Abstract
Fatty acid receptors in the mouth and gut are implicated in the appetite for fat-rich foods. The role of lipolysis in oral- and postoral-based fat preferences of C57BL/6J mice was investigated by inhibiting lipase enzymes with orlistat. Experiment 1 showed that postoral lipolysis is required: mice learned to prefer (by 70%) a flavored solution paired with intragastric infusions of 5% soybean oil but not a flavor paired with soybean oil + orlistat (4 mg/g fat) infusions. Experiments 2–4 tested the oral attraction to oil in mice given brief choice tests that minimize postoral effects. In experiment 2, the same low orlistat dose did not reduce the strong (83–94%) preference for 2.5 or 5% soybean oil relative to fat-free vehicle in 3-min tests. Mice in experiment 3 given choice tests between two fat emulsions (2% triolein, corn oil, or soybean oil) with or without orlistat at a high dose (250 mg/g fat) preferred triolein (72%) and soybean oil (67%) without orlistat to the oil with orlistat but were indifferent to corn oil with and without orlistat. In experiment 4, mice preferred 2% triolein (62%) or soybean oil (89%) to vehicle when both choices contained orlistat (250 mg/g fat). Fatty acid receptors are thus essential for postoral but not oral-based preferences. Both triglyceride and fatty acid taste receptors may mediate oral fat preferences.
Keywords: corn oil, intragastric infusions, orlistat, soybean oil, triolein oil
INTRODUCTION
Laboratory rodents, like humans and other species, are attracted to fat-rich foods. The source of this attraction has been the subject of extensive research, implicating both oral and postoral sensory processes (2). The attractive flavor of fat was long attributed primarily to its textural and olfactory components, but there is evidence that fatty acid taste receptors contribute to fat preference in rodents (2, 4, 6). Dietary fat, however, is primarily in the form of triglycerides. Kawai and Fushiki (8) reported that lingual lipase can rapidly generate significant amounts of fatty acid from triglyceride in the oral cavity of rats and proposed that the released fatty acids allow rats to taste dietary fat. This was supported by their findings that in brief two-bottle tests rats preferred a triglyceride emulsion (2% triolein) to the same emulsion containing the lipase inhibitor orlistat.
A substantial postoral contribution to fat appetite is documented by the ability of intragastric (IG) fat infusions to stimulate intake and condition flavor preferences in rodents by a process referred to as appetition, to distinguish it from fat-induced satiation that suppresses intake (2, 16). Postoral fat appetition can be quite rapid: food-restricted mice trained 1/h day to consume a flavored solution [the conditioned stimulus (CS+)] paired with IG soybean oil infusions increased their intake of the CS+ within 15 min in the first session (3, 33). This rapid appetition response suggests the involvement of fatty acid sensors in the gut. Consistent with this view, IG fat infusions were much less effective in stimulating CS+ intake and preference in double knockout (DoKO) mice missing G protein-coupled receptor (GPR)40 and GPR120 fatty acid receptors in the gut (24). To activate these GPR receptors, the infused fat must be converted in the gut to fatty acids by lipases. An important role for lipolysis in fat conditioning was suggested by our finding that rats trained 0.5 h/day to consume flavored fat sources (vegetable shortening) with or without orlistat developed a preference for the flavor of the orlistat-free fat source (1). This was taken as evidence that fat digestion is essential for postoral fat conditioning. However, the subsequent report of Kawai and Fushiki (8) cited above suggests that the addition of orlistat might have reduced the orosensory attractiveness of the flavored fat. Yet, we found that naïve rats equally preferred fat sources with and without added orlistat in initial short-term choice tests and only with repeated testing did they show a preference for the orlistat-free fat (1). This finding is not inconsistent with Kawai and Fushiki (8) because while they reported that rats preferred triolein oil to triolein oil + orlistat, no preference was observed with a different fat source, i.e., corn oil versus corn oil + orlistat. Taken together, these findings raise questions about the role of fat lipolysis in orosensory and postoral fat sensing.
The present experiments addressed this issue by determining the effects of inhibiting triglyceride lipolysis with orlistat on both oral and postoral influences on fat preference. Experiment 1 examined orlistat inhibition of postoral appetition produced by IG soybean oil infusions using the methods of previous studies (3, 33). Experiments 2, 3, and 4 tested oral fat preference as measured in brief-access choice tests with a triglyceride oil + orlistat versus vehicle or versus the same oil without orlistat. The subjects of these experiments were C57BL/6J (B6) mice, which display rapid flavor conditioning with IG fat infusions, strong oral preferences for fat emulsions in brief and long-term tests, and disruptions in fat preferences with genetic deletion of oral and/or postoral fat-sensing components (3, 7, 20, 23, 33).
Experiment 1: Effects of Orlistat on Postoral Fat Appetition
In this experiment, we compared the flavor conditioning response of B6 mice to IG infusions of soybean oil with or without orlistat. We used a 1 h/day conditioning protocol that produces rapid stimulation of intake and flavor preferences in mice and an orlistat dose that altered fat preference in our prior rat study (1, 3, 33).
Experiment 2: Effects of Orlistat on Soybean Oil Preference
In this experiment, the effect of orlistat on the oral preference of naïve B6 mice for soybean oil emulsions was determined using brief-access, two-bottle tests to minimize postoral factors. The animals were tested with 2.5 and 5% soybean oil emulsions using the dose of orlistat (4 mg/g of fat) that blocked postoral fat conditioning in the first experiment. To minimize the oral textural effects of fat, xanthan gum was added to the soybean oil and vehicle emulsions (8).
Experiment 3: Effects of Orlistat on Triolein, Corn Oil and Soybean Oil Preference
In a widely cited paper supporting the importance of lipolysis in oral fat preference, Kawai and Fushiki (8) gave rats 5-min choice tests between 2% fat versus the same fat containing orlistat. They reported that the animals significantly preferred triolein oil to triolein oil + orlistat but showed no preference for corn oil versus corn oil + orlistat. An unusual feature of this experiment is that the rats, before the 5-min tests, were trained to discriminate between triolein oil and mineral oil in overnight one-bottle sessions “so that the rats could learn to distinguish nutritive from nonnutritive oil.” Consequently, the animals were highly familiar with triolein oil but not triolein oil + orlistat or with corn oil before the 5-min choice tests, which may have influenced the outcome of the tests. Given this feature and the failure of orlistat to block soybean oil preference in experiment 2, experiment 3 determined if orlistat would selectively alter the fat preference in naïve mice tested with triolein oil, corn oil, or soybean oil. In particular, B6 mice were given choice tests with triolein oil versus triolein oil + orlistat, followed by tests with corn oil versus corn oil + orlistat and soybean oil versus soybean oil + orlistat. Following Kawai and Fushiki, the two-choice tests were 5 min in duration, the 2% oils were presented as suspensions in xanthan gum, and the orlistat concentration was 0.5%, which corresponds to 250 mg/g of fat, a dose over 60 times that used in experiments 1 and 2.
