Abstract
The objective of the present study is to review current knowledge regarding the bioaccumulation potential of IOCs, with a focus on the availability of empirical data for fish. Aspects of the bioaccumulation potential of IOCs in fish that can be characterized relatively well include the pH-dependence of gill uptake and elimination, uptake in the gut, and sorption to phospholipids (membrane-water partitioning). Key challenges include the lack of empirical data for biotransformation and binding in plasma. Fish possess a diverse array of proteins which may transport IOCs across cell membranes. Except in a few cases, however, the significance of this transport for uptake and accumulation of environmental contaminants is unknown. Two case studies are presented. The first describes modeled effects of pH and biotransformation on bioconcentration of organic acids and bases, while the second employs an updated model to investigate factors responsible for accumulation of perfluoroalkylated acids (PFAA). The PFAA case study is notable insofar as it illustrates the likely importance of membrane transporters in the kidney and highlights the potential value of read across approaches. Recognizing the current need to perform bioaccumulation hazard assessments and ecological and exposure risk assessment for IOCs, we provide a tiered strategy that progresses (as needed) from conservative assumptions (models and associated data) to more sophisticated models requiring chemical-specific information. This article is protected by copyright. All rights reserved
Keywords: Bioaccumulation, Toxicokinetics, Ecological risk assessment, Ionizable organic chemicals
INTRODUCTION
The behaviour of ionizable organic chemicals (IOCs) in humans has long been of interest to the pharmaceutical industry, and in silico tools to simulate the uptake, distribution, and elimination of such substances are widely available (e.g., [1–3]). This is because many active pharmaceutical ingredients (APIs) are ionized at physiological pH values [4,5]; thus, understanding the impact of speciation (i.e., the fractional amount of neutral and ionized forms) on pharmacokinetics is important. Increasingly, environmental chemists, toxicologists and ecological risk assessors have begun to focus their attention on IOCs. The main drivers of this interest include the detection of perfluorinated alkyl acids (PFAAs) in humans and wildlife around the globe [6,7], concerns regarding the potential impacts of APIs in aquatic environments [8,9], and the recognition that IOCs make up a substantial portion of the chemicals in commerce requiring assessment under various regulatory schemes (e.g., REACH) [10]. Because it is not feasible to directly test all chemicals of interest, there is a need to develop and build confidence in models that can be used to support hazard and risk assessment of IOCs. Whereas numerous modeling tools are available to support the assessment of neutral organic chemicals, generic tools designed for IOCs have only recently been developed (e.g., [11–14]) and are not yet in widespread use.
The main objective of this paper is to review current knowledge regarding the bioaccumulation potential of IOCs, with a focus on the availability of empirical data for fish and identification of issues potentially important for future model development. This effort can be traced to a workshop on IOCs which was held November 5–7, 2014, in Vancouver, BC (Experts Workshop on the Ecotoxicological Risk Assessment of Ionizable Organic Chemicals: Towards a Science-Based Framework for Chemical Assessment). Since that time, additional work has been performed to evaluate existing models for accumulation of IOCs in fish, providing further insights into the key factors influencing uptake and elimination processes. Special emphasis is placed on chemical behaviors which distinguish IOCs from neutral substances, including preferential sorption to membranes (phospholipids) and plasma proteins, the influence of pH on uptake and elimination rates, and the greater potential importance of active transport mechanisms. Biotransformation pathways and possible differences in the type and abundance of enzymes responsible for these reactions in fish and mammals are also discussed. Due to a lack of empirical data, this review does not address the potential accumulation of IOCs by aquatic invertebrates. However, many of the key considerations for fish will also be relevant for these organisms. Factors determining the uptake and accumulation of IOCs in terrestrial plants have been investigated and reviewed elsewhere (e.g., [15–18]). A brief summary of this information is provided here as Supplemental Data (Section S1).
GENERAL CONSIDERATIONS
Bioaccumulation is an integral aspect of hazard assessment (e.g., Persistence, Bioaccumulation, and Toxicity categorization) and exposure/risk assessment [19]. An understanding of the bioaccumulation of IOCs is also useful for interpreting toxicity data (e.g., relating external concentrations to internal body burdens). Bioaccumulation potential is commonly characterized using metrics such as the whole-body bioconcentration factor (BCF), the bioaccumulation factor (BAF), the biomagnification factor (BMF) and the trophic magnification factor (TMF) (see Supplemental Data, Section S2, for definitions) [19,20]. For regulatory purposes, priority is typically given to BCFs and BAFs, and numerical thresholds range from 500 L/kg (CLP/Globally Harmonized System of Classification and Labelling of Chemicals) to 5000 L/kg (e.g., REACH vB criterion, Stockholm Convention B criterion). For exposure/risk assessment, chemical concentrations in blood or plasma have also been proposed as a key metric for IOCs [21,22].
As with neutral organic compounds, the bioaccumulation potential of IOCs is generally expected to be a function of hydrophobicity and susceptibility to biotransformation [23]. For example, despite substantial scatter in the empirical BCF data for IOCs [11,24], there are significant positive relationships between measured BCFs, octanol-water partition coefficients for the neutral form of the compound (KOW,N), and octanol-water distribution ratios (DOW) (i.e., the pH-dependent weighted averages of the partition coefficients of the neutral and charged form). Ionizable organic compounds also exhibit an inverse relationship between bioavailability, defined as the freely-dissolved concentration in the water column, and hydrophobicity (DOW) [25–27]. Thus, our knowledge of the behaviour of neutral organic chemicals already provides a basic conceptual understanding of the general behaviour of IOCs, and the main challenge is to modify available models to account for any important deviations that may exist.
Both regression-based [24,28,29] and mechanistic [11,30–32] models for predicting the bioconcentration of IOCs in aquatic organisms are available. Regression-based BCF models are derived through a statistical analysis of available data (‘training set’), typically using physical-chemical properties (e.g., KOW,N or DOW and pKa) as the independent variables (predictors). Mechanistic BCF models are those that directly estimate and/or include estimated rate constants describing key uptake and elimination processes (e.g., uptake and elimination across the gills, biotransformation, fecal egestion, growth dilution). These models typically require physicalchemical property data, biological characteristics (e.g., body size, lipid content) and environmental conditions (e.g., temperature, alkalinity/buffering capacity, dissolved oxygen content) as inputs. The organism may be represented as a single well-mixed compartment or multiple compartments representing different tissues and organs. Model performance is generally evaluated by comparing predicted BCFs to empirical data. For both basic model types, predicted BCF values are often within a factor of three of observations on average, although substantially larger discrepancies can occur for some chemicals (e.g., [11,24]).
The main advantages of mechanistic models include greater flexibility and applicability (e.g., to different species and experimental/environmental conditions), and the ability to more readily identify critical processes and important data gaps. While regression-based BCF models can be useful for regulatory purposes and exposure/risk assessment, the lack of process information (i.e., outputs describing the underlying toxicokinetics) limits their utility for addressing the objectives of this review. For this reason, they are not examined in detail here. Regardless of the model type, a key concern for bioaccumulation assessment is the likelihood of Type I (‘false positive’/overestimation of true BCF) and Type II (‘false negative’/underestimation of true BCF) errors, with Type II errors given priority in the regulatory domain. While the performance of available BCF models for IOCs is encouraging, factor of three errors can have important regulatory implications (e.g., ‘vB’, ‘B’ or ‘not B’ under REACH; subject to or not subject to the provisions of the CLP/Globally Harmonized System of Classification and Labelling of Chemicals). The larger discrepancies observed for certain chemicals are more problematic in this context as well as with respect to exposure/risk assessment. Accordingly, research supporting the development of improved BCF models and parameterization tools (e.g., for estimating biotransformation rate constants) is warranted.
The following sections describe various toxicokinetic processes underlying the bioaccumulation of IOCs. For consistency with the broader literature, the discussion topics are divided into sections that address Absorption, Distribution, Metabolism (Biotransformation) and Excretion (ADME). Following the general ADME discussion, two case studies are presented in order to explore key issues related to the bioaccumulation of IOCs in aquatic organisms. Because of the importance of gill exchange for the bioaccumulation of IOCs in aquatic organisms, the first case study focuses on the influence of bulk water pH on gill uptake, gill elimination and bioconcentration of IOCs. The influence of biotransformation is also considered. The second case study focuses specifically on PFAAs and the role of renal clearance. The mechanistic models applied in these case studies ([30,32] are summarized briefly in the Supplemental Data (Section S3). Two considerations thus far little explored in aquatic organisms but potentially relevant for IOCs are the presence of mucus at epithelial exchange surfaces (gills, gut, and skin) and the role of membrane transporters. These considerations are introduced first because both may influence several ADME processes.
Presence of mucus on exterior surfaces of aquatic organisms and epithelium
The presence and possible functions of mucus on the epithelial surfaces of aquatic organisms has received substantial attention in the ecological literature (e.g., [33–35]). Mucus is composed of high-molecular weight macromolecules and water, and forms a gel-like coating estimated to be approximately 10–500 μm thick. The predominant macromolecules in the mucus of vertebrates are glycoproteins (mucins), whereas mucopolysacchrides are more prevalent in the mucus of invertebrates. Because glycoproteins contain acidic functional groups (e.g., sialic acid), the mucus coating can act as a cation exchanger [33,34] and therefore engage in attractive interactions with organic bases and repulsive interactions with organic acids. We are not aware of any systematic studies exploring how mucus influences chemical sorption and uptake/elimination at epithelial surfaces, including the skin, gills, and gastrointestinal tract. Given that the passive flux of many IOCs across biological membranes is dominated by diffusion of the neutral form [30,31,36,37], it is possible that the presence of mucus is only relevant for chemicals that are strongly dissociated at environmental and physiological pH values.
Potential role of membrane transporters/active transport
Biological membranes act as diffusion barriers for inorganic ions and polar and/or charged organic and inorganic molecules, including energy substrates and structural components (e.g. sugars, fatty acids, and amino acids), signaling molecules (e.g. hormones and neurotransmitters), and substances that regulate cell motility, osmotic pressure and pH (e.g., various organic and inorganic ions, and water). As such, these membranes provide for compartmentalization and control of biological function at several levels of biological organization. The transport of ions and polar molecules across biological membranes is largely controlled by membrane-bound channel and transporter proteins. These proteins may facilitate the movement of an ion or molecule down its electrochemical gradient or transport a substance against its electrochemical gradient via some type of active mechanism. The energy required for this active transport may be provided by hydrolysis of ATP (primary active mechanism) or by coupling the electrochemical potential of one solute to the transmembrane flux of a second solute (secondary active mechanism) [38–41].
Membrane transporters play a critical role in determining the pharmacokinetics of some APIs in humans [39,40,42]. This is true in part because many drugs are structurally similar to endogenous substances (indeed, this is frequently the basis for their activity). Based on a large and growing literature, it is clear that the major transporter families responsible for transmembrane flux of both drugs and toxicologically relevant compounds are:
- the SLC (solute carrier) family [44]; (capital letters are used to denote human transporter proteins, fish transporter protein names are written as capitals followed by lower case letters), including:
-
∘OATPs/Oatps (Organic anion transporter polypeptides) [SLCO family]
-
∘OATs/Oats (organic anion transporters) [SLC22A superfamily]
-
∘OCTs/Octs (organic cation transporters) [SLC22A superfamily]
-
∘
ATP-binding cassette transporters, including the well-known multixenobiotic resistance (MXR)- or multidrug resistance (MDR)-related transporters, act via the primary active mechanism and generally operate as ―efflux pumps‖ to keep intracellular concentrations of substrates low. Solute carrier proteins participate in both uptake and efflux of chemical substrates, although most are considered to be uptake transporters. The SLC transporter-mediated entry of ions into cells is a secondary active process linked to the transmembrane electrochemical potential of other ions [45].
A considerable number of studies on genetic sequences, tissue expression patterns and toxicologically and physiologically relevant roles of ABC transporters in teleosts have been published in recent years, much of it focusing on MXR-related transporters [46]. These studies have demonstrated functional similarities between mammals and teleosts for some MXR-related orthologs, although questions remain concerning the occurrence of multiple isoforms of orthologs that exist in mammals only as one isoform [46,47]. The expression of MXR-related transporters has been demonstrated in teleost embryos, suggesting that they play a role in protecting developing embryos against contaminants dissolved in water [48,49]. Additional research with isolated renal proximal tubules has shown that ABC transporters secrete xenobiotics in the teleost kidney [50–54].
In contrast to ABC transporters, there have been relatively few studies of SLCs in fish. Teleostean Oatps have been phylogenetically characterized [55–57], and functional studies performed using in vitro expression systems suggest a potential role in transporting pollutants and other environmentally relevant compounds [55,58–61]. Whole animal studies conducted using classical substrates for mammalian transporters also indicate the occurrence and activity of Oats and Oatps in fish liver and kidney. Compared to the data available for mammals, knowledge of membrane transporters and their potential role in determining the bioaccumulation potential of IOCs in fish is limited. For this reason, most of the currently available bioaccumulation models for IOCs in aquatic organisms do not explicitly address the potential role of membrane transporters/active transport.
ABSORPTION
Uptake across the gills.
The passive absorption of an organic chemical at fish gills involves several sequential steps. Chemical is pumped through lamellar channels, diffuses to the epithelial surface, crosses multiple membranes, diffuses away from the epithelium into blood, and becomes distributed among the various components of blood (e.g., plasma, plasma proteins, red blood cells). As such, passive absorption depends on the rate at which chemical is made available to the gills in ventilated water, the permeability of various aqueous and membrane layers, and the rate at which blood flow removes chemical [62–70].
