Abstract
Lesions of the anteroventral third ventricle (AV3V region) are known to prevent many forms of experimental hypertension, including mineralocorticoid [deoxycorticosterone acetate (DOCA)-salt] hypertension in the rat. However, AV3V lesions include the organum vasculosum of the lamina terminalis (OVLT), portions of the median preoptic nucleus, and efferent fibers from the subfornical organ (SFO), thereby limiting the ability to define the individual contribution of these structures to the prevention of experimental hypertension. Having previously reported that the SFO does not play a significant role in the development of DOCA-salt hypertension, the present study was designed to test the hypothesis that the OVLT is necessary for DOCA-salt hypertension in the rat. In uninephrectomized OVLT-lesioned (OVLTx; n = 6) and sham-operated (n = 4) Sprague-Dawley rats consuming a 0.1% NaCl diet and 0.9% NaCl drinking solution, 24-h mean arterial pressure (MAP) was recorded telemetrically 5 days before and 21 days after DOCA implantation (100 mg sc per rat). No differences in control MAP were observed between groups. The chronic pressor response to DOCA was attenuated in OVLTx rats such that MAP increased to 133 ± 3 mmHg in sham-operated rats by day 21 of DOCA compared with 120 ± 4 mmHg (means ± SE) in OVLTx rats. These results support the hypothesis that the OVLT is an important brain site of action for the pathogenesis of DOCA-salt hypertension in the rat.
Keywords: DOCA-salt hypertension, myocardial infarction
INTRODUCTION
Hypertension affects nearly 700 million people worldwide (16) and is a known risk factor for myocardial infarction and stroke, the first and third most frequent causes of death, respectively, in Europe and the United States (56). Despite decades of research, the underlying cause of hypertension in most cases remains unknown, and furthermore, most individuals who are afflicted remain either untreated or suffer from ineffective treatment (31). Effective control of high blood pressure is severely limited by availability, cost, and adverse effects of antihypertensive medication (11) resulting in nearly one-half of hypertensive patients being noncompliant with their treatment recommendations (44). Therefore, further studies utilizing animal models elucidating the complex pathophysiology of hypertension are necessary to lead to novel and cost-effective treatment strategies.
Deoxycorticosterone acetate (DOCA)-salt hypertension in the rat has been studied as a model of human hypertension for decades. There is much evidence to suggest a role of the central nervous system and downstream activation of sympathetic nervous system activity in chronic DOCA hypertension in the rat. For example, chronic intracerebroventricular infusion of aldosterone produces hypertension (22). Additionally, intracerebroventricular mineralocorticoid receptor antagonists or epithelial sodium channel blockers prevent aldosterone or DOCA hypertension (1, 21, 35). Furthermore, mineralocorticoid receptors and epithelial sodium channels are present throughout the lamina terminalis in the brain (4, 33). Finally, renal or splanchnic denervation attenuates DOCA hypertension in the rat (5, 26, 28). Despite this evidence, the exact location of DOCA activity in the lamina terminalis to cause downstream sympathetic nervous system activity changes and eventual hypertension remains to be fully determined.
The anteroventral area of the third ventricle (AV3V region) of the hypothalamus has been known for years to be involved in and necessary for the development of many forms of experimental hypertension (7) including the hypertensive response to DOCA in the rat (10, 45). This area contains the median preoptic nucleus (MnPO), efferent fibers of the subfornical organ (SFO), and the organum vasculosum of the lamina terminalis (OVLT; 8, 9). Our laboratory has focused on dissecting the roles of these individual components of the AV3V in hormonally induced chronic hypertension. Although we have demonstrated a role of the MnPO in the central and chronic hypertensive effects of angiotensin II (ANG II; 40–42), we have primarily focused on the circumventricular organs of the AV3V and their mitigation of central hormonal effects. Both the SFO and OVLT are known circumventricular organs lacking the blood-brain barrier and therefore are accessible and responsive to circulating substances, including hormones (27). As such, both of these areas have been investigated in their homeostatic roles in response to changes in osmolarity, as well as circulating hormones such as ANG II and aldosterone (27). Indeed, we have demonstrated a role for both the SFO and OVLT in the chronic hypertensive response to ANG II in the rat (12, 13, 25, 37, 54).
In contrast, we reported that the SFO does not play a significant role in the development of DOCA-salt hypertension (38). Since lesions of the entire AV3V region are known to mitigate DOCA-salt hypertension (45), the present study was designed to test the hypothesis that an intact OVLT is necessary for the development and maintenance of DOCA-salt hypertension. To test this hypothesis, telemetric recordings of blood pressure were measured in chronic DOCA-salt-treated OVLT-lesioned and sham-operated rats.
MATERIALS AND METHODS
All experimental protocols and procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee and conducted according to guidelines of the National Institutes of Health. Adult male Sprague-Dawley rats (250–275 g; Charles River Laboratories, Wilmington, MA) were used in all experiments. Animals were housed in a facility approved and monitored by the Institutional Animal Care and Use Committee with a 12-h day-night light cycle (lights on at 7 AM).
Surgical procedures.
The rats were randomly selected to receive either electrolytic lesion of the organum vasculosum of the lamina terminalis (OVLTx; n = 6) or sham operation (n = 4) as previously described (13, 54). Rats were anesthetized with an intraperitoneal injection of ketamine (75 mg/kg) and xylazine (10 mg/kg). After achieving surgical anesthesia, rats were placed in a stereotaxic apparatus with the head level and fixed. A dorsal midline incision was made in the skin of the skull. Bregma and lambda were exposed and repositioned to be on the same horizontal level, and a 2.0-mm hole was drilled into the skull. An 0.008-in.-diameter Teflon-coated monopolar tungsten electrode, with 0.5 mm bared at the tip, was inserted into the brain using the following coordinates: +0.6 mm from bregma, on the midline and −8.00 mm below the dura mater. Electrolytic lesions were performed using a cathode current (1.0 mA for 20 s). Sham rats underwent the same surgical procedures, with the exception that ventral coordinates were 4.0 mm less so as not to damage the OVLT, and no current was passed. The hole in the skull was repaired with bone wax, and the skin was closed with 3-0 (0.2 mm) silk suture. After surgery, rats were given an injection of the antibiotic gentamicin (2.5 mg im) and the analgesic butorphanol tartrate (0.075 mg sc).
