluated using simple mean effects tests. Within group differences in Intralipid and water intakes were evaluated using ANOVAs with fluid and concentration as within-group factors.
In the 3-min tests, licks were averaged over the last 2 days at each concentration, i.e., over four sessions since there were two 3-min sessions each day. Group differences in licks and fat preferences were evaluated as described above.
Preliminary analyses revealed no sex differences in intakes, licks, or fat preferences in experiments 1, 2, 3, and 5, and therefore, data for male and female mice were combined. In experiment 4, male mice in both KO and WT groups consumed more soybean oil emulsion than female mice, but there were no group × sex interactions and no sex differences in soybean oil preferences. Therefore, the data for male and female mice were combined in this experiment as well.
RESULTS
Experiment 1: 24-h Intralipid preferences in CD36 KO and WT mice.
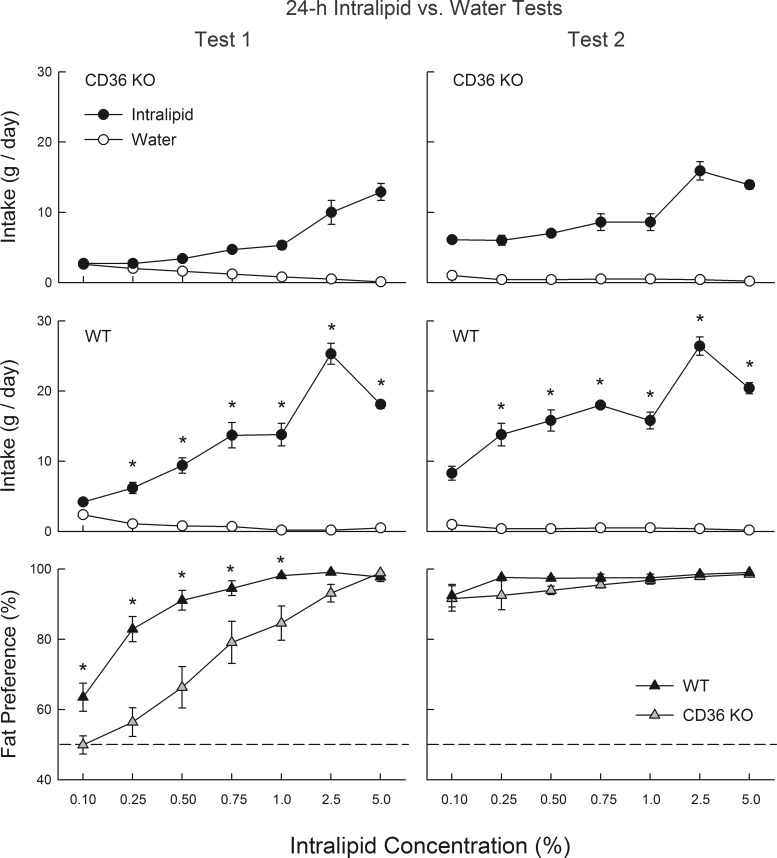
In test 1, CD36 KO mice consumed less Intralipid emulsion than WT mice at all but the lowest concentration [group × concentration interaction, F(6,114) = 20.9, P < 0.001; Fig. 1]. Overall, Intralipid emulsion intake increased and then decreased with concentration [F(6,114) = 139.8, P < 0.001], but when expressed as energy, fat intake simply increased with concentration (data not presented). With respect to percent fat intake, CD36 KO mice displayed lower Intralipid preferences than did WT mice at 0.1 to 1% concentrations [group × concentration interaction, F(6,114) = 9.3, P < 0.001]. Within group analyses indicated that WT mice consumed more (P < 0.05) Intralipid emulsion than water at 0.25% and higher concentrations, whereas CD36 KO mice consumed significantly more (P < 0.05) at 0.75% and higher concentrations.
Fig. 1.
Experiment 1: intake (means ± SE) of Intralipid emulsions and water for CD36 knockout (KO) mice (n = 11; top), wild-type (WT) mice (n = 10; middle), and percent fat preference for both groups (bottom) during 24-h 2-bottle Intralipid emulsions versus water choice tests. In test 1 (left), naïve mice were tested with 0.1–5% Intralipid and water. In test 2 (right), the same mice were retested with Intralipid emulsions and water. *Significantly (P < 0.05) higher values in the WT than CD36 KO group.
In test 2, CD36 KO and WT mice did not differ in their percent Intralipid intakes and both groups displayed strong fat preferences (>90%) at all concentrations. Within group comparisons indicated that CD36 KO mice increased their percent Intralipid intakes from tests 1 to 2 at concentrations 0.1–1% [F(6,60) = 20.7, P < 0.001] and WT mice increased their percent intakes at 0.1 and 0.25% concentrations from the first to second test [F(6,54) = 22.1, P < 0.001]. Despite their strong fat preference, CD36 KO mice again consumed less Intralipid emulsion than WT mice at all concentrations except 0.1% [group × concentration interaction; F(6,114) = 9.3, P < 0.001; Fig. 1]. The reduced fat emulsion intakes of CD36 KO mice in tests 1 and 2 were not secondary to their lower body weights because KO and WT intake differences persisted when emulsion intakes were expressed on a body weight basis (intake/20 g body wt; data not presented).