For IOCs, the different properties of the neutral and charged forms further complicate the situation. Due to the low membrane permeability of organic ions, increased ionization (i.e., fraction of chemical in charged form) is expected to reduce gill absorption rates. However, Saarikoski et al. [71] noted that this decrease was not proportional to the extent of ionization in the exposure water, and considered how the charged species may contribute to absorption. To address these potential mechanisms, Erickson et al. [30,31] modified a finite element model given previously by Erickson and McKim [69] for uptake of neutral organic compounds. The revised model accounts for the possibility that:
Ions contribute to diffusion to and from membranes, which facilitates absorption by maintaining steep gradients of the neutral form across the membranes.
The pH at the gill surface is reduced compared to the bulk water pH due to elimination of metabolically-derived acid.
There is some permeation of ions through membranes, alone and/or in association with counter-ions.
The model given by Erickson et al. [30,31] explained important aspects of the effects of pH and buffering on gill absorption rates for several chlorinated phenols in large rainbow trout, but there were deviations of the data from model predictions indicative of the need for better understanding of partitioning and permeability of ionizable chemicals in biological tissues. A second version of the model, parameterized for fathead minnows, successfully described pH effects on bioconcentration of diphenhydramine, a weakly basic pharmaceutical [72], in short-term waterborne exposures. The broader utility of this model is limited, however, by the need to specify about 20 parameters regarding fish morphology/physiology and chemical partitioning/permeability.
To more simply predict effects of ionization on gill absorption, Armitage et al. [11] modified the Gobas and Mackay [67] gill uptake equation for neutral compounds by adjusting the resistance to chemical transport in the organic phase based on the ratio of the total concentration to that of the neutral form, consistent with consideration (i) above (see Supplemental Data, Section S4 for details). Calculation of this ratio requires estimation of gill surface pH, consistent with the reduction of pH at the gill surface due to elimination of metabolically-derived acid, i.e., consideration (ii) above. In addition, the model accounts for direct absorption of ions across membranes via paracellular transport, which broadly addresses consideration (iii) above.
A preliminary comparison of the gill models given by Armitage et al. [11] and Erickson et al. [30] was performed as part of the present work by simulating data given by Erickson et al. [30,31] for rainbow trout (Oncorhynchus mykiss) and Saarikoski et al. [71] for guppies (Poecilia reticulata). To perform this comparison, the Erickson et al. [30] model was used to predict the average pH at the gill surface, which was then used as an input to the Armitage et al. [11] model. Thus, in general, differences in predictions generated by the two models do not relate to differences in pH at the gill epithelium. The results of this model inter-comparison are presented in the Supplemental Data (Section S5). Both models successfully describe data sets provided by Erickson et al. [30,31]. However, the model provided by Erickson et al. [30] performs better when simulating data from Saarikoski et al. [71], particularly at elevated pH (see Supplemental Data, Figure S1). The generally good performance of both models suggests that the presence of mucus at the gills is not an influential factor, at least for the IOCs considered.
Uptake in the gastrointestinal tract (GIT)
As with uptake at the gills, passive uptake of organic chemicals in the GIT can be described using advective transport terms (i.e., the flow of digesta through the GIT and transport away from the GIT in blood) and diffusive transport terms (i.e., passive transport across aqueous and organic layers) [73,74]. Micelle-mediated transport across the unstirred water layer in the lumen has also been proposed as an important process facilitating uptake [75]. However, only the advective and passive transport processes are considered in most generic gut uptake models. A key factor distinguishing uptake across the gut wall from uptake at the gills is the residence time, which is on the order of hours in the gut compared to fractions of a second in gill lamellar channels. For neutral organic chemicals, reported chemical uptake efficiencies in the gut (ED) tend to be fairly constant over a broad range of hydrophobicity (KOW), with reduced uptake efficiencies observed only for very hydrophilic (log KOW < 0) and superhydrophic (log KOW > 7– 8) chemicals [73,74]. The reduction in chemical uptake efficiencies for very hydrophilic chemicals is due to increased membrane resistance (i.e., diffusion across organic layers) whereas the transport terms for advection in the gut (‘ingestion flow delay’) and diffusion across aqueous layers become rate-limiting as hydrophobicity increases [73,74]. Biotransformation within the gastrointestinal tract may substantially reduce a compound’s uptake efficiency regardless of its hydrophobicity [76–78].
Arnot and Quinn [79] recently published a critical review of dietary uptake studies of organic compounds in fish. Of the 400 observations, approximately 7% are for IOCs that are appreciably charged in the gut including per- and polyfluorinated alkyl substances, pesticides (e.g., metconazole, fipronil) and industrial chemicals (e.g., pentachlorophenol). Reported or derived chemical uptake efficiencies for IOCs range from 0.7–125%, with the upper value clearly erroneous (100% being the maximum). Although there is considerable scatter and inconsistency in the data, there is no indication that uptake efficiencies of the IOCs are greatly reduced in comparison to neutral organic chemicals with similar properties (e.g., KOW). For example, Xiao et al. [80] reported apparent and benchmarked chemical uptake efficiencies for pentachlorophenol, an organic acid (log KOW,N = 5.1, pKa = 4.7), that were within 10% of values for pentachlorobenzene, a neutral compound (log KOW = 5.2). These results are consistent with the analysis of chemical uptake efficiencies of drugs in humans published by Abraham et al. [37], which suggested that there was no strong relationship between degree of speciation and uptake efficiency. Abraham et al. [37] further concluded that chemical uptake efficiency of IOCs could, as a first approximation, be reasonably estimated from properties of the neutral species. A similar conclusion was reached by O’Connor et al. [74]. While the potential importance of specific uptake mechanisms for IOCs in fish is poorly understood, uptake efficiencies of IOCs in the gut are not expected to be greatly reduced as a consequence of speciation unless the ratio of charged to neutral species is very large. Additional studies on the chemical uptake efficiencies of quaternary amine compounds or other highly dissociated IOCs (e.g., linear alkylbenzene sulfonates, LAS) would therefore be valuable.
Dermal uptake.
For neutral organic compounds in water, the relative importance of dermal uptake compared to gill uptake is largely a function of the size of the organism. For example, dermal uptake may account for up to 50% of total uptake in waterborne exposures with smaller fish/early fish life stages (< 4 g), whereas experiments with larger fish (500–1000 g) indicate only a minor contribution (≤10%) [81–83]. Factors accounting for the increased importance of dermal uptake in smaller fish include a larger skin/branchial surface area ratio, a larger skin surface area/body size ratio, and a thinner, more vascularized skin tissue (i.e., shorter diffusion distance). Dermal uptake rates also may depend to some extent on chemical solubility in the outermost layers of the skin, although in general this appears to be less true for fish than it is for mammals [83].
Empirical data characterizing the dermal uptake of IOCs is limited, but suggest that this process can contribute to total uptake from the water phase, at least for smaller fish. Tovell et al. [84] estimated that dermal uptake accounted for approximately 20% of total absorption of sodium lauryl sulfate (an anionic surfactant) in goldfish (3.8–105 g), while Saarikoski et al. [71] reported that dermal uptake of pentachlorophenol (pKa = 4.7) at pH 5 and 9 accounted for 24% and 38% of total absorption, respectively in guppies (60–100 mg). The presence of negatively charged mucus does not appear to be a limiting factor in these cases.
DISTRIBUTION
The internal distribution of organic chemicals in biota is influenced by the rate of chemical delivery to tissues in blood, transport across biological membranes separating blood from the interior of cells, and the relative affinity (sorption capacity) of different tissues for specific substances. For many neutral chemicals, passive diffusion is the dominant mechanism of membrane transport, and steady-state distribution may be adequately predicted from the total lipid content of individual tissues [85,86]. Under these conditions, lipid-normalized concentrations in different tissues will tend to the same value, as has been observed for hydrophobic chemicals of concern [87,88]. More complex approaches distinguish between different types of lipids and other biological macromolecules (e.g., storage lipids, phospholipids, plasma proteins, structural proteins) [89], but the conceptual approach and assumptions are essentially the same.
The internal distribution of IOCs is driven by the same basic considerations but is complicated by the different behaviour of charged and neutral species. For example, interactions between storage lipids (e.g., triglycerides) and charged compounds are not favourable [36] and hence adipose is not an important reservoir for strongly dissociated IOCs (e.g., PFAAs) [90,91]. The key factors underlying the passive tissue distribution of IOCs are phospholipid content and plasma protein content [92–95]. Accordingly, tissues with relatively high phospholipid content (e.g., liver, kidney) and/or plasma protein content (e.g., blood) are expected to exhibit larger wet weight concentrations of IOCs, all else being equal. Acidic phospholipids (e.g., phosphatidylserine) will interact favourably only with bases, while zwitterionic (neutral) phospholipids (e.g., phosphatidylcholine) are important for both acids and bases [36,95,96]. Specific binding interactions that saturate at high substrate concentrations are also possible. This is particularly true for plasma binding of APIs that resemble ionized endogenous molecules. Weakly acidic drugs generally bind to albumin while basic compounds bind to alpha-1-acid glycoprotein (α1AGp). Because this type of binding involves specific interactions with molecular binding sites, it is more difficult to predict from simple partitioning properties [97]. Fish are known to possess both albumin and α1AGp; however, binding data for IOCs in fish plasma are scarce and the potential variability between species is not well characterized.
An additional consideration for organic bases that are substantially charged at physiological pH (i.e., pKa > 8) is lysosomal sequestration [98,99], which is an intracellular example of the general phenomenon known as the ‘ion trap’ effect. Assuming the concentration of the neutral form in both fluids is equal, the large pH gradient between fluid within lysosomes (pH = 4–5) and cytoplasm (pH = 7.2) means that the concentration of the charged form of a monoprotic base could be ~2–3 orders of magnitude larger in the lysosome, assuming that pH-dependent partitioning holds. The potential effect is even larger for multiprotic bases. Partitioning to phospholipids within the lysosomes would also be greater relative to the cytoplasm [98]. Following from these considerations, cells with an abundance of lysosomes (e.g., liver, kidney, spleen, lung) have a greater inherent capacity to accumulate organic bases compared to tissues with relatively few lysosomes (e.g., muscle) [98,100]. Determining the relative importance of lysosomal sequestration compared to general sorption to phospholipids in vivo is challenging [98–100], and the implications for assessing the bioaccumulation potential of organic bases in aquatic organisms at the whole-body level are unclear. However, the results published by Daniel and Wojcikowski [100] suggest that while important, lysosomal sequestration is secondary to general sorption to phospholipids in determining tissue partitioning, at least for the monoprotic bases and experimental conditions considered in that study.
Active transport mechanisms for membrane transport of IOCs could potentially result in tissue distribution patterns which differ from those predicted by simple partitioning considerations. However, we are not aware of any systematic studies in aquatic organisms that could be used to assess the potential importance of these processes in a generic manner. In practical terms, it may be difficult to distinguish the influence of active transport on internal distribution because of measurement uncertainties inherent to in vitro approaches used to derive tissue/water and tissue/plasma partition coefficients. For example, tissue/plasma distribution ratios predicted by Schmitt [95] assuming equilibrium partitioning differed from measured values by a factor of approximately 1.5–5.0, depending on the tissue. Clearly, expectations based on simple equilibrium partitioning need to be better defined to inform discussions of the potential role of active transporters. Additional data characterizing the phospholipid and plasma protein content of tissues in aquatic organisms are needed as are more data quantifying sorption affinities for IOCs (e.g., phospholipid/water distribution ratios and protein binding affinities). Such data can readily be incorporated into the existing mechanistic models that already distinguish among these different biological phases (e.g., [11,32]).
METABOLISM (BIOTRANSFORMATION)
Phase I biotransformation reactions, including those catalyzed by cytochrome P450 monooxygenase (CYP) enzymes, tend to increase a compound’s polarity by adding or exposing a polar atom, while the major phase II pathways further enhance a compound’s polarity by conjugation with a polar endogenous molecule. Acting alone or in concert, these pathways generally increase a compound’s water solubility and may result in products that are substrates for specific membrane transporters.
In mammals, neutral compounds secreted to bile or filtered at the kidney tend to be reabsorbed. For this reason, most ionizable drugs possessing a log DOW greater than 1.0 at pH 7.4 require biotransformation to facilitate their elimination from humans. Reabsorption in the liver and kidney also occurs in fish; however, fish can efficiently eliminate neutral compounds across the gills, provided that they exhibit only moderate hydrophobicity (log KOW < 3). For such compounds, biotransformation is not required to facilitate elimination, and even fast rates of metabolism are predicted to have little impact on accumulation of the parent compound [95]. Modeled effects of biotransformation on accumulation of IOCs by fish are presented below as part of the gill uptake case study (Case Study 1: The relationship of the pH-dependence of BCFs to gill exchange processes and biotransformation). This assessment is complicated by uncertainties relating to branchial flux of IOCs (e.g., pH changes at the gill surface). Nevertheless, biotransformation is predicted to have little impact on accumulation of an IOC that possesses moderate hydrophobicity as the neutral form (log KOW,N < 3), unless the compound is very strongly dissociated.
Because of this fundamental difference between fish and mammals, it is possible that CYPs which transform xenobiotic substances have evolved somewhat differently in the two taxa. Based on sequence homology as well as functional studies, it is clear that fish possess counterparts to the CYPs responsible for metabolizing relatively hydrophobic compounds in mammals (primarily CYP1A and CYP3A forms). In contrast, fish do not appear to possess orthologs for CYP2C9 and CYP2D6, both of which transform a number of ionizable drugs in humans [101,102]. Limited functional studies with prototypical CYP2C9 and CYP2D6 substrates also indicate that trout liver S9 fractions possess little (if any) activity towards these compounds [103].