Experiment 4. Is Lipolysis Essential for Triolein and Soybean Preferences?
Kawai and Fushiki (8) interpreted their triolein + orlistat findings as evidence that fatty acid released in the mouth by lipolysis acts on fatty acid taste receptors and is responsible for the fat preference of animals. However, this is not necessarily the case. Rats and mice may prefer triolein or soybean oil to oil + orlistat because orlistat adds an aversive taste to the oil, although this does not explain the corn oil findings. Also Kawai and Fushiki (8) reported that rats equally preferred fatty acid emulsions with and without orlistat, suggesting that the drug did not have an “unpleasant flavor.” In any event, the results obtained with the high dose of orlistat in experiment 3 and by Kawai and Fushiki (8) do not mean that the same orlistat dose would block oil preferences relative to a fat-free vehicle. Experiment 4 therefore evaluated the effect of the 0.5% orlistat dose on the preference of naïve mice for triolein and soybean versus vehicle. To eliminate the possibility that an orlistat off-taste might influence preference, orlistat was added to both the oil and vehicle choices.
METHODS
Experiment 1
Subjects.
Adult male C57BL/6J (B6) mice (n = 20, 10 wk old) bred in the laboratory from Jackson Laboratories (Bar Harbor, ME) stock were singly housed in plastic tub cages kept in a test room maintained at 22°C with a 12:12-h light-dark cycle. The mice were maintained on chow (LabDiet 5001; PMI Nutrition International, Brentwood, MO) before food restriction. When food restricted during testing, they were fed fixed-size chow pellets (0.5 or 1 g, Bio-Serv, Frenchtown, NJ), which allowed for precise adjustment of daily food rations. The mice were fitted with a chronic gastric catheter and harness while anesthetized with isoflurane (2%) inhalation as previously described (19). Analgesia was provided by a subcutaneous injection of long-acting buprenorphine SR (1 mg/kg; ZooPharm, Windsor, CO) at the time of surgery.
Experimental protocols were approved by the Institutional Animal Care and Use Committee at Brooklyn College and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Apparatus.
IG infusion testing was conducted in plastic test cages with one or two stainless-steel sipper spouts as previously described (20). Licking behavior was monitored with electronic lickometers (model ENV-250B; Med Associates) interfaced to a computer that operated a syringe pump (A-99; Razel Scientific, Stamford, CT) that infused liquid into the gastric catheters as the animals licked. The pump rate was nominally 0.5 ml/min, but the animal controlled the overall infusion rate and volume by its licking response. The oral-to-infusion intake ratio was maintained at ~1:1 by adjusting the number of licks required per 3-s pump activation. Daily oral fluid intakes were measured to the nearest 0.1 g, and IG infusions were recorded to the nearest 0.5 ml.
Test solutions.
The CS solutions contained 0.025% sodium saccharin (Sigma Chemical, St. Louis, MO) flavored with 0.02% ethyl acetate or propyl acetate (Sigma) dissolved in deionized water. For half the mice, the CS+ contained ethyl acetate and the CS− contained propyl acetate; these flavor assignments were reversed for the remaining mice. These odors are readily discriminated by mice and have been used in many conditioning studies, e.g., Refs. 3, 21, 24, 33. The CS− solution was paired with IG infusion of water while the CS+ solution was paired with IG infusion of 5% soybean oil emulsion (Crisco vegetable oil; J. M. Smucker, Orrville, OH) in the control group. For the orlistat group, the emulsion contained 0.02% orlistat (Sigma), a dose (4 mg/g of fat) that altered fat preference in rats (1). The emulsions were prepared using deionized water and 0.15% Emplex (sodium stearoyl lactylate; American Products, Kansas City, MO) and were homogenized using an Ultra-Turrax T25 disperser (IKA-Works, Cincinnati, OH) at high speed for 5 min. Emplex rather than xanthan gum, as used in prior work (8), was used to prepare the emulsion because an oil-gum mixture would not pass through the infusion tubing.
Procedure.
pretraining.
Two weeks after surgery, the mice were trained (1 h/day) in the test cages to drink unflavored 0.025% saccharin while water deprived for two sessions; they were given 1-h access to water in home cages after the sessions. The mice were then trained for four sessions while given unlimited water but restricted food rations that kept them at 85–90% of their ad libitum body weight. Saccharin intakes were paired with matched volume infusions of water during pretraining sessions.
flavor conditioning.
The mice were offered CS solutions in daily 1-h sessions during training and testing. In the first three sessions, the mice drank the CS− solution paired with IG water infusions. The mice were divided into two groups (n = 10 each) equated for their licks and intakes during the last two CS− sessions. In the next three sessions (tests 1–3), the mice drank the CS+ solution paired with IG infusions of soybean oil emulsion with (Orlistat group) or without orlistat (control group). This was followed by four alternating sessions designed to facilitate discrimination of the CS+ and CS− flavors in the two-bottle tests. In these four sessions, the mice drank the CS−, CS+, CS−, and CS+, in that order, with each CS paired with its respective infusion. In the third and fourth alternating sessions, a second sipper tube was available containing water that was not paired with IG infusions; this familiarized the mice to the presence of two sipper tubes in the subsequent two-bottle test. The two-bottle test, with the CS+ and CS− solutions no longer paired with IG infusions, was conducted over the next four sessions. The positions of the CS+ and CS− bottles alternated daily during two-bottle testing.
postconditioning testing.
After the two-bottle tests, the mice were given six daily 1-h one-bottle test sessions with the CS+ paired with IG oil infusions. During all six tests the orlistat mice were infused with 5% soybean oil + orlistat as they drank the CS+. The control mice were infused IG with 5% soybean oil without orlistat during the first three tests and with 5% soybean oil + orlistat during the last three tests. The purpose of these tests was to determine if orlistat suppressed CS+ licking in the control mice to the level of that of orlistat mice.
Data analysis.