One week after lesion or sham operation, rats underwent right unilateral nephrectomy and were instrumented with radiotelemetric pressure transducers (model no. TA11PAC40; Data Sciences International, St. Paul, MN) for continuous 24-h (500 Hz for 10 s/min) sampling of mean arterial pressure (MAP) and heart rate (HR). Rats were anesthetized with 2% isoflurane. The right ureter and renal vessels were isolated and cut between two ligatures, and the kidney was removed. Subsequently, the descending aorta was exposed and clamped proximally. A small hole was punctured in the aorta, and the tip of the telemetry device was advanced cranially and glued in place. The device was anchored to the internal abdominal wall with 3-0 silk, and the incision was closed with surgical staples. Rats recovered on a heating pad and were given antibiotics and analgesics as described above.
Experimental protocol.
After 2 wk of recovery, all rats were housed individually in metabolic cages (Nalge Nunc, Rochester, NY) and placed on 0.1% NaCl diet and ad libitum 0.9% NaCl drinking solution. After 5 days of control measurements, rats were briefly anesthetized with isoflurane, and a 100-mg pellet of DOCA was implanted subcutaneously in each rat. Daily measurements were made of blood pressure, HR, saline and food intake, and urine output for 5 days of control and 21 days after DOCA implantation (see Fig. 1).
Fig. 1.
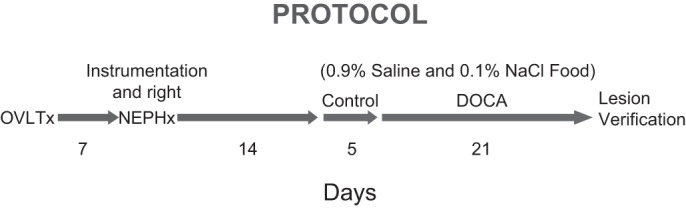
Timeline of study protocol and surgical procedures. DOCA, deoxycorticosterone acetate; NEPHx, nephrectomy; OVLTx, electrolytic ablation of the organum vasculosum of the lamina terminalis.
The daily food and water intake and urine output were measured gravimetrically. Sodium intake was calculated as the sum of the sodium received from saline intake (0.154 meq/ml) plus the product of the food intake and the sodium content of the food (0.1% NaCl, 0.0175 mmol/g). Urinary sodium concentration was measured with an ion-specific electrode (Beckman-Coulter AU680; Beckman-Coulter, Brea, CA). The daily urinary sodium excretion was calculated as the product of the urine output and urinary sodium concentration. The daily sodium and water balances were calculated as the difference between intake and urinary excretion of sodium and water, respectively.
At the end of the experiment, rats were deeply anesthetized with a lethal dose of pentobarbital sodium and perfused transcardially with 4% paraformaldehyde in PBS. Brains were collected, frozen, cut coronally (50-µm sections), stained with cresyl violet, and analyzed by light microscopy to confirm the site of the OVLT lesion.
Statistical analysis.
The results are reported as means ± SE. Two-way ANOVA combined with a Student-Newman-Keuls test was used for comparisons. Differences were considered significant at P < 0.05.
RESULTS
Histology.
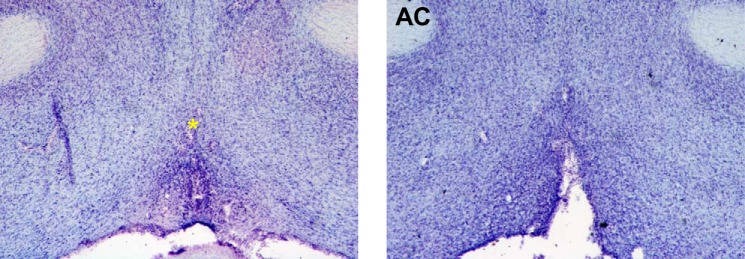
Six of 16 performed lesions were considered complete (≥90% ablation) with no or minimal damage to periventricular structures surrounding the OVLT, and only these were included in the following analyses. Figure 2 demonstrates representative coronal forebrain sections of complete OVLT lesion (Fig. 2, right) and of sham operation (Fig. 2, left).
Fig. 2.
Photomicrographs of representative cresyl violet-stained 50-µm hypothalamic coronal sections from a sham-operated rat showing an intact organum vasculosum of the lamina terminalis (OVLT, *directly above OVLT, left) and from an OVLT-lesioned rat illustrating area of lesion (right). AC, anterior commissure.
Effect of OVLT lesion on DOCA hypertension.
The response of MAP (Fig. 3, top) and HR (Fig. 3, bottom) to 21 days of DOCA can be seen in Fig. 3. MAP was not statistically different between OVLTx and sham rats during 5 days of baseline measurements (average for 5 days: 104 ± 1 and 108 ± 3 mmHg, respectively). Both groups of rats demonstrated increased MAP during 21 days of DOCA treatment. MAP was lower in OVLTx rats on days 1, 3, and 9–21 of DOCA treatment compared with sham rats such that MAP was 120 ± 4 in OVLTx rats and 133 ± 3 by day 21 of DOCA treatment. HR was not different between OVLTx and sham groups during the 5-day control period. Both groups responded similarly with decreased HR throughout 21 days of DOCA treatment.
Fig. 3.
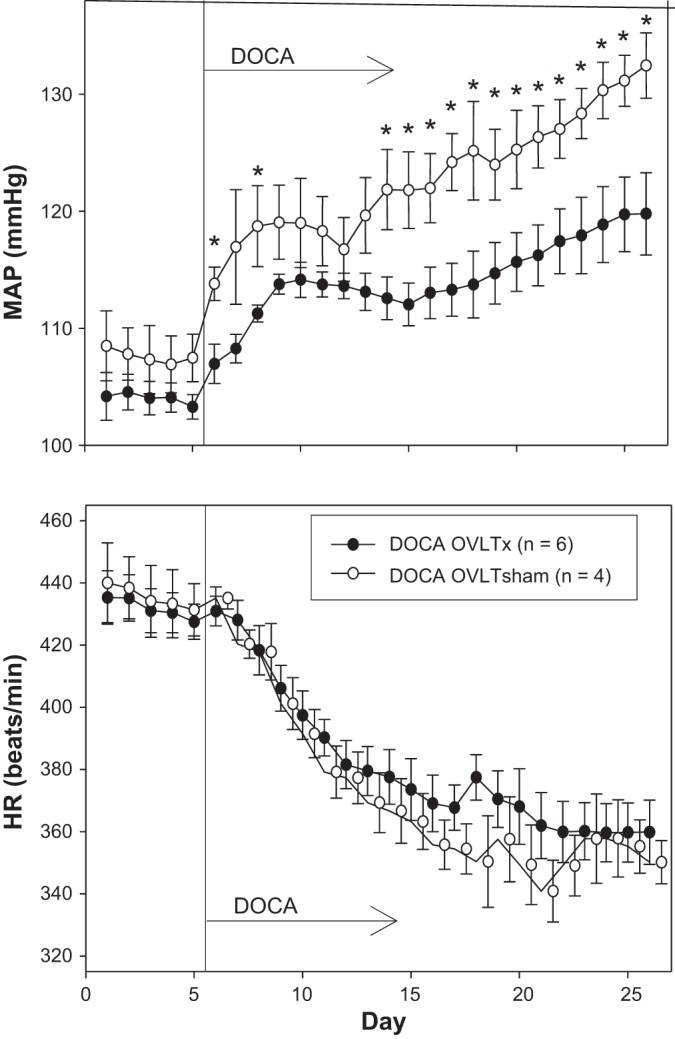
Average 24-h mean arterial pressure (MAP, top) and heart rate (HR, bottom) recorded during 5 days of control period (rats began drinking saline) followed by 21 days after DOCA implantation (100-mg pellet sc per rat) in organum vasculosum of the lamina terminalis (OVLT)-lesioned (OVLTx) and sham-operated rats (OVLTsham). *P < 0.05 between lesioned and sham rats.