Some IOCs are metabolized in fish by phase II conjugation reactions [101,104]. Existing studies indicate that fish glucuronidate a number of resin acids and phenolic compounds, while other work suggests that they metabolize some carboxylic acids by amino acid conjugation. Sulfonation may also be a relevant process for certain acidic functional groups. In general, however, the role that these pathways play in limiting the accumulation of IOCs is poorly known. More research is needed to characterize the biotransformation of IOCs by fish, as this will help clarify the extent to which information from mammals can be “read across” (see READ ACROSS FOR BIOACCUMULATION/ADME). The validation of in vitro test systems (e.g., liver S9 or hepatocytes) for IOCs would greatly facilitate such efforts [105]. Robust methods to extrapolate in vitro data for IOCs to the in vivo/whole body situation are also required.
EXCRETION
Elimination across the gills
All current approaches used to model the absorption of IOCs at fish gills assume that uptake occurs by passive diffusion in response to a chemical activity gradient [11,30,31]. Because the process of passive diffusion is inherently non-saturable and bidirectional, these models have equal utility for describing elimination. Active transport is not considered in available modeling approaches for gill exchange and the potential relevance of such processes is discussed below (see Evidence for transporter-mediated chemical flux in the gills and gut).
Fecal elimination
Passive elimination of organic chemicals through fecal egestion requires the diffusion of the chemical across the gut wall back into the lumen, where sorption to the remaining gut contents will occur. Some bioaccumulation models assume that transport terms describing gut uptake and elimination are equal whereas others assume that processes such as micelle-mediated transport result in larger uptake rates compared to elimination [73]. The key drivers of fecal elimination are the chemical gradient between blood and feces (based on concentration and sorption capacity) and the efficiency with which the chemical can diffuse across the gut wall. Following from the discussion of the intestinal absorption, it is reasonable to assume that diffusive transport can be modeled as a first approximation using the properties of neutral form. Again, IOCs that are very strongly dissociated could be exceptions and are therefore priorities for additional research.
Evidence for transporter-mediated chemical flux in the gills and gut
ATP-binding cassette transporters are expressed in teleost gill tissue [106,107] and could contribute to branchial flux of specific chemical substrates. Stott et al. [108] employed a primary gill culture system to examine transport of seven pharmaceuticals under various experimental conditions. The authors reported that there was some evidence for active transport; however, passive transport was the primary determinant of exchange across the interface (e.g., active transport accounted for approximately 10% of total transport for propranolol and imipramine). Additional studies indicate that transporters may play a role in the fish intestine similar to that in mammals, where MXR pumps act as ―gate keepers‖ prohibiting uptake of certain compounds from food [106,107,109]. It is unclear whether any of these processes can substantially impact the accumulation of a xenobiotic substance in fish. Additional research is needed to determine whether active transport rates are comparatively faster than passive transport rates and if so, delineate the circumstances under which these differences are important for bioaccumulation assessment. Currently available mechanistic BCF models (e.g., [11,30]) do not account for active transport in the gill and gut. Accordingly, if significant active transport can be unambiguously demonstrated, predictive models that consider only passive flux at the gills and/or gut would have to be revised.
Biliary elimination.
In mammals, SLC transporters present in the basolateral membrane of liver hepatocytes promote the uptake of organic anions from plasma, while ABC transporters in the canalicular membrane actively secrete organic anions, cations, and conjugated products of phase II biotransformation into bile [110–112]. Both of these processes play an important role in supporting the liver’s normal biological functions (e.g., secretion of bile salts), and both have been shown to promote the elimination of ionized xenobiotics.
Although fish express a diverse array of membrane transporters in liver tissue, there have been relatively few studies demonstrating their functional role in the intact animal. Biliary secretion of phenol red has been studied in several species [113–115]. Hagfish, flounder, and catfish eliminated phenol red primarily as the parent compound, while in trout, skates and sharks, much of the phenol red was glucuronidated before its secretion to bile. Rainbow trout eliminated sulfobromophthalein (BSP) to bile, both as the parent compound and as one or more conjugated products [116,117]. Indocyanine green was secreted to bile in hagfish, flounder, skates and sharks [114]. Phenol red, BSP, and indocyanine green have been used extensively to study biliary secretion in mammals, and all three compounds exist predominantly as anions at physiological pH values.
Conjugates secreted to bile may be cleaved in the GIT by microbial enzymes, releasing the parent compound. If this compound is reabsorbed, the cycle can be repeated, resulting in a process called enterohepatic circulation. Limited studies indicate that enterohepatic circulation can occur in fish [118–120]; however, the toxicological significance of this process is largely unknown and this process is not explicitly incorporated into currently available mechanistic models for aquatic organisms.
Renal elimination.
In fish as in mammals, the chemical content of urine reflects the net result of glomerular filtration, tubular secretion, and reabsorption [121,122]. Importantly, the urine flow rate of a fish is profoundly impacted by the environment within which it lives. In general, seawater-adjusted fish produce small quantities of relatively concentrated urine while freshwater fish produce large volumes of dilute urine.
The urine has very little capacity for neutral hydrophobic organic chemicals relative to that of kidney tissue. For this reason, neutral hydrophobic compounds filtered at the glomerulus are largely reabsorbed. In contrast, renal clearance may be an important route of elimination for some IOCs. Chemicals that are freely dissolved in blood plasma will be filtered at the glomerulus. Once in urine, these substances will dissociate in accordance with the local pH environment, and if they remain ionized will tend to be retained in urine. Curtis and Wood [123] determined the pH of trout urine to be 7.3, which is somewhat lower than that of plasma (typically 7.8 to 7.9), and showed that this value could be adjusted upward by infusing animals with NaHCO3. In humans, the pH of urine can be manipulated with acid (e.g., oral vitamin C) or base (infused NaHCO3) to ―trap‖ ionized drugs, facilitating their elimination, or promote formation of the neutral form, causing them to be retained. We are not aware of any research demonstrating pH effects on renal clearance of IOCs by fish.
The glomerular filtration rate (GFR) determined for trout is somewhat less than 1% of total blood flow to the kidney [124], and establishes an upper limit on renal clearance that can be achieved by simple filtration. For some IOCs, however, active secretion into urine can result in clearance rates that substantially exceed the GFR. This is because renal tubular secretion operates against the entire kidney blood flow. In addition, chemicals bound in plasma can desorb and become available to membrane transport proteins. Under these circumstances, the renal clearance of a chemical becomes rate-limited by blood flow to the kidney, which in trout approaches 60% of total cardiac output [125]. The potential significance of tubular secretion as a mechanism for chemical elimination was highlighted by the classic work of Pritchard and James [126], which showed that in winter flounder renal clearance of phenoxyacetic acid (2,4-D; pKa = 3.1), a known substrate for OATs/Oats, was 500 times higher than the measured GFR. Additional whole-animal studies with fish have demonstrated renal clearance of DDA (2,2-bis(p-chlorophenyl)-acetic acid, the acetic acid derivative of DDT; [127]), as well as ionized phase II conjugates of phenol [124] and several benzo[a]pyrene metabolites [128]. In a recent study with rainbow trout, Consoer et al. [129] found that urinary elimination of perfluorooctanoate (PFOA) substantially exceeded the rate predicted from glomerular filtration, and was the primary route of chemical clearance. This finding is consistent with mammalian studies which show that membrane transporters (primarily OATPs/Oats and OATPs/Oatps) control renal clearance of PFOA and are largely responsible for observed species differences in elimination half-life (see Case Study 2: Toxicokinetics of Perfluorinated Alkyl Acids (PFAAs)). Additional work with trout suggests that perfluorooctane sulfonate (PFOS) is not secreted to urine [130]. Sulfonated PFAAs tend to accumulate in fish to a greater extent than carboxylated derivatives with the same number of perfluorinated carbons. One possible explanation for this finding is that the perfluorocarboxylates are much better substrates for membrane transporters in the fish kidney compared to perfluorinated sulfonates. Most currently available mechanistic BCF models do not account for renal clearance. Whereas passive elimination across the gills is expected to dominate over renal clearance occurring as a result of simple filtration, it is clear that active renal clearance can be a key elimination process for certain chemicals and therefore deserves further exploration.
READ ACROSS FOR BIOACCUMULATION/ADME
Environmental risk assessors commonly ―read across‖ existing information to untested conditions of interest. When applied to in vivo toxicity testing data, this approach assumes that different compounds sharing the same mode of action will have similar effects in the same or different species if their unbound concentration at the site of action is the same. This mode of action assumption implies, in turn, that relevant molecular targets are present in all species of interest and that the biological function of these targets is conserved. Because the unbound concentration at the site of action is determined by ADME considerations, use of the read-across approach to extrapolate among species requires consideration of the manner in which compounds are handled by different organisms.
With respect to predicting the effects of IOCs in fish, the ―read across hypothesis‖ articulated by Huggett et al. [21] is of special interest. This hypothesis states that a drug is likely to cause pharmacological effects in fish if the steady-state concentration in plasma approaches that associated with a therapeutic response in humans. In order to predict the drug concentration in plasma from that in water, Huggett et al. [21] employed an empirically-based equation given by Fitzsimmons et al. [131]. This equation was derived using data for neutral organic chemicals and predicts blood(plasma)-water partitioning based on non-specific interactions with organic constituents using a compound’s log KOW value. Several authors have adapted the Fitzsimmons et al. [115] equation for use with ionizable drugs by replacing log KOW with the log DOW calculated for an assumed pH value [22,132–134]. Unfortunately, this simple modification may result in underestimation of plasma binding because DOW is not a representative surrogate for the interactions of IOCs with key biological macromolecules (e.g., [36,72,97]). Experimental support for the read across hypothesis proposed by Huggett et al. [21] has been provided by several studies (e.g., [134,135]). To date, however, there have been very few studies with fish in which drug concentrations in plasma were measured, and for which measured endpoints can be related to human therapeutic effects [136].
The accumulation of ionizable drugs by fish will be impacted by ADME processes as discussed throughout this paper. Development of a predictive capability for fish requires, therefore, that we learn how to read across not only therapeutic plasma levels, but also basic ADME information from human drug development efforts. For some processes, such as plasma and tissue binding, it may be possible to quantitatively read across measured values. For example, Nichols et al. [72] found that the ionized form of diphenhydramine binds extensively in plasma of fathead minnows. Measured concentrations in whole animals also suggested a high level of tissue binding. These binding relationships could not have been predicted from nonspecific partitioning of neutral diphenhydramine. An examination of the drug literature suggested, however, that the apparent volume of distribution of diphenhydramine in fathead minnows (3 L/kd; calculated as the whole body-plasma concentration ratio) was similar to that in humans (3 L/kg to 8 L/kg) [137]. Had the authors adopted mammalian binding values as initial inputs to their steady-state model, they would have accurately predicted observed levels of accumulation.
For other processes, such as biotransformation or the involvement of membrane transporters, it is less likely that quantitative information from mammals can be read across to fish. Indeed, even among mammals there are large species differences in biotransformation (rates and predominant products) and the activities of different transporters. Nevertheless, it may be possible to use information from mammals to identify processes of likely importance in fish and make qualitative predictions regarding their relative magnitude (e.g., fast, slow, and no metabolism). In a recent effort, Berninger et al. [138] compiled ADME data (therapeutic CMAX, whole-body clearance, VD, and T1/2) for approximately 1000 drugs and used this information to rank the environmental hazard associated with these substances. Although not employed for this purpose, the values assembled by these authors could be combined with other information (e.g., pKa and log KOW,N) to assess the potential for individual drugs to accumulate in aquatic species.
CASE STUDY 1: THE RELATIONSHIP OF THE PH-DEPENDENCE OF BCFS TO GILL EXCHANGE PROCESSES AND BIOTRANSFORMATION
Previous sections described how chemical properties, environmental variables, and gill physiology can affect uptake and elimination rates for IOCs at fish gills. However, the net accumulation of chemical in a fish will usually be of more interest than these separate exchange rates. Also, the factors that determine this net accumulation might differ from those which impact the rates. For example, the contribution of ions to diffusive flux in aqueous phases will generally affect both uptake and elimination rates similarly, and thus affect the rate at which steady state is reached, but might be of little or no consequence to steady-state accumulation. This case study addresses the pH-dependence of steady-state accumulation, and how this may be affected not only by processes controlling gill uptake and elimination, but also by biotransformation of IOCs.
This case study was not intended to provide a complete, definitive model for IOC bioaccumulation, to comprehensively compare existing models, or to assess model performance over a broad data set. Instead, we sought to identify and illustrate processes important to the pH-dependence of IOC accumulation that warrant attention in further research and model development. Relevant predictions (see below) were obtained using the gill model of Erickson et al. [30,31], as implemented by Erickson et al. [30] and Nichols et al. [72] for trout and fathead minnows, respectively. In all cases, the steady state blood:water bioconcentration factor (BCFBW) was used to examine the dependence of IOC accumulation on external pH, because this is directly linked to the processes at the gill regulating this dependence. Absent specific mechanisms which could impact the internal distribution of a compound (e.g., membrane transporters operating in specific tissues and/or tissue-specific binding), whole-body accumulation is expected to be proportional to BCFBW and thus have the same pH-dependence. To be consistent with current regulatory criteria for assessing bioaccumulation potential [19], the focus here will be on the accumulation of the parent compound only.
