Abstract
Left ventricular (LV) twist mechanics differ between men and women during acute physiological stress, which may be partly mediated by sex differences in autonomic control. While men appear to have greater adrenergic control of LV twist, the potential contribution of vagal modulation to sex differences in LV twist remains unknown. Therefore, the present study examined the role of vagal control on sex differences in LV twist during graded lower body negative pressure (LBNP) and supine cycling. On two separate visits, LV mechanics were assessed using two-dimensional speckle-tracking echocardiography in 18 men (22 ± 2 yr) and 17 women (21 ± 4 yr) during −40- and −60-mmHg LBNP and 25% and 50% of peak supine cycling workload with and without glycopyrrolate (vagal blockade). LV twist was not different at baseline but was greater in women during −60 mmHg in both control (women: 16.0 ± 3.4° and men: 12.9 ± 2.3°, P = 0.004) and glycopyrrolate trials (women: 17.7 ± 5.9° and men: 13.9 ± 3.3°, P < 0.001) due to greater apical rotation during control (women: 11.9 ± 3.6° and men: 7.8 ± 1.5°, P < 0.001) and glycopyrrolate (women: 11.6 ± 4.9° and men: 7.1 ± 3.6°, P = 0.009). These sex differences in LV twist consistently coincided with a greater LV sphericity index (i.e., ellipsoid geometry) in women compared with men. In contrast, LV twist did not differ between the sexes during exercise with or without glycopyrrolate. In conclusion, women have augmented LV twist compared with men during large reductions to preload, even during vagal blockade. As such, differences in vagal control do not appear to contribute to sex differences in the LV twist responses to physiological stress, but they may be related to differences in ventricular geometry.
NEW & NOTEWORTHY This is the first study to specifically examine the role of vagal autonomic control on sex-related differences in left ventricular (LV) mechanics. Contrary to our hypothesis, vagal control does not appear to primarily determine sex differences in LV mechanical or hemodynamic responses to acute physiological stress. Instead, differences in LV geometry may be a more important contributor to sex differences in LV mechanics.
Keywords: autonomic nervous system, echocardiography, exercise, left ventricular mechanics, sex differences
INTRODUCTION
Vagal autonomic activity is an important regulator of cardiac function. Reflexive adjustments to vagal (muscarinic) and sympathetic (adrenergic) activation constitutively adjust cardiac chronotropy and contractility, particularly in response to hemodynamic challenges (24, 39, 42). This sympathovagal control has been reported to differ between men and women, as studies assessing heart rate (HR) variability at rest demonstrated augmented indexes of increased parasympathetic activity in women and increased sympathetic mediation in men (2, 12, 13). Moreover, there is evidence for sex differences in the autonomic responses to acute stressors such as during graded reductions to preload and increasing exercise intensities, both of which involve continuous vagal withdrawal with reciprocal sympathetic activation (39, 42). During these acute challenges, men generally have larger increases in muscle sympathetic nerve activity (28) and circulating catecholamines (8, 14, 38), whereas women often have augmented HR responses that have been suggested to reflect greater vagal withdrawal (14, 28, 38). However, it is not known whether the progressive adjustments to sympathovagal balance differ between men and women during graded levels of acute physiological stress.
Variations in vagal or sympathetic activation could have important implications for sex-related differences in cardiac mechanics and hemodynamic responses to acute stressors. Left ventricular (LV) mechanics (rotation, twist, and strain) fundamentally support the cardiac responses to such challenges, as increases in LV twist counter the decline in stroke volume (SV) during lower body negative pressure (LBNP) (18, 41) and augment SV during dynamic exercise (11, 29). Our group has demonstrated that women have greater LV twist and faster untwisting velocities than men during higher levels of LBNP (41), although potential sex differences in LV twist mechanics during dynamic exercise have not yet been characterized. Given that the LV responses to LBNP and exercise are critically dependent on progressive adjustments to sympathovagal balance, it is feasible that sex differences in vagal or sympathetic cardiac control could contribute to differences in LV twist mechanics among men and women during LBNP and exercise. Indeed, adrenergic stimulation is a key modulator of LV twist mechanics (1, 10, 26), and we have recently presented evidence that LV twist is more sensitive to altered β1-adrenergic stimulation in men compared with women (40), yet these differences in adrenergic control do not explain the augmented LV twist responses in women compared with men during LBNP. It remains to be determined whether sex-related differences in vagal activity and/or withdrawal contribute to the larger responses of LV twist in women compared with men during acute stressors.
To date, no studies have investigated the role of vagal control on LV twist mechanics. As such, we aimed to determine the influence of vagal autonomic control on sex differences in LV twist during graded reductions to preload with LBNP and dynamic exercise using the muscarinic receptor antagonist glycopyrrolate (GLY). Assuming that greater vagal withdrawal contributes to the augmented LV twist responses during acute physiological stress in women, we tested the hypothesis that women would have larger LV twist than men during −60-mmHg LBNP and exercise at 50% of peak power output (POpeak) and that these sex differences in LV twist would be minimized after the administration of GLY.
METHODS
Study Participants
Participants aged 19–39 yr were recruited from the local university community. Exclusion criteria included history of cardiovascular, respiratory, or musculoskeletal disease, a body mass index of >30 kg/m2, resting systolic blood pressure of ≥140 or ≤100 mmHg, current smokers, performance of ≥5 h of moderate-intensity exercise or ≥3 bouts/wk of high-intensity training (25, 34), inadequate imaging windows, or inability to tolerate −60-mmHg LBNP. The study was approved by The University of British Columbia (H15-02490) and Cardiff Metropolitan University (15/9/03S) research ethics boards and informed consent was obtained from all participants.
Study Design
Participants visited the laboratory on three occasions and refrained from caffeine, exercise, and alcohol for ≥24 h before each visit. In the initial visit, participants were screened for adequate imaging windows, resting blood pressure, and LBNP tolerance as previously described (41). Participants able to tolerate LBNP then completed an incremental exercise test to volitional fatigue to determine POpeak on a supine cycle ergometer.
For the second and third visits, participants completed control (CON) or vagal blockade (GLY) experiments on separate days. During both visits, participants were sealed at the level of the iliac crests in an LBNP chamber as previously described (41). LBNP was applied at −40 and −60 mmHg, and images were acquired once HR and mean arterial pressure (MAP) had stabilized. After LBNP, participants rested for ≥20 min before exercising at 25% and 50% of POpeak in the left lateral supine position [exercise (EX) trials]. Images were acquired during exercise once steady state was achieved (i.e., change in HR of <5 beats/min).
For GLY experiments, baseline (preinfusion) imaging was performed in the LBNP chamber. Participants then received stepwise infusions of 0.2 mg GLY through an indwelling venous catheter every 2 min until HR was unchanged to consecutive 0.2-mg doses (27). Postinfusion images were collected at rest before LBNP. An additional 0.2 mg was administered before GLY-EX trials to ensure full blockade throughout the protocol. Total doses were 1.0 ± 0.3 mg for women and 1.1 ± 0.3 mg for men (P = 0.34).
Specific Methodology
Supine incremental exercise testing.
Incremental cycling tests started at 75 W for men and 50 W for women and were performed to volitional exhaustion on a supine cycle ergometer (Lode, Angio, Groningen, The Netherlands) using a ramp protocol of 20 W/min for women and 25 W/min for men.
Transthoracic echocardiography.
All imaging was performed by an experienced sonographer using a commercially available ultrasound system (Vivid E9, GE Healthcare) and M5S 1.5- to 4.6-MHz and 4V 1.5- to 4.0-MHz transducers. All images were acquired with the participant tilted left laterally toward the sonographer. Two-dimensional, triplane, and pulsed Doppler recordings were acquired at end expiration for the assessment of LV structure and function in accordance with current guidelines (22). During exercise, particular care was taken to ensure that images were acquired during a transient breath-hold at end expiration (~2–3 s), with participants cycling at a low cadence of ~40 revolutions/min to reduce torso movement and image blur. Measurements of LV hemodynamics [end-diastolic volume (EDV), end-systolic volume (ESV), SV, and ejection fraction (EF)] and structure [posterior wall thickness (PWT), interventricular septal wall thickness, LV end-diastolic internal diameter (LVIDd), and LV length (lengthd)] were performed as previously described (40, 41). The LV sphericity index was calculated as LV lengthd/LVIDd, and relative wall thickness was calculated as 2 × PWT/LVIDd. To account for sex-related differences in LV size, LV dimensions were scaled allometrically to body surface area0.5, and LV volumes were scaled to body surface area1.5 (3).
Speckle-tracking analysis.
A single experienced sonographer performed all analyses and was blinded to participant sex and experimental condition with all identifiers removed from the imaging files. Images for speckle-tracking analysis were acquired at >70 frames/s and at least ≥90 frames/s during exercise for adequate time resolution during analysis. Measurements of LV rotation and strain were performed for three cardiac cycles as previously described (41). Rotational parameters were assessed in short-axis views at the level of the LV base (mitral valve) and at the apex just proximal to the point of luminal obliteration (33). Circumferential strain was also assessed in the short-axis views, and longitudinal strain was assessed in the apical four-chamber view. Images with inadequate tracking in ≥2 myocardial segments were excluded from analysis of the respective mechanics parameters (EchoPAC v.113, GE, Fairfield, CT). Individual curves for speckle-tracking data were time aligned and transformed to 1,200 points using cubic spline interpolation (2D Strain Analysis Tool, Stuttgart, Germany). Twist data were calculated by subtracting time-aligned basal data from apical data, and torsion was calculated as peak twist/lengthd. Peak data represent maximum values across the cardiac cycle.