CS− licks and total intakes (oral + IG infusate) during the last two 1 h/day sessions were averaged. The data from these two sessions, referred to as test 0, and the licks and intakes during the next three CS+ sessions (tests 1–3) were analyzed using a mixed model ANOVA with a group factor and repeated measure factor (tests 0–3). The licks during the alternating sessions with CS− and CS+ were averaged and evaluated in a separate ANOVA. The licks during the first two and second two CS+ versus CS− two-bottle sessions were averaged (tests 1 and 2), which controlled for possible side preferences, and the percent preferences in these tests were calculated (CS+ licks/total licks × 100). These data were evaluated in separate ANOVAs.
Experiment 2
Apparatus.
Brief-access two-bottle lick tests were conducted in 10 plastic cages with stainless-steel perforated floors (35). Fluid was available from two stainless-steel sipper spouts through slots (5 × 20 mm, 32 mm apart) in a stainless steel plate at the front of the cage. The sipper spouts had a 2.5-mm hole and were attached to 50-ml glass tubes mounted on motorized bottle holders (ENV-252M; Med Associates, Georgia, VT). The bottle holders positioned the spouts 1 mm in front of the cage at the start of a session and retracted them away from the cage 3 min after the animal had emitted 10 licks. Licking behavior was monitored with electronic lickometers (ENV-250B, Med Associates) interfaced to a microcomputer.
Test solutions.
The soybean oil emulsions were prepared as in the first experiment using 2.5 and 5% soybean oil concentrations. Note that the 5% soybean oil emulsion infused in experiment 1 was diluted to 2.5% in the stomach by the ingested CS+ solution. After the soybean oil and 0.15% Emplex was processed in the Ultra-Turrax T25 disperser, 0.3% xanthan gum (Sigma) was added to the emulsion and mixed with a handheld blender (Braun Model 4169) for 5 min. A vehicle emulsion was prepared in the same manner but contained no soybean oil. The orlistat and control groups were tested with soybean oil emulsions with and without orlistat (4 mg/g of fat), respectively. In pretraining sessions, the mice were given a 4% sucrose solution (Domino Foods, Yonkers, NY) containing 0.3% xanthan gum and a gum vehicle (0.3% xanthan gum in water). These sessions served to train the animals to sample both spouts and accustom them to gum while keeping them naïve to oil.
Procedure.
Naive, adult male B6 mice (n = 20) were singly housed as in the first experiment. The mice were water deprived overnight and trained to drink water in the test cages from two sipper tubes in a 5-min session. They were then trained 3 min/day to drink 4% sucrose-gum versus gum for 4 days. After the 5-min and first 3-min sessions, the mice were given 1-h access to water in their home cages and then following all subsequent sessions they were given unlimited water and a food ration in their home cages that maintained them at 85–90% of ad libitum body weight. The mice were divided into two groups (n = 10 each) equated for their sucrose preference during the last two sessions. The control group was given two 3 min/day sessions with 2.5% soybean oil versus vehicle followed by another two sessions with 5% soybean oil versus vehicle. The orlistat group was similarly tested but with orlistat added to the soybean oil emulsions.
Experiment 3
Test solutions.
The oil suspensions were prepared using 2% triolein oil (Sigma), corn oil (Mazola oil; ACH Food, Oakbridge Terrace, IL), and soybean oil. The oil was mixed with 0.3% xanthan gum (Sigma) and deionized water using the Ultra-Turrax T25 disperser at high speed for 5 min. The oil plus 0.5% orlistat suspensions were prepared by first mixing orlistat in the oil and then adding it to the gum + water suspension and mixing it with the Ultra-Turrax T25 disperser. The triolein oil (>99% oleic acid triglyceride), was stored at −20°C as recommended by the supplier; the food-grade corn oil and soybean oil were stored at room temperature. In pretraining sessions, the mice were offered sucrose + 0.3% gum versus gum suspensions at sugar concentrations of 4% and 2%.
Procedure.
Naïve, adult male B6 mice (n = 10) were singly housed in tub cages and tested in lick cages as in experiment 2. The mice were water deprived overnight and trained to drink water in the test cages from two sipper tubes in 5-min sessions. They were given 4% sucrose/gum versus gum for four sessions; two while water restricted and two while food restricted. Daily water and food rations were given as in experiment 2. The food-restricted mice were then given two sessions (5 min/day) with 2% sucrose/gum versus gum. This was followed by a series of two-choice tests (5 min/day, 2 sessions each) with triolein versus triolein + orlistat, corn oil versus corn oil + orlistat, and soybean oil versus soybean oil + orlistat.
Experiment 4
Experiment 4A.
Naïve B6 mice were trained (5 min/day) to drink 4% sucrose/gum versus gum and then 2% sucrose/gum versus gum as in experiment 3. They were then divided into two groups equated for their 2% sucrose preferences. The orlistat group (n = 10) was given a two-choice test (5 min/day, 2 sessions each) with triolein oil + orlistat versus vehicle + orlistat and the control group (n = 10) was given the choice of triolein oil versus vehicle without the drug. The 2% triolein oil + gum emulsion was prepared as in experiment 3 with or without the addition of 0.5% orlistat. The gum emulsion was prepared as in experiment 2 with or without the addition of 0.5% orlistat.
Experiment 4B.
Naïve B6 mice were trained and tested as in experiment 4A except that the orlistat and control groups (n = 10 each) were tested with 2% soybean oil versus vehicle with or without the addition of 0.5% orlistat.
RESULTS AND DISCUSSION
Experiment 1
Flavor conditioning.
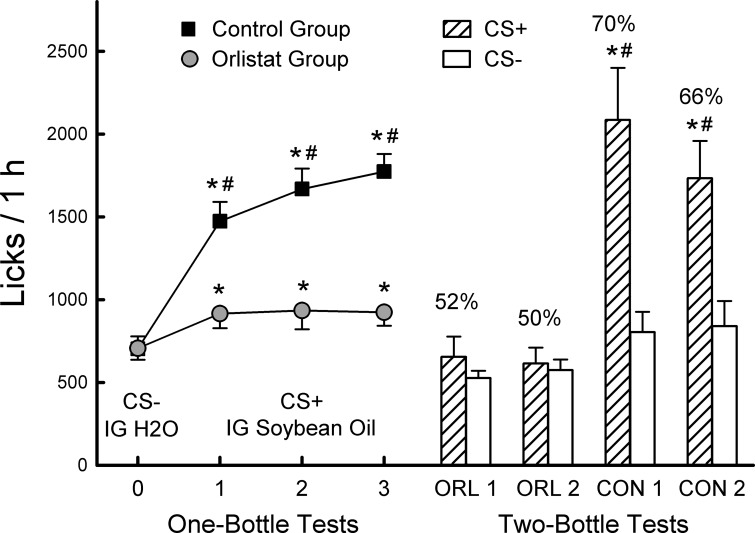
The groups did not differ in their CS− licks (test 0) paired with IG water infusions and increased their licks when switched to the CS+ paired with IG soybean oil infusions (tests 1–3) but by different amounts [group × test interaction, F(3,54) = 24.5, P < 0.001; Fig. 1]. In particular, the control mice increased their licks from CS− test 0 to CS+ test 3 by 174% while the orlistat mice increased their licks by only 36%. In addition, the control mice licked more for the CS+ than did the orlistat mice in each of the three CS+ tests. The control group also increased their total fluid intakes substantially from CS− test 0 to CS+ test 3 (2.1 to 3.8 g/h) whereas the orlistat group showed a smaller, nonsignificant increase [2.0 to 2.4 g/h; group × test interaction, F(3,54) = 15.4, P < 0.001]. During the alternating training sessions (data not shown), the control mice licked more for the CS+ than CS− (1,718 vs. 1,498 licks, P < 0.01) whereas the orlistat group did not (829 vs. 860 licks).