Experiment 2: 24-h Intralipid preferences in CALHM1 KO and WT mice.
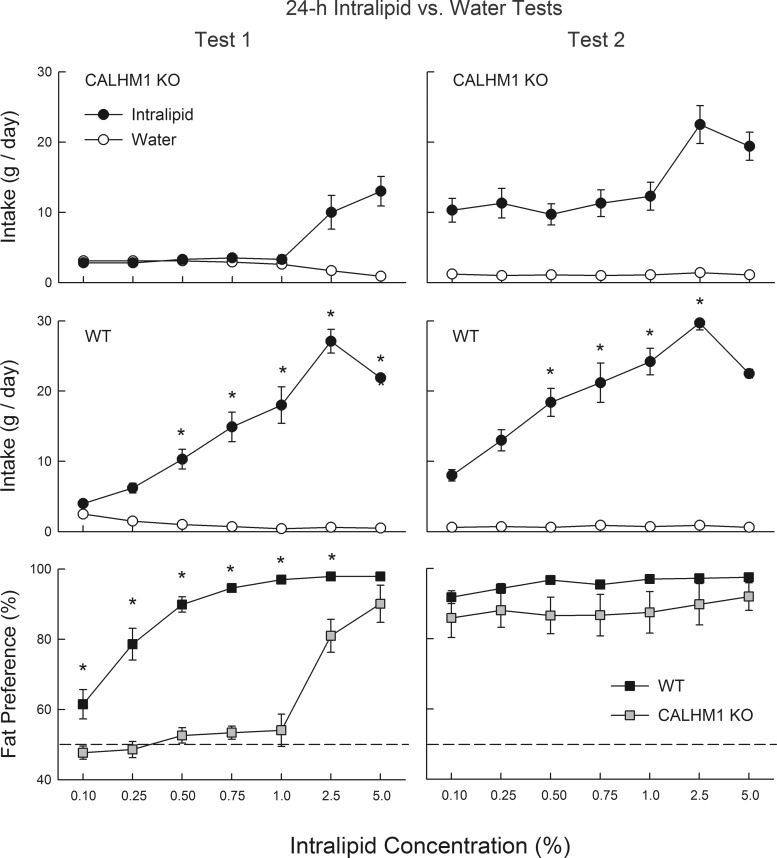
In the first test series, CALHM1 KO mice consumed less Intralipid emulsion than WT mice at all concentrations except 0.1% and 0.25% [group × concentration, F(6,102) = 13.8, P < 0.0001; Fig. 2]. Overall, Intralipid emulsion intake increased and then decreased with concentration [F(6,102) = 58.0, P < 0.001] but fat energy intake simply increased with concentration (data not presented). The reduced fat emulsion intakes of CALHM1 KO mice were not secondary to their lower body weights because the KO and WT intake differences persisted when intakes were expressed on a body weight basis (data not presented). The percent fat preferences of CALHM1 KO mice were lower than those of WT mice at all concentrations except for the highest [5%; group × concentration interaction, F(6,102) = 13.1, P < 0.001; Fig. 2]. Furthermore, whereas WT mice consumed more (P < 0.05) Intralipid emulsion than water at 0.5% and higher concentrations, CALHM1 KO mice consumed significantly more (P < 0.05) Intralipid emulsion only at 2.5% and 5% concentrations.
Fig. 2.
Experiment 2: intake (means ± SE) of Intralipid emulsions and water for the CALHM1 knockout (KO) mice (n = 9; top), WT mice (n = 10; middle), and percent fat preference for both groups (bottom) during 24-h 2-bottle Intralipid emulsion versus water choice tests. In test 1 (left), naïve mice were tested with 0.1–5% Intralipid emulsions and water. In test 2 (right), the same mice were retested with Intralipid emulsions and water. *Significantly (P < 0.05) higher values in the WT than CALHM1 KO group.
In the second test series, CALHM1 KO mice consumed less Intralipid emulsion than WT mice at the intermediate concentrations of 0.5–2.5% [group × concentration interaction, F(6,102) = 11.1, P < 0.001; Fig. 2]. In contrast, the two groups did not significantly differ in their fat preferences, although the percent Intralipid intakes of KO mice were slightly below those of WT mice (Fig. 2). Within group comparisons indicated that KO mice increased their percent intakes from test 1 to 2 at all concentrations except 5% [test × concentration interaction, F(6,48) = 4.4, P < 0.01]. The WT mice increased their percent fat intakes from test 1 to 2 at the 0.1–0.5% concentrations [test × concentration interaction, F(6,54) = 26.1, P < 0.001].