Statistical Analysis and Power Calculation
For clarity, all data are presented as means ± SD. Normalcy was determined using the Shapiro-Wilk test. For normally distributed data, dependent variables were compared between the sexes using independent t-tests. A 2 (group) × 3 (level) ANOVA was performed to independently assess the effects of sex (between-subjects factor) and LBNP or exercise (within-subjects factor). When a positive effect was detected, pairwise comparisons were made using Fisher’s least-significant-difference test. For non-normally distributed data, dependent variables were assessed using Mann-Whitney U-tests. Friedman repeated-measures ANOVA on ranks was used to detect within-group differences during exercise and LBNP, and pairwise differences were assessed using the Wilcoxon matched-pairs test. Dependent variables were compared between preinfusion and postinfusion (GLY-LBNP) separately from ANOVA using paired-samples t-tests (normally distributed data) or Wilcoxon signed-rank tests (nonparametric data). Linear regression analysis was used to determine individual slopes of the changes to LVSV versus EDV (modified Frank-Starling relationship) during LBNP, and mean slopes were compared between men and women using an independent t-test (14). All statistical analyses were performed using STATISTICA (version 8.0, StatSoft, Tulsa, OK) with α set a priori to 0.05.
Previously, we reported a 5.6° difference in LV twist between the sexes with SD = 5.9° during −60-mmHg LBNP (41). Thus, with α = 0.05 and β = 0.80, we required 18 subjects/group to detect this effect in the present study. No data were available regarding sex differences in LV twist during exercise; however, Stohr et al. (29) have reported SD = 6.0° during exercise at 50% POpeak. With 18 subjects/group, we had 80% power to detect a 5.8° difference in LV twist between sexes.
RESULTS
Participant Recruitment and Characteristics
Of the 19 men and 18 women recruited, 1 man did not tolerate the LBNP screening and 1 woman withdrew after visit 1. Additionally, two men and one woman withdrew before completing visit 3. Thus, the final n of participants with data collected for each respective trial was 17 women and 17 men in the CON-LBNP trial, 16 women and 18 men in the GLY-LBNP trial, 17 women and 16 men in the CON-EX trial, and 16 women and 17 men in the GLY-EX trial.
During the maximal incremental supine cycling test, men attained a larger absolute POpeak (P < 0.001) than women (Table 1). However, women had a greater percent predicted POpeak compared with men (P < 0.001) (20).
Table 1.
Participant characteristics
| Men | Women | |
|---|---|---|
| Participant characteristics | ||
| Age, yr | 22 ± 2 | 21 ± 4 |
| Height, m | 1.79 ± 0.08 | 1.66 ± 0.08* |
| Weight, kg | 75.8 ± 9.7 | 65.8 ± 7.7* |
| Body mass index, kg/m2 | 23.7 ± 3.1 | 23.8 ± 2.4 |
| Body surface area, m2 | 1.94 ± 0.14 | 1.74 ± 0.13* |
| Supine cycling performance | ||
| POpeak, W | 251 ± 29 | 201 ± 31* |
| Percent predicted POpeak† | 100 ± 14 | 123 ± 17* |
| Resting hemodynamics | ||
| Heart rate, beats/min | 61 ± 7 | 61 ± 9 |
| Systolic blood pressure, mmHg | 124 ± 8 | 111 ± 8* |
| Diastolic blood pressure, mmHg | 73 ± 9 | 62 ± 8* |
| Mean arterial pressure, mmHg | 89 ± 8 | 77 ± 7* |
| LV ejection fraction, % | 54 ± 4 | 56 ± 4 |
| Resting LV structure and geometry | ||
| End-diastolic length, cm | 8.8 ± 0.3 | 8.4 ± 0.4* |
| End-diastolic length × body surface area−0.5, cm/m | 6.3 ± 0.2 | 6.4 ± 0.2 |
| LV end-diastolic internal diameter, mm | 49 ± 3 | 44 ± 3* |
| LV end-diastolic internal diameter × body surface area−0.5, mm/m | 35 ± 3 | 34 ± 2 |
| Sphericity index | 1.81 ± 0.11 | 1.90 ± 0.11* |
| Interventricular septum thickness, mm | 10 ± 2 | 9 ± 1 |
| Interventricular septum thickness × body surface area−0.5, mm/m | 7 ± 1 | 7 ± 1 |
| Posterior wall thickness, mm | 10 ± 1 | 9 ± 1 |
| Posterior wall thickness × body surface area−0.5, mm/m | 7 ± 1 | 7 ± 1 |
| Relative wall thickness | 0.39 ± 0.05 | 0.41 ± 0.05 |
Values are means ± SD; n = 18 men and 17 women. LV, left ventricular; POpeak, peak power output.
P < 0.05 vs. men;
Predicted values were derived from Ref. 20 for POpeak in the upright position.
At baseline, blood pressure was higher in men than women (P < 0.001), but HR did not differ between the sexes (Table 1). Absolute LV lengthd (P = 0.001) and LVIDd (P < 0.001) were larger in men, but there were no sex differences in allometrically scaled dimensions. The sphericity index was larger in women (P = 0.026), but PWT, septal wall thickness, and relative wall thickness did not differ between the sexes.
Effect of GLY Infusion on Baseline LV Mechanics, Hemodynamics, Structure, and Geometry
Compared with preinfusion, LV basal rotation, twist, torsion, and untwisting velocity were increased postinfusion (P < 0.01 for all; Table 2), whereas apical rotation was unchanged after GLY infusion in both sexes. Longitudinal strain and basal circumferential strain were reduced postinfusion in both sexes (women: P = 0.036 and men: P < 0.001], but apical circumferential strain was unaltered. There were no sex differences in rotation, twist, or strain parameters pre- or postinfusion.
Table 2.
Left ventricular mechanics during LBNP with and without vagal blockade
| CON-LBNP |
GLY-LBNP |
||||||
|---|---|---|---|---|---|---|---|
| Rest | −40 mmHg | −60 mmHg | Preinfusion | Postinfusion | −40 mmHg | −60 mmHg | |
| Twist, ° | |||||||
| Men | 10.5 ± 2.7a | 12.2 ± 3.1†a | 12.9 ± 2.3†a | 10.7 ± 3.7a | 13.6 ± 2.6§a | 13.5 ± 3.3a | 13.9 ± 3.3a |
| Women | 11.2 ± 3.2a | 12.7 ± 3.7†a | 16.0 ± 3.4*†‡a | 10.6 ± 3.4a | 13.5 ± 3.3§a | 15.2 ± 3.5a | 17.7 ± 5.9*†‡a |
| Torsion, °/cm | |||||||
| Men | 1.19 ± 0.33a | 1.43 ± 0.40†a | 1.58 ± 0.31†‡a | 1.22 ± 0.43a | 1.59 ± 0.35§a | 1.68 ± 0.45a | 1.75 ± 0.43a |
| Women | 1.34 ± 0.39a | 1.60 ± 0.52†a | 2.11 ± 0.44*†‡a | 1.27 ± 0.42a | 1.67 ± 0.46§a | 1.97 ± 0.49a | 2.36 ± 0.86*†‡a |
| Apical rotation, ° | |||||||
| Men | 7.1 ± 1.8a | 7.7 ± 2.4a | 7.8 ± 1.5a | 7.0 ± 2.5a | 8.0 ± 2.8a | 7.7 ± 3.5a | 7.1 ± 3.6a |
| Women | 8.4 ± 3.6a | 8.7 ± 2.6a | 11.9 ± 3.6*†‡a | 7.5 ± 3.4a | 8.4 ± 3.7a | 10.4 ± 3.5*†a | 11.6 ± 4.9*†a |
| Basal rotation, ° | |||||||
| Men | −3.8 ± 1.6 | −5.3 ± 1.4 | −5.5 ± 1.2† | −4.2 ± 1.7 | −6.2 ± 2.2§ | −6.2 ± 3.4 | −7.5 ± 3.3 |
| Women | −3.1 ± 1.8 | −4.7 ± 2.4† | −5.4 ± 2.0† | −3.4 ± 1.0 | −5.5 ± 2.4§ | −5.6 ± 2.3 | −7.1 ± 2.5 |
| Untwisting velocity, °/s | |||||||
| Men | −97 ± 30 | −98 ± 35 | −114 ± 34† | −91 ± 36a | −134 ± 47§a | −146 ± 50a | −162 ± 38†a |
| Women | −99 ± 26 | −113 ± 21 | −132 ± 36† | −91 ± 28a | −138 ± 35§a | −158 ± 41a | −162 ± 54a |
| Longitudinal strain, % | |||||||
| Men | −17.2 ± 1.5 | −14.7 ± 1.7 | −13.1 ± 1.7† | −17.4 ± 1.6 | −15.8 + 1.7§ | −13.0 ± 1.7† | −12.2 ± 1.3† |
| Women | −18.3 ± 1.8* | −15.2 ± 1.2 | −13.3 ± 1.7† | −18.3 ± 1.4 | −16.6 ± 1.7§ | −14.5 ± 2.0†* | −12.5 ± 1.9†‡ |
| Circumferential strain (base), % | |||||||
| Men | −19.3 ± 2.4a | −16.4 ± 2.2†a | −15.9 ± 2.2†a | −19.4 ± 2.8 | −15.8 ± 2.5§ | −11.5 ± 3.4† | −11.4 ± 3.1† |
| Women | −22.1 ± 3.4*a | −17.0 ± 3.8†a | −15.3 ± 3.2†‡a | −20.6 ± 3.5 | −16.9 ± 3.6§ | −12.3 ± 3.7† | −14.1 ± 3.4*† |
| Circumferential strain (apex), % | |||||||
| Men | −27.0 ± 3.0 | −23.4 ± 3.3† | −23.4 ± 4.0† | −26.1 ± 3.6 | −24.7 ± 3.7 | −25.3 ± 4.8† | −22.8 ± 4.6†‡ |
| Women | −29.2 ± 3.1* | −27.7 ± 3.2* | −25.7 ± 4.5† | −28.6 ± 4.1 | −27.3 ± 4.3 | −27.5 ± 4.3 | −24.3 ± 3.2‡ |
Values are means ± SD; n = 17 women and 17 men in the control (CON)-lower body negative pressure (LBNP) group and 16 women and 18 men in the glycopyrrolate (GLY)-LBNP group.