Fig. 1.
Experiment 1. Licks (means ± SE) for conditioned stimulus (CS− and CS+) flavored solutions paired with intragastric (IG) infusions of water and 5% soybean oil, respectively, during 1-bottle tests and without IG infusions during 2-bottle tests. Left: the mice drank (1 h/day) a CS− flavored solution paired with IG water infusions in test 0 (mean of the last 2 CS- sessions) and a CS+ flavored solution paired with IG 5% soybean oil infusions in tests 1–3. The orlistat (ORL) group (n = 10) was infused with oil + orlistat and the control (CON) group (n = 10) was infused with oil without orlistat. Right: 1-h licks (means +SE) of CS− and CS+ during the 2-day 2-bottle tests 1 (ORL1, CON 1) and 2 (ORL 2, CON 2). Number above bar represents mean percent preference for the CS+ solution. #Significant differences (P < 0.05) between 1-bottle tests 0 and tests 1–3 licks and between 2-bottle CS+ vs. CS− licks. *Significant difference (P < 0.05) between orlistat and control group CS+ licks.
In the two-bottle choice tests (Fig. 1), the control mice licked significantly more for the CS+ than CS− whereas the orlistat group licked similarly for the two flavors [group × CS interaction, F(1,18) = 6.9, P < 0.05]. Percent CS+ preferences for the control group exceeded those of the orlistat group [F(1,18) = 8.4, P < 0.01], and both groups displayed only small declines from the first to second test. However, CS+ licks declined more from tests 1 to 2 in the control group than the orlistat group [group × CS × test interaction, F(1,18) = 4.6, P < 0.05] which was not unexpected given that the two-bottle tests were “extinction” sessions, in that the CS+ was no longer paired with IG fat infusions.
Postconditioning testing.
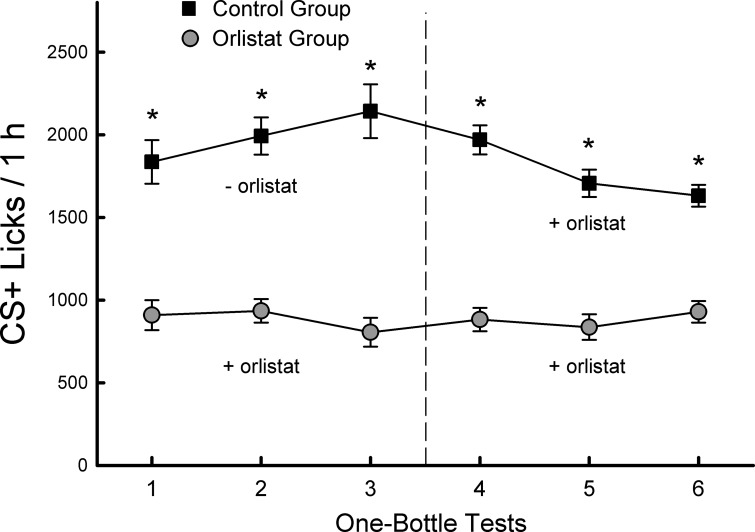
In the IG fat reinforced one-bottle sessions that followed the two-bottle tests, the control mice licked substantially more for the CS+ than did the orlistat mice [F(1,18) = 76.3, P < 0.001] (Fig. 2). Whereas the licking response of the orlistat mice during the six sessions was stable, the response of the control mice varied [group × session interaction, F(5,90) = 6.9, P < 0.001]. In particular, the control mice increased their CS+ licking response during the first three sessions that were paired with IG soybean oil infusions. They then decreased their CS+ licks in sessions 4–6 when infused with IG soybean oil + orlistat. However, their licks during the last two sessions (5, 6) with orlistat did not significantly differ from session 1 (without orlistat) and remained above that of the orlistat group. Consequently, the control mice self-infused significantly more oil + orlistat in sessions 5 and 6 than did the orlistat mice [1.6 vs. 1.2 ml/h, t(18) = 3.4, P < 0.01]. The control mice consumed more CS+ (and oil + orlistat) in last two sessions than did the orlistat mice [3.3 vs. 2.2 g/h, t(18) = 6.3, P < 0.001] and their CS+ IG intakes in these sessions did not differ from their intakes during the last two alternating training sessions when infused with oil without orlistat (3.3 vs. 3.6 g/h).
Fig. 2.
Experiment 1. Licks (means ± SE) for CS+ flavored solutions paired with intragastric (IG) infusions of 5% soybean oil during daily 1-bottle tests of postconditioning test sessions. The orlistat group (n = 10) was infused with soybean oil + orlistat during all tests. The control group (n = 10) was infused with soybean oil without orlistat in tests 1–3 and with soybean oil + orlistat in tests 4–6. *Significant difference (P < 0.05) between orlistat and control group CS+ licks.
The stimulation of CS+ licks and the conditioned CS+ preference produced by the IG 5% soybean oil infusions in the control mice are consistent with prior results obtained with IG infusions of 3.2 and 6.4% Intralipid (a commercial soybean oil emulsion) (3, 33). The new findings here are that adding orlistat to the oil infusion substantially reduced the stimulation of CS+ intake during one-bottle sessions and blocked CS+ preference conditioning as revealed in two-bottle tests. Conceivably, the soybean oil + orlistat infusions may have had aversive gastrointestinal consequences in the orlistat mice. However, the finding that the soybean oil + orlistat infusions in the orlistat mice did not decrease their CS+ licking over one-bottle sessions and did not produce CS+ avoidance in the two-bottle tests strongly indicates that oil + orlistat infusion did not have aversive effects. This is further supported by the finding that the control mice showed only a relatively small reduction in their elevated CS+ lick rates when infused with soybean oil + orlistat in the postconditioning tests.