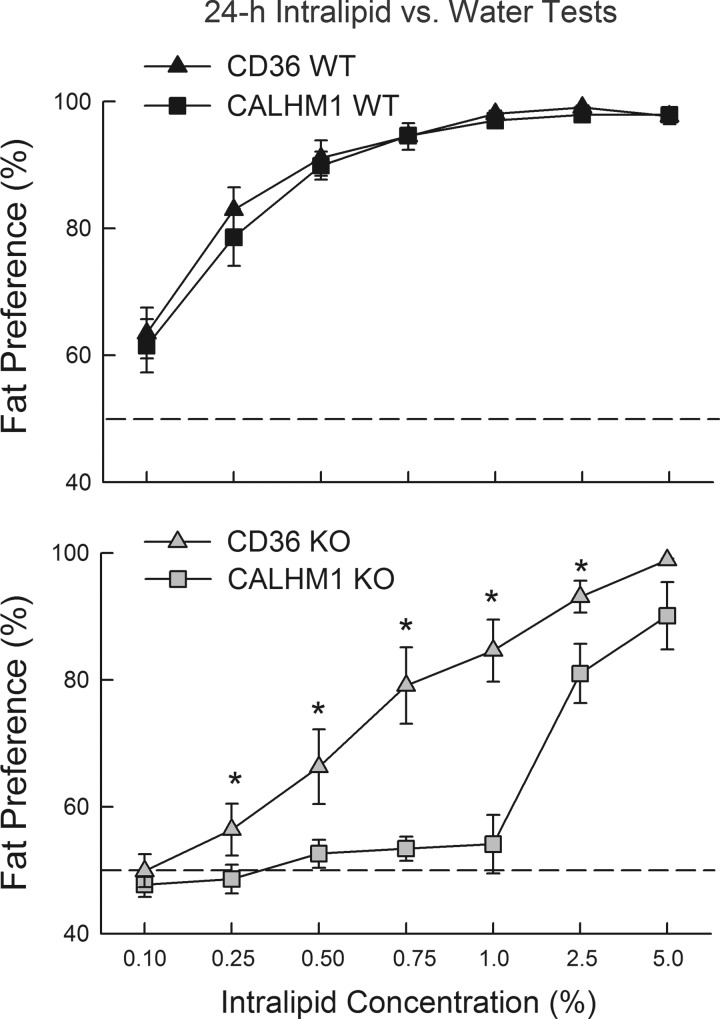
The CALHM1 KO mice displayed a much greater fat preference deficit than did the CD36 KO mice of the first experiment. As shown in Fig. 3, the Intralipid preferences of WT mice from the two experiments were nearly identical, whereas the preferences of CALHM1 KO were significantly lower than those of CD36 KO mice at 0.25 to 2.5% concentrations [KO strain × concentration interaction, F(6,108) = 5.8, P < 0.001].
Fig. 3.
Percent preferences (means ± SE) for Intralipid emulsions over water in test 1 for CD36 wild-type (WT) and CALHM1 WT mice (top) and CD36 knockout (KO) and CALHM1 KO mice (bottom) from experiments 1 and 2. *Significantly (P < 0.05) higher values in the CD36 KO than CALHM1 KO mice.
Experiment 3: short-term Intralipid preferences in CALHM1 KO and WT mice.
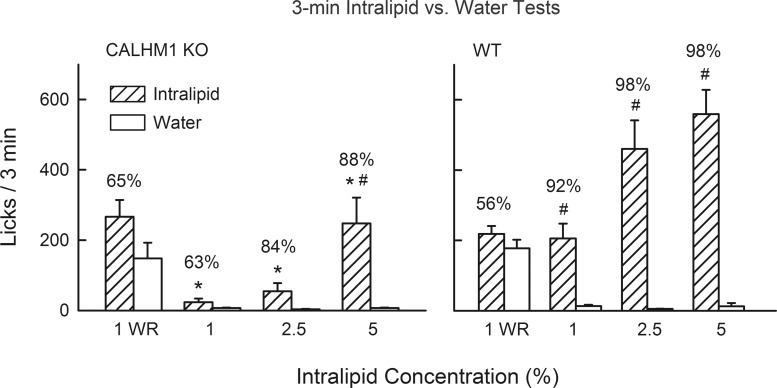
When water restricted, KO and WT mice did not differ in their 3-min licks for 1% Intralipid, water, or percent Intralipid licks; neither group licked significantly more for Intralipid emulsion than for water (Fig. 4). However, when food restricted, the KO mice licked significantly less for Intralipid emulsion [F(1,17) = 21.3, P < 0.001] and displayed weaker fat preferences [F(1,17) = 10.9, P < 0.01] than did WT mice. Food-restricted WT mice licked more (P < 0.05) for Intralipid emulsion than water at all three concentrations, but for KO mice this difference was significant (P < 0.05) only at the 5% concentration. When compared with WT mice, the KO mice licked very little for 2.5% Intralipid; 8 of the 10 mice emitted <100 licks for fat emulsion. Nevertheless, all KO mice licked more for 2.5% Intralipid than water, which accounts for the 84% Intralipid intake.
Fig. 4.
Experiment 3: licks (means + SE) for Intralipid (1–5%) and water during 3-min 2-bottle tests conducted with CALHM1 KO mice (n = 10, left) and WT mice (n = 9, right). Leftmost bars: 1% Intralipid emulsion and water licks when animals were water restricted (WR). Right bars: 1–5% Intralipid emulsion and water licks when the animals were food restricted. Numbers atop bars represent mean percent preference for Intralipid emulsion. *Significantly (P < 0.05) lower licks in the CALHM1 KO than WT groups. #Significantly (P < 0.05) higher licks for Intralipid emulsion vs. water within groups.