P < 0.05 vs. men;
P < 0.05 vs. rest in the CON-LBNP or postinfusion in the GLY-LBNP group;
P < 0.05 vs. −40 mmHg;
P < 0.05 vs. preinfusion in the GLY-LBNP group.
P values derived from 2 (sex) × 3 (LBNP) ANOVA examining main effects for sex, LBNP level, and interaction (sex × LBNP) are provided in Supplemental Table S1 in the Supplemental Material (Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website).
Data analyzed with nonparametric statistics; medians and interquartile ranges are provided in Supplemental Table S3.
HR and MAP were increased in both sexes after GLY infusion (P < 0.001; Table 3). LVEDV, SV, and EF were reduced postinfusion (P < 0.001); however, ESV was unchanged. The sphericity index (P < 0.01) and relative wall thickness (P < 0.05) increased in both sexes postinfusion but were not different between the sexes pre- or postinfusion.
Table 3.
LV hemodynamics during LBNP with and without vagal blockade
| CON-LBNP |
GLY-LBNP |
||||||
|---|---|---|---|---|---|---|---|
| Rest | −40 mmHg | −60 mmHg | Preinfusion | Postinfusion | −40 mmHg | −60 mmHg | |
| Heart rate, beats/min | |||||||
| Men | 61 ± 7 | 73 ± 8† | 83 ± 11†‡ | 64 ± 9 | 113 ± 10§ | 125 ± 11† | 133 ± 13†‡ |
| Women | 61 ± 9 | 71 ± 15† | 86 ± 17†‡ | 63 ± 11 | 110 ± 11§ | 122 ± 15† | 134 ± 16†‡ |
| Mean arterial pressure, mmHg | |||||||
| Men | 89 ± 8a | 90 ± 6a | 89 ± 7a | 89 ± 6a | 97 ± 7§a | 93 ± 11†a | 88 ± 11†‡a |
| Women | 77 ± 7*a | 80 ± 7*a | 76 ± 8*a | 82 ± 8*a | 95 ± 7§a | 92 ± 7†a | 93 ± 11†‡a |
| End-diastolic volume, ml | |||||||
| Men | 135 ± 16 | 114 ± 12† | 99 ± 13†‡ | 133 ± 15 | 115 ± 14§ | 99 ± 13† | 90 ± 11†‡ |
| Women | 110 ± 15* | 91 ± 16*† | 78 ± 15*†‡ | 113 ± 15* | 99 ± 16*§ | 83 ± 14*† | 72 ± 11*†‡ |
| End-diastolic volume, ml/m | |||||||
| Men | 49 ± 7 | 42 ± 5† | 36 ± 5†‡ | 50 ± 7 | 43 ± 6§ | 37 ± 5† | 33 ± 4†‡ |
| Women | 48 ± 5 | 40 ± 6† | 34 ± 5†‡ | 49 ± 4 | 43 ± 4§ | 36 ± 4† | 30 ± 3*†‡ |
| End-systolic volume, ml | |||||||
| Men | 62 ± 8 | 57 ± 8† | 52 ± 9†‡ | 62 ± 8 | 61 ± 8 | 54 ± 8† | 50 ± 7†‡ |
| Women | 48 ± 8* | 44 ± 9*† | 40 ± 8*†‡ | 52 ± 9* | 49 ± 10* | 44 ± 9*† | 40 ± 7*†‡ |
| End-systolic volume, ml/m | |||||||
| Men | 23 ± 3 | 21 ± 3† | 19 ± 3†‡ | 23 ± 3 | 23 ± 3 | 20 ± 3† | 19 ± 2†‡ |
| Women | 21 ± 3 | 19 ± 3† | 17 ± 3†‡ | 22 ± 3 | 21 ± 3 | 19 ± 3† | 17 ± 3†‡ |
| Stroke volume, ml | |||||||
| Men | 72 ± 11 | 57 ± 6† | 47 ± 6†‡ | 72 ± 9 | 54 ± 7§ | 44 ± 5† | 40 ± 5†‡ |
| Women | 62 ± 9* | 47 ± 8*† | 38 ± 8*†‡ | 61 ± 8* | 49 ± 7§ | 39 ± 6*† | 31 ± 6*†‡ |
| Stroke volume, ml/m | |||||||
| Men | 27 ± 5 | 21 ± 3† | 17 ± 3†‡ | 27 ± 5 | 20 ± 3§ | 17 ± 2† | 15 ± 2†‡ |
| Women | 27 ± 4 | 21 ± 3† | 17 ± 3†‡ | 27 ± 3 | 22 ± 2§ | 17 ± 2† | 13 ± 2*†‡ |
| Ejection fraciton, % | |||||||
| Men | 54 ± 4 | 50 ± 3† | 48 ± 4†‡ | 54 ± 9a | 47 ± 7§a | 45 ± 5a | 44 ± 5†a |
| Women | 56 ± 4 | 52 ± 5† | 49 ± 4†‡ | 54 ± 2a | 50 ± 3*§a | 48 ± 49*†a | 44 ± 5†‡a |
| Cardiac output, l/min | |||||||
| Men | 4.40 ± 0.54 | 4.09 ± 0.45† | 3.86 ± 0.41† | 4.56 ± 0.47 | 6.15 ± 0.89§ | 5.54 ± 0.52† | 5.27 ± 0.66† |
| Women | 3.73 ± 0.66* | 3.31 ± 0.60*† | 3.17 ± 0.67*† | 3.84 ± 0.76* | 5.40 ± 0.70*§ | 4.76 ± 0.69*† | 4.16 ± 0.66*†‡ |
| Total peripheral resistance, mmHg·l−1· min−1 | |||||||
| Men | 20.5 ± 3.0 | 22.2 ± 3.4 | 23.2 ± 3.0† | 19.7 ± 2.5a | 16.1 ± 2.6§a | 17.0 ± 2.6a | 17.0 ± 2.9a |
| Women | 21.3 ± 3.8 | 24.8 ± 4.3† | 25.2 ± 5.4† | 22.1 ± 4.2a | 17.8 ± 1.7*§a | 19.3 ± 2.5*a | 20.5 ± 4.9*a |
Values are means ± SD; n = 17 women and 17 men in the control (CON)-lower body negative pressure (LBNP) group and 16 women and 18 men in the glycopyrrolate (GLY)-LBNP group.
P < 0.05 vs. men;
P < 0.05 vs. rest in the CON-LBNP group or vs. postinfusion in the GLY-LBNP group;
P < 0.05 vs. −40 mmHg;
P < 0.05 vs. preinfusion in the GLY-LBNP group.
P values derived from 2 (sex) × 3 (LBNP) ANOVA examining main effects for sex, LBNP level, and interaction (sex × LBNP) are provided in Supplemental Table S1.
Data analyzed with nonparametric statistics; medians and interquartile ranges are provided in Supplemental Table S3.
LV Mechanics During LBNP With and Without Vagal Blockade
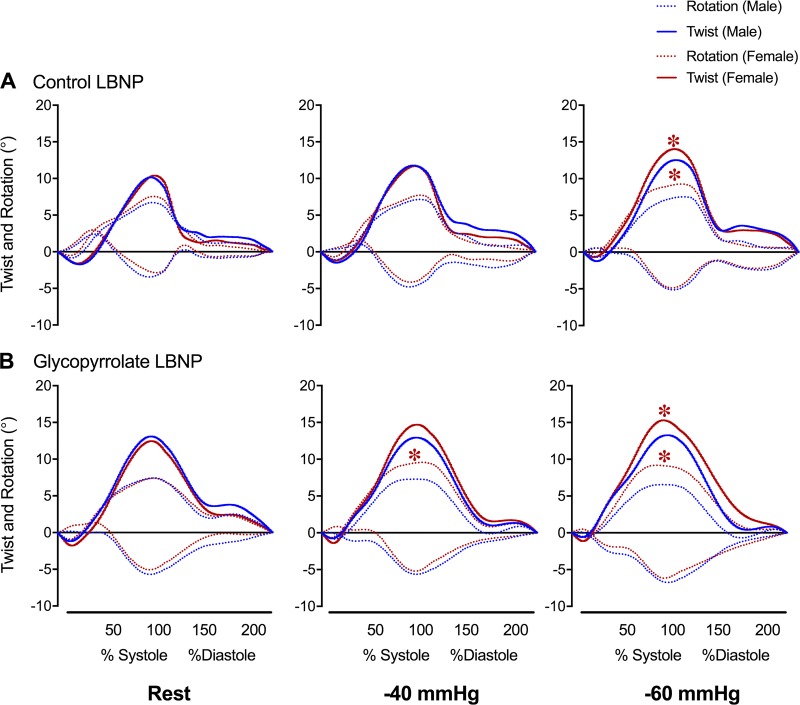
During the CON-LBNP trial, women had greater increases to LV twist (women: 4.8 ± 2.6° and men: 2.4 ± 2.7°, P = 0.02) and torsion (women: 0.74 ± 0.34°/cm and men: 0.39 ± 0.33°/cm, P = 0.008) compared with men from baseline to −60 mmHg. The larger twist (P = 0.004) and torsion (P < 0.001) in women during −60 mmHg was attributable to greater rotation at the apex (P = 0.003) compared with men (Fig. 1A and Table 2); however, basal rotation and untwisting velocity did not differ between the sexes during the CON-LBNP trial. Longitudinal strain (P = 0.047), apical circumferential strain (P < 0.001), and basal circumferential strain (P < 0.001) were reduced in both sexes during the CON-LBNP trial. Only apical circumferential strain was larger in women during −40 mmHg (P < 0.001); otherwise, LV strain was not different between the sexes during −60-mmHg LBNP.