Effect of OVLT lesion on sodium and water balance.
Figure 4 displays saline intake, urine output, and water balance. Although there were several days during which OVLTx rats drank more than sham rats, this was compensated for by increased urinary output such that there was no statistical significance in overall water balance between lesioned and sham-operated rats. Figure 5 displays sodium intake, sodium excretion, and sodium balance. Similar to water balance, there was no statistical significance in sodium balance between groups during the entire protocol.
Fig. 4.
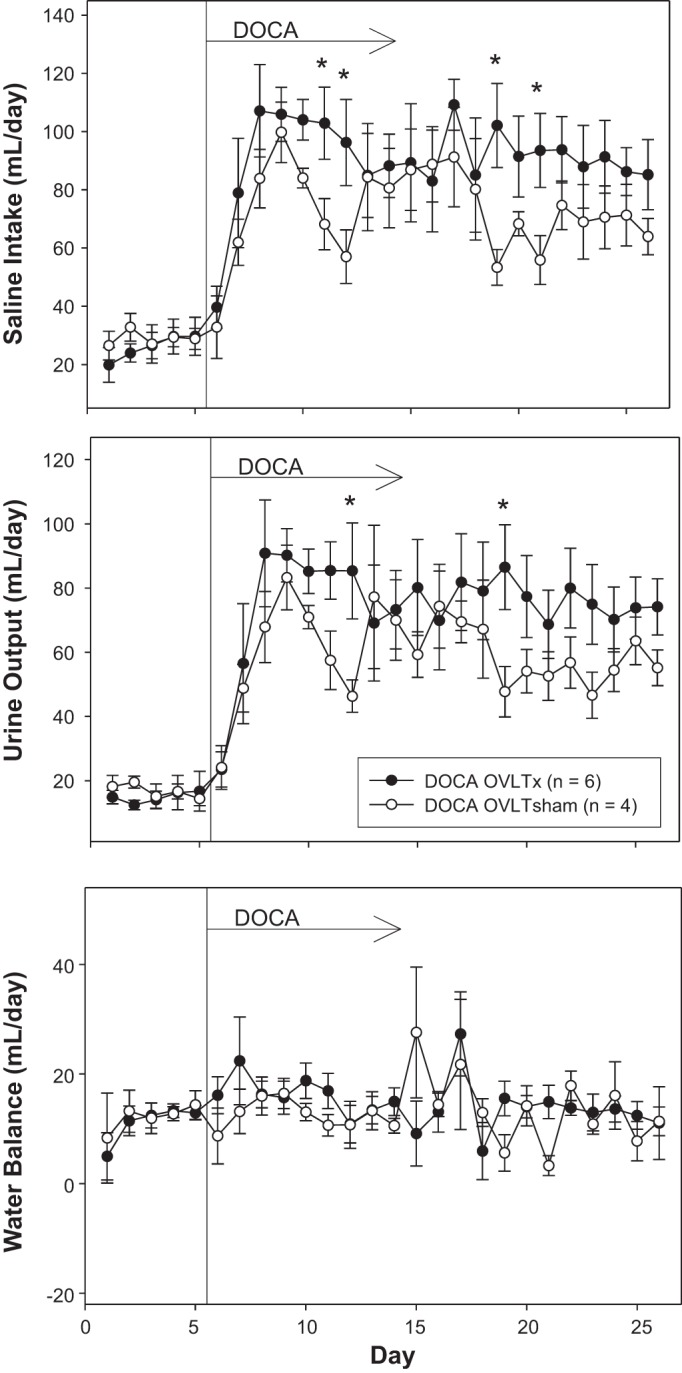
Average 24-h saline intake (top), urine output (middle), and water balance (bottom) during 5 days of control period (rats began drinking saline) followed by 21 days after DOCA implantation (100-mg pellet sc per rat) in organum vasculosum of the lamina terminalis (OVLT)-lesioned (OVLTx) and sham-operated rats (OVLTsham). *P < 0.05 between lesioned and sham rats.
Fig. 5.