The KO mice had very low lick rates for 1 and 2.5% Intralipid emulsions, indicating that they were only weakly attracted to fat emulsion at these concentrations. The KO mice did lick for and prefer 5% Intralipid, although their response matched that of the WT mice offered more dilute 1% Intralipid. This finding suggests that the significant 24-h preference displayed by KO mice for 5% Intralipid in experiment 2 was not due to the postoral actions of the lipid. However, whether the attraction of KO mice to 5% Intralipid was due to its taste or some other orosensory property, e.g., texture, is not certain. This issue was addressed in experiments 4 and 5, which used xanthan gum to partially mask the texture properties of soybean oil.
Experiment 4: 24-h soybean oil in gum preferences in CALHM1 KO and WT mice.
In test 1, CALHM1 KO mice consumed less soybean oil emulsion than did WT mice at all concentrations [group, F(1,20) = 66.2, P < 0.0001; Fig. 5]. Overall, soybean oil emulsion intake increased and then decreased with concentration in the WT group but simply increased in the KO group [group × concentration interaction, F(3,60) = 33.4, P < 0.001]; fat energy intake increased with concentration in both groups (data not presented). With respect to fat preference, percent soybean oil intakes of CALHM1 KO mice were lower than those of WT mice at all concentrations except 5% [group × concentration, F(3,60) = 11.3, P < 0.001; Fig. 5]. Furthermore, whereas WT mice consumed more (P < 0.05) soybean oil emulsion than vehicle at all concentrations, CALHM1 KO mice consumed significantly (P < 0.05) more soybean oil emulsion only at the 2.5 and 5% concentrations.
Fig. 5.
Experiment 4: intake (means ± SE) of soybean oil emulsions and vehicle for CALHM1 knockout (KO) mice (n = 12; top), wild-type (WT) mice (n = 10, middle), and percent fat preference for both groups (bottom) during 24-h 2-bottle soybean oil emulsion versus vehicle choice tests. The vehicle contained emulsifier and xanthan gum as did the soybean oil emulsions. In test 1 (left), naïve mice were tested with 0.5–5% soybean oil emulsions and vehicle. In test 2 (right), the same mice were retested with soybean oil emulsions and vehicle. *Significantly (P < 0.05) higher values in the WT than CALHM1 KO group.
In test 2, CALHM1 KO mice consumed less soybean oil emulsion than WT mice at the intermediate concentrations of 1% and 2.5% [group × concentration interaction, F(3,60) = 16.1, P < 0.001; Fig. 5]. In contrast, the two groups did not differ in their percent soybean oil preferences and displayed strong preferences (>90%) for all concentrations (Fig. 5). Within group comparisons indicated that KO mice increased their percent intakes from test 1 to 2 at 0.5–2.5% concentrations [test × concentration interaction, F(3,33) = 16.2, P < 0.01]. WT mice increased their percent fat intakes from test 1 to 2 only at the 0.5 concentration [test × concentration interaction, F(3,27) = 8.3, P < 0.001].
The preferences displayed by CALHM1 KO and WT mice for soybean oil/gum emulsion versus vehicle in test 1 were similar to those observed in experiment 2 for Intralipid emulsion versus water. This suggests that the added glycerol and phospholipids in the Intralipid emulsions and the texture differences between Intralipid emulsions versus water did not appreciably contribute to the 24-h fat preferences displayed by the mice.
Experiment 5: short-term soybean oil preferences in CALHM1 KO and WT mice.
When water restricted, the KO and WT mice did not differ in their 3-min licks for 1% soybean oil or vehicle or percent soybean oil licks; neither group licked more for soybean oil emulsion than for vehicle (Fig. 6). However, when food restricted, the KO mice licked significantly less for soybean oil emulsion than did WT mice at all concentrations [F(1,17) = 37.7, P < 0.001]. The soybean oil preferences of KO mice were also lower than those of WT mice at all concentrations [F(1,17) = 4.2, P < 0.05]. Food-restricted WT mice licked more (P < 0.05) for soybean oil emulsion than vehicle at 2.5–10% concentrations. In contrast, KO mice licked significantly more (P < 0.05) for soybean oil emulsion than vehicle only at the 10% concentration.
Fig. 6.
Experiment 5: licks (means + SE) for soybean oil emulsions (1–10%) and vehicle during 3-min, 2-bottle tests conducted with CALHM1 knockout (KO) mice (n = 9; left) and wild-type (WT) mice (n = 10; right). The vehicle contained emulsifier and xanthan gum as did the soybean oil emulsions. Leftmost bars: 1% soybean oil emulsion and vehicle licks when animals were water restricted (WR). Right bars: 1–10% soybean oil emulsion and vehicle licks when the animals were food restricted. Numbers atop bars represent mean percent preference for soybean oil emulsion. *Significantly (P < 0.05) lower licks in the CALHM1 KO than WT groups. #Significantly (P < 0.05) higher licks for soybean oil emulsion vs. vehicle within groups.
Compared with WT mice, the KO mice licked very little for the 1–5% soybean oil emulsions in the 3-min tests. Yet, in the 24-h tests (experiment 4), their intake and preference for 5% soybean oil was nearly identical to that of the WT mice. This suggests that the 24-h intake and preferences for 5% soybean oil were driven in part by the postoral actions of fat. In contrast, the significant preference for 10% soybean oil displayed by KO mice in the 3-min tests indicates that they found the orosensory properties of the concentrated fat emulsion attractive, although not as much as did WT mice.