Fig. 1.
Graphical representation left ventricular twist mechanics during rest (left), −40-mmHg (middle), and −60-mmHg (right) lower body negative pressure (LBNP) in control (A) and glycopyrrolate (B) experiments. Blue and red lines represent mean data for men and women, respectively. Solid lines represent left ventricular twist, and dotted lines represent rotation (base: negative and apex: positive). SD, n, and statistical analyses for peak data are shown in Table 2. *P < 0.05, men vs. women.
During the GLY-LBNP trial, men did not have significant alterations to LV basal rotation, apical rotation, twist, or torsion compared with postinfusion baseline. In contrast, women increased LV twist (women: 3.9 ± 5.5° and men: 0.3 ± 3.9°, P = 0.04) and torsion (women: 0.87 ± 0.64°/cm and men: 0.17 ± 0.47°/cm, P = 0.004) from postinfusion baseline to −60 mmHg. The greater LV twist and torsion in women again resulted from a larger apical rotation compared with men (P < 0.05 for all; Fig. 1B), but there were no sex differences in basal rotation or untwisting velocity during the GLY-LBNP trial. It is important to note that LV twist was similar at −60mmHg in the GLY-LBNP and CON-LBNP trials for both men and women. All strain parameters were reduced during the GLY-LBNP trial in both sexes (P < 0.001 for all); however, women had larger longitudinal strain during −40 mmHg only (P = 0.026) and larger basal circumferential strain during −60 mmHg only (P = 0.039). There were no sex differences in apical circumferential strain during the GLY-LBNP trial.
LV Hemodynamics During LBNP With and Without Vagal Blockade
During the CON-LBNP trial, HR and EF did not differ between the sexes; however, MAP was consistently augmented in men compared with women (P < 0.001). There were no sex differences in scaled LV volumes or in the relative reductions to EDV (women: −30 ± 7% and men: −26 ± 6%), ESV (women: −18 ± 11% and men: −17 ± 9%), and SV (women: −39 ± 9% and men: −34 ± 7%). The slopes of the Frank-Starling relationship (women: 0.70 ± 0.17 and men: 0.71 ± 0.13) also did not differ between the sexes during the CON-LBNP trial.
During the GLY-LBNP trial, HR and MAP were not different between men and women. Scaled LVEDV and SV were lower in women than men during −60 mmHg, and thus women had greater relative reductions to EDV (women: −29 ± 4% and men: −22 ± 4%, P < 0.001) and SV (women: −38 ± 9% and men: −27 ± 5%, P < 0.001) during the GLY-LBNP trial. Nonetheless, slopes of the Frank-Starling relationship were not different between the sexes (women: 0.71 ± 0.13 and men: 0.74 ± 0.09). EF was larger in women compared with men during −40 mmHg (P = 0.038) but not −60 mmHg.
LV Structure and Geometry During LBNP With and Without Vagal Blockade
The sphericity index increased in both sexes during the CON-LBNP and GLY-LBNP trials (P < 0.01 for all; Table 4) but was larger in women during the CON-LBNP trial at −60 mmHg (P = 0.014) and during the GLY-LBNP trial at −40 mmHg (P = 0.019) and −60 mmHg (P = 0.003). Relative wall thickness also increased in both sexes during the CON-LBNP and GLY-LBNP trials (P < 0.01 for all). Relative wall thickness was not different between the sexes during the CON-LBNP but was larger in women during the GLY-LBNP trial at −40 mmHg (P = 0.049) and −60 mmHg (P = 0.021).
Table 4.
Left ventricular structure and geometry during LBNP with and without vagal blockade
| CON-LBNP |
GLY-LBNP |
||||||
|---|---|---|---|---|---|---|---|
| Rest | −40 mmHg | −60 mmHg | Preinfusion | Postinfusion | −40 mmHg | −60 mmHg | |
| End-diastolic length, mm | |||||||
| Men | 87.9 ± 2.8 | 85.5 ± 3.1† | 82.1 ± 4.2†‡ | 87.9 ± 3.0 | 86.0 ± 3.7§ | 82.0 ± 3.8† | 79.7 ± 3.8†‡ |
| Women | 83.8 ± 3.9* | 79.6 ± 4.4*† | 76.9 ± 3.9*†‡ | 83.9 ± 3.9* | 81.5 ± 5.0*§ | 77.8 ± 4.7*† | 75.2 ± 4.3*†‡ |
| LV end-diastolic internal diameter, mm | |||||||
| Men | 48.7 ± 3.5 | 44.7 ± 3.3† | 42.3 ± 3.4†‡ | 49.0 ± 3.5 | 45.4 ± 3.3§ | 41.2 ± 3.0† | 40.5 ± 3.3† |
| Women | 44.0 ± 2.9* | 40.6 ± 3.6*† | 37.0 ± 3.4*†‡ | 44.7 ± 3.3* | 41.8 ± 3.9*§ | 37.3 ± 3.9*† | 35.0 ± 3.5*†‡ |
| Sphericity index | |||||||
| Men | 1.82 ± 0.10 | 1.92 ± 0.12† | 1.95 ± 0.14† | 1.80 ± 0.11 | 1.90 ± 0.12§ | 1.98 ± 0.09† | 1.98 ± 0.13† |
| Women | 1.91 ± 0.11* | 1.97 ± 0.16† | 2.08 ± 0.13*†‡ | 1.89 ± 0.15 | 1.96 ± 0.18§ | 2.10 ± 0.17*† | 2.17 ± 0.19*† |
| Relative wall thickness | |||||||
| Men | 0.39 ± 0.05 | 0.44 ± 0.05† | 0.49 ± 0.06†‡ | 0.40 ± 0.05a | 0.44 ± 0.07§a | 0.48 ± 0.06†a | 0.51 ± 0.07†a |
| Women | 0.41 ± 0.05 | 0.46 ± 0.06† | 0.51 ± 0.08†‡ | 0.40 ± 0.04a | 0.45 ± 0.06§a | 0.54 ± 0.09*†a | 0.58 ± 0.09*†‡a |
Values are means ± SD; n = 17 women and 17 men in the control (CON)-lower body negative pressure (LBNP) group and 16 women and 18 men glycopyrrolate (GLY)-LBNP group.
P < 0.05 vs. men;
P < 0.05 vs. rest in the CON-LBNP group or postinfusion in the GLY-LBNP group;
P < 0.05 vs. −40 mmHg;
P < 0.05 vs. preinfusion.
P values derived from 2 (sex) × 3 (LBNP) ANOVA examining main effects for sex, LBNP level, and interaction (sex × LBNP) are provided in Supplemental Table S1.
Data analyzed with nonparametric statistics; medians and interquartile ranges are provided in Supplemental Table S3.
LV Mechanics During Exercise With and Without Vagal Blockade
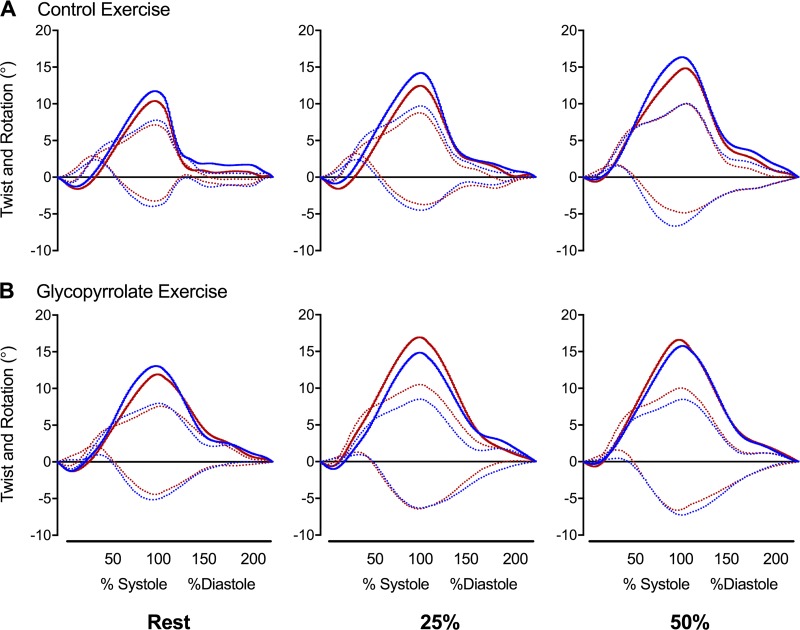
During the CON-EX trial, LV basal rotation, apical rotation, twist, torsion, and untwisting velocity increased in both sexes (P < 0.001 for all; Fig. 2 and Table 5). However, there were no sex differences in these parameters or in the net increases to LV twist (women: 5.0 ± 3.0° and men: 5.4 ± 2.5°) and torsion (women: 0.60 ± 0.37°/cm and men: 0.60 ± 0.32°/cm) from baseline to 50% POpeak. Longitudinal strain, basal circumferential strain, and apical circumferential strain also increased during exercise in both sexes (P < 0.001 for all). Longitudinal strain was greater in women at 25% (P = 0.01) and 50% POpeak (P < 0.001) compared with men, but circumferential strain at the base and apex were not different between the sexes during exercise.
Fig. 2.