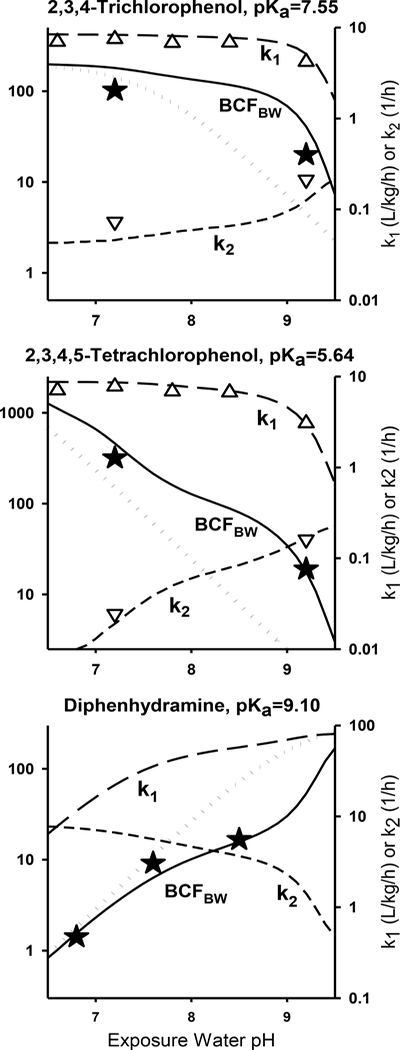
Initially, we considered how IOC bioaccumulation reflects the processes affecting gill uptake (k1) and elimination (k2) rates. For three example chemicals, Figure 1 illustrates how BCFBW varies with pH, and compares these changes to pH-dependent changes in k1 and k2. Simulations and data are provided for two weak acids, 2,3,4-trichlorophenol and 2,3,4,5-tetrachlorophenol, for which k1 and k2 were measured for large rainbow trout at multiple bulk water pH values [30]. The BCFBW values given in both panels were estimated as the ratio of k1 to k2. Additional simulations and data are given for a weak base, diphenhydramine, for which the BCFBW was measured in fathead minnows [72].
Figure 1.
Model [30] predictions and observed values for the gill uptake rate constant (k1, upright triangles), gill elimination rate constant (k2, inverted triangles) and steady-state blood/water concentration factor (BCFBW, stars) as a function of pH, for the weak acids 2,3,4-trichlorophenol (log KOW,N = 4.0) and 2,3,4,5-tetrachlorophenol (log KOW,N =4.2) and the weak base diphenhydramine (log KOW,N = 3.6). Solid, long-dashed, and short-dashed lines show predicted values for BCFBW, k1, and k2 respectively, where the modeled BCFBW is the ratio of k1 and k2. The dotted line denotes what the model-calculated BCFBW would be in the absence of any pH reduction in the gills.
The model-predicted BCFBW values shown in Figure 1 agree well with measured values, and can be understood in terms of the factors regulating k1 and k2 as presented in Absorption, Uptake across the gills. For all three chemicals, the BCFBW varies much more with pH than does either k1 or k2, due to k1 decreasing and k2 increasing with increased ionization. Also for all three chemicals, there are large differences between modeled BCFBW values (solid lines) and model simulations generated assuming no pH reduction at the gill surface (dotted lines). Generally, a reduction in gill pH tends to increase the bioaccumulation potential of weak acids and decrease the bioaccumulation potential of weak bases. These trends occur because the reduction in gill pH results in a larger fraction of an organic acid being present in neutral form at the gill surface compared to bulk water. The opposite pattern is predicted for organic bases.
For 2,3,4-trichlorophenol, the BCFBW varies by less than two-fold from pH 6.5 to 8.5, due to both k1 and k2 being fairly constant. The constancy of k1 reflects efficient extraction of chemical even at rather high ionization in the exposure water due to the mechanisms discussed previously (see Absorption, Uptake across the gills). The constancy of k2 is due to the pH at the gill surface being near or below this chemical’s pKa, so that the fraction of chemical that is ionized does not change greatly over this pH range. At pH>8.5, chemical ionization becomes large enough to substantially reduce k1 and increase k2, resulting in large decreases in the expected BCFBW.
In contrast to 2,3,4-trichlorophenol, the BCFBW for 2,3,4,5-tetrachlorophenol is predicted to vary more than ten-fold from pH 6.5 to 8.5. While k1 is still roughly constant in this range and is very close to that of 2,3,4-trichlorophenol, k2 declines markedly with decreasing pH due to changes in ionization associated with this chemicals’s lower pKa value. The predicted decline in k1 at higher pH values is moderately greater than for 2,3,4-trichlorophenol, but the combined effects of k1 and k2 on the BCFBW above pH 9 are similar for the two chemicals. Because of the limited number of measurements for k2, the modeled shape of the BCFBW vs. pH curve is not empirically established, but it is clear that the effect of pH is greater for 2,3,4,5-tetrachlorophenol than for 2,3,4-trichlorophenol, consistent with model predictions.
As for the weak acids, the modeled k1 for diphenhydramine (a weak base) decreases with increasing ionization in a less-than-proportional manner; however, this lack of proportionality is less pronounced than for the weak acids because pH reductions at the gill increase ionization for this weak base. The lower gill pH is also responsible for relatively small changes in the modeled k2 at pH < 9 because chemical at the lamellar surface is > 90% ionized over this entire range. The modeled and measured BCFBW values agree well, and both show a large deviation above pH 8 from the BCFBW expected in the absence of pH reductions in the gill.
Next, we considered possible impacts of biotransformation on the pH-dependence of IOC bioaccumulation. This effort was not intended to provide predictions about biotransformation rates as a function of pKa, hydrophobicity, and other chemical properties, but rather to consider whether the pH-dependence of IOC bioaccumulation should differ for chemicals that have significant biotransformation rates from those chemicals that have negligible biotransformation. The influence of biotransformation on steady-state accumulation depends on the magnitude of the whole-body biotransformation rate constant (kM) relative to k2, such that, for the same kM, biotransformation for non-ionizable chemicals becomes more important with increased chemical hydrophobicity (because of smaller k2). Because ionization can affect k2 (Figure 1), this raises the question of whether biotransformation would change the pH-dependence of the BCFBW for IOCs and be more or less important than it is for non-ionizable chemicals with the same hydrophobicity as the neutral forms of IOCs. There are no empirical data available to directly address this question, so the calculations here are hypothetical; however, the relative magnitudes of kM and k2 will govern the consequences of biotransformation, and this exercise can define its likely impact on the pH-dependency of IOC accumulation.
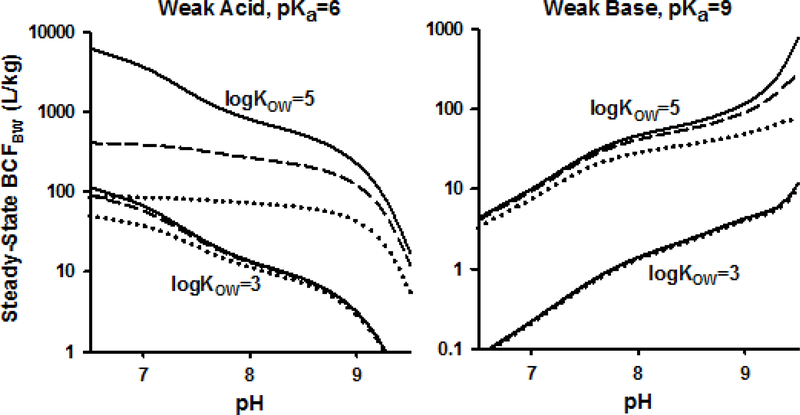
Figure 2 illustrates how pH effects on BCFBW would be expected to change due to biotransformation. These simulations were generated with the same model that was used to obtain Figure 1 (parameterized for trout [30]). In this case, however, predictions were obtained for four hypothetical chemicals with log KOW of 3 and 5 for the neutral form, two being weak acids with pKa = 6 and the other two being weak bases with pKa = 9. The figure compares BCFBW values predicted in the absence of biotransformation to those expected assuming moderate (0.02/h) and fast (0.1/h) rates of biotransformation. Because the purpose of this exercise was to illustrate the impact of a total rate of biotransformation on accumulation, there was no need to consider how biotransformation is affected by internal ionization or partitioning; however, this biotransformation must be considered to occur in tissues other than the gills because biotransformation in the gills [139–143] would directly impact apparent k1 and k2 values. A model developed to describe the effects of gill metabolism on chemical accumulation would therefore require modification of the gill description itself.
Figure 2.
Modeled [30] effects of biotransformation on steady-state blood/water bioconcentration factors (BCFBW) for hypothetical weak acids (left panel) and weak bases (right panel) with pKa and log KOW,N values as indicated. No biotransformation - solid lines; moderate (0.02/h) biotransformation - dashed lines; fast (0.1/h) biotransformation - dotted lines. Figure 3: Proposed interim workflow for screening and prioritization of IOCs for bioaccumulation hazard assessment and ecological exposure and risk assessment. For the latter, the ―B question‖ would be replaced by the results and decision-contexts for the exposure and risk estimates.
For the organic acid with log KOW,N = 3, setting kM to 0.1/h reduces accumulation compared to no biotransformation by a factor of 3.0 at pH 6 (where k2 is 0.05/h), but only by 7% at pH 9 (where k2 is 1.0/h), thereby reducing the total variation of accumulation across pH. Using the same model, a non-ionizable chemical with log KOW = 3 would have a k2 = 0.45/h across the entire range of pH values and a kM equal to 0.1/h would reduce the BCFBW by 18%. Thus, not only should biotransformation alter the pH-dependence of the bioaccumulation potential of acidic IOCs, but also, depending on pH, the relative importance of biotransformation could be more or less than it is for a non-ionizable chemical of similar hydrophobicity.
The dampening effect of biotransformation on the pH-dependence of BCFBW becomes very evident for the more hydrophobic organic acid (log KOW,N = 5) in Figure 2. When kM is set equal to 0.02/h, the change in predicted BCFBW over the pH 6–9 range becomes relatively small because the biotransformation rate constant dominates elimination and the pH-dependence of k2 is less relevant. This effect is even more pronounced at the larger kM of 0.1/h. Setting kM equal to 0.02/h reduces the BCFBW by a factor of 20 and 1.7 at pH 6 and 9, respectively compared to simulations assuming no biotransformation. For a non-ionizable chemical with a log KOW = 5, setting kM to 0.02/h is predicted to reduce the BCFBW by a factor of 5.0.
The model output for the two hypothetical bases indicates that, under the model assumptions, biotransformation will perturb the pH-dependence of the BCFBW less so than for the organic acids. For example, in Figure 2, biotransformation has a negligible influence on the pH-dependence of the BCFBW of the hypothetical base with log KOW = 3. This pattern is observed because the gill elimination rate constants for these bases are much larger than the assumed biotransformation rate constants across the entire pH range. Biotransformation exerts a more substantial influence on the model output for the more hydrophobic base (log KOW = 5), particularly at elevated bulk water pH (pH > 8). This occurs because, as bulk water pH increases, the predicted gill elimination rate constant decreases (Figure 1) to the point where it is similar to the assumed biotransformation rate constant. In summary, because the relative impact of biotransformation on overall bioaccumulation increases as the rates from other elimination processes decrease, it is expected that the relative effect of pH on IOC accumulation will change as biotransformation rates change and thus be an important aspect of interpreting data on the pHdependence of IOC accumulation.
The case study presented here highlights some important needs for better assessing the accumulation and effects of IOCs. First, although there are considerable data on the bioaccumulation of various IOCs [11,24,144], little of this systematically addresses the effects of pH for the same chemical and species. However, from both collective data on IOC accumulation and specific data sets addressing pH effects, the general trend is clear: the bioaccumulation potential of organic acids is inversely correlated with bulk water pH and the opposite is true for organic bases, but not in proportion to the extent of ionization in exposure water. These trends can be related to various processes at the gills, but better data are needed regarding this to develop more rigorous models. Second, a critical factor in predicting the effect of pH on accumulation of IOCs is the reduction in pH at the gill surface, which can vary depending on ambient pH and buffering strength, as well as the organism’s morphology and physiology. More information on this pH reduction under laboratory and realistic field conditions is greatly needed. This could be obtained by direct measurement or by inference, based on the dependence of accumulation on pH and pKa. Third, many IOCs are subject to biotransformation, which can affect the pH-dependence of the bioaccumulation potential of the parent compound. Studies that examine pH effects on accumulation could be combined with independently obtained estimates of kM (e.g., by extrapolating biotransformation rate constant data from an in vitro system [145]) to corroborate modeled predictions.
CASE STUDY 2: Toxicokinetics of Perfluorinated Alkyl Acids (PFAAs)
PFAAs can be considered ―extreme‖ IOCs, in the sense that they have very low pKa values (<1) and hence are more than 99% ionized at environmentally relevant pH values [146,147]. Nevertheless, field and laboratory studies have shown that long-chain PFAAs (i.e., those with at least 8 perfluorinated carbons) can accumulate in fish to the same extent as some neutral hydrophobic compounds (e.g., BCFs and BAFs on the order of 104, similar to mid-chlorinated PCBs) [148].
Studies with both fish and mammals indicate that with respect to chemical concentration, PFAAs accumulate preferentially in blood and liver (e.g., [90,149,150]). In vitro studies have shown that PFAAs bind strongly to serum albumin [151–155] and liver fatty acid binding protein (L-FABP) [156–158], which may at least partly explain their tissue-specific accumulation. Elimination half-lives of PFAAs vary widely among species, and for some, such as the rat, between genders [159–162]. In mammals, this elimination is largely controlled by the rate of renal clearance, which has been linked to the interactions of PFAAs with membrane transport proteins. Cellular uptake and competitive inhibition studies indicate that membrane transport of several long-chain PFAAs can be mediated by OATs/Oats and OATPs/Oatps [163,164]. Some of these transporters appear to secrete PFAAs to urine while others promote their reabsorption. For any given compound and species, the rate of renal elimination may reflect a balance of these two competing processes [161].
Based on these observations, Ng and Hungerbuehler [32] developed a model to predict the bioaccumulation of PFAAs in fish. This model explicitly describes the interactions of PFAAs with serum albumin, L-FABP in liver cytosol, and membrane transporters in the kidney. Although the model can be adapted to different species, it was parameterized to predict bioconcentration factors (BCFs) and patterns of tissue distribution for several PFAAs in rainbow trout (Oncorhynchus mykiss), in order to compare predicted values with data from an earlier study by Martin et al. [90].