In contrast to the decreased CS+ licking displayed by the control mice when they were switched from soybean oil to soybean oil + orlistat infusions, Kleberg et al. (9) reported that mice self-infusing IG soybean oil by licking a dry sipper tube increased their rate of licking when orlistat was added to the soybean oil infusion. In the study of Kleberg et al., however, the mice self-infused 30% soybean oil and the orlistat was administered as a preload before the soybean oil infusion. This route of administration is likely less effective than mixing the orlistat in the soybean oil infusion as in the present experiment. Consequently, the orlistat treatment in the study of Kleberg et al. may have reduced rather than blocked the reinforcing action of the soybean oil infusion. Consistent with this interpretation, mice licking a dry sipper tube for IG infusions of concentrated soybean oil emulsions (20–30%) increase their rate of licking when infused with more dilute emulsions (5–15%) (9, 23, 27). In contrast, we reported that mice self-infusing 5% soybean oil decreased their licking rate when infused with water instead (23), which is consistent with the results obtained with the control mice switched to the soybean oil + orlistat infusions.
The CS+ preference results confirm the findings of a preliminary study in which naïve B6 mice were given six alternating training sessions with a CS+ paired with IG 5% soybean oil and a CS− paired with IG water followed by a two-bottle choice test. Mice infused with soybean oil containing doses of orlistat at 4 or 8 mg/g of fat (8 mice/group) failed to prefer the CS+ (47% and 51% CS+ percent intakes), whereas control mice (n = 7) infused with soybean oil without orlistat displayed an 88% CS+ preference. Taken together, these findings demonstrate that lipolysis of infused triglyceride to fatty acids is essential for postoral fat conditioning in mice. We next turned to examination of oral preferences for oil when lipolysis is inhibited.
Experiment 2
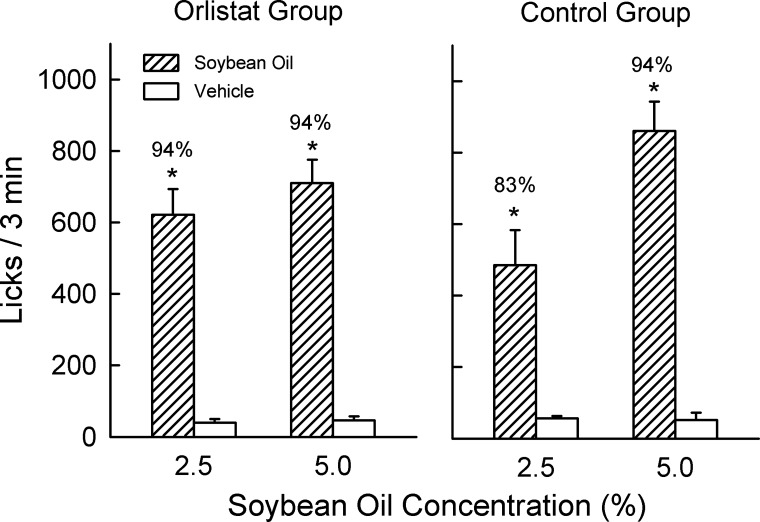
When given the choice between soybean oil and vehicle, the orlistat and control groups both licked more for soybean oil than vehicle [F(1,18) = 144.9, P < 0.001] and increased their licks when oil concentration increased from 2.5 to 5% [F(1,18) = 26.3, P < 0.001; Fig. 3]. The two groups did not differ in their soybean oil licks or percent preference. The somewhat lower licks and preference for 2.5% oil displayed by the control group were due largely to one mouse that emitted very few licks (24 licks/3 min) for the 2.5% oil but who responded strongly for the 5% oil emulsion (810 licks/3 min).
Fig. 3.
Experiment 2. Licks (means ± SE) for 2.5 or 5% soybean oil and vehicle during 3-min, 2-bottle tests. The orlistat group (n = 10) drank soybean oil with orlistat (4 mg/g of fat), and the control group (n = 10) drank soybean oil without orlistat. Number above bar represents mean percent preference for soybean oil. *Significant differences (P < 0.05) between soybean oil and vehicle licks within each test.
These findings indicate that orlistat at a dose that blocked flavor preference conditioning by IG soybean oil infusions in Experiment 1 had no effect on the oral preference of B6 mice for 2.5 or 5% soybean oil. It is conceivable, however, that the orlistat dose was too low to block oral fat preferences. Subsequent experiments addressed this issue.
Experiment 3
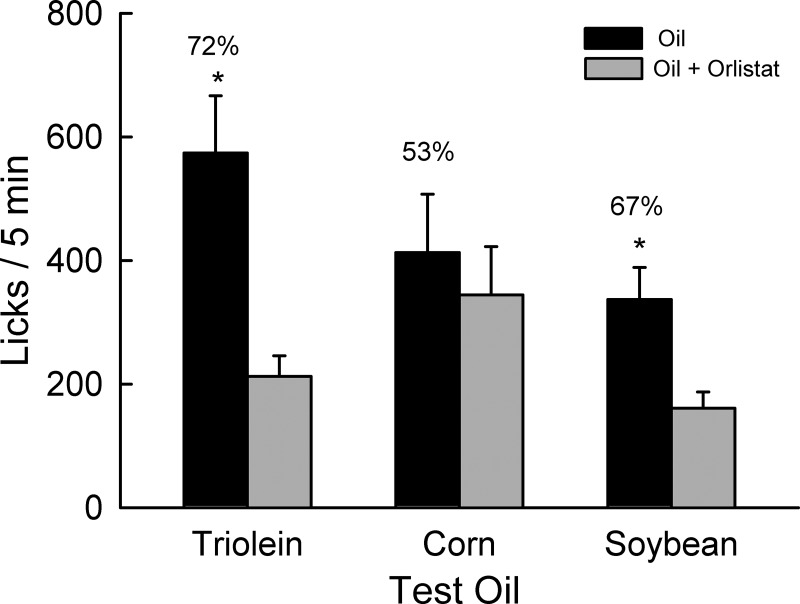
Overall, the mice consumed more of the orlistat-free oil than oil containing orlistat [F(1,9) = 15.1, P < 0.01], but the magnitude of this difference varied as a function of the type of oil [oil × drug interaction, F(2,8) = 7.2, P < 0.01]. In particular, the mice licked more (P < 0.01) for triolein than triolein + orlistat and more for soybean oil than soybean oil + orlistat (P < 0.01), but they did not differ significantly in their licks for corn oil versus corn oil + orlistat (Fig. 4). Furthermore, the preferences for triolein and soybean oil over their orlistat alternatives did not differ (72 vs. 67%) but exceeded (P < 0.05) the percent intake of corn oil versus corn oil + orlistat [53%, F(2,18) = 8.2, P < 0.01]. Total licks varied across the three tests [F(2,18) = 4.6, P < 0.05] in that the mice licked less (P < 0.05) for soybean oil (498.1) than for triolein oil (786.2) and corn oil (757.0).