Left ventricular twist mechanics at rest (left), 25% (middle), and 50% (right) of peak power during exercise in control (A) and glycopyrrolate (B) experiments. Blue and red lines represent mean data for men and women, respectively. Solid lines represent left ventricular twist, and dotted lines represent rotation (base: negative and apex; positive). SD, n, and statistical analyses for peak data are shownin Table 5. *P < 0.05, men vs. women.
Table 5.
Left ventricular mechanics during submaximal exercise with and without vagal blockade
| CON-EX |
GLY-EX |
|||||
|---|---|---|---|---|---|---|
| Rest | 25% | 50% | Rest | 25% | 50% | |
| Twist, ° | ||||||
| Men | 11.9 ± 2.7 | 14.5 ± 3.0† | 17.7 ± 3.2†‡ | 13.3 ± 3.8a | 15.7 ± 2.5a | 17.2 ± 3.7†a |
| Women | 11.2 ± 3.3 | 13.7 ± 3.3† | 16.4 ± 4.2†‡ | 12.2 ± 3.5a | 17.2 ± 4.9†a | 18.1 ± 4.9†a |
| Torsion, °/cm | ||||||
| Men | 1.36 ± 0.33 | 1.64 ± 0.39† | 2.01 ± 0.42†‡ | 1.56 ± 0.52a | 1.81 ± 0.34a | 2.04 ± 0.45†‡a |
| Women | 1.35 ± 0.40 | 1.65 ± 0.41† | 1.95 ± 0.49†‡ | 1.50 ± 0.47a | 2.12 ± 0.62†a | 2.19 ± 0.60†a |
| Apical rotation, ° | ||||||
| Men | 8.1 ± 2.2a | 10.0 ± 2.4†a | 11.2 ± 1.8†a | 8.4 ± 2.8a | 9.25 ± 1.9a | 9.6 ± 3.3a |
| Women | 8.0 ± 2.4a | 9.9 ± 2.6†a | 11.2 ± 2.8†‡a | 8.0 ± 3.3a | 11.2 ± 4.4†a | 11.3 ± 3.6†a |
| Basal rotation, ° | ||||||
| Men | −4.2 ± 1.3 | −4.8 ± 1.4 | −7.0 ± 1.9†‡ | −5.5 ± 1.6 | −6.7 ± 2.3 | −8.2 ± 2.7†‡ |
| Women | −3.6 ± 1.5 | −4.3 ± 1.5 | −6.2 ± 2.2†‡ | −4.7 ± 1.5 | −6.8 ± 1.7† | −7.5 ± 2.6† |
| Untwisting velocity, °/s | ||||||
| Men | −107 ± 30 | −145 ± 46† | −195 ± 50†‡ | −134 ± 48a | −161 ± 47a | −214 ± 49†‡a |
| Women | −106 ± 24 | −135 ± 43† | −199 ± 53†‡ | −121 ± 40a | −192 ± 61†a | −231 ± 75†‡a |
| Longitudinal strain, % | ||||||
| Men | −17.1 ± 1.4 | −19.9 ± 1.4† | −20.7 ± 1.4†‡ | −17.1 ± 1.5 | −18.5 ± 2.3† | −19.7 ± 2.2†‡ |
| Women | −19.0 ± 1.9* | −21.3 ± 1.4*† | −23.0 ± 1.3*†‡ | −18.5 ± 1.4* | −20.0 ± 1.3*† | −21.2 ± 1.9†‡ |
| Circumferential strain (base), % | ||||||
| Men | −20.2 ± 1.9 | −22.3 ± 3.6† | −23.0 ± 3.4† | −18.0 ± 2.0 | −18.6 ± 2.4 | −19.2 ± 4.0 |
| Women | −20.3 ± 3.9 | −20.2 ± 3.7 | −22.6 ± 3.6† | −16.9 ± 3.7 | −19.2 ± 4.1† | −21.0 ± 3.5† |
| Circumferential strain (apex), % | ||||||
| Men | −27.1 ± 3.2a | −31.0 ± 3.4†a | −33.9 ± 4.4†‡a | −26.6 ± 3.2 | −29.7 ± 4.5† | −31.1 ± 4.0†‡ |
| Women | −29.5 ± 3.7*a | −33.1 ± 4.3†a | −36.3 ± 3.3†‡a | −27.7 ± 4.5 | −31.6 ± 3.7† | −35.6 ± 3.4*†‡ |
Values are means ± SD; n = 17 women and 16 men in the control-exercise (CON-EX) group and 16 women and 17 men in the glycopyrrolate-exercise (GLY-EX) group.
P < 0.05 vs. men;
P < 0.05 vs. rest;
P < 0.05 vs. 25%.
P values derived from 2 (sex) × 3 (exercise) ANOVA examining main effects for sex, exercise level, and interaction (sex × exercise) are provided in Supplemental Table S2.
Data analyzed with nonparametric statistics; medians and interquartile ranges are provided in Supplemental Table S4.
During the GLY-EX trial, there were no sex differences in LV rotation, twist, torsion, or untwisting velocity, and the net increases to LV twist (women: 5.8 ± 3.8° and men: 3.9 ± 3.8°) and torsion (women: 0.69 ± 0.42°/cm and men: 0.44 ± 0.45°/cm) also did not differ between the sexes. It is also important to note that for both men and women, LV twist was similar at 50% POpeak during the GLY-EX trial compared with the CON-EX trial. Longitudinal and apical circumferential strain increased in both sexes during exercise (P < 0.001 for both), but basal circumferential strain only increased in women (P = 0.013) and not men. Longitudinal strain was larger in women during 25% POpeak (P = 0.037) and tended to be greater during 50% POpeak (P = 0.056). Apical circumferential strain was larger in women during 50% POpeak in the GLY-EX trial, but basal circumferential strain was not different between the sexes.
LV Hemodynamics During Exercise With and Without Vagal Blockade
During the CON-EX trial, there were no sex differences in HR, but MAP was consistently higher in men compared with women (P < 0.001; Table 6). Scaled LV volumes were not different between the sexes; however, women tended have larger increases to LVEDV (women: 10 ± 6% and men: 6 ± 6%, P = 0.089) and SV (women: 26 ± 8% and men: 20 ± 10%, P = 0.055) during exercise. LVEF was also larger in women compared with men during exercise at 25% (P = 0.046) and 50% POpeak (P = 0.02).
Table 6.
Left ventricular hemodynamics during exercise with and without vagal blockade
| CON-EX |
GLY-EX |
|||||
|---|---|---|---|---|---|---|
| Rest | 25% | 50% | Rest | 25% | 50% | |
| Heart rate, beats/min | ||||||
| Men | 63 ± 8 | 95 ± 10† | 122 ± 16†‡ | 109 ± 9a | 127 ± 13†a | 151 ± 15†‡a |
| Women | 61 ± 10 | 96 ± 8† | 122 ± 12†‡ | 108 ± 15a | 126 ± 15†a | 145 ± 16†‡a |
| Mean arterial pressure, mmHg | ||||||
| Men | 89 ± 7 | 96 ± 7† | 103 ± 8†‡ | 88 ± 7 | 89 ± 9 | 95 ± 8†‡ |
| Women | 77 ± 8* | 85 ± 7*† | 91 ± 9*†‡ | 82 ± 10* | 80 ± 6* | 87 ± 9*†‡ |
| End-diastolic volume, ml | ||||||
| Men | 136 ± 18 | 143 ± 20† | 144 ± 21† | 125 ± 16 | 128 ± 19† | 130 ± 18† |
| Women | 109 ± 15* | 118 ± 18*† | 119 ± 17*† | 106 ± 15* | 113 ± 18*† | 118 ± 20†‡ |
| End-diastolic volume, ml/m | ||||||
| Men | 49 ± 7 | 54 ± 7† | 53 ± 8† | 46 ± 7 | 48 ± 7 | 49 ± 6† |
| Women | 47 ± 4 | 52 ± 5† | 52 ± 5† | 46 ± 5 | 49 ± 6† | 51 ± 5†‡ |
| End-systolic volume, ml | ||||||
| Men | 63 ± 9 | 59 ± 10 | 57 ± 9†‡ | 64 ± 8 | 58 ± 10† | 56 ± 11† |
| Women | 49 ± 9* | 47 ± 8*† | 44 ± 10*† | 51 ± 8* | 50 ± 10* | 49 ± 10 |
| End-systolic volume, ml/m | ||||||
| Men | 23 ± 3 | 22 ± 3† | 21 ± 3† | 24 ± 3 | 22 ± 3† | 21 ± 3† |
| Women | 21 ± 3 | 20 ± 3† | 19 ± 3†‡ | 22 ± 3 | 22 ± 3 | 22 ± 3 |
| Stroke volume, ml | ||||||
| Men | 73 ± 11 | 83 ± 11† | 87 ± 14†‡ | 60 ± 9 | 70 ± 10† | 74 ± 9†‡ |
| Women | 60 ± 7* | 72 ± 12*† | 76 ± 9*†‡ | 55 ± 7 | 63 ± 10*† | 68 ± 12†‡ |
| Stroke volume, ml/m | ||||||
| Men | 27 ± 5 | 31 ± 4† | 32 ± 6†‡ | 23 ± 5 | 26 ± 3† | 27 ± 3† |
| Women | 26 ± 2 | 31 ± 4† | 33 ± 3†‡ | 24 ± 4 | 27 ± 3† | 30 ± 3†‡ |
| Ejection fraciton, % | ||||||
| Men | 53 ± 3 | 58 ± 2† | 60 ± 3†‡ | 48 ± 2 | 55 ± 3† | 57 ± 4†‡ |
| Women | 55 ± 4 | 61 ± 3*† | 64 ± 4*†‡ | 52 ± 3* | 56 ± 3† | 58 ± 4†‡ |
| Cardiac output, l/min | ||||||
| Men | 4.47 ± 0.39 | 7.87 ± 0.98† | 10.54 ± 1.33†‡ | 6.57 ± 1.00 | 8.82 ± 0.83† | 11.11 ± 0.89†‡ |
| Women | 3.66 ± 0.58* | 6.88 ± 1.05*† | 9.23 ± 0.96*†‡ | 5.92 ± 0.91 | 7.87 ± 1.13*† | 9.80 ± 1.40*†‡ |
| Total peripheral resistance, mmHg·l−1· min−1 | ||||||
| Men | 20.2 ± 2.7 | 12.4 ± 1.7† | 10.0 ± 1.8†‡ | 13.7 ± 2.2 | 10.2 ± 1.4† | 8.6 ± 0.9†‡ |
| Women | 21.5 ± 3.2 | 12.6 ± 1.9† | 10.0 ± 1.3†‡ | 14.1 ± 2.8 | 10.3 ± 1.3† | 9.0 ± 1.6†‡ |
Values are means ± SD; n = 17 women and 16 men in the control-exercise (CON-EX) group and 16 women and 17 men in the glycopyrrolate-exercise (GLY-EX) group.