DISCUSSION
The present results provide new information on the role of gustation in fat preference and acceptance. Prior studies implicated CD36 as a critical fatty acid taste receptor based on failure of CD36 KO mice to prefer a linoleic acid to a vehicle fluid (12, 15, 27). In one study, CD36 KO mice also displayed reduced preferences for soybean oil emulsions, but in other tests in the same study, CD36 KO mice displayed normal preferences (27). The normal fat preferences were observed in KO mice that had prior experience with soybean oil or fatty acids, which could have normalized their subsequent fat preference. Fat-experienced CD36 KO mice also displayed normal preferences for linoleic acid (27). The present study revealed that naïve CD36 KO mice have significant preference deficits for Intralipid emulsions compared with naïve WT controls but that this deficit disappeared when the KO and WT mice were given a second Intralipid test. Mice missing the downstream taste signaling channel CALHM1 also displayed fat preference deficits that disappeared in a second test. The Intralipid preference deficits observed in CALHM1 KO mice were more severe than those seen in CD36 KO mice, which, as discussed below, suggests that CD36 is not the only taste receptor that mediates fat preference.
In experiment 1, the Intralipid preferences of naïve CD36 KO mice in the first test were significantly below those of WT mice at 0.10% to 1% concentrations. The CD36 KO mice did not differ from WT controls, however, in their preference for 2.5 and 5% Intralipid. At these concentrations, Intralipid has postoral flavor conditioning effects, which may account for the preference displayed by the KO mice (2, 27). In particular, we previously reported that CD36 KO mice, like WT mice, learn significant preferences for a flavored solution paired with intragastric infusions of 5% Intralipid, which was diluted to 2.5% in the stomach by the ingested solution (27). In test 2, CD36 KO mice, like WT mice, displayed strong preferences for all Intralipid concentrations, which confirms our previous findings that experience with nutritive oils induces a strong preference for Intralipid in CD36 KO mice (27). The results of tests 1 and 2 demonstrate that CD36 KO mice can distinguish dilute (0.1–0.5%) Intralipid emulsions from water but are not inherently attracted to these concentrations. Conceivably, intake of concentrated Intralipid concentrations in test 1 conditioned a preference for the odor or other orosensory properties of Intralipid that produced the preferences for dilute Intralipid emulsions in test 2. This possibility is suggested by our previous observations that sweet-ageusic T1R3 KO mice are indifferent to dilute sucrose solutions in an initial preference test series but after experience with concentrated sucrose solutions prefer dilute as well as concentrated sugar in a second test series. However, removal of the olfactory bulbs in sugar-experienced T1R3 KO mice blocked their preference for dilute sucrose solutions in a second test, suggesting that odor cues mediated the experience-induced preference for dilute sucrose (43). The role of odor cues in the preference for dilute Intralipid emulsions displayed by CD36 KO mice in test 2 is open to question, however. A recent study reported that CD36 is localized on select olfactory receptor neurons and CD36 KO mice display reduced attraction to fatty acid odor cues (39). Further research is needed to elucidate the role of olfactory CD36 in the preference of naive and experienced mice for fat emulsions.
Consistent with reports of impaired soybean oil preferences in TRPM5 KO and P2X2/P2X3 DoKO mice (25, 33), the present study revealed that CALHM1 KO mice show substantial reductions in fat preference and intake. A recent study also reported that CALHM1 KO mice, unlike WT mice, did not prefer 0.2% linoleic acid or canola oil to a gum vehicle (35). In the initial 24 h/day tests of experiment 2, fat preferences of naïve CALHM1 KO mice were significantly below those of WT mice at 0.1 to 2.5% concentrations. Furthermore, CALHM1 KO mice consumed 60% less 2.5% Intralipid than did WT mice, which resembles the 75% reduction in Intralipid intake displayed by P2X2/P2X3 DoKO mice (25). In test 2, however, CALHM1 KO mice, like CD36 KO mice in experiment 1 and P2X2/P2X3 DoKO mice in our earlier study (25), displayed strong preferences for all Intralipid concentrations, although they consumed less fat than did WT mice. Experiment 4 revealed similar reductions in fat preference and intake in CALHM1 KO mice tested 24 h/day with soybean oil/gum emulsions vs. gum vehicle, although the preference differences disappeared in test 2.
In the 3-min choice tests of experiments 3 and 5, CALHM1 KO mice were indifferent to fat at dilute concentrations, although they preferred soybean oil at the highest concentrations offered (5% or 10%), suggesting that KO mice are inherently attracted to some nontaste orosensory features of concentrated fat. Although experiment 5 used xanthan gum to minimize fat texture cues, gum does not mimic all the texture qualities of oil (e.g., slipperiness) (17) and thus residual texture cues may have contributed to the fat preference of CALHM1 KO mice. Olfactory cues may also have been a factor. A recent report localized CALHM1 in nasal epithelial cells (38), but there is as yet no evidence for its involvement in olfactory receptor neuron function. The similar reductions in fat preference and acceptance displayed by naïve CALHM1 KO and P2X2/P2X3 DoKO mice implicate ATP as the primary neurotransmitter mediating fat taste preference, although other neurotransmitters (e.g., serotonin) may also be involved (8).