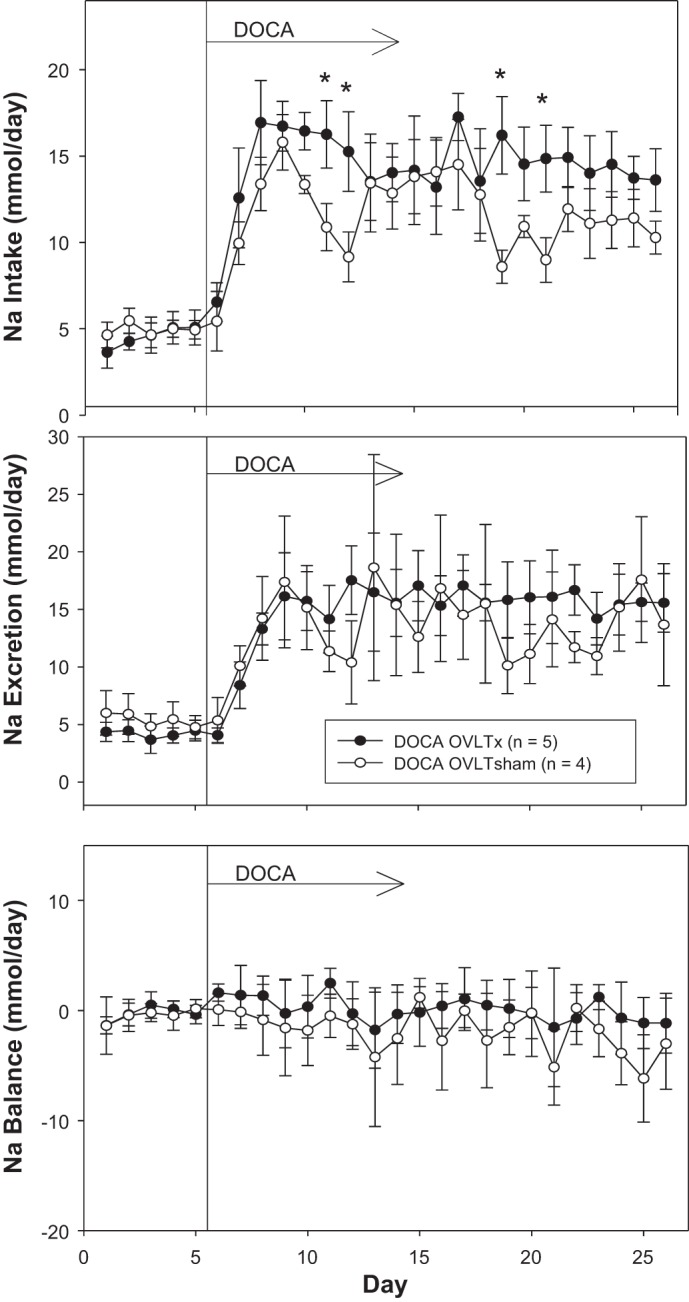
Average 24-h sodium intake (top), sodium output (middle), and sodium balance (bottom) during 5 days of control period (rats began drinking saline) followed by 21 days after DOCA implantation (100-mg pellet sc per rat) in organum vasculosum of the lamina terminalis (OVLT)-lesioned (OVLTx) and sham-operated rats (OVLTsham). *P < 0.05 between lesioned and sham rats.
DISCUSSION
The AV3V has been implicated in the pathogenesis of nearly all forms of experimental hypertension (7), including DOCA-salt hypertension (45). This critical homeostatic area of the brain comprises the OVLT, the MnPO, and efferent fibers of the SFO (8, 9). Since study of each of these areas presents a unique opportunity and contributes understanding to the overall investigation of neurogenic hypertension, our laboratory has spent considerable effort dissecting these individual components and their respective roles in hypertension. Herein, we tested the hypothesis that the OVLT is necessary for the development and maintenance of DOCA-salt hypertension in the rat. We observed that rats with discrete lesions of the OVLT had a markedly attenuated hypertensive response to chronic DOCA-salt treatment that was significant throughout most of the protocol (days 9–21). On the basis of these results, we conclude that the OVLT is necessary and plays a significant role in the pathogenesis of DOCA-salt hypertension in the rat.
Mineralocorticoid-salt-induced hypertension has been extensively studied and produced in many species of experimental animal models including mice (36), rats (26, 38, 47), dogs (6), and pigs (32) and has often focused on the well-known effects of mineralocorticoids at the renal tubules to increase water and sodium reabsorption. Mineralocorticoids, such as aldosterone or deoxycorticosterone, the steroid resulting from the deacetylation of DOCA, bind to renal tubular mineralocorticoid receptors of the distal collecting duct and cause transcriptional activation and, ultimately, increased synthesis and insertion of epithelial sodium channels into the plasma membrane leading to augmented reabsorption of sodium and water from the tubular lumen to the vasculature (20).
In contrast to the classic renal actions of mineralocorticoids, there is sufficient evidence to suggest a central and sympathetic nervous system component to the chronic hypertension produced by DOCA-salt treatment. Indirect and direct measures of sympathetic nervous system activity support the notion that it is amplified during DOCA-salt hypertension (15, 47). Furthermore, in terms of the peripheral sympathetic nervous system, removal of the renal or splanchnic sympathetic innervation has been shown to attenuate chronic DOCA-salt hypertension in the rat (5, 26, 28). Finally, lesions of the well-described AV3V region prevent DOCA-salt hypertension (10, 45). Our findings further support this notion of central actions of DOCA-salt as we presently report a definitive and prominent role of one specific component of the AV3V, namely, the OVLT, in mediating the central hypertensive actions of DOCA-salt. Consistent with the idea that the OVLT mediates central hypertensive effects via the sympathetic nervous system are its known efferent projections to sympathetic regulatory centers. The OVLT projects to downstream neurons of the paraventricular nucleus, which send excitatory efferent projections to the rostral ventrolateral medulla (RVLM; 2). Taken together, it is possible that the combination of DOCA and salt in the brain acts at OVLT mineralocorticoid receptors to increase expression of epithelial sodium channels, changes in sodium transport, and activation of osmosensitive sympathoexcitatory pathways leading to downstream hypertension.
Not only do our present results support the idea of a central and sympathetic nervous system component mediating the chronic hypertensive effects DOCA-salt, but also our results are strikingly similar to some of the aforementioned studies in terms of the pattern and magnitude of the attenuated hypertensive response, suggesting a common pathway from the brain to the peripheral sympathetic nervous system. For example, Abrams et al. demonstrated a chronic attenuated response to a 42-day protocol of DOCA-salt in intracerebroventricular benzamil-treated rats (1). In that paper, they describe initiation, developmental, and maintenance phases of the arterial pressure response to DOCA-salt. Both groups responded similarly with increases in arterial pressure and fluid intake for 4–5 days of the initiation period followed by a developmental phase of ~15 days where the groups diverge and the observed attenuated response of arterial pressure in the benzamil-treated rats becomes apparent. Finally, both groups’ pressures stabilized and plateaued at different levels during the maintenance phase. Our present data mimic these data with the exception that we did not observe the maintenance phase because our protocol was not as long (21 days) and neither group’s pressures were seen to have stably reached a plateau. Nevertheless, we observed an almost identical pattern in an initial 5–6-day rise in arterial pressure followed by a developmental phase in which the two groups’ pressures continued to rise but were markedly and significantly attenuated in the OVLT-lesioned rats perhaps supporting the concept that the developmental phase (approximately days 6–21 of either protocol) is indeed mediated centrally via mineralocorticoid receptors in the OVLT.