P < 0.05 vs. men;
P < 0.05 vs. rest;
P < 0.05 vs. 25%.
P values derived from 2 (sex) × 3 (exercise) ANOVA examining main effects for sex, exercise level, and interaction (sex × exercise) are provided in Supplemental Table S2.
Data analyzed with nonparametric statistics; medians and interquartile ranges are provided in Supplemental Table S4.
During the GLY-EX trial, HR was not different between the sexes, but MAP was higher in men compared with women (P < 0.05). There were no sex differences in LVEF, scaled LV volumes, or relative increases to LVSV (women: 25 ± 14% and men: 22 ± 11%), yet LVEDV increased to a greater extent in women (women: 11 ± 9% and men: 4 ± 7%, P = 0.02), whereas men had greater reductions to LVESV (women: −3 ± 11% and men: −13 ± 9%, P = 0.01) from rest to 50% POpeak in the GLY-EX trial.
LV Structure and Geometry During Exercise With and Without Vagal Blockade
The sphericity index and relative wall thickness were unchanged in both men and women during exercise and were not different between the sexes during the CON-EX or GLY-EX trials (Table 7).
Table 7.
Left ventricular structure and geometry during exercise with and without vagal blockade
| CON-EX |
GLY-EX |
|||||
|---|---|---|---|---|---|---|
| Rest | 25% | 50% | Rest | 25% | 50% | |
| End-diastolic length, mm | ||||||
| Men | 88.1 ± 2.9 | 89.1 ± 3.1† | 89.3 ± 4.2† | 86.6 ± 4.1 | 87.1 ± 4.5 | 86.6 ± 4.6 |
| Women | 83.7 ± 3.9* | 83.1 ± 4.5* | 83.9 ± 3.9* | 81.8 ± 4.7* | 81.8 ± 4.7* | 82.6 ± 4.3* |
| LV end-diastolic internal diameter, mm | ||||||
| Men | 49.4 ± 3.3 | 49.3 ± 3.9 | 48.9 ± 3.7 | 48.1 ± 4.2 | 47.5 ± 3.7 | 47.3 ± 3.7 |
| Women | 44.9 ± 3.0* | 44.8 ± 3.6* | 45.8 ± 3.5*†‡ | 43.8 ± 3.6* | 43.5 ± 4.3* | 44.2 ± 4.4* |
| Sphericity index | ||||||
| Men | 1.79 ± 0.09 | 1.82 ± 0.13 | 1.84 ± 0.15† | 1.81 ± 0.17 | 1.84 ± 0.14 | 1.83 ± 0.12 |
| Women | 1.87 ± 0.12* | 1.87 ± 0.18 | 1.84 ± 0.15 | 1.88 ± 0.16 | 1.89 ± 0.17 | 1.88 ± 0.18 |
| Relative wall thickness | ||||||
| Men | 0.40 ± 0.05a | 0.40 ± 0.07a | 0.40 ± 0.05a | 0.42 ± 0.07a | 0.41 ± 0.06a | 0.41 ± 0.05a |
| Women | 0.41 ± 0.05a | 0.41 ± 0.05a | 0.40 ± 0.05a | 0.44 ± 0.07a | 0.44 ± 0.06a | 0.42 ± 0.07a |
Values are means ± SD; n = 17 women and 16 men in the control-exercise (CON-EX) group and 16 women and 17 men in the glycopyrrolate-exercise (GLY-EX) group.
P < 0.05 vs. men;
P < 0.05 vs. rest;
P < 0.05 vs. 25%.
P values derived from 2 (sex) × 3 (exercise) ANOVA examining main effects for sex, exercise level, and interaction (sex × exercise) are provided in Supplemental Table S2.
Data analyzed with nonparametric statistics; medians and interquartile ranges are provided in Supplemental Table S4.
DISCUSSION
This is the first study to assess the influence of vagal control on sex-related differences in LV mechanics and hemodynamics. Contrary to our hypothesis, vagal blockade had no effect on sex differences in LV twist during LBNP and exercise. Additionally, vagal blockade did not impact the responses of LV twist to −60 mmHg or 50% POpeak in either men or women, despite sex-related differences in the relative alterations to LVEDV and ESV during exercise. These data provide novel evidence supporting that vagal cardiac control does not primarily determine sex differences in LV twist, nor does it play a major role in regulating the responses of LV twist during acute physiological stress in men or women.
Vagal Control of LV Mechanics During LBNP
Findings from the present study have reproduced data from our previous work (41) in an entirely new cohort of men and women, confirming that women have augmented LV twist and torsion compared with men during large reductions to preload. We have further demonstrated that these sex differences in LV twist mechanics occur even when vagal stimulation to the heart is blocked with GLY. Although vagal blockade initially augmented LV twist at rest, it did not alter the response of LV twist in either of the sexes during high levels of LBNP, supporting that sex differences in LV twist mechanics are not a consequence of differences in vagal cardiac control.
Our group has recently demonstrated that LV twist appears to be more sensitive to adrenergic stimulation in men compared with women (40). However, we have consistently observed augmented LV twist in women during LBNP in the present and previous studies (40, 41); therefore, it is unlikely that differences in sympathetic or adrenergic regulation significantly contribute to these sex differences in LV twist mechanics during large reductions to preload. Considering the findings in the present study that sex differences in LV twist persist during vagal blockade with GLY, the data from the present and previous studies collectively support that cardiac autonomic control is not a key regulator of sex differences in the responses of LV twist to large reductions in preload.
Sex differences in LV twist mechanics occurred in the absence of differences in HR, both during control and GLY experiments. This finding is in contrast to previous studies (14, 28, 41) that have demonstrated greater increases to HR in women compared with men during large reductions to preload and suggested that a greater vagal withdrawal in women may produce sex differences in LV hemodynamics (14, 28). In the present study, vagal withdrawal may not have differed between the sexes given the comparable chronotropic responses to LBNP both with and without vagal blockade. While we excluded endurance-trained individuals, predicted POpeak was higher in women than men within the present study cohort (123% vs. 100%, respectively), so it is possible that small differences in aerobic fitness may have influenced any sex-related differences in vagal withdrawal. Increased cardiorespiratory fitness has been linked to greater resting cardiac vagal control and blunted vagal withdrawal during acute stressors (31) as well as attenuated HR responses in women to progressive LBNP (19). Nevertheless, even without any apparent sex differences in vagal withdrawal or HR, we demonstrated that women have a greater LV twist response to preload reduction, which supports that sex differences in LV twist are not a result of differences in vagal cardiac control.
While much of the prior literature has used measures of HR variability to examine sex differences in cardiac autonomic control (2, 12, 13), our present and prior studies (40, 41) have provided novel data regarding the sympathovagal control of LV mechanics and hemodynamics in addition to HR dynamics alone. This focus on LV function and mechanics is especially important in the context of vagal activation, given that it exerts different degrees of control on cardiac chronotropy versus myocardial contractility. Unlike β-adrenergic receptors, which are more uniformly distributed across the atrial and ventricular tissues (5), muscarinic receptor densities are much greater in the human atria versus the ventricles (9). Moreover, cholinergic innervation is substantially greater in atrial and nodal tissues compared with the ventricles as well as at the LV base versus the apex (21, 37). These regional heterogeneities in innervation and receptor distribution indicate a stronger vagal control of nodal firing and conduction (23) and relatively weaker effects on ventricular contractility. This might account for why vagal blockade only had minimal effects on LV twist mechanics at rest and did not alter the absolute twist or torsion responses to LBNP or exercise in either of the sexes.
The regional variances in myocardial muscarinic receptor and cholinergic innervation densities could also explain the regional alterations to LV rotation and strain after GLY infusion and explain why GLY did not impact sex differences in LV twist. Specifically, the sparse cholinergic innervation and muscarinic receptor densities of the LV apex compared with the base should likely lead to a greater effect of GLY on basal mechanics rather than apical mechanics. This was apparent as LV twist was augmented in both sexes postinfusion as a result of increased rotation at the base but not the apex. Likewise, circumferential strain increased in both sexes postinfusion at the base but not the apex. As the augmented LV twist in women during LBNP results from greater rotation at the apex, GLY likely had a minimal effect on apical contractility and thus did not impact or minimize the sex differences in LV twist. To our knowledge, there are currently no data regarding sex-related differences in vagal innervation or receptor densities; however, our findings suggest that any sex-related variations in those factors are unlikely to contribute to sex differences in LV twist mechanics.