Comparisons of the Intralipid preferences observed in experiments 1 and 2 revealed much greater deficits in CALHM1 KO than in CD36 KO mice. The differential preference deficits of the two KO models strongly suggest that CD36 is not the only taste receptor that mediates fat preference. As previously discussed, prior research by Cartoni et al. (5) indicated that GPR40 and GPR120 receptors mediate fatty acid preference, but subsequent studies do not support this proposal (3, 9, 32). Taken together, these results indicate that receptors other than CD36, GPR40, and GPR120 contribute to the preference for fat. It has been assumed that fat is detected by fatty acid receptors activated by fatty acids released in the mouth by the action of lipase enzymes on triglycerides, the primary constituents of dietary fat (10). However, we recently observed that inhibiting oral lipolysis with orlistat did not block the preference of mice for fat emulsions (soybean oil, triolein) versus a nonfat control vehicle (26). This suggests the possibility that triglyceride receptors as well as fatty acid receptors mediate the preference for dietary fat. It is also possible that minor constituents and impurities may contribute to the attractive flavor of fats (17, 19, 22). We also found that mice missing α-gustducin, a G protein that mediates sweet and umami tastes and is colocalized with CD36 in taste cells, display normal preferences for soybean oil (33). Thus the identities of fatty acid or triglyceride receptor(s) other than CD36 and a G protein other than α-gustducin that contribute to fat preference and acceptance remain to be identified.
As previously noted, the fat preference deficits of CD36 KO and CALHM1 KO mice disappeared in the second series of 24-h two-bottle tests, which replicates prior findings obtained with CD36 KO, P2X2/P2X3, and TRPM5 KO mice (25, 27, 33). Similar experience-induced sugar preferences were displayed by sweet taste-deficient T1R3, TRPM5, and CALHM1 KO mice (41–43). We attribute these “recovered” taste preferences to KO mice associating residual fat and sugar orosensory cues with the postoral reinforcing actions of the nutrients. Consistent with this interpretation, normal as well as taste-impaired KO mice readily learn to prefer arbitrarily flavored solutions (e.g., grape) that are paired with intragastric infusions of fat or sugar (27, 28, 30, 40). The ability of rodents to acquire nutrient-conditioned flavor preferences might seem to question whether fat and sugar taste receptors are needed to drive intake of these nutrients. However, for flavor-postoral nutrient conditioning to occur, the animals must first consume the flavor to begin with and this is facilitated if the flavor is inherently attractive to the animal. Preferences can be conditioned in the laboratory to neutral or even unpalatable flavors (e.g., bitter or sour) but only by providing the solutions as the sole source of fluid (23, 29, 30). Furthermore, the intake of these conditionally preferred solutions remains rather low. Rodents will more readily learn to prefer an already attractive flavor (e.g., saccharin-sweetened grape solution) and consume much more of it than an initially neutral or aversive flavor (29, 30). Thus the reduced fat intakes displayed by the KO mice in test 2 of the present study and in prior studies can be attributed to the absence of an inherent attraction to the flavor of the fat emulsions. The adaptive advantage of fat taste receptors is that it motivates animals to consume fat-containing foods and fluids, which, in turn, facilitates postoral nutrient conditioning and stimulation of appetite.
Perspectives and Significance
Dietary fat is highly attractive to rodents. In the present study, peak 24-h intakes of 2.5% soybean oil matched or exceeded the body weight of WT mice. We obtained similar results in C57BL/6 mice in an earlier study, which also revealed that peak Intralipid intakes were comparable to peak intakes of sucrose solutions (30). The present and prior results demonstrate that the strong preferences for and high intakes of fat emulsions displayed by experimentally naïve mice are driven in large part by the gustatory system. CD36 KO mice (experiment 1; Ref. 27), CALHM1 KO mice (experiments 2 and 4; Ref. 35), TRPM5 KO mice (33), and P2X2/P2X3 DoKO mice (25) all displayed significantly reduced fat preferences and intakes compared with WT controls. The same studies demonstrate, however, that these gustatory components are less essential in fat-experienced animals, which learn to use other fat flavor stimuli based on postoral nutritive effects. Humans are also attracted to fat-rich foods, and recent findings indicate that they have a taste for fatty acids (21). However, the role of taste for fat preferences in humans is open to question. In particular, whereas rodents are attracted to pure fatty acids, humans are not (21). A role of taste, and the CD36 receptor in particular, for the human taste and preference for dietary fats (triglycerides) has been suggested, but this requires further investigation (6, 11, 16). The identification of fat taste receptors other than CD36, suggested to exist in rodents by the present and other recent animals studies (26), should help to elucidate the involvement of gustation in fat detection and preference by humans (18).
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-031135.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S. and K.A. conceived and designed research; A.S. analyzed data; A.S. and K.A. interpreted results of experiments; A.S. prepared figures; A.S. and K.A. drafted manuscript; A.S. and K.A. edited and revised manuscript; A.S. and K.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Nada A. Abumrad and Philippe Marambaud for providing the breeding stock of CD36 KO, CALHM1 KO, and CALHM1 WT mice. The technical assistance of Austin S. Vural, Martin Zartarian, and Mohammed Riad, who performed the experiments, is gratefully acknowledged.