Fig. 4.
Experiment 3. Licks (means ± SE) for 2% triolein oil, 2% corn oil, and 2% soybean oil with and without orlistat (0.5%, 250 mg/g of fat) during 5-min, 2-bottle tests (n = 10). Number above bar represents mean percent preference for oil without orlistat. *Significant differences (P < 0.05) between oil vs. oil + orlistat licks within each test.
The findings that B6 mice preferred 2% triolein to 2% triolein plus 0.5% orlistat but were indifferent to corn oil with or without orlistat are similar to results obtained with rats (8). In fact, the percent triolein and corn oil preferences obtained with the rats and mice were nearly identical (73 vs. 72% and 56 vs. 53%, respectively), which is remarkable given the species difference and the prior training the rats had with triolein oil. The mice in the present experiment also significantly preferred 2% soybean oil to soybean oil + orlistat. With respect to their different findings with triolein and corn oil, Kawai and Fushiki (8) speculated that commercial corn oil may contain “some attractive ingredients” other than triglyceride or fatty acid that accounts for the rats’ indifference to corn oil with and without orlistat. Corn oil has about three times more phytosterols than soybean oil (866 vs. 298 mg/100 g; Ref. 31), and these plus tocopherols are <1% by weight of the oils, so each is >99% triglyceride. Attraction to these nontriglyceride components of vegetable oils has not been studied. Their higher level in corn oil than soybean oil may account for the present results, though they would be present in only miniscule amounts in the 2% oils.
It is possible that differences in trace amounts of free fatty acid found in triolein (oleic acid only) and corn oil (oleic, linoleic, and linolenic) could account for the differential effect of orlistat on the preferences for the two oils. When attraction to 1% preparations of fatty acids were compared, rats preferred linolenic to linoleic, which in turn was preferred to oleic (30). However, this would predict that soybean oil, which contains 7% by weight linolenic acid, should have a more attractive flavor than corn oil with only 1%, and both should be more attractive than all-oleic triolein. Further research is required to account for the orlistat effects obtained with triolein, corn, and soybean oil.
The differences in total licks displayed by the mice in the three tests may represent differences in the attractiveness of the different oils (but see experiment 4 below) or the lesser intake of soybean oil in the third test may represent cumulative effects of orlistat on fat acceptance as observed in experiment 1 (see Fig. 2) and in our earlier rat study (1).
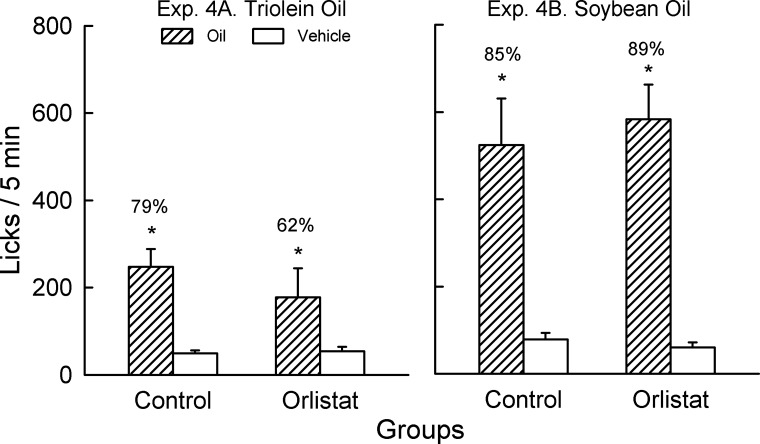
Experiment 4
Experiment 4A.
In the two-bottle choice test with triolein oil versus vehicle, both the orlistat and control groups licked more for oil than vehicle [F(1,18) = 19.0, P < 0.001] and there were no group differences (Fig. 5). The percent triolein preference was numerically greater in the control than orlistat group but the difference (79 vs. 62%) was not significantly different.
Fig. 5.
Experiment 4. Licks (means ± SE) for 2% oil vs. gum vehicle in control and orlistat groups (n = 10 each) during 5-min, 2-bottle tests. Left: experiment 4A: 2% triolein oil. Right: experiment 4B: 2% soybean oil. Orlistat (0.5%, 250 mg/g of fat) was added to the oil and vehicle in the orlistat group. Number above bar represents mean percent preference for oil. *Significant differences (P < 0.05) between oil vs. vehicle licks within each test.
Experiment 4B.
Both the orlistat and control groups licked more for soybean oil than vehicle [F(1,18) = 57.6, P < 0.001], and there were no group differences (Fig. 5). The percent soybean oil preferences were similar in the control and orlistat groups (85 vs. 89%).
Consistent with the results of experiment 2, the control groups significantly preferred 2% soybean and triolein oil to the gum vehicle. The new finding is that the addition of 0.5% orlistat, a dose 60 times higher than used in the second experiment, did not significantly reduce the preference for triolein or soybean oil relative to the vehicle. Thus, although the mice in experiment 3 preferred the triolein and soybean oil to the same oil containing 0.5% orlistat, inhibiting lipolysis with this high dose did not block the attractiveness of the oils.
Contrary to the results obtained in experiment 3, the mice tested with soybean oil (with or without orlistat) licked substantially more than did the mice given triolein. The animals in experiments 4A and 4B did not differ in age, body weight or their licks for 2% sucrose before the oil test. Conceivably, soybean oil may be more palatable to naïve mice than triolein oil. This was not confirmed, however, in 5-min choice tests of 2% oil conducted with naïve mice (n = 12). In an initial test, the mice did not significantly differ in their licks for soybean oil versus triolein oil (311.7 vs. 349.3 licks/5 min). The mice also did not differ in their licks for soybean oil versus vehicle (369.2 vs. 72.0 licks/5 min) or triolein versus vehicle (431.9 vs. 71.6 licks/5 min) in tests conducted in a counterbalanced order.