Likewise, when comparing the present results with those observed in renal denervated and celiac ganglionectomized rats during chronic DOCA-salt hypertension, the results are strikingly similar in both the initiation and developmental phases. With regard to renal denervated rats, Jacob et al. reported a biphasic response to DOCA in that there was an initial rapid rise in arterial pressure during the first few days of DOCA that tapered by day 5, followed by a more slowly developing chronic hypertension such that by day 20 of DOCA, arterial pressure had risen only 13 ± 2 mmHg in renal denervated rats compared with 23 ± 5 mmHg in sham rats (26). This essentially is the developmental phase of the hypertension and is noticeably similar to what we observed in the 21-day protocol used in this study whereby at day 21 we reported a rise in arterial pressure of 25 ± 4 mmHg in OVLT sham-operated rats compared with the attenuated increase of 16 ± 3 mmHg in OVLT-lesioned rats. However, despite this similarity in the chronic attenuated responses to DOCA-salt in OVLT-lesioned rats and renal denervated rats suggesting a common central pathway to the peripheral sympathetic nervous system mediating the hypertensive response to DOCA, recent evidence suggests that the major component of the renal nerve effects is mediated by afferent renal nerve activity, as rats with specific afferent renal denervation responded with the same fully attenuated hypertensive response to DOCA as rats with total renal denervation (5). Although Jacob et al. observed similar attenuations to the hypertensive effects of DOCA in total renal denervated and afferent denervated rats, it should be noted that they also reported an increase in T cell infiltration and multiple inflammatory cytokine mediators in DOCA-treated rats that was greatly reduced only in the total renal denervated group of rats, as opposed to marked inflammation still present in the afferent renal denervated rats (5). Therefore, it is possible that the OVLT and efferent renal nerve component of the hypertension is more related to the rise in inflammation at the kidney, which, in turn, theoretically drives the afferent renal nerve activity ultimately resulting in the hypertension (5).
Furthermore, as mentioned above, our present results also closely mimic the observations of the hypertensive response to DOCA-salt in sham and celiac ganglionectomized rats. Kandlikar and Fink demonstrated similar initiation and developmental phases as described above during 28 days of DOCA-salt treatment (28). MAP rose rapidly over the course of 5–6 days in both groups, followed by a more slowly developing hypertension that was attenuated in celiac ganglionectomized rats, such that by day 28 of DOCA-salt, MAP had risen 25.6 ± 2.2 mmHg in sham rats compared with 15.6 ± 2.2 mmHg in celiac ganglionectomized rats. These data again are almost identical to the results of the present study and are consistent with the possibility of a final common mediating pathway from the brain (OVLT) to the peripheral nervous system (in this case, the splanchnic sympathetic nervous system). Indeed, although Kandlikar and Fink attributed this attenuated response to the removal of the splanchnic sympathetic innervation, indicators of sympathetic activity such as whole body norepinephrine spillover were not different between groups, and nonhepatic splanchnic norepinephrine spillover was not different between DOCA and sham groups (28). They concluded that despite a lack of indirect evidence of increased sympathetic activity as measured by norepinephrine levels, these results could be explained by the fact that there is evidence to suggest increased mesenteric arterial constrictor responsiveness to norepinephrine (52), enhanced sympathetic neurotransmission to mesenteric arteries (39), and increased venous tone (18) due to an augmented reactivity of mesenteric veins to norepinephrine during the development of DOCA-salt hypertension (57).
Despite the compelling comparisons of these previous studies, consistent with the notion of a common central pathway to the peripheral sympathetic nervous system mediating the hypertensive response to DOCA, we cannot rule out the possibility of the involvement of other factors, such as the hormone vasopressin, in playing a role in the attenuated response to DOCA OVLT-lesioned rats. Indeed, vasopressin has been shown to be involved in the hypertensive response to DOCA, as rats with diabetes insipidus were reported to be unresponsive to the hypertensive effects of DOCA whereas control rats had elevated blood pressures, urinary vasopressin, and depressor responses to analogs of vasopressin that block the pressor response to vasopressin (14). Importantly, the OVLT has also been reported to be involved in the rise in vasopressin levels in response to both osmotic stimuli (intravenous hypertonic saline) and ANG II (49). Unfortunately, vasopressin levels were not measured in the present study, and therefore we cannot rule out the possibility that lesion of the OVLT affected vasopressin levels in response to DOCA and mediated some of the attenuated hypertensive effects of DOCA in the OVLT-lesioned rats. Interestingly, Banek et al. proposed the involvement of vasopressin secretion in the aforementioned studies involving the mechanism of action of afferent renal nerves and the hypertensive response to DOCA (5).
Furthermore, although the DOCA-salt model has been used for decades as a model of salt-dependent hypertension, it is not simply a model of mineralocorticoid excess, and some of its limitations are due to the historical nature of this presumption, conflicting reports as to the overall mineralocorticoid and glucocorticoid activity of deoxycorticosterone (DOC), and the complexities of steroid synthesis and regulation (23, 53). When the inactive steroid DOCA is administered, it is assumed to be rapidly deacetylated by nonspecific esterases leading to formation of the free alcohol, DOC, although this well-accepted assumption has not been widely verified (24). Additionally, DOC itself is not considered to be as physiologically relevant a hormone as normal secretion rates are much lower than those of either corticosterone or aldosterone (29, 43). Yet historically, DOCA was one of the first corticosteroid derivatives to be synthesized and shown to be able to maintain adrenalectomized dogs (48) and be effective in the treatment of adrenocortical insufficiency or Addison’s disease (17). Therefore, it is clearly converted to active steroid even in the setting of a dysfunctional or absent adrenal gland, a different setting from the present model with intact adrenal glands. In terms of mineralocorticoid activity itself, DOC not only has been reported to be much weaker than aldosterone in some studies (20, 46) but also has been shown by others to have effects on sodium and potassium levels similar to those of aldosterone (3). Importantly, it should be noted that despite some controversy as to its level of mineralocorticoid activity, DOC itself also has potent glucocorticoid activity that has also been debated over many years. Nevertheless, clear glucocorticoid activity has been demonstrated in that administration of DOCA in adrenalectomized cats restored normal liver glycogen (34) and stimulated both gluconeogenesis and glycogenolysis in isolated rat skeletal muscle (53). Finally, to complicate matters further as to the overall effects of DOCA administration, DOC can also be further converted to corticosterone by 11β-hydroxylase or, ultimately, to aldosterone as well. Aldosterone synthase is a successive processing enzyme, whose normal substrate DOC is sequentially hydroxylated to corticosterone, to 18-hydroxycorticosterone, and then to an unstable diol that spontaneously is dehydrated to aldosterone (23). In the DOCA-salt model, this is particularly relevant as the high salt intake itself can modulate and decrease aldosterone synthase activity (55), thus exacerbating the already excessive and potential glucocorticoid effects of DOC both peripherally and centrally, by also increasing corticosterone levels. These complexities of the DOCA-salt model, sparingly mentioned here, therefore need to be taken into consideration when interpreting results of studies from this frequently used model of salt-dependent hypertension, as it is not merely a mineralocorticoid-salt model, but truly a model of both mineralocorticoid- and glucocorticoid-induced effects.