REFERENCES
- 1.Ackroff K, Sclafani A. Oral and post-oral determinants of dietary fat appetite. In: Fat Detection: Taste, Texture, and Post Ingestive Effects, edited by Montmayeur JP, le Coutre J. Boca Raton, FL: Taylor & Francis, 2010, p. 295–321. [PubMed] [Google Scholar]
- 2.Ackroff K, Sclafani A. Post-oral fat stimulation of intake and conditioned flavor preference in C57BL/6J mice: a concentration-response study. Physiol Behav 129: 64–72, 2014. doi: 10.1016/j.physbeh.2014.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancel D, Bernard A, Subramaniam S, Hirasawa A, Tsujimoto G, Hashimoto T, Passilly-Degrace P, Khan NA, Besnard P. The oral lipid sensor GPR120 is not indispensable for the orosensory detection of dietary lipids in mice. J Lipid Res 56: 369–378, 2015. doi: 10.1194/jlr.M055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besnard P, Passilly-Degrace P, Khan NA. Taste of fat: a sixth taste modality? Physiol Rev 96: 151–176, 2016. doi: 10.1152/physrev.00002.2015. [DOI] [PubMed] [Google Scholar]
- 5.Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci 30: 8376–8382, 2010. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daoudi H, Plesník J, Sayed A, Šerý O, Rouabah A, Rouabah L, Khan NA. Oral fat sensing and CD36 gene polymorphism in Algerian lean and obese teenagers. Nutrients 7: 9096–9104, 2015. doi: 10.3390/nu7115455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuwatari T, Kawada T, Tsuruta M, Hiraoka T, Iwanaga T, Sugimoto E, Fushiki T. Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett 414: 461–464, 1997. doi: 10.1016/S0014-5793(97)01055-7. [DOI] [PubMed] [Google Scholar]
- 8.Gilbertson TA, Khan NA. Cell signaling mechanisms of oro-gustatory detection of dietary fat: advances and challenges. Prog Lipid Res 53: 82–92, 2014. doi: 10.1016/j.plipres.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Godinot N, Yasumatsu K, Barcos ME, Pineau N, Ledda M, Viton F, Ninomiya Y, le Coutre J, Damak S. Activation of tongue-expressed GPR40 and GPR120 by non caloric agonists is not sufficient to drive preference in mice. Neuroscience 250: 20–30, 2013. doi: 10.1016/j.neuroscience.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 10.Kawai T, Fushiki T. Importance of lipolysis in oral cavity for orosensory detection of fat. Am J Physiol Regul Integr Comp Physiol 285: R447–R454, 2003. doi: 10.1152/ajpregu.00729.2002. [DOI] [PubMed] [Google Scholar]
- 11.Keller KL. Genetic influences on oral fat perception and preference: Presented at the symposium “The Taste for Fat: New Discoveries on the Role of Fat in Sensory Perception, Metabolism, Sensory Pleasure and Beyond” held at the Institute of Food Technologists 2011 Annual Meeting, New Orleans, LA, June 12, 2011. J Food Sci 77: S143–S147, 2012. doi: 10.1111/j.1750-3841.2011.02585.x. [DOI] [PubMed] [Google Scholar]
- 12.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest 115: 3177–3184, 2005. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu P, Shah BP, Croasdell S, Gilbertson TA. Transient receptor potential channel type M5 is essential for fat taste. J Neurosci 31: 8634–8642, 2011. doi: 10.1523/JNEUROSCI.6273-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Z, Siebert AP, Cheung KH, Lee RJ, Johnson B, Cohen AS, Vingtdeux V, Marambaud P, Foskett JK. Calcium homeostasis modulator 1 (CALHM1) is the pore-forming subunit of an ion channel that mediates extracellular Ca2+ regulation of neuronal excitability. Proc Natl Acad Sci USA 109: E1963–E1971, 2012. doi: 10.1073/pnas.1204023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin C, Passilly-Degrace P, Gaillard D, Merlin JF, Chevrot M, Besnard P. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS One 6: e24014, 2011. doi: 10.1371/journal.pone.0024014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong HH, Tan YN, Say YH. Fatty acid translocase gene CD36 rs1527483 variant influences oral fat perception in Malaysian subjects. Physiol Behav 168: 128–137, 2017. doi: 10.1016/j.physbeh.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez I. Chemoreception for fat: do rats sense triglycerides directly? Appetite 18: 193–206, 1992. doi: 10.1016/0195-6663(92)90197-E. [DOI] [PubMed] [Google Scholar]
- 18.Reed DR, Xia MB. Recent advances in fatty acid perception and genetics. Adv Nutr 6: 353S–360S, 2015. doi: 10.3945/an.114.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rice HB, Greenberg D, Corwin RL. Different preferences for oils with similar fatty acid profiles. Physiol Behav 68: 755–759, 2000. doi: 10.1016/S0031-9384(99)00255-3. [DOI] [PubMed] [Google Scholar]
- 20.Roper SD, Chaudhari N. Taste buds: cells, signals and synapses. Nat Rev Neurosci 18: 485–497, 2017. doi: 10.1038/nrn.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Running CA, Mattes RD. A review of the evidence supporting the taste of non-esterified fatty acids in humans. J Am Oil Chem Soc 93: 1325–1336, 2016. doi: 10.1007/s11746-016-2885-7. [DOI] [Google Scholar]