At the time of its development, few data existed for the interactions of PFAAs with fish plasma proteins, and none for fish-specific L-FABP. There were also no studies that had looked specifically at renal elimination of these chemicals in fish. However, binding to fish plasma proteins had been observed [165] while other studies had shown that fish possess Oats and Oatps, and that these proteins transport a variety of organic anions (see Potential role of membrane transporters/active transport). The model therefore used existing data from studies with rat and human proteins to parameterize protein binding interactions and renal clearance by membrane transporters in the trout kidney.
The recent publication of two detailed kinetic studies [129,130] presents an opportunity to test how model predictions based on protein interactions derived from mammalian systems compare to observed rates of uptake and elimination for PFOA and PFOS. Consoer et al. [129,130] used large trout confined to respirometer-metabolism chambers to directly determine renal and branchial clearance rates for both compounds, injected as a bolus dose. Additional studies were then performed to estimate gill uptake rates during continuous waterborne exposures. Here we summarize our efforts to simulate the results of these studies using the model given by Ng and Hungerbuehler [32]. Adjustments to the original model required to perform these comparisons, along with a complete description of modeled results, are presented as Supplemental Data (Section S6).
In comparison with the experimental results of Consoer et al. [129,130], the original model overestimated measured rates of gill uptake (k1; L/kg/d) and elimination (k2; 1/d) for both PFOA and PFOS. A better match between the measured and predicted values was obtained by adjusting gill permeability constants downward by a factor of 15 (Table 1). The total elimination half-life (T1/2) value for PFOA (2.67 d) predicted by the adjusted model using the median observed KA value for albumin binding is one-fifth that determined by Consoer et al. (12.6 d) [129]. However, 12.6 d is well within the range of values predicted using all available albumin binding data (2 to 43 d). For PFOS, the predicted T1/2 using the median KA (68.7 d) is very close to that (86.9 d) determined by Consoer et al. [130]. Renal elimination rates (kR; 1/d) predicted by both the original and adjusted models were in good agreement with reported values. Thus, renal clearance appears to have been well-described using information derived from studies on mammalian cell lines.
Table 1.
Measured and modeled kinetic constants for perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) in large rainbow trout
| Ng and Hungerbuehler [32]1, adjusted2 | ||||
|---|---|---|---|---|
| Consoer et al. [129] PFOA mean (SD) |
Consoer et al. [130] PFOS mean (SD) |
PFOA med3 (range) | PFOS med3 (range) | |
| k1 (L/kg/d) | 0.19 (0.09) | 0.85 (0.29) | 0.126 (0.03 – 0.46) | 1.75 (0.19 – 2.43) |
| k2 (1/d) | 0.030 (0.011) | 0.027 (0.010) | 0.010 (0.0085 – 0.014) | 0.014 (0.014 – 0.092) |
| kR (1/d) | 0.330 (0.236) | 0.007 (0.003) | 0.25 (0.024 – 0.33) | 0.023 (0.023 – 0.33) |
| T1/24 | 12.6 (7.2) | 86.9 (14.1) | 2.67 (2.02 – 43.3) | 68.7 (2.01 – 184) |
Abbreviations: k1 – gill uptake rate constant; k2 – gill (branchial) elimination rate constant; kR – renal elimination rate constant; T1/2 – whole-body elimination half-life.
Model parameterized for a 1.1 kg rainbow trout using the urine flow rate (UFR) given by Consoer et al.[129]
Adjusted by reducing gill permeability by a factor of 15.
Model predictions obtained using median (med), maximum and minimum reported values for equilibrium association constants with albumin (KA).
Understanding the bioaccumulation of chemicals that deviate from expected behavior requires that we capitalize on existing toxicokinetic data, and better understand the strengths and limitations of read-across approaches as they apply to bioaccumulation mechanisms. In this case study, a convincing argument can be made for the ability to read across certain ADME processes from mammals to fish. That is, protein binding and active-transport-mediated renal elimination were identified as key processes in mammals, and the comparisons we have made here indicate these processes are also important in rainbow trout. Some of the parameters derived using mammalian cell lines and proteins (e.g., binding affinities and active transport rates) worked well within the trout model, while others (e.g., equating the permeability of mammalian cells to gill permeability) were less successful. Given the importance of protein interactions to the toxicokinetics of PFAAs as a chemical class, we see the need for a better understanding of transporter-mediated uptake and elimination mechanisms in different organisms.
SUMMARY AND RECOMMENDATIONS
Key conclusions stemming from this review are as follows:
Uptake and accumulation of IOCs from water may vary substantially with pH. Factors that influence these relationships include the identity of the compound itself (acid or base), its physicochemical properties (pKa and log KOW,N), and pH reductions at the gill surface due to elimination of metabolically-derived acid. Existing gill models describe observed patterns with respect to pH effects on accumulation; however, data are needed to further refine these models. Active transport and biotransformation at the gills are not explicitly included in currently available mechanistic models. The relative importance of these processes remains poorly known
Generally, pH effects on uptake and accumulation from water are predicted to be minor unless the extent of ionization in bulk water exceeds 90%. When the extent of ionization is < 90%, models that consider only the hydrophobicity of the neutral form may be sufficient to describe accumulation.
Biotransformation is predicted to have a dampening effect on the pH-dependence of bioconcentration for IOCs; however, the relative importance of biotransformation compared to gill elimination may differ substantially depending on whether the compound is an acid or base, as well as the hydrophobicity of the neutral form.
Biotransformation is expected to play a crucial role in bioaccumulation of IOCs with large log KOW,N values. As with neutral organics, there are few measured biotransformation rates for IOCs in fish and other ecological receptors. Integrated testing strategies (e.g., in vitro studies with S9 [105] or hepatocytes) should be developed to strategically select IOCs for measurement of biotransformation rates in aquatic species.
For many (if not most) IOCs, uptake efficiency from the diet is not expected to be significantly impacted by the extent of ionization and can be adequately predicted from log KOW,N. Active transport and biotransformation in the gut are not explicitly included in currently available mechanistic models, but could potentially be addressed by adjusting the chemical uptake efficiency (ED) estimated using current approaches (e.g., [11,166]).
For many IOCs, distribution to tissues is determined by non-specific sorption to phospholipids and may be predicted using relatively simple models. By comparison, plasma binding of IOCs is more difficult to predict and more likely to involve specific interactions with transporter proteins such as albumin and α1AGp. In general, binding interactions of IOCs with plasma proteins derived from fish are poorly known.
For some IOCs we can expect interactions with proteins that transport ionized substances across biological membranes. In such cases, biliary or renal elimination may control the overall rate of elimination. Efflux transporters in the gut (and possibly gills) have the potential to limit uptake from the diet (and possibly water). While fish are known to possess a diverse array of transporters, there have been few studies evaluating their role as determinants of whole-animal chemical accumulation. Additional studies employing likely substrates for these transporters are needed. Currently, the potential importance of membrane transporters for each IOC must be assessed on a case-by-case basis.
Read across approaches that employ information from mammals to inform ADME in fish may be useful for predicting the distribution of IOCs. This is especially true for ionized pharmaceuticals, which tend to be data-rich compounds. Limited data suggest that information pertaining to distribution (e.g., tissue and plasma binding) is most amenable to this approach. Information pertaining to metabolism and excretion are likely to be useful for identifying important processes in fish (e.g., the possibility of biotransformation or a likely role for membrane transporters), and may in some cases provide quantitative estimates of critical modeling parameters (e.g., renal tubular secretion of PFAAs).
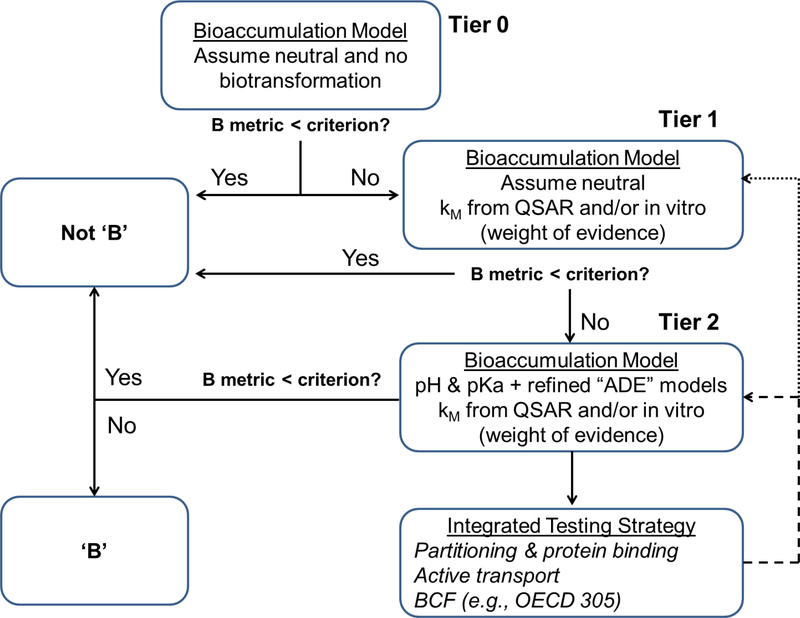
Based on the foregoing and recognizing that there are regulatory demands to make decisions in the present, we recommend that interim methods for bioaccumulation assessment of IOCs follow the tiered approach outlined in Armitage et al. [1] using an appropriate bioaccumulation model (Figure 3). The workflow shown in Figure 3 is appropriate for a bioaccumulation hazard assessment prior to the generation of or in the absence of sufficient empirical data; however, the same approach could be applied when parameterizing models for exposure and risk assessment. For exposure and risk assessment, the decision-making context to potentially proceed to additional tiers would be driven by risk estimates (or margin of safety/exposure) rather than bioaccumulation assessment criteria. The general strategy is to initially use conservative assumptions and progress to more realistic and sophisticated assumptions (models and associated data requirements) as required. In Tier 0, the IOC is treated as though it is a neutral compound, and biotransformation is assumed to be negligible. Tier 1 incorporates biotransformation rate estimates from QSAR modeling (if within the applicability domain of the model) and/or from in vitro to in vivo extrapolation of measured biotransformation rates. Tier 2 incorporates refined gill uptake and elimination models and may consider site-specific exposure conditions (e.g., pH and water hardness). Empirical or in silico data characterizing the sorption behaviour of the IOCs of interest are used directly in Tier 2 but can also be used to complement assessments in lower Tiers. The need for additional refinements would be informed by supporting test information (e.g., in vitro data) and/or existing ADME information from mammals, especially data indicating the importance of membrane/active transporters. Even if in vitro and ADME data from mammals cannot be used quantitatively in the bioaccumulation or exposure assessment, they can still serve as a qualitative flag indicating that uncertainty in the assessment is greater than for other chemicals. As discussed here and elsewhere [144], the bioaccumulation potential of acidic IOCs is inversely related to pH whereas it is directly related for basic IOCs. Accordingly, Tier 2 bioaccumulation hazard assessments for acidic IOCs and basic IOCs should be conducted at pH 8.1 for marine systems and at the lowest and highest relevant bulk water pH respectively for freshwater systems. Following from this, bulk water pH and test water characteristics (alkalinity, hardness) should always be reported and used to interpret in vivo bioaccumulation data used for B categorization. Generic exposure and risk assessments should also be conducted across the relevant range of pHs whereas site-specific assessments should be based on knowledge of the local conditions.
Figure 3:
Proposed interim workflow for screening and prioritization of IOCs for bioaccumulation hazard assessment and ecological exposure and risk assessment. For the latter, the ―B question‖ would be replaced by the results and decision-contexts for the exposure and risk estimates.
Supplementary Material
Acknowledgment—
Participation in the workshop and contributions to this manuscript by JMA and JAA were supported by the CEFIC-LRI ECO21 project (http://cefic-lri.org/projects/) and ECETOC (http://www.ecetoc.org/). We also thank Environment and Climate Change Canada, the ILSI Health and Environmental Sciences Institute (HESI), the European Chemical Industry Council (CEFIC) and the Society of Environmental Toxicology and Chemistry (SETAC) for providing logistical and/or financial support for the Experts Workshop on the Ecotoxicological Risk Assessment of Ionizable Organic Chemicals: Towards a Science-Based Framework for Chemical Assessment.
Footnotes
Disclaimer—The views expressed in the present article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency.
Data Availability—Modeling tools applied in the current manuscript are based on publications in Environmental Toxicology and Chemistry, as indicated in the manuscript. See publications on modeling tools identified in manuscript for data.
This article includes online-only Supplemental Data.