Vagal Control of LV Mechanics During Submaximal Exercise
Vagal blockade did not alter the LV twist response to submaximal exercise in either of the sexes, nor were there sex differences in LV twist mechanics during exercise with or without vagal blockade. In the present study, men and women had similar chronotropic responses to exercise with and without GLY, which was unexpected considering the notable evidence for greater vagal cardiac control (2, 12, 13) and chronotropic responses to acute stress in women compared with men (14, 28, 38, 41) as well as sex differences in the hemodynamic responses to exercise (16, 38). Although the alterations to LV volumes were not significantly different between the sexes during exercise without vagal blockade, we observed that LV hemodynamics did differ between men and women during exercise with vagal blockade. Notably, women primarily increased LVEDV, whereas men predominantly reduced LVESV to increase SV during exercise. Despite these differences in the responses of LV hemodynamics, there were no sex differences in LV twist or torsion. This suggests that women used the Frank-Starling mechanism to augment LV twist, whereas men increased twist by augmenting contractility, supporting our previous finding that LV twist is mediated to a greater extent by adrenergic stimulation in men than in women (40).
Potential Role for LV Geometry in Determining Sex Differences in LV Mechanics
An intriguing finding separate from autonomic control is that a greater LV apical rotation and twist in women consistently coincides with a larger sphericity index compared with men during high levels of LBNP with and without vagal blockade, and there was a significant sex × LBNP interaction, indicating larger increases to sphericity index in women during progressive LBNP. These data replicate our previous observations (41) and are notable because LV sphericity index has been identified as an independent predictor of LV twist (32). In a cohort of healthy participants, van Dalen and colleagues (32) identified a parabolic relationship for LV sphericity with both LV twist and apical rotation but not basal rotation. The authors noted that a sphericity index of ~2.1 was associated with the highest LV twist at rest and that lower or higher sphericity indexes were associated with lesser LV twist. Those results are closely aligned with data from the present study where women had greater LV twist than men during LBNP with sphericity indexes of ~2.08 to ~2.17 compared with ~1.95 to ~1.98 in men, and the sex differences in LV twist predominantly resulted from differences in rotation at the apex and not at the base. An ellipsoid LV geometry likely provides more optimal myofiber configurations and cross-fiber interactions (6, 30) to ultimately generate greater LV apical rotation and twist mechanics (32). This hypothesis is further supported by a computational modeling study that demonstrated increased active fiber stress and myofiber shortening in a more ellipsoid versus spherical ventricle (6).
While van Dalen et al. (32) have highlighted the important influence of LV geometry on LV twist mechanics in the resting state, the present study has identified sex differences in sphericity index and LV twist mechanics in response to an acute hemodynamic challenge. High levels of LBNP augment adrenergic stimulation (7, 14), so the increased myocardial contractility may further accentuate the impact of sphericity index on sex differences in LV twist (i.e., result in larger increases to LV twist for a given change in sphericity index). The consistent finding of a greater sphericity index in women during LBNP in the present and previous study (41) suggests that LV geometry may be a key contributor to sex differences in LV twist rather than autonomic control. This might further provide some explanation for the lack of sex differences in LV twist during exercise, as LV sphericity index was similar in men and women during exercise with and without vagal blockade. Future studies should consider either controlling for or directly addressing the potential influence of LV geometry on sex-related differences in LV twist mechanics.
A role for sex-related differences in LV chamber size (i.e., dimensions and volume) might also be considered. There is some evidence to suggest that a smaller LV is associated with augmented load-independent systolic function (15); however, when accounting for sex differences in LV chamber volume and dimensions, we have previously reported that women have larger increases to LV torsion (i.e., twist scaled to LV length) for a given reduction in allometrically scaled EDV with LBNP (41). Likewise, in the present study, LV torsion was augmented in women compared with men during LBNP despite similar allometrically scaled LV volumes in both sexes. As torsion accounts for major differences in LV size between men and women, we believe it is reasonable to suggest that sex differences in LV twist are not just a phenomenon of LV size alone but that other factors likely contribute more to the higher LV twist in women during LBNP.
Influences of LV Preload and Afterload on Sex Differences in LV Mechanics
While the present study primarily manipulated vagal control, because of the integrative nature of cardiovascular regulation, concurrent alterations to LV preload and afterload may have influenced the observed responses of LV twist in men and women. Preload and afterload are key regulators of LV twist mechanics; both reductions and increases to LV preload can augment LV twist (17, 35), and LV twist is reduced in the face of augmented afterload (10, 36). In the present and previous (41) studies, men and women had similar relative reductions to LVEDV during LBNP without GLY. Fu et al. (14) have also reported similar reductions to pulmonary capillary wedge pressure (a surrogate of LV end-diastolic pressure) in men and women during high levels of LBNP. It is therefore unlikely that the augmented LV twist mechanics in women during LBNP result from sex differences in alterations to LV preload. While we did not specifically measure indexes of LV afterload in the present study, we have previously demonstrated augmented twist mechanics in women compared with men during β1-adrenergic receptor blockade despite no differences in end-systolic wall stress (a surrogate of LV afterload) (40). Furthermore, in the present and previous study (41), MAP was unaltered from baseline in both sexes during LBNP, suggesting that alterations to, and sex differences in, LV twist mechanics were not primarily determined by systemic afterload or blood pressure. Owing to the highly complex and integrative nature of LV physiology and mechanics, it is challenging, if not impossible, to delineate the exact contributions of each regulator of LV twist in these studies; however, it seems unlikely that sex differences in LV twist mechanics are largely attributable to sex-related differences in LV preload or afterload.
Limitations
While we used GLY to directly manipulate vagal control, we cannot discount the possibility of any reflexive or compensatory alterations to adrenergic stimulation during vagal blockade. However, it is unlikely that our findings were significantly influenced by changes to adrenergic stimulation during vagal blockade, as previous reports have shown no impact of vagal blockade on baseline levels or increases to epinephrine and norepinephrine both during progressive LBNP up to −60 mmHg (42) and dynamic exercise (4).
Conclusions
Vagal autonomic control of the heart does not appear to contribute to sex differences in the responses of LV twist mechanics to acute hemodynamic challenges. Women have greater increases to LV twist and torsion compared with men during large reductions to preload, even during vagal blockade with GLY. Vagal blockade also does not appear to alter the responses of LV twist during LBNP and exercise in either of the sexes. These findings suggest that vagal stimulation has a relatively minimal influence on LV mechanics, notably at the apex, likely because of lower muscarinic receptor and cholinergic innervation densities in the LV myocardium. Collectively, our data provide novel evidence that vagal control is not a primary determinant of sex differences in the responses of LV twist mechanics to acute physiological stress.
GRANTS
This study was funded by the Natural Sciences and Engineering Research Council of Canada Grant 371950. A. M. Williams was supported by Natural Sciences and Engineering Research Council of Canada Grants CGSD2-460367-2014 and CGS-MSFSS 477373. N. D. Eves was supported by Michael Smith Foundation for Health Research Grant 7085.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.M.W., R.E.S., J.M.C., and N.D.E. conceived and designed research; A.M.W., J.M.C., H.W., and B.R.-S. performed experiments; A.M.W. and N.D.E. analyzed data; A.M.W., R.E.S., and N.D.E. interpreted results of experiments; A.M.W. prepared figures; A.M.W. and N.D.E. drafted manuscript; A.M.W., R.E.S., J.M.C., H.W., B.R.-S., and N.D.E. edited and revised manuscript; A.M.W., R.E.S., J.M.C., H.W., B.R.-S., and N.D.E. approved final version of manuscript.
Supplemental Data
ACKNOWLEDGMENTS
The authors thank Sarah Mckevitt and Rebecca O’Connor for contributing time to data collection.