- 22.Sasaki K, Mitsumoto M. Role of unsaponifiable fraction on the preference for beef tallow in C57Black/6 mice. Physiol Behav 81: 665–670, 2004. doi: 10.1016/j.physbeh.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Sclafani A. Conditioned food preferences and appetite. Appetite 17: 71–72, 1991. doi: 10.1016/0195-6663(91)90087-9. [DOI] [PubMed] [Google Scholar]
- 24.Sclafani A. Fat and sugar flavor preference and acceptance in C57BL/6J and 129 mice: experience attenuates strain differences. Physiol Behav 90: 602–611, 2007. doi: 10.1016/j.physbeh.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Sclafani A, Ackroff K. Maltodextrin and fat preference deficits in “taste-blind” P2X2/P2X3 knockout mice. Chem Senses 39: 507–514, 2014. doi: 10.1093/chemse/bju019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sclafani A, Ackroff K. Fat preference in mice: lipolysis is critical for postoral but not oral fat preferences. Am J Physiol Regul Integr Comp Physiol. First published April 18, 2018. doi: 10.1152/ajpregu.00014.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol 293: R1823–R1832, 2007. doi: 10.1152/ajpregu.00211.2007. [DOI] [PubMed] [Google Scholar]
- 28.Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol 299: R1643–R1650, 2010. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sclafani A, Glendinning JI. Flavor preferences conditioned in C57BL/6 mice by intragastric carbohydrate self-infusion. Physiol Behav 79: 783–788, 2003. doi: 10.1016/S0031-9384(03)00174-4. [DOI] [PubMed] [Google Scholar]
- 30.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol 289: R712–R720, 2005. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- 31.Sclafani A, Touzani K, Ackroff K. Intragastric fat self-administration is impaired in GPR40/120 double knockout mice. Physiol Behav 147: 141–148, 2015. doi: 10.1016/j.physbeh.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sclafani A, Zukerman S, Ackroff K. GPR40 and GPR120 fatty acid sensors are critical for postoral but not oral mediation of fat preferences in the mouse. Am J Physiol Regul Integr Comp Physiol 305: R1490–R1497, 2013. doi: 10.1152/ajpregu.00440.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Fat and carbohydrate preferences in mice: the contribution of α-gustducin and Trpm5 taste-signaling proteins. Am J Physiol Regul Integr Comp Physiol 293: R1504–R1513, 2007. doi: 10.1152/ajpregu.00364.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith Richards BK, Belton BN, York B, Volaufova J. Mice bearing Acads mutation display altered postingestive but not 5-s orosensory response to dietary fat. Am J Physiol Regul Integr Comp Physiol 286: R311–R319, 2004. doi: 10.1152/ajpregu.00488.2003. [DOI] [PubMed] [Google Scholar]
- 35.Subramaniam S, Ozdener MH, Abdoul-Azize S, Saito K, Malik B, Maquart G, Hashimoto T, Marambaud P, Aribi M, Tordoff MG, Besnard P, Khan NA. ERK1/2 activation in human taste bud cells regulates fatty acid signaling and gustatory perception of fat in mice and humans. FASEB J 30: 3489–3500, 2016. doi: 10.1096/fj.201600422R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 495: 223–226, 2013. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tordoff MG, Ellis HT, Aleman TR, Downing A, Marambaud P, Foskett JK, Dana RM, McCaughey SA. Salty taste deficits in CALHM1 knockout mice. Chem Senses 39: 515–528, 2014. doi: 10.1093/chemse/bju020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Workman AD, Carey RM, Chen B, Saunders CJ, Marambaud P, Mitchell CH, Tordoff MG, Lee RJ, Cohen NA. CALHM1-mediated ATP release and ciliary beat frequency modulation in nasal epithelial cells. Sci Rep 7: 6687, 2017. doi: 10.1038/s41598-017-07221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xavier AM, Ludwig RG, Nagai MH, de Almeida TJ, Watanabe HM, Hirata MY, Rosenstock TR, Papes F, Malnic B, Glezer I. CD36 is expressed in a defined subpopulation of neurons in the olfactory epithelium. Sci Rep 6: 25507, 2016. doi: 10.1038/srep25507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zukerman S, Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol Regul Integr Comp Physiol 301: R1635–R1647, 2011. doi: 10.1152/ajpregu.00425.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol Regul Integr Comp Physiol 296: R866–R876, 2009. doi: 10.1152/ajpregu.90870.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. Impact of T1r3 and Trpm5 on carbohydrate preference and acceptance in C57BL/6 mice. Chem Senses 38: 421–437, 2013. doi: 10.1093/chemse/bjt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zukerman S, Touzani K, Margolskee RF, Sclafani A. Role of olfaction in the conditioned sucrose preference of sweet-ageusic T1R3 knockout mice. Chem Senses 34: 685–694, 2009. doi: 10.1093/chemse/bjp055. [DOI] [PMC free article] [PubMed] [Google Scholar]