REFERENCES
- 1.Parrott N, Jones H, Paquereau N, Lavé T. 2005. Application of Full Physiological Models for Pharmaceutical Drug Candidate Selection and Extrapolation of Pharmacokinetics to Man. Basic Clin. Pharmacol. Toxicol 96:193–199. [DOI] [PubMed] [Google Scholar]
- 2.De Buck SS, Sinha VK, Fenu LA, Gilissen RA, Mackie CE, Nijsen MJ. 2007. Theprediction of drug metabolism, tissue distribution, and bioavailability of 50 structurally diverse compounds in rat using mechanism-based absorption, distribution, and metabolism prediction tools. Drug Metab. Dispos 35:649–59. [DOI] [PubMed] [Google Scholar]
- 3.Jones HM, Gardner IB, Watson KJ. 2009. Modelling and PBPK Simulation in Drug Discovery. The AAPS Journal 11:155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manallack DT. 2009. The acid-base profile of a contemporary set of drugs: implications for drug discovery. SAR QSAR Environ. Res 20:611–55. [DOI] [PubMed] [Google Scholar]
- 5.Charifson PS, Walters WP. 2014. Acidic and Basic Drugs in Medicinal Chemistry: A Perspective. J. Med. Chem 57:9701–9717. [DOI] [PubMed] [Google Scholar]
- 6.Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SP. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr. Environ. Assess. Manag 7:513–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giesy JP, Kannan K. 2001. Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ. Sci. Technol 35:1339–1342. [DOI] [PubMed] [Google Scholar]
- 8.Arnold KE, Brown AR, Ankley GT, Sumpter JP. 2014. Medicating the environment: assessing risks of pharmaceuticals to wildlife and ecosystems. Philosophical Transactions of the Royal Society B: Biological Sciences 369:20130569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kümmerer K 2009. The presence of pharmaceuticals in the environment due to human use – present knowledge and future challenges. J. Environ. Manage 90:2354–2366. [DOI] [PubMed] [Google Scholar]
- 10.Franco A, Ferranti A, Davidsen C, Trapp S. 2010. An unexpected challenge: ionizable compounds in the REACH chemical space. The International Journal of Life Cycle Assessment 15:321–325. [Google Scholar]
- 11.Armitage JM, Arnot JA, Wania F, Mackay D. 2013. Development and evaluation of a mechanistic bioconcentration model for ionogenic organic chemicals in fish. Environ. Toxicol. Chem 32:115–28. [DOI] [PubMed] [Google Scholar]
- 12.Franco A, Trapp S. 2010. A multimedia activity model for ionizable compounds: Validation study with 2,4-dichlorophenoxyacetic acid, aniline, and trimethoprim. Environ. Toxicol. Chem 29:789–799. [DOI] [PubMed] [Google Scholar]
- 13.Trapp S, Franco A, Mackay D. 2010. Activity-Based Concept for Transport and Partitioning of Ionizing Organics. Environ. Sci. Technol 44:6123–6129. [DOI] [PubMed] [Google Scholar]
- 14.Gobas FAPC Burkhard LP, Doucette WJ, Sappington KG, Verbruggen EMJ Hope BK, Bonnell MA Arnot JA, Tarazona JV. 2016. Review of existing terrestrial bioaccumulation models and terrestrial bioaccumulation modeling needs for organic chemicals. Integr. Environ. Assess. Manage 12:123–134. [DOI] [PubMed] [Google Scholar]
- 15.Malchi T, Maor Y, Tadmor G, Shenker M, Chefetz B. 2014. Irrigation of root vegetables with treated wastewater: evaluating uptake of pharmaceuticals and the associated human health risks. Environ. Sci. Technol 48:9325–33. [DOI] [PubMed] [Google Scholar]
- 16.Prosser RS, Lissemore L, Topp E, Sibley PK. 2014. Bioaccumulation of triclosan and triclocarban in plants grown in soils amended with municipal dewatered biosolids. Environ. Toxicol. Chem 33:975–84. [DOI] [PubMed] [Google Scholar]
- 17.Wu X, Dodgen LK, Conkle JL, Gan J. 2015. Plant uptake of pharmaceutical and personal care products from recycled water and biosolids: a review. Sci. Total Environ 536:655–666. [DOI] [PubMed] [Google Scholar]
- 18.Miller EL, Nason SL, Karthikeyan KG, Pedersen JA. 2016. Root Uptake of Pharmaceuticals and Personal Care Product Ingredients. Environ. Sci. Technol 50:525541. [DOI] [PubMed] [Google Scholar]
- 19.Gobas FAPC, de Wolf W, Burkhard LP, Verbruggen E, Plotzke K 2009. Revisiting Bioaccumulation Criteria for POPs and PBT Assessments. Integr. Environ. Assess. Manage 5:624–637. [DOI] [PubMed] [Google Scholar]
- 20.Burkhard LP, Arnot JA, Embry MR, Farley KJ, Hoke RA, Kitano M, Leslie HA, Lotufo GR, Parkerton TF, Sappington KG, Tomy GT, Woodburn KB. 2012. Comparing laboratory and field measured bioaccumulation endpoints. Integr. Environ. Assess. Manage 8:17–31. [DOI] [PubMed] [Google Scholar]
- 21.Huggett DB, Cook JC, Ericson JF, Williams RT. 2003. A Theoretical Model for UtilizingMammalian Pharmacology and Safety Data to Prioritize Potential Impacts of Human Pharmaceuticals to Fish. Human and Ecological Risk Assessment: An International Journal 9:1789–1799. [Google Scholar]
- 22.Schreiber R, Gundel U, Franz S, Kuster A, Rechenberg B, Altenburger R. 2011. Using the fish plasma model for comparative hazard identification for pharmaceuticals in the environment by extrapolation from human therapeutic data. Regul. Toxicol. Pharmacol 61:261–75. [DOI] [PubMed] [Google Scholar]
- 23.Arnot JA, Gobas FAPC. 2006. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev 14:257–297. [Google Scholar]
- 24.Fu W, Franco A, Trapp S. 2009. Methods for estimating the bioconcentration factor of ionizable organic chemicals. Environ. Toxicol. Chem 28:1372–9. [DOI] [PubMed] [Google Scholar]
- 25.Droge S, Goss K-U. 2012. Effect of Sodium and Calcium Cations on the Ion-Exchange Affinity of Organic Cations for Soil Organic Matter. Environ. Sci. Technol 46:5894–5901. [DOI] [PubMed] [Google Scholar]
- 26.Franco A, Fu W, Trapp S. 2009. Influence of soil pH on the sorption of ionizable chemicals: Modeling advances. Environ. Toxicol. Chem 28:458–464. [DOI] [PubMed] [Google Scholar]
- 27.Franco A, Trapp S. 2008. Estimation of the soil–water partition coefficient normalized to organic carbon for ionizable organic chemicals. Environ. Toxicol. Chem 27:1995–2004. [DOI] [PubMed] [Google Scholar]
- 28.Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ. Res 16:531–54. [DOI] [PubMed] [Google Scholar]
- 29.Dimitrov S, Dimitrova N, Georgieva D, Vasilev K, Hatfield T, Straka J, Mekenyan O. 2012. Simulation of chemical metabolism for fate and hazard assessment. III. New developments of the bioconcentration factor base-line model. SAR QSAR Environ. Res 23:17–36. [DOI] [PubMed] [Google Scholar]
- 30.Erickson RJ, McKim JM, Lien GJ, Hoffman AD, Batterman SL. 2006. Uptake and elimination of ionizable organic chemicals at fish gills: I. Model formulation, parameterization, and behavior. Environ. Toxicol. Chem 25:1512–1521. [DOI] [PubMed] [Google Scholar]
- 31.Erickson RJ, McKim JM, Lien GJ, Hoffman AD, Batterman SL. 2006. Uptake and elimination of ionizable organic chemicals at fish gills: II. Observed and predicted effects of pH, alkalinity, and chemical properties. Environ. Toxicol. Chem 25:1522–1532. [DOI] [PubMed] [Google Scholar]
- 32.Ng CA, Hungerbühler K. 2013. Bioconcentration of Perfluorinated Alkyl Acids: How Important Is Specific Binding? Environ. Sci. Technol 47:7214–7223. [DOI] [PubMed] [Google Scholar]
- 33.Marshall WS. 1978. On the involvement of mucous secretion in teleost osmoregulation. Can. J. Zool 56:1088–1091. [Google Scholar]
- 34.Schlichter LC. 1982. Unstirred Mucus Layers: Ion Exchange Properties and Effect On Ion Regulation in Lymnaea Stagnalis. J. Exp. Biol 98:363–372. [Google Scholar]
- 35.Shephard KL. 1994. Functions for fish mucus. Rev. Fish Biol. Fish 4:401–429. [Google Scholar]
- 36.Avdeef A, Box KJ, Comer JEA, Hibbert C, Tam KY. 1998. pH-Metric logP 10. Determination of Liposomal Membrane-Water Partition Coefficients of lonizable Drugs. Pharm. Res 15:209–215. [DOI] [PubMed] [Google Scholar]
- 37.Abraham MH, Zhao YH, Le J, Hersey A, Luscombe CN, Reynolds DP, Beck G, Sherborne B, Cooper I. 2002. On the mechanism of human intestinal absorption. Eur. J. Med. Chem 37:595–605. [DOI] [PubMed] [Google Scholar]
- 38.Leslie EM, Deeley RG, Cole SP. 2005. Multidrug resistance proteins: role of Pglycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol 204:216–37. [DOI] [PubMed] [Google Scholar]
- 39.Dobson PD, Kell DB. 2008. Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule? Nat. Rev. Drug Discov 7:205–20. [DOI] [PubMed] [Google Scholar]
- 40.Sugano K, Kansy M, Artursson P, Avdeef A, Bendels S, Di L, Ecker GF, Faller B, Fischer H, Gerebtzoff G, Lennernaes H, Senner F. 2010. Coexistence of passive and carrier-mediated processes in drug transport. Nat. Rev. Drug Discov 9:597–614. [DOI] [PubMed] [Google Scholar]
- 41.Hagenbuch B, Stieger B. 2013. The SLCO (former SLC21) superfamily of transporters. Mol. Aspects Med. 34:396–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith D, Artursson P, Avdeef A, Di L, Ecker GF, Faller B, Houston JB, Kansy M, Kerns EH, Kramer SD, Lennernas H, van de Waterbeemd H, Sugano K, Testa B. 2014. Passive lipoidal diffusion and carrier-mediated cell uptake are both important mechanisms of membrane permeation in drug disposition. Mol. Pharm 11:1727–38. [DOI] [PubMed] [Google Scholar]
- 43.Deeley RG, Westlake C, Cole SP. 2006. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol. Rev 86:849–99. [DOI] [PubMed] [Google Scholar]
- 44.Roth M, Obaidat A, Hagenbuch B. 2012. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br. J. Pharmacol 165:1260–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nigam SK, Bush KT, Martovetsky G, Ahn SY, Liu HC, Richard E, Bhatnagar V, Wu W. 2015. The organic anion transporter (OAT) family: a systems biology perspective. Physiol. Rev 95:83–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luckenbach T, Fischer S, Sturm A. 2014. Current advances on ABC drug transporters in fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol 165:28–52. [DOI] [PubMed] [Google Scholar]
- 47.Annilo T, Chen ZQ, Shulenin S, Costantino J, Thomas L, Lou H, Stefanov S, Dean M. 2006. Evolution of the vertebrate ABC gene family: analysis of gene birth and death. Genomics 88:1–11. [DOI] [PubMed] [Google Scholar]
- 48.Long Y, Li Q, Wang Y, Cui Z. 2011. MRP proteins as potential mediators of heavy metal resistance in zebrafish cells. Comp. Biochem. Physiol. C Toxicol. Pharmacol 153:310–7. [DOI] [PubMed] [Google Scholar]
- 49.Fischer S, Kluver N, Burkhardt-Medicke K, Pietsch M, Schmidt AM, Wellner P, Schirmer K, Luckenbach T. 2013. Abcb4 acts as multixenobiotic transporter and active barrier against chemical uptake in zebrafish (Danio rerio) embryos. BMC Biol. 11:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller DS. 1995. Daunomycin secretion by killfish renal proximal tubules. Am. J. Physiol 269:R370–9. [DOI] [PubMed] [Google Scholar]
- 51.Sussman-Turner C, Renfro JL. 1995. Heat-shock-stimulated transepithelial daunomycin secretion by flounder renal proximal tubule primary cultures. Am. J. Physiol 268:F135–44. [DOI] [PubMed] [Google Scholar]
- 52.Miller DS, Pritchard JB. 1997. Dual pathways for organic anion secretion in renal proximal tubule. J. Exp. Zool 279:462–70. [DOI] [PubMed] [Google Scholar]
- 53.Masereeuw R, Terlouw SA, van Aubel RA, Russel FG, Miller DS. 2000. Endothelin B receptor-mediated regulation of ATP-driven drug secretion in renal proximal tubule. Mol. Pharmacol 57:59–67. [PubMed] [Google Scholar]
- 54.Reichel V, Masereeuw R, van den Heuvel JJ, Miller DS, Fricker G. 2007. Transport of a fluorescent cAMP analog in teleost proximal tubules. Am. J. Physiol. Regul. Integr. Comp. Physiol 293:R2382–9. [DOI] [PubMed] [Google Scholar]
- 55.Aslamkhan AG, Thompson DM, Perry JL, Bleasby K, Wolff NA, Barros S, Miller DS, Pritchard JB. 2006. The flounder organic anion transporter fOat has sequence, function, and substrate specificity similarity to both mammalian Oat1 and Oat3. Am. J. Physiol. Regul. Integr. Comp. Physiol 291:R1773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Popovic M, Zaja R, Smital T. 2010. Organic anion transporting polypeptides (OATP) in zebrafish (Danio rerio): Phylogenetic analysis and tissue distribution. Comp. Biochem. Physiol. A Mol. Integr. Physiol 155:327–35. [DOI] [PubMed] [Google Scholar]