General Discussion
The present findings indicate that inhibiting lipolysis with orlistat prevents the postoral intake stimulating and flavor conditioning actions of IG soybean oil infusions in mice. In contrast, orlistat at low or high doses did not block the oral preferences for soybean or triolein oil emulsions over a fat-free emulsion. However, orlistat reduced the preference for soybean or triolein oil relative to the same oil without the drug, but this was not case for corn oil.
The postoral flavor conditioning actions of fat are well documented in rodents (2, 4). Recent mouse studies demonstrate that IG soybean oil infusions rapidly stimulate the intake of a CS+ solution and condition a robust preference over a CS− solution paired with IG water infusions (3, 20, 33). This appetition effect is greatly attenuated in GPR40/GPR120 DoKO mice, indicating a critical role for intestinal fatty acid receptors in postoral fat conditioning. Consistent with these findings, experiment 1 revealed that adding orlistat to an IG soybean oil infusion greatly attenuated the intake stimulation and blocked the development of a flavor preference. The IG conditioning results support our early suggestion that orlistat reduces the postoral reinforcing action of fat in rats (1). In experiment 2, the same dose of orlistat that blocked IG fat conditioning did not attenuate the oral preference and acceptance of mice for soybean oil over the fat-free emulsion. This finding is also compatible with our early finding that the same orlistat dose did not influence the initial short-term fat preference of rats. Taken together, the results suggest that lipolysis-released fatty acids are essential for the postoral but not the oral preference for fats.
Experiment 3 further investigated the actions of orlistat on fat preference using three different fats and the high dose of orlistat (0.5%) utilized by Kawai and Fushiki (8). Consistent with their findings obtained with rats, B6 mice significantly preferred triolein oil to triolein oil + orlistat but showed no such preference for corn oil with and without orlistat. The same mice also preferred food-grade soybean oil to soybean oil + orlistat. As previously noted, why orlistat differentially altered the preferences for triolein and soybean oils but not corn oil is not certain, and might reflect differential amounts of non-triglyceride components. Nevertheless, experiment 4 revealed that the high orlistat dose did not block the preferences naïve mice displayed for triolein or soybean oil versus a fat-free vehicle. These findings, along with those of experiment 2, indicate that lipolysis of fat in the mouth is not essential for the expression of fat preferences in mice. Whether the same is true for rats remains to be determined in that Kawai and Fushiki (8) did not investigate the effects of orlistat on the rats’ preference for fat versus a fat-free vehicle.
It could be argued that the fat preferences displayed by the orlistat groups in experiments 2 and 4 were due to the nongustatory orosensory features of 2–2.5% soybean and triolein oil, e.g., odor or oily texture not masked by the gum. This is not supported, however, by our findings (17) obtained with ageusic CALHM1 KO mice, which are indifferent to sweet, umami, or bitter tastants in brief-access tests (26). Unlike wild-type controls, CALHM1 KO mice failed to prefer 2.5% soybean oil to a fat-free gum vehicle in 3-min choice tests. They did, however, prefer a 10% soybean oil emulsion, indicating that nongustatory oral stimuli can support preferences for concentrated fat sources.
From our CALHM1 KO findings, the significant fat preferences displayed by the orlistat groups in the present study suggest that, in addition to fatty acid receptors, triglyceride taste receptors contribute to fat preferences in mice. Some human findings also suggest the existence of triglyceride receptors (Refs. 10, 13 but see Refs. 12, 32 for an opposing view). Additional evidence that fatty acid receptors do not fully account for the attraction of mice for fat is our finding that B6 mice consume four times more 2% soybean oil emulsion than 2% linoleic acid emulsion in 24-h tests versus vehicle (18).
Perspectives and Significance
The proposal for the existence of multiple types of fat taste receptors is not unprecedented in light of the evidence for multiple carbohydrate taste receptors. We long ago proposed (14) that rodents have maltodextrin taste receptors distinct from sugar (sweet) receptors, which is now well documented by a variety of findings including results obtained with various taste knockout mice (25, 28, 29, 35). There is also some evidence for a starch taste receptor distinct from maltodextrin and sugar receptors (25, 36). Furthermore, in parallel with the present orlistat findings, we have observed similar results with acarbose, a drug that inhibits carbohydrate digestion by blocking salivary and pancreatic amylase enzymes. That is, the addition of acarbose to a maltodextrin solution counteracted its postoral flavor conditioning actions but not its attractive taste (5, 14, 15, 22). Subsequent studies indicated that intestinal glucose sensors, including SGLT1, but not the T1r2+T1r3 sweet receptor, mediate postoral carbohydrate preference conditioning (19, 21, 34). Recent findings suggest that fat appetite in mice is mediated, in part, by fatty acid taste receptors (CD36) (11, 17, 18) and intestinal fatty acid receptors (GPR40 and GPR120) (23, 24) and the present results suggest the involvement of triglyceride taste receptors as well.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-031135.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S. and K.A. conception and design of research; A.S. analyzed data; A.S. and K.A. interpreted results of experiments; A.S. prepared figures; A.S. and K.A. drafted, edited and revised manuscript and approved final version of manuscript; K.M., A.S.V., and M.Z. performed experiments.
ACKNOWLEDGMENTS
We thank Kwame McCartney, Austin S. Vural, and Martin Zartarian for technical assistance.