REFERENCES
- 1.Akagawa E, Murata K, Tanaka N, Yamada H, Miura T, Kunichika H, Wada Y, Hadano Y, Tanaka T, Nose Y, Yasumoto K, Kono M, Matsuzaki M. Augmentation of left ventricular apical endocardial rotation with inotropic stimulation contributes to increased left ventricular torsion and radial strain in normal subjects: quantitative assessment utilizing a novel automated tissue tracking technique. Circ J 71: 661–668, 2007. doi: 10.1253/circj.71.661. [DOI] [PubMed] [Google Scholar]
- 2.Barantke M, Krauss T, Ortak J, Lieb W, Reppel M, Burgdorf C, Pramstaller PP, Schunkert H, Bonnemeier H. Effects of gender and aging on differential autonomic responses to orthostatic maneuvers. J Cardiovasc Electrophysiol 19: 1296–1303, 2008. doi: 10.1111/j.1540-8167.2008.01257.x. [DOI] [PubMed] [Google Scholar]
- 3.Batterham AM, George KP, Mullineaux DR. Allometric scaling of left ventricular mass by body dimensions in males and females. Med Sci Sports Exerc 29: 181–186, 1997. doi: 10.1097/00005768-199702000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Boushel R, Calbet JAL, Rådegran G, Sondergaard H, Wagner PD, Saltin B. Parasympathetic neural activity accounts for the lowering of exercise heart rate at high altitude. Circulation 104: 1785–1791, 2001. doi: 10.1161/hc4001.097040. [DOI] [PubMed] [Google Scholar]
- 5.Brodde OE, Bruck H, Leineweber K, Seyfarth T. Presence, distribution and physiological function of adrenergic and muscarinic receptor subtypes in the human heart. Basic Res Cardiol 96: 528–538, 2001. doi: 10.1007/s003950170003. [DOI] [PubMed] [Google Scholar]
- 6.Choi HF, Rademakers FE, Claus P. Left-ventricular shape determines intramyocardial mechanical heterogeneity. Am J Physiol Heart Circ Physiol 301: H2351–H2361, 2011. doi: 10.1152/ajpheart.00568.2011. [DOI] [PubMed] [Google Scholar]
- 7.Convertino VA, Ludwig DA, Cooke WH. Stroke volume and sympathetic responses to lower-body negative pressure reveal new insight into circulatory shock in humans. Auton Neurosci 111: 127–134, 2004. doi: 10.1016/j.autneu.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol Regul Intergr Comp Physiol 275: R1909–R1920, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Deighton NM, Motomura S, Borquez D, Zerkowski HR, Doetsch N, Brodde OE. Muscarinic cholinoceptors in the human heart: demonstration, subclassification, and distribution. Naunyn Schmiedebergs Arch Pharmacol 341: 14–21, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Dong SJ, Hees PS, Huang WM, Buffer SA Jr, Weiss JL, Shapiro EP. Independent effects of preload, afterload, and contractility on left ventricular torsion. Am J Physiol Heart Circ Physiol 277: H1053–H1060, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Doucende G, Schuster I, Rupp T, Startun A, Dauzat M, Obert P, Nottin S. Kinetics of left ventricular strains and torsion during incremental exercise in healthy subjects: the key role of torsional mechanics for systolic-diastolic coupling. Circ Cardiovasc Imaging 3: 586–594, 2010. doi: 10.1161/CIRCIMAGING.110.943522. [DOI] [PubMed] [Google Scholar]
- 12.Dutra SGV, Pereira APM, Tezini GCSV, Mazon JH, Martins-Pinge MC, Souza HCD. Cardiac autonomic modulation is determined by gender and is independent of aerobic physical capacity in healthy subjects. PLoS One 8: e77092, 2013. doi: 10.1371/journal.pone.0077092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS, Leonelli FM, Knapp CF. Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J Appl Physiol 91: 2611–2618, 2001. doi: 10.1152/jappl.2001.91.6.2611. [DOI] [PubMed] [Google Scholar]
- 14.Fu Q, Arbab-Zadeh A, Perhonen MA, Zhang R, Zuckerman JH, Levine BD. Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol Heart Circ Physiol 286: H449–H457, 2004. doi: 10.1152/ajpheart.00735.2002. [DOI] [PubMed] [Google Scholar]
- 15.Hayward CS, Kalnins WV, Kelly RP. Gender-related differences in left ventricular chamber function. Cardiovasc Res 49: 340–350, 2001. doi: 10.1016/S0008-6363(00)00280-7. [DOI] [PubMed] [Google Scholar]
- 16.Higginbotham MB, Morris KG, Coleman RE, Cobb FR. Sex-related differences in the normal cardiac response to upright exercise. Circulation 70: 357–366, 1984. doi: 10.1161/01.CIR.70.3.357. [DOI] [PubMed] [Google Scholar]
- 17.Hodt A, Hisdal J, Stugaard M, Stranden E, Atar D, Steine K. Reduced preload elicits increased LV twist in healthy humans: an echocardiographic speckle-tracking study during lower body negative pressure. Clin Physiol Funct Imaging 31: 382–389, 2011. doi: 10.1111/j.1475-097X.2011.01029.x. [DOI] [PubMed] [Google Scholar]
- 18.Hodt A, Hisdal J, Stugaard M, Stranden E, Atar D, Steine K. Increased LV apical untwist during preload reduction in healthy humans: an echocardiographic speckle tracking study during lower body negative pressure. Physiol Rep 3: e12330, 2015. doi: 10.14814/phy2.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudson DL, Smith ML, Raven PB. Physical fitness and hemodynamic response of women to lower body negative pressure. Med Sci Sports Exerc 19: 375–381, 1987. doi: 10.1249/00005768-198708000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Jones NL, Makrides L, Hitchcock C, Chypchar T, McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis 131: 700–708, 1985. [DOI] [PubMed] [Google Scholar]
- 21.Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels 18: 32–39, 2003. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- 22.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J-U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28: 1–39.e14, 2015. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Levy MN. Sympathetic-parasympathetic interactions in the heart. Circ Res 29: 437–445, 1971. doi: 10.1161/01.RES.29.5.437. [DOI] [PubMed] [Google Scholar]
- 24.Lewis ME, Al-Khalidi AH, Bonser RS, Clutton-Brock T, Morton D, Paterson D, Townend JN, Coote JH. Vagus nerve stimulation decreases left ventricular contractility in vivo in the human and pig heart. J Physiol 534: 547–552, 2001. doi: 10.1111/j.1469-7793.2001.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin WH III, Spina RJ, Korte E, Ogawa T. Effects of chronic and acute exercise on cardiovascular β-adrenergic responses. J Appl Physiol 71: 1523–1528, 1991. doi: 10.1152/jappl.1991.71.4.1523. [DOI] [PubMed] [Google Scholar]
- 26.Moon MR, Ingels NB Jr, Daughters GT II, Stinson EB, Hansen DE, Miller DC. Alterations in left ventricular twist mechanics with inotropic stimulation and volume loading in human subjects. Circulation 89: 142–150, 1994. doi: 10.1161/01.CIR.89.1.142. [DOI] [PubMed] [Google Scholar]
- 27.Ogoh S, Fisher JP, Dawson EA, White MJ, Secher NH, Raven PB. Autonomic nervous system influence on arterial baroreflex control of heart rate during exercise in humans. J Physiol 566: 599–611, 2005. doi: 10.1113/jphysiol.2005.084541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol 281: H2028–H2035, 2001. doi: 10.1152/ajpheart.2001.281.5.H2028. [DOI] [PubMed] [Google Scholar]
- 29.Stöhr EJ, González-Alonso J, Shave R. Left ventricular mechanical limitations to stroke volume in healthy humans during incremental exercise. Am J Physiol Heart Circ Physiol 301: H478–H487, 2011. doi: 10.1152/ajpheart.00314.2011. [DOI] [PubMed] [Google Scholar]
- 30.Taber LA, Yang M, Podszus WW. Mechanics of ventricular torsion. J Biomech 29: 745–752, 1996. doi: 10.1016/0021-9290(95)00129-8. [DOI] [PubMed] [Google Scholar]
- 31.Tulppo MP, Mäkikallio TH, Seppänen T, Laukkanen RT, Huikuri HV. Vagal modulation of heart rate during exercise: effects of age and physical fitness. Am J Physiol Heart Circ Physiol 274: H424–H429, 1998. [DOI] [PubMed] [Google Scholar]
- 32.van Dalen BM, Kauer F, Vletter WB, Soliman OII, van der Zwaan HB, Ten Cate FJ, Geleijnse ML. Influence of cardiac shape on left ventricular twist. J Appl Physiol 108: 146–151, 2010. doi: 10.1152/japplphysiol.00419.2009. [DOI] [PubMed] [Google Scholar]
- 33.van Dalen BM, Vletter WB, Soliman OII, ten Cate FJ, Geleijnse ML. Importance of transducer position in the assessment of apical rotation by speckle tracking echocardiography. J Am Soc Echocardiogr 21: 895–898, 2008. doi: 10.1016/j.echo.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Weiner RB, DeLuca JR, Wang F, Lin J, Wasfy MM, Berkstresser B, Stöhr E, Shave R, Lewis GD, Hutter AM Jr, Picard MH, Baggish AL. Exercise-induced left ventricular remodeling among competitive athletes: a phasic phenomenon. Circ Cardiovasc Imaging 8: e003651, 2015. doi: 10.1161/CIRCIMAGING.115.003651. [DOI] [PubMed] [Google Scholar]
- 35.Weiner RB, Weyman AE, Khan AM, Reingold JS, Chen-Tournoux AA, Scherrer-Crosbie M, Picard MH, Wang TJ, Baggish AL. Preload dependency of left ventricular torsion: the impact of normal saline infusion. Circ Cardiovasc Imaging 3: 672–678, 2010. doi: 10.1161/CIRCIMAGING.109.932921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiner RB, Weyman AE, Kim JH, Wang TJ, Picard MH, Baggish AL. The impact of isometric handgrip testing on left ventricular twist mechanics. J Physiol 590: 5141–5150, 2012. doi: 10.1113/jphysiol.2012.236166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wharton J, Polak JM, Gordon L, Banner NR, Springall DR, Rose M, Khagani A, Wallwork J, Yacoub MH. Immunohistochemical demonstration of human cardiac innervation before and after transplantation. Circ Res 66: 900–912, 1990. doi: 10.1161/01.RES.66.4.900. [DOI] [PubMed] [Google Scholar]
- 38.Wheatley CM, Snyder EM, Johnson BD, Olson TP. Sex differences in cardiovascular function during submaximal exercise in humans. Springerplus 3: 445, 2014. doi: 10.1186/2193-1801-3-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White DW, Raven PB. Autonomic neural control of heart rate during dynamic exercise: revisited. J Physiol 592: 2491–2500, 2014. doi: 10.1113/jphysiol.2014.271858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams AM, Shave RE, Cheyne WS, Eves ND. The influence of adrenergic stimulation on sex differences in left ventricular twist mechanics. J Physiol 595: 3973–3985, 2017. doi: 10.1113/JP273368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams AM, Shave RE, Stembridge M, Eves ND. Females have greater left ventricular twist mechanics than males during acute reductions to preload. Am J Physiol Heart Circ Physiol 311: H76–H84, 2016. doi: 10.1152/ajpheart.00057.2016. [DOI] [PubMed] [Google Scholar]
- 42.Wray DW, Formes KJ, Weiss MS, O-Yurvati AH, Raven PB, Zhang R, Shi X. Vagal cardiac function and arterial blood pressure stability. Am J Physiol Heart Circ Physiol 281: H1870–H1880, 2001. doi: 10.1152/ajpheart.2001.281.5.H1870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.