- 57.Verri T, Terova G, Romano A, Barca A, Pisani P, Storelli C, Saroglia M. 2012. The SoLute Carrier (SLC) Family Series in Teleost Fish in Functional Genomics in Aquaculture. Wiley-Blackwell; pp 219–320 [Google Scholar]
- 58.Popovic M, Zaja R, Fent K, Smital T. 2013. Molecular characterization of zebrafish Oatp1d1 (Slco1d1), a novel organic anion-transporting polypeptide. J. Biol. Chem 288:33894–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Popovic M, Zaja R, Fent K, Smital T. 2014. Interaction of environmental contaminants with zebrafish organic anion transporting polypeptide, Oatp1d1 (Slco1d1). Toxicol. Appl. Pharmacol 280:149–58. [DOI] [PubMed] [Google Scholar]
- 60.Steiner K, Hagenbuch B, Dietrich DR. 2014. Molecular cloning and functional characterization of a rainbow trout liver Oatp. Toxicol. Appl. Pharmacol 280:534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boaru DA, Dragoş N, Schirmer K. 2006. Microcystin-LR induced cellular effects in mammalian and fish primary hepatocyte cultures and cell lines: A comparative study. Toxicology 218:134–148. [DOI] [PubMed] [Google Scholar]
- 62.Norstrom RJ, McKinnon AE, deFreitas ASW. 1976. A Bioenergetics-Based Model for Pollutant Accumulation by Fish. Simulation of PCB and Methylmercury Residue Levels in Ottawa River Yellow Perch (Perca flavescens). Journal of the Fisheries Research Board of Canada 33:248–267. [Google Scholar]
- 63.Neely WB. 1979. Estimating rate constants for the uptake and clearance of chemicals by fish. Environ. Sci. Technol 13:1506–1510. [Google Scholar]
- 64.Bruggeman WA, Martron LBJM, Kooiman D, Hutzinger O. 1981. Accumulation and elimination kinetics of di-, tri- and tetra chlorobiphenyls by goldfish after dietary and aqueous exposure. Chemosphere 10:811–832. [Google Scholar]
- 65.McKim J, Schmieder P, Veith G. 1985. Absorption dynamics of organic chemical transport across trout gills as related to octanol-water partition coefficient. Toxicol. Appl. Pharmacol 77:1–10. [DOI] [PubMed] [Google Scholar]
- 66.Opperhulzen A, Volde EWvd, Gobas FAPC, Liem DAK, Steen JMDvd, Hutzinger O. 1985. Relationship between bioconcentration in fish and steric factors of hydrophobic chemicals. Chemosphere 14:1871–1896. [Google Scholar]
- 67.Gobas FAPC, MacKay D 1987. Dynamics of hydrophobic organic chemical bioconcentration in fish. Environ. Toxicol. Chem 6:495–504. [Google Scholar]
- 68.Barber MC, Suérez LA, Lassiter RR. 1988. Modeling bioconcentration of nonpolar organic pollutants by fish. Environ. Toxicol. Chem 7:545–558. [Google Scholar]
- 69.Erickson RJ, McKim JM. 1990. A model for exchange of organic chemicals at fish gills: flow and diffusion limitations. Aquat. Toxicol 18:175–197. [Google Scholar]
- 70.Hayton WL, Barron MG. 1990. Rate-limiting barriers to xenobiotic uptake by the gill. Environ. Toxicol. Chem 9:151–157. [Google Scholar]
- 71.Saarikoski J, Lindstrom R, Tyynela M, Viluksela M. 1986. Factors affecting the absorption of phenolics and carboxylic acids in the guppy (Poecilia reticulata). Ecotoxicol. Environ. Saf 11:158–73. [DOI] [PubMed] [Google Scholar]
- 72.Nichols JW, Du B, Berninger JP, Connors KA, Chambliss CK, Erickson RJ, Hoffman AD, Brooks BW. 2015. Observed and modeled effects of pH on bioconcentration of diphenhydramine, a weakly basic pharmaceutical, in fathead minnows. Environ. Toxicol. Chem 34:1425–1435. [DOI] [PubMed] [Google Scholar]
- 73.Kelly BC, Gobas FA, McLachlan MS. 2004. Intestinal absorption and biomagnification of organic contaminants in fish, wildlife, and humans. Environ. Toxicol. Chem 23:2324–36. [DOI] [PubMed] [Google Scholar]
- 74.O’Connor IA, Huijbregts MA, Ragas AM, Hendriks AJ. 2013. Predicting the oral uptake efficiency of chemicals in mammals: combining the hydrophilic and lipophilic range. Toxicol. Appl. Pharmacol 266:150–6. [DOI] [PubMed] [Google Scholar]
- 75.Drouillard KG, Norstrom RJ. 2000. Dietary absorption efficiencies and toxicokinetics of polychlorinated biphenyls in ring doves following exposure to aroclor® mixtures. Environ. Toxicol. Chem 19:2707–2714. [Google Scholar]
- 76.Van Veld PA, Patton JS, Lee RF. 1988. Effect of preexposure to dietary benzo[a]pyrene (BP) on the first-pass metabolism of BP by the intestine of toadfish (Opsanus tau): in vivo studies using portal vein-catheterized fish. Toxicol. Appl. Pharmacol 92:255–65. [DOI] [PubMed] [Google Scholar]
- 77.Kleinow KM, James MO, Tong Z, Venugopalan CS. 1998. Bioavailability and biotransformation of benzo(a)pyrene in an isolated perfused In situ catfish intestinal preparation. Environ. Health Perspect 106:155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lo JC, Campbell DA, Kennedy CJ, Gobas FAPC. 2015. Somatic and gastrointestinal in vivo biotransformation rates of hydrophobic chemicals in fish. Environ. Toxicol. Chem 34:2282–2294. [DOI] [PubMed] [Google Scholar]
- 79.Arnot JA, Quinn CL. 2015. Development and Evaluation of a Database of Dietary Bioaccumulation Test Data for Organic Chemicals in Fish. Environ. Sci. Technol 49:4783–4796. [DOI] [PubMed] [Google Scholar]
- 80.Xiao R, Adolfsson-Erici M, Akerman G, McLachlan MS, MacLeod M. 2013. A benchmarking method to measure dietary absorption efficiency of chemicals by fish. Environ. Toxicol. Chem. 32:2695–700. [DOI] [PubMed] [Google Scholar]
- 81.Lien GJ, McKim JM. 1993. Predicting branchial and cutaneous uptake of 2,2′,5,5′-tetrachlorobiphenyl in fathead minnows (Pimephales promelas) and Japanese medaka (Oryzias latipes): Rate limiting factors. Aquat. Toxicol 27:15–31. [Google Scholar]
- 82.McKim JM, Nichols JW, Lien GJ, Hoffman AD, Gallinat CA, Stokes GN. 1996. Dermal absorption of three waterborne chloroethanes in rainbow trout (Oncorhynchus mykiss) and channel catfish (Ictalurus punctatus). Fundam. Appl. Toxicol 31:218–228. [DOI] [PubMed] [Google Scholar]
- 83.Nichols JW, McKim JM, Lien GJ, Hoffman AD, Bertelsen SL, Elonen CM. 1996. A physiologically based toxicokinetic model for dermal absorption of organic chemicals by fish. Fundam. Appl. Toxicol 31:229–42. [DOI] [PubMed] [Google Scholar]
- 84.Tovell PW, Howes D, Newsome CS. 1975. Absorption, metabolism and excretion by goldfish of the anionic detergent sodium lauryl sulphate. Toxicology 4:17–29. [DOI] [PubMed] [Google Scholar]
- 85.Mackay D 2001. Multimedia Environmental Models: The Fugacity Approach, Second Edition. CRC Press, Boca Raton, FL, USA. [Google Scholar]
- 86.Cahill TM, Cousins I, Mackay D. 2003. Development and application of a generalized physiologically based pharmacokinetic model for multiple environmental contaminants. Environ. Toxicol. Chem 22:26–34. [PubMed] [Google Scholar]
- 87.Gauthier JM, Metcalfe CD, Sears R. 1997. Validation of the blubber biopsy technique for monitoring of organochlorine contaminants in balaenopterid whales. Mar. Environ. Res 43:157–179. [Google Scholar]
- 88.Jahnke A, Mayer P, Adolfsson-Erici M, McLachlan MS. 2011. Equilibrium sampling of environmental pollutants in fish: comparison with lipid-normalized concentrations and homogenization effects on chemical activity. Environ. Toxicol. Chem 30:1515–21. [DOI] [PubMed] [Google Scholar]
- 89.Endo S, Brown TN, Goss K-U. 2013. General Model for Estimating Partition Coefficients to Organisms and Their Tissues Using the Biological Compositions and Polyparameter Linear Free Energy Relationships. Environ. Sci. Technol 47:6630–6639. [DOI] [PubMed] [Google Scholar]
- 90.Martin JW, Mabury SA, Solomon KR, Muir DCG. 2003. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem 22:196–204. [PubMed] [Google Scholar]
- 91.Conder JM, Hoke RA, Wolf Wd, Russell MH, Buck RC. 2008. Are PFCAs Bioaccumulative? A Critical Review and Comparison with Regulatory Criteria and Persistent Lipophilic Compounds. Environ. Sci. Technol 42:995–1003. [DOI] [PubMed] [Google Scholar]
- 92.Poulin P, Theil FP. 2000. A priori prediction of tissue:plasma partition coefficients of drugs to facilitate the use of physiologically-based pharmacokinetic models in drug discovery. J. Pharm. Sci 89:16–35. [DOI] [PubMed] [Google Scholar]
- 93.Rodgers T, Leahy D, Rowland M. 2005. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J. Pharm. Sci 94:1259–76. [DOI] [PubMed] [Google Scholar]
- 94.Rodgers T, Rowland M. 2006. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J. Pharm. Sci 95:1238–57. [DOI] [PubMed] [Google Scholar]
- 95.Schmitt W 2008. General approach for the calculation of tissue to plasma partition coefficients. Toxicol. In Vitro 22:457–67. [DOI] [PubMed] [Google Scholar]
- 96.Bittermann K, Spycher S, Endo S, Pohler L, Huniar U, Goss K-U, Klamt A. 2014. Prediction of Phospholipid–Water Partition Coefficients of Ionic Organic Chemicals Using the Mechanistic Model COSMOmic. The Journal of Physical Chemistry B 118:14833–14842. [DOI] [PubMed] [Google Scholar]
- 97.Henneberger L, Goss K-U, Endo S. 2016. Equilibrium Sorption of Structurally Diverse Organic Ions to Bovine Serum Albumin. Environ. Sci. Technol 50:5119–5126. [DOI] [PubMed] [Google Scholar]
- 98.Macintyre AC, Cutler DJ. 1988. The potential role of lysosomes in tissue distribution of weak bases. Biopharm. Drug Disposition 9:513–526. [DOI] [PubMed] [Google Scholar]
- 99.Kazmi F, Hensley T, Pope C, Funk RS, Loewen GJ, Buckley DB, Parkinson A. 2013. Lysosomal sequestration (trapping) of lipophilic amine (cationic amphiphilic) drugs in immortalized human hepatocytes (Fa2N-4 cells). Drug Metab. Dispos 41:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Daniel WA, Wojcikowski J. 1997. Contribution of lysosomal trapping to the total tissue uptake of psychotropic drugs. Pharmacol. Toxicol 80:62–8. [DOI] [PubMed] [Google Scholar]
- 101.Schlenk D, Celander M, Gallagher EP, George S, James M, Kullman S, van den Hurk P, Willett W. 2008. Biotransformation in Fishes in The Toxicology of Fishes. CRC Press; pp 153–234 [Google Scholar]
- 102.Goldstone JV, McArthur AG, Kubota A, Zanette J, Parente T, Jonsson ME, Nelson DR, Stegeman JJ. 2010. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genomics 11:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Connors KA, Du B, Fitzsimmons PN, Hoffman AD, Chambliss CK, Nichols JW, Brooks BW. 2013. Comparative pharmaceutical metabolism by rainbow trout (Oncorhynchus mykiss) liver S9 fractions. Environ. Toxicol. Chem 32:1810–1818. [DOI] [PubMed] [Google Scholar]
- 104.James MO, Marth CJ, Rowland-Faux L. 2012. Slow O-demethylation of methyl triclosan to triclosan, which is rapidly glucuronidated and sulfonated in channel catfish liver and intestine. Aquat. Toxicol 124–125:72–82. [DOI] [PubMed] [Google Scholar]
- 105.Chen Y, Hermens JLM, Jonker MTO, Arnot JA, Armitage JM, Brown T, Nichols JW, Fay KA, Droge STJ. 2016. Which molecular features affect the intrinsic hepatic clearance rate of ionizable organic chemicals in fish? Environ. Sci. Technol [DOI] [PubMed] [Google Scholar]
- 106.Loncar J, Popovic M, Zaja R, Smital T. 2010. Gene expression analysis of the ABC efflux transporters in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. C Toxicol. Pharmacol 151:209–15. [DOI] [PubMed] [Google Scholar]
- 107.Fischer S, Loncar J, Zaja R, Schnell S, Schirmer K, Smital T, Luckenbach T. 2011. Constitutive mRNA expression and protein activity levels of nine ABC efflux transporters in seven permanent cell lines derived from different tissues of rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol 101:438–446. [DOI] [PubMed] [Google Scholar]
- 108.Stott LC, Schnell S, Hogstrand C, Owen SF, Bury NR. 2015. A primary fish gill cell culture model to assess pharmaceutical uptake and efflux: Evidence for passive and facilitated transport. Aquat. Toxicol 159:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bieczynski F, De Anna JS, Pirez M, Brena BM, Villanueva SS, Luquet CM. 2014. Cellular transport of microcystin-LR in rainbow trout (Oncorhynchus mykiss) across the intestinal wall: possible involvement of multidrug resistance-associated proteins. Aquat. Toxicol 154:97–106. [DOI] [PubMed] [Google Scholar]