Finally, in contrast to the present results from OVLT-lesioned rats demonstrating a marked attenuation of DOCA-salt hypertension, we have previously reported no effect of lesion of the SFO, another circumventricular organ of the lamina terminalis, on the chronic effects of DOCA-salt in the rat (38). Notably, in the ANG II model of hypertension, we have actually previously reported a role for both the SFO and the OVLT in mediating the chronic hypertensive effects of ANG II in rats consuming a normal-salt diet (12, 25, 37, 54). Interestingly, in a chronic ANG II-salt-dependent model of hypertension, in which rats were consuming a high-salt diet combined with ANG II treatment, we recently reported a prominent role of the OVLT in this response (13), yet lesion of the SFO had a minimal effect in attenuating the hypertensive response (37). Our present and previous results in OVLT- and SFO-lesioned rats treated with chronic DOCA-salt are consistent with these previous reports examining ANG II hypertension in that 1) DOCA-salt hypertension is a form of salt-dependent hypertension and 2) it would appear that the OVLT has a more prominent role in mediating the synergistic effects of salt and the inciting hormonal stimulus in these models. Indeed, in our previous report demonstrating an attenuated hypertensive response to ANG II-salt in OVLT-lesioned rats, we also reported lower baseline pressures in OVLT-lesioned rats consuming a high-salt diet during the control period (13). Although it was not statistically significant in the present study, we did observe overall lower baseline MAP in OVLT-lesioned rats compared with sham-operated rats consuming high salt during the control period. This finding is consistent with a study showing that the OVLT is an important site for mediating the effects of chronic high salt intake on increased excitability of sympathetic premotor neurons in the RVLM (2). Our findings support this view and suggest that this increased excitability of RVLM premotor neurons in rats on high salt results in a modest, but measurable, increase in arterial pressure that is dependent on an intact OVLT.
In summary, we presently report a prominent role of the OVLT in mediating the chronic hypertensive response to DOCA-salt in the rat. This marked level of attenuation of the hypertensive response was not previously seen in rats with lesions of the SFO. These findings are in agreement with the idea that the OVLT has a prominent role as the central osmoreceptor site of the lamina terminalis as has been previously reported owing to their role in mediating sympathetic nerve responses to increased plasma osmolality (50, 51). As briefly mentioned above, it has been shown that rats on a high-salt diet have an increased sympathoexcitatory response to activation of sympathetic premotor neurons in the RVLM and this response is abolished by lesion of the OVLT (2). This is consistent with our observation that lesion of the OVLT decreases basal levels of arterial pressure in rats consuming a high-salt diet and, more importantly, that the OVLT appears to be the dominant circumventricular organ involved in the pathogenesis of salt-dependent forms of hypertension, as we have demonstrated its definitive role in the development of both the ANG II-salt-dependent and DOCA-salt models of hypertension. Finally, on the basis of our present results, we therefore conclude that the delayed neurogenic or “maintenance” phase of hypertension induced by DOCA-salt administration is mediated in part by the OVLT.
Perspectives and Significance
The results of the present study support the notion that the OVLT is a major osmosensitive and hormonally responsive forebrain area that mediates a great amount of the synergistic hypertensive effects of chronic DOCA-salt in the rat. However, OVLT lesion did not prevent DOCA-salt hypertension entirely. One possibility is that the delayed maintenance or “neurogenic” phase of DOCA-salt hypertension is also mediated, in part, by nonneurogenic mechanisms (i.e., salt and water retention). We do not believe this to be the case as we did not observe any changes in chronic water and/or sodium balance in our studies. Another possibility is that no single site in the AV3V region is solely responsible for mediating neurogenic hypertension in the DOCA-salt model. Along these lines, it is possible that other circumventricular organs, such as the SFO, assume a greater role in the pathogenesis of DOCA-salt hypertension in OVLTx animals even though we previously reported no effect of SFO lesion on DOCA-salt hypertension. This is illustrated in a study by McKinley et al., in which they reported minimal effects on the drinking response to intravenous hypertonic saline in animals with individual lamina terminalis lesions of the SFO, MnPO, or OVLT (30). However, the drinking effects were markedly and synergistically attenuated with combined lesions of two of these structures, and only after lesion of all three structures was the drinking response to intracerebroventricular hypertonic saline abolished (30). Indeed, this study highlights the fact that with lesion studies of this nature, these possibilities need to be taken into consideration. Therefore, despite the dominant role of the OVLT, we cannot conclude that it is the sole forebrain site responsible for the hypertensive effects of DOCA-salt.
GRANTS
This study was funded by University of Minnesota Grant-In-Aid 107426 to J. P. Collister and National Heart, Lung, and Blood Institute Grant K99-HL-141650 to C. T. Banek.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.P.C. conceived and designed research; J.P.C., D.B.N., C.E.W., and C.T.B. performed experiments; J.P.C., D.B.N., and C.E.W. analyzed data; J.P.C. interpreted results of experiments; D.B.N. and R.H. prepared figures; J.P.C. drafted manuscript; J.P.C., R.H., C.T.B., and J.W.O. edited and revised manuscript; J.P.C., D.B.N., R.H., C.T.B., and J.W.O. approved final version of manuscript.