REFERENCES
- 1.Ackroff K, Sclafani A. Effects of the lipase inhibitor orlistat on intake and preference for dietary fat in rats. Am J Physiol Regul Integr Comp Physiol 271: R48–R54, 1996. doi: 10.1152/ajpregu.1996.271.1.R48. [DOI] [PubMed] [Google Scholar]
- 2.Ackroff K, Sclafani A. Oral and post-oral determinants of dietary fat appetite. In: Fat Detection: Taste, Texture, and Post Ingestive Effects, edited by Montmayeur JP, le Coutre J. Boca Raton, FL: Taylor & Francis, 2010, p. 295–321. [PubMed] [Google Scholar]
- 3.Ackroff K, Sclafani A. Post-oral fat stimulation of intake and conditioned flavor preference in C57BL/6J mice: a concentration-response study. Physiol Behav 129: 64–72, 2014. doi: 10.1016/j.physbeh.2014.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besnard P, Passilly-Degrace P, Khan NA. Taste of fat: a sixth taste modality? Physiol Rev 96: 151–176, 2016. doi: 10.1152/physrev.00002.2015. [DOI] [PubMed] [Google Scholar]
- 5.Elizalde G, Sclafani A. Starch-based conditioned flavor preferences in rats: influence of taste, calories and CS-US delay. Appetite 11: 179–200, 1988. doi: 10.1016/S0195-6663(88)80002-3. [DOI] [PubMed] [Google Scholar]
- 6.Gilbertson TA, Khan NA. Cell signaling mechanisms of oro-gustatory detection of dietary fat: advances and challenges. Prog Lipid Res 53: 82–92, 2014. doi: 10.1016/j.plipres.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Glendinning JI, Feld N, Goodman L, Bayor R. Contribution of orosensory stimulation to strain differences in oil intake by mice. Physiol Behav 95: 476–483, 2008. doi: 10.1016/j.physbeh.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Kawai T, Fushiki T. Importance of lipolysis in oral cavity for orosensory detection of fat. Am J Physiol Regul Integr Comp Physiol 285: R447–R454, 2003. doi: 10.1152/ajpregu.00729.2002. [DOI] [PubMed] [Google Scholar]
- 9.Kleberg K, Jacobsen AK, Ferreira JG, Windeløv JA, Rehfeld JF, Holst JJ, de Araujo IE, Hansen HS. Sensing of triacylglycerol in the gut: different mechanisms for fatty acids and 2-monoacylglycerol. J Physiol 593: 2097–2109, 2015. doi: 10.1113/jphysiol.2014.285635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni BV, Mattes RD. Lingual lipase activity in the orosensory detection of fat by humans. Am J Physiol Regul Integr Comp Physiol 306: R879–R885, 2014. doi: 10.1152/ajpregu.00352.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest 115: 3177–3184, 2005. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pepino MY, Love-Gregory L, Klein S, Abumrad NA. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J Lipid Res 53: 561–566, 2012. doi: 10.1194/jlr.M021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed DR, Xia MB. Recent advances in fatty acid perception and genetics. Adv Nutr 6: 353S–360S, 2015. doi: 10.3945/an.114.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sclafani A. Carbohydrate taste, appetite, and obesity: an overview. Neurosci Biobehav Rev 11: 131–153, 1987. doi: 10.1016/S0149-7634(87)80019-2. [DOI] [PubMed] [Google Scholar]
- 15.Sclafani A. Carbohydrate appetite in rats: taste and postingestive factors. Appetite 11, Suppl 1: 20–25, 1988. doi: 10.1016/S0195-6663(88)80042-4. [DOI] [PubMed] [Google Scholar]
- 16.Sclafani A. Gut-brain nutrient signaling. Appetition vs. satiation. Appetite 71: 454–458, 2013. doi: 10.1016/j.appet.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sclafani A, Ackroff K. Greater reductions in fat preferences in CALHM1 than CD36 knockout mice. Am J Physiol Regul Integr Comp Physiol. In press. doi: 10.1152/ajpregu.00015.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol 293: R1823–R1832, 2007. doi: 10.1152/ajpregu.00211.2007. [DOI] [PubMed] [Google Scholar]
- 19.Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol 299: R1643–R1650, 2010. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol 289: R712–R720, 2005. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- 21.Sclafani A, Koepsell H, Ackroff K. SGLT1 sugar transporter/sensor is required for post-oral glucose appetition. Am J Physiol Regul Integr Comp Physiol 310: R631–R639, 2016. doi: 10.1152/ajpregu.00432.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sclafani A, Nissenbaum JW. Oral versus postingestive origin of polysaccharide appetite in the rat. Neurosci Biobehav Rev 11: 169–172, 1987. doi: 10.1016/S0149-7634(87)80022-2. [DOI] [PubMed] [Google Scholar]
- 23.Sclafani A, Touzani K, Ackroff K. Intragastric fat self-administration is impaired in GPR40/120 double knockout mice. Physiol Behav 147: 141–148, 2015. doi: 10.1016/j.physbeh.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sclafani A, Zukerman S, Ackroff K. GPR40 and GPR120 fatty acid sensors are critical for postoral but not oral mediation of fat preferences in the mouse. Am J Physiol Regul Integr Comp Physiol 305: R1490–R1497, 2013. doi: 10.1152/ajpregu.00440.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Fat and carbohydrate preferences in mice: the contribution of α-gustducin and Trpm5 taste-signaling proteins. Am J Physiol Regul Integr Comp Physiol 293: R1504–R1513, 2007. doi: 10.1152/ajpregu.00364.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 495: 223–226, 2013. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tellez LA, Ferreira JG, Medina S, Land BB, DiLeone RJ, de Araujo IE. Flavor-independent maintenance, extinction, and reinstatement of fat self-administration in mice. Biol Psychiatry 73: 851–859, 2013. doi: 10.1016/j.biopsych.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Treesukosol Y, Blonde GD, Spector AC. T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol 296: R855–R865, 2009. doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treesukosol Y, Smith KR, Spector AC. Behavioral evidence for a glucose polymer taste receptor that is independent of the T1R2+3 heterodimer in a mouse model. J Neurosci 31: 13527–13534, 2011. doi: 10.1523/JNEUROSCI.2179-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuruta M, Kawada T, Fukuwatari T, Fushiki T. The orosensory recognition of long-chain fatty acids in rats. Physiol Behav 66: 285–288, 1999. doi: 10.1016/S0031-9384(98)00299-6. [DOI] [PubMed] [Google Scholar]
- 31.United States Department of Agriculture Agricultural Research Service USDA Food Composition Databases. https://ndb.nal.usda.gov/ndb/search/ [2016].
- 32.Voigt N, Stein J, Galindo MM, Dunkel A, Raguse JD, Meyerhof W, Hofmann T, Behrens M. The role of lipolysis in human orosensory fat perception. J Lipid Res 55: 870–882, 2014. doi: 10.1194/jlr.M046029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zukerman S, Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol Regul Integr Comp Physiol 301: R1635–R1647, 2011. doi: 10.1152/ajpregu.00425.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zukerman S, Ackroff K, Sclafani A. Postoral appetite stimulation by sugars and nonmetabolizable sugar analogs. Am J Physiol Regul Integr Comp Physiol 305: R840–R853, 2013. doi: 10.1152/ajpregu.00297.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol Regul Integr Comp Physiol 296: R866–R876, 2009. doi: 10.1152/ajpregu.90870.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. Impact of T1r3 and Trpm5 on carbohydrate preference and acceptance in C57BL/6 mice. Chem Senses 38: 421–437, 2013. doi: 10.1093/chemse/bjt011. [DOI] [PMC free article] [PubMed] [Google Scholar]