REFERENCES
- 1.Abrams JM, Engeland WC, Osborn JW. Effect of intracerebroventricular benzamil on cardiovascular and central autonomic responses to DOCA-salt treatment. Am J Physiol Regul Integr Comp Physiol 299: R1500–R1510, 2010. doi: 10.1152/ajpregu.00431.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams JM, Bardgett ME, Stocker SD. Ventral lamina terminalis mediates enhanced cardiovascular responses of rostral ventrolateral medulla neurons during increased dietary salt. Hypertension 54: 308–314, 2009. doi: 10.1161/HYPERTENSIONAHA.108.127803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal MK. Perspectives in receptor-mediated mineralocorticoid hormone action. Pharmacol Rev 46: 67–87, 1994. [PubMed] [Google Scholar]
- 4.Amin MS, Wang HW, Reza E, Whitman SC, Tuana BS, Leenen FH. Distribution of epithelial sodium channels and mineralocorticoid receptors in cardiovascular regulatory centers in rat brain. Am J Physiol Regul Integr Comp Physiol 289: R1787–R1797, 2005. doi: 10.1152/ajpregu.00063.2005. [DOI] [PubMed] [Google Scholar]
- 5.Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y, Osborn JW. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 68: 1415–1423, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bravo EL, Tarazi RC, Dustan HP. Multifactorial analysis of chronic hypertension induced by electrolyte-active steroids in trained, unanesthetized dogs. Circ Res 40, Suppl 1: I140–I145, 1977. [PubMed] [Google Scholar]
- 7.Brody MJ. Central nervous system and mechanisms of hypertension. Clin Physiol Biochem 6: 230–239, 1988. [PubMed] [Google Scholar]
- 8.Brody MJ, Johnson AK. Role of the anteroventral third ventricle region in fluid and electrolyte balance, arterial pressure regulation and hypertension. In: Frontiers in Neuroendocrinology, edited by Martini L and Ganong WF. New York: Raven, 1980, vol. 6, p. 249–292. [Google Scholar]
- 9.Brody MJ, Fink GD, Buggy J, Haywood JR, Gordon FJ, Johnson AK. The role of the anteroventral third ventricle (AV3V) region in experimental hypertension. Circ Res 43: 1–13, 1978. [DOI] [PubMed] [Google Scholar]
- 10.Buggy J, Fink GD, Johnson AK, Brody MJ. Prevention of the development of renal hypertension by anteroventral third ventricular tissue lesions. Circ Res 40, Suppl 1: I110–I117, 1977. [PubMed] [Google Scholar]
- 11.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee . Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 12.Collister JP, Hendel MD. Chronic effects of angiotensin II and at1 receptor antagonists in subfornical organ-lesioned rats. Clin Exp Pharmacol Physiol 32: 462–466, 2005. doi: 10.1111/j.1440-1681.2005.04212.x. [DOI] [PubMed] [Google Scholar]
- 13.Collister JP, Olson MK, Nahey DB, Vieira AA, Osborn JW. OVLT lesion decreases basal arterial pressure and the chronic hypertensive response to ANG II in rats on a high-salt diet. Physiol Rep 1: e00128, 2013. doi: 10.1002/phy2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crofton JT, Share L, Shade RE, Lee-Kwon WJ, Manning M, Sawyer WH. The importance of vasopressin in the development and maintenance of DOC-salt hypertension in the rat. Hypertension 1: 31–38, 1979. doi: 10.1161/01.HYP.1.1.31. [DOI] [PubMed] [Google Scholar]
- 15.de Champlain J, Eid H, Drolet G, Bouvier M, Foucart S. Peripheral neurogenic mechanisms in deoxycorticosterone acetate–salt hypertension in the rat. Can J Physiol Pharmacol 67: 1140–1145, 1989. doi: 10.1139/y89-181. [DOI] [PubMed] [Google Scholar]
- 16.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ; Comparative Risk Assessment Collaborating Group . Selected major risk factors and global and regional burden of disease. Lancet 360: 1347–1360, 2002. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 17.Ferrebee JW, Ragan C, Atchley DW, Loeb RF. Desoxycorticosterone esters. Certain effects in the treatment of Addison’s disease. J Am Med Assoc 113: 1725–1731, 1939. doi: 10.1001/jama.1939.02800440029009. [DOI] [Google Scholar]
- 18.Fink GD, Johnson RJ, Galligan JJ. Mechanisms of increased venous smooth muscle tone in desoxycorticosterone acetate-salt hypertension. Hypertension 35: 464–469, 2000. doi: 10.1161/01.HYP.35.1.464. [DOI] [PubMed] [Google Scholar]
- 19.Fuller PJ, Young MJ. Mechanisms of mineralocorticoid action. Hypertension 46: 1227–1235, 2005. doi: 10.1161/01.HYP.0000193502.77417.17. [DOI] [PubMed] [Google Scholar]
- 20.Genest J. The present status of aldosterone in clinical medicine. Can Med Assoc J 73: 876–883, 1955. [PMC free article] [PubMed] [Google Scholar]
- 21.Gómez-Sánchez EP, Fort CM, Gómez-Sánchez CE. Intracerebroventricular infusion of RU28318 blocks aldosterone-salt hypertension. Am J Physiol Endocrinol Metab 258: E482–E484, 1990. doi: 10.1152/ajpendo.1990.258.3.E482. [DOI] [PubMed] [Google Scholar]
- 22.Gomez-Sanchez EP. Intracerebroventricular infusion of aldosterone induces hypertension in rats. Endocrinology 118: 819–823, 1986. doi: 10.1210/endo-118-2-819. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H, Gomez-Sanchez EP. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol 383: 111–117, 2014. doi: 10.1016/j.mce.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grekin RJ, Terris JM, Bohr DF. Electrolyte and hormonal effects of deoxycorticosterone acetate in young pigs. Hypertension 2: 326–332, 1980. doi: 10.1161/01.HYP.2.3.326. [DOI] [PubMed] [Google Scholar]
- 25.Hendel MD, Collister JP. Contribution of the subfornical organ to angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 288: H680–H685, 2005. doi: 10.1152/ajpheart.00823.2004. [DOI] [PubMed] [Google Scholar]
- 26.Jacob F, Clark LA, Guzman PA, Osborn JW. Role of renal nerves in development of hypertension in DOCA-salt model in rats: a telemetric approach. Am J Physiol Heart Circ Physiol 289: H1519–H1529, 2005. doi: 10.1152/ajpheart.00206.2005. [DOI] [PubMed] [Google Scholar]
- 27.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J 7: 678–686, 1993. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 28.Kandlikar SS, Fink GD. Splanchnic sympathetic nerves in the development of mild DOCA-salt hypertension. Am J Physiol Heart Circ Physiol 301: H1965–H1973, 2011. doi: 10.1152/ajpheart.00086.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katzung BG. Basic and Clinical Pharmacology. New York: McGraw-Hill, 2004. [Google Scholar]
- 30.McKinley MJ, Mathai ML, Pennington G, Rundgren M, Vivas L. Effect of individual or combined ablation of the nuclear groups of the lamina terminalis on water drinking in sheep. Am J Physiol Regul Integr Comp Physiol 276: R673–R683, 1999. doi: 10.1152/ajpregu.1999.276.3.R673. [DOI] [PubMed] [Google Scholar]
- 31.Mensah GA. The global burden of hypertension: good news and bad news. Cardiol Clin 20: 181–185, 2002. doi: 10.1016/S0733-8651(02)00002-4. [DOI] [PubMed] [Google Scholar]
- 32.Miller AW II, Bohr DF, Schork AM, Terris JM. Hemodynamic responses to DOCA in young pigs. Hypertension 1: 591–597, 1979. doi: 10.1161/01.HYP.1.6.591. [DOI] [PubMed] [Google Scholar]
- 33.Miller RL, Wang MH, Gray PA, Salkoff LB, Loewy AD. ENaC-expressing neurons in the sensory circumventricular organs become c-Fos activated following systemic sodium changes. Am J Physiol Regul Integr Comp Physiol 305: R1141–R1152, 2013. doi: 10.1152/ajpregu.00242.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montigel C, Verzár F. Untersuchungen über den Kohlenhydratstoffwechsel nach Adrenalektomie, II Mitteilung: Glykogenbildung unter dem Einfluss von Desoxycorticosteron. Helv Phys Acta 1: 137–141, 1943. [Google Scholar]
- 35.Nishimura M, Ohtsuka K, Nanbu A, Takahashi H, Yoshimura M. Benzamil blockade of brain Na+ channels averts Na+-induced hypertension in rats. Am J Physiol Regul Integr Comp Physiol 274: R635–R644, 1998. doi: 10.1152/ajpregu.1998.274.3.R635. [DOI] [PubMed] [Google Scholar]
- 36.Obst M, Gross V, Luft FC. Systemic hemodynamics in non-anesthetized l-NAME- and DOCA-salt-treated mice. J Hypertens 22: 1889–1894, 2004. doi: 10.1097/00004872-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Osborn JW, Hendel MD, Collister JP, Ariza-Guzman PA, Fink GD. The role of the subfornical organ in angiotensin II-salt hypertension in the rat. Exp Physiol 97: 80–88, 2012. doi: 10.1113/expphysiol.2011.060491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osborn JW, Jacob F, Hendel M, Collister JP, Clark L, Guzman PA. Effect of subfornical organ lesion on the development of mineralocorticoid-salt hypertension. Brain Res 1109: 74–82, 2006. doi: 10.1016/j.brainres.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 39.Park J, Galligan JJ, Fink GD, Swain GM. Alterations in sympathetic neuroeffector transmission to mesenteric arteries but not veins in DOCA-salt hypertension. Auton Neurosci 152: 11–20, 2010. doi: 10.1016/j.autneu.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ployngam T, Collister JP. An intact median preoptic nucleus is necessary for chronic angiotensin II-induced hypertension. Brain Res 1162: 69–75, 2007. doi: 10.1016/j.brainres.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ployngam T, Collister JP. Role of the median preoptic nucleus in chronic angiotensin II-induced hypertension. Brain Res 1238: 75–84, 2008. doi: 10.1016/j.brainres.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 42.Ployngam T, Katz SS, Collister JP. Role of the median preoptic nucleus in the chronic hypotensive effect of losartan in sodium-replete normal rats. Clin Exp Pharmacol Physiol 37: e7–e13, 2010. doi: 10.1111/j.1440-1681.2009.05307.x. [DOI] [PubMed] [Google Scholar]
- 43.Romanoff LP, Baxter MN. The secretion rates of deoxycorticosterone and corticosterone in young and elderly men. J Clin Endocrinol Metab 41: 630–633, 1975. doi: 10.1210/jcem-41-3-630. [DOI] [PubMed] [Google Scholar]
- 44.Shaw E, Anderson JG, Maloney M, Jay SJ, Fagan D. Factors associated with noncompliance of patients taking antihypertensive medication. Hosp Pharm 30: 201–203, 1995. [PubMed] [Google Scholar]
- 45.Songu-Mize E, Bealer SL, Caldwell RW. Effect of AV3V lesions on development of DOCA-salt hypertension and vascular Na+-pump activity. Hypertension 4: 575–580, 1982. doi: 10.1161/01.HYP.4.5.575. [DOI] [PubMed] [Google Scholar]
- 46.Tait SA, Tait JF. The correspondence of S.A.S. Simpson and J.F. Tait with T. Reichstein during their collaborative work on the isolation and elucidation of the structure of electrocortin (later aldosterone). Steroids 63: 440–453, 1998. doi: 10.1016/S0039-128X(98)00050-6. [DOI] [PubMed] [Google Scholar]
- 47.Takeda K, Buñag RD. Augmented sympathetic nerve activity and pressor responsiveness in DOCA hypertensive rats. Hypertension 2: 97–101, 1980. doi: 10.1161/01.HYP.2.1.97. [DOI] [PubMed] [Google Scholar]
- 48.Thorn GW, Engle LL, Eisenberg H. The effect of corticosterone and related compounds on the renal excretion of electrolytes. J Exp Med 68: 161–171, 1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thrasher TN, Keil LC. Regulation of drinking and vasopressin secretion: role of organum vasculosum laminae terminalis. Am J Physiol Regul Integr Comp Physiol 253: R108–R120, 1987. doi: 10.1152/ajpregu.1987.253.1.R108. [DOI] [PubMed] [Google Scholar]
- 50.Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand 177: 43–55, 2003. doi: 10.1046/j.1365-201X.2003.01046.x. [DOI] [PubMed] [Google Scholar]
- 51.Toney GM, Stocker SD. Hyperosmotic activation of CNS sympathetic drive: implications for cardiovascular disease. J Physiol 588: 3375–3384, 2010. doi: 10.1113/jphysiol.2010.191940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsuda K, Kuchii M, Nishio I, Masuyama Y. Neurotransmitter release, vascular responsiveness and their suppression by Ca-antagonist in perfused mesenteric vasculature of DOCA-salt hypertensive rats. Clin Exp Hypertens A 8: 259–275, 1986. doi: 10.3109/10641968609074775. [DOI] [PubMed] [Google Scholar]
- 53.Verzár F, Wang FC. Reversal of glycogenetic to glycogenolytic action of desoxycorticosterone in rats. Nature 165: 114, 1950. doi: 10.1038/165114a0. [DOI] [PubMed] [Google Scholar]
- 54.Vieira AA, Nahey DB, Collister JP. Role of the organum vasculosum of the lamina terminalis for the chronic cardiovascular effects produced by endogenous and exogenous ANG II in conscious rats. Am J Physiol Regul Integr Comp Physiol 299: R1564–R1571, 2010. doi: 10.1152/ajpregu.00034.2010. [DOI] [PubMed] [Google Scholar]
- 55.Vinson GP. The mislabelling of deoxycorticosterone: making sense of corticosteroid structure and function. J Endocrinol 211: 3–16, 2011. doi: 10.1530/JOE-11-0178. [DOI] [PubMed] [Google Scholar]
- 56.Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, Kastarinen M, Poulter N, Primatesta P, Rodríguez-Artalejo F, Stegmayr B, Thamm M, Tuomilehto J, Vanuzzo D, Vescio F. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA 289: 2363–2369, 2003. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- 57.Xu H, Fink GD, Galligan JJ. Increased sympathetic venoconstriction and reactivity to norepinephrine in mesenteric veins in anesthetized DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol 293: H160–H168, 2007. doi: 10.1152/ajpheart.01414.2006. [DOI] [PubMed] [Google Scholar]