- 110.Diaz GJ. 2000. Basolateral and canalicular transport of xenobiotics in the hepatocyte: A review. Cytotechnology 34:225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Faber KN, Muller M, Jansen PL. 2003. Drug transport proteins in the liver. Adv Drug Deliv Rev 55:107–24. [DOI] [PubMed] [Google Scholar]
- 112.Pfeifer ND, Hardwick RN, Brouwer KL. 2014. Role of hepatic efflux transporters in regulating systemic and hepatocyte exposure to xenobiotics. Annu. Rev. Pharmacol. Toxicol 54:509–35. [DOI] [PubMed] [Google Scholar]
- 113.Bungay PM, Dedrick RL, Guarino AM. 1976. Pharmacokinetic modeling of the dogfish shark (Squalus acanthias): distribution and urinary and biliary excretion of phenol red and its glucuronide. J. Pharmacokinet. Biopharm 4:377–88. [DOI] [PubMed] [Google Scholar]
- 114.Pritchard JB, Anderson JB, Rall DP, Guarino AM. 1980. Comparative hepatic and renal handling of phenol red and indocyanine green by cyclostome, elasmobranch and teleost fish. Comp. Biochem. Physiol. C 65:99–104. [DOI] [PubMed] [Google Scholar]
- 115.Plakas SM, Stehly GR, Khoo L. 1992. Pharmacokinetics and excretion of phenol red in the channel catfish. Xenobiotica 22:551–7. [DOI] [PubMed] [Google Scholar]
- 116.Schmidt DC, Weber LJ. 1973. Metabolism and Biliary Excretion of Sulfobromophthalein by Rainbow Trout (Salmo gairdneri). Journal of the Fisheries Research Board of Canada 30:1301–1308. [Google Scholar]
- 117.Gingerich WH, Weber LJ, Larson RE. 1977. Hepatic accumulation, metabolism and biliary excretion of sulfobromophthalein by rainbow trout (Salmo gairdneri). Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 58:113–120. [DOI] [PubMed] [Google Scholar]
- 118.Layiwola PJ, Linnecar DF, Knights B. 1983. Hydrolysis of the biliary glucuronic acid conjugate of phenol by the intestinal mucus/flora of goldfish (Carassius auratus). Xenobiotica 13:27–9. [DOI] [PubMed] [Google Scholar]
- 119.James MO. 1987. Conjugation of organic pollutants in aquatic species. Environ. Health Perspect 71:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fricker G, Wossner R, Drewe J, Fricker R, Boyer JL. 1997. Enterohepatic circulation of scymnol sulfate in an elasmobranch, the little skate (Raja erinacea). Am. J. Physiol 273:G1023–30. [DOI] [PubMed] [Google Scholar]
- 121.Pritchard JB, Bend JR. 1984. Mechanism controlling the renal excretion of xenobiotics in fish: effects of chemical structure. Drug Metab. Rev 15:655–71. [DOI] [PubMed] [Google Scholar]
- 122.Pritchard JB, Renfro JL. 1984. Interactions of xenobiotics with teleost renal function in Weber LJ, ed, Aquatic Toxicology, volume 2 Raven Press, NY, NY, USA: pp 51–106 [Google Scholar]
- 123.James-Curtis B, Wood CM. 1992. Kidney and urinary bladder responses of freshwater rainbow trout to isosmotic NaCl and NaHCO3 infusion. J. Exp. Biol 173:181–203. [Google Scholar]
- 124.McKim JM, Kolanczyk RC, Lien GJ, Hoffman AD. 1999. Dynamics of renal excretion of phenol and major metabolites in the rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol 45:265–277. [Google Scholar]
- 125.Nichols JW, McKim JM, Andersen ME, Gargas ML, Clewell HJ 3rd, Erickson RJ. 1990. A physiologically based toxicokinetic model for the uptake and disposition of waterborne organic chemicals in fish. Toxicol. Appl. Pharmacol 106:433–47. [DOI] [PubMed] [Google Scholar]
- 126.Pritchard JB, James MO. 1979. Determinants of the renal handling of 2, 4-dichlorophenoxyacetic acid by winter flounder. J. Pharmacol. Exp. Ther 208:280–6. [PubMed] [Google Scholar]
- 127.Pritchard JB, Karnaky KJ, Jr., Guarino AM, Kinter WB. 1977. Renal handling of the polar DDT metabolite DDA (2,2-bis[p-chlorophenyl] acetic acid) by marine fish. Am. J. Physiol 233:F126–32. [DOI] [PubMed] [Google Scholar]
- 128.Pritchard JB, Bend JR. 1991. Relative roles of metabolism and renal excretory mechanisms in xenobiotic elimination by fish. Environ. Health Perspect 90:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Consoer DM, Hoffman AD, Fitzsimmons PN, Kosian PA, Nichols JW. 2014. Toxicokinetics of perfluorooctanoate (PFOA) in rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol 156:65–73. [DOI] [PubMed] [Google Scholar]
- 130.Consoer DM, Hoffman AD, Fitzsimmons PN, Kosian PA, Nichols JW. 2016. Toxicokinetics of perfluorooctane sulfonate in rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem 35:717–27. [DOI] [PubMed] [Google Scholar]
- 131.Fitzsimmons PN, Fernandez JD, Hoffman AD, Butterworth BC, Nichols JW. 2001. Branchial elimination of superhydrophobic organic compounds by rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol 55:23–34. [DOI] [PubMed] [Google Scholar]
- 132.Winter MJ, Lillicrap AD, Caunter JE, Schaffner C, Alder AC, Ramil M, Ternes TA, Giltrow E, Sumpter JP, Hutchinson TH. 2008. Defining the chronic impacts of atenolol on embryo-larval development and reproduction in the fathead minnow (Pimephales promelas). Aquat. Toxicol 86:361–9. [DOI] [PubMed] [Google Scholar]
- 133.Berninger JP, Du B, Connors KA, Eytcheson SA, Kolkmeier MA, Prosser KN, Valenti TW Jr., Chambliss CK, Brooks BW. 2011. Effects of the antihistamine diphenhydramine on selected aquatic organisms. Environ. Toxicol. Chem 30:2065–72. [DOI] [PubMed] [Google Scholar]
- 134.Margiotta-Casaluci L, Owen SF, Cumming RI, de Polo A, Winter MJ, Panter GH, Rand-Weaver M, Sumpter JP. 2014. Quantitative cross-species extrapolation between humans and fish: the case of the anti-depressant fluoxetine. PLoS One 9:e110467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Valenti TW, Gould GG, Berninger JP, Connors KA, Keele NB, Prosser KN, Brooks BW. 2012. Human therapeutic plasma levels of the selective serotonin reuptake inhibitor (SSRI) sertraline decrease serotonin reuptake transporter binding and shelter-seeking behavior in adult male fathead minnows. Environ. Sci. Technol 46:2427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rand-Weaver M, Margiotta-Casaluci L, Patel A, Panter GH, Owen SF, Sumpter JP. 2013. The read-across hypothesis and environmental risk assessment of pharmaceuticals. Environ. Sci. Technol 47:11384–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Madan A, O’Brien Z, Wen J, O’Brien C, Farber RH, Beaton G, Crowe P, Oosterhuis B, Garner RC, Lappin G, Bozigian HP. 2009. A pharmacokinetic evaluation of five H(1) antagonists after an oral and intravenous microdose to human subjects. Br. J. Clin. Pharmacol 67:288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Berninger JP, LaLone CA, Villeneuve DL, Ankley GT. 2015. Prioritization of pharmaceuticals for potential environmental hazard through leveraging a large-scale mammalian pharmacological dataset. Environ. Toxicol. Chem [DOI] [PubMed] [Google Scholar]
- 139.Andersson T, Pärt P. 1989. Benzo[a]pyrene metabolism in isolated perfused rainbow trout gills. Mar. Environ. Res 28:3–7. [Google Scholar]
- 140.Barron MG, Schultz IR, Hayton WL. 1989. Presystemic branchial metabolism limits di-2-ethylhexyl phthalate accumulation in fish. Toxicol. Appl. Pharmacol 98:49–57. [DOI] [PubMed] [Google Scholar]
- 141.Leguen I, Carlsson C, Perdu-Durand E, Prunet P, Pärt P, Cravedi JP. 2000. Xenobiotic and steroid biotransformation activities in rainbow trout gill epithelial cells in culture. Aquat. Toxicol 48:165–176. [DOI] [PubMed] [Google Scholar]
- 142.Zhou B, Liu C, Wang J, Lam PK, Wu RS. 2006. Primary cultured cells as sensitive in vitro model for assessment of toxicants--comparison to hepatocytes and gill epithelia. Aquat. Toxicol 80:109–18. [DOI] [PubMed] [Google Scholar]
- 143.Gomez CF, Constantine L, Huggett DB. 2010. The influence of gill and liver metabolism on the predicted bioconcentration of three pharmaceuticals in fish. Chemosphere 81:1189–95. [DOI] [PubMed] [Google Scholar]
- 144.Rendal C, Kusk KO, Trapp S. 2011. Optimal choice of pH for toxicity and bioaccumulation studies of ionizing organic chemicals. Environ. Toxicol. Chem 30:2395–406. [DOI] [PubMed] [Google Scholar]
- 145.Nichols JW, Schultz IR, Fitzsimmons PN. 2006. In vitro-in vivo extrapolation of quantitative hepatic biotransformation data for fish. I. A review of methods, and strategies for incorporating intrinsic clearance estimates into chemical kinetic models. Aquat. Toxicol 78:74–90. [DOI] [PubMed] [Google Scholar]
- 146.Goss KU. 2008. The pKa values of PFOA and other highly fluorinated carboxylic acids. Environ. Sci. Technol 42:456–8. [DOI] [PubMed] [Google Scholar]
- 147.Vierke L, Berger U, Cousins IT. 2013. Estimation of the acid dissociation constant of perfluoroalkyl carboxylic acids through an experimental investigation of their water-to-air transport. Environ. Sci. Technol 47:11032–9. [DOI] [PubMed] [Google Scholar]
- 148.Ng CA, Hungerbuhler K. 2014. Bioaccumulation of perfluorinated alkyl acids: observations and models. Environ. Sci. Technol 48:4637–48. [DOI] [PubMed] [Google Scholar]
- 149.Ahrens L, Siebert U, Ebinghaus R. 2009. Total body burden and tissue distribution of polyfluorinated compounds in harbor seals (Phoca vitulina) from the German Bight. Mar. Pollut. Bull 58:520–5. [DOI] [PubMed] [Google Scholar]
- 150.Bogdanska J, Sundstrom M, Bergstrom U, Borg D, Abedi-Valugerdi M, Bergman A, DePierre J, Nobel S. 2014. Tissue distribution of 35S-labelled perfluorobutanesulfonic acid in adult mice following dietary exposure for 1–5 days. Chemosphere 98:28–36. [DOI] [PubMed] [Google Scholar]
- 151.Han X, Snow TA, Kemper RA, Jepson GW. 2003. Binding of perfluorooctanoic acid to rat and human plasma proteins. Chem. Res. Toxicol 16:775–81. [DOI] [PubMed] [Google Scholar]
- 152.Chen YM, Guo LH. 2009. Fluorescence study on site-specific binding of perfluoroalkyl acids to human serum albumin. Arch. Toxicol 83:255–61. [DOI] [PubMed] [Google Scholar]
- 153.Bischel HN, Macmanus-Spencer LA, Luthy RG. 2010. Noncovalent interactions of long-chain perfluoroalkyl acids with serum albumin. Environ. Sci. Technol 44:5263–9. [DOI] [PubMed] [Google Scholar]
- 154.Hebert PC, MacManus-Spencer LA. 2010. Development of a fluorescence model for the binding of medium- to long-chain perfluoroalkyl acids to human serum albumin through a mechanistic evaluation of spectroscopic evidence. Anal. Chem 82:6463–71. [DOI] [PubMed] [Google Scholar]
- 155.MacManus-Spencer LA, Tse ML, Hebert PC, Bischel HN, Luthy RG. 2010. Binding of perfluorocarboxylates to serum albumin: a comparison of analytical methods. Anal. Chem 82:974–81. [DOI] [PubMed] [Google Scholar]
- 156.Luebker DJ, Hansen KJ, Bass NM, Butenhoff JL, Seacat AM. 2002. Interactions of fluorochemicals with rat liver fatty acid-binding protein. Toxicology 176:175–85. [DOI] [PubMed] [Google Scholar]
- 157.Woodcroft MW, Ellis DA, Rafferty SP, Burns DC, March RE, Stock NL, Trumpour KS, Yee J, Munro K. 2010. Experimental characterization of the mechanism of perfluorocarboxylic acids’ liver protein bioaccumulation: the key role of the neutral species. Environ. Toxicol. Chem 29:1669–77. [DOI] [PubMed] [Google Scholar]
- 158.Zhang L, Ren XM, Guo LH. 2013. Structure-based investigation on the interaction of perfluorinated compounds with human liver fatty acid binding protein. Environ. Sci. Technol 47:11293–301. [DOI] [PubMed] [Google Scholar]
- 159.Olsen GW, Chang SC, Noker PE, Gorman GS, Ehresman DJ, Lieder PH, Butenhoff JL. 2009. A comparison of the pharmacokinetics of perfluorobutanesulfonate (PFBS) in rats, monkeys, and humans. Toxicology 256:65–74. [DOI] [PubMed] [Google Scholar]
- 160.Chang SC, Noker PE, Gorman GS, Gibson SJ, Hart JA, Ehresman DJ, Butenhoff JL. 2012. Comparative pharmacokinetics of perfluorooctanesulfonate (PFOS) in rats, mice, and monkeys. Reprod. Toxicol 33:428–40. [DOI] [PubMed] [Google Scholar]
- 161.Han X, Nabb DL, Russell MH, Kennedy GL, Rickard RW. 2012. Renal elimination of perfluorocarboxylates (PFCAs). Chem. Res. Toxicol 25:35–46. [DOI] [PubMed] [Google Scholar]
- 162.Sundstrom M, Chang SC, Noker PE, Gorman GS, Hart JA, Ehresman DJ, Bergman A, Butenhoff JL. 2012. Comparative pharmacokinetics of perfluorohexanesulfonate (PFHxS) in rats, mice, and monkeys. Reprod. Toxicol 33:441–51. [DOI] [PubMed] [Google Scholar]
- 163.Han X, Yang CH, Snajdr SI, Nabb DL, Mingoia RT. 2008. Uptake of perfluorooctanoate in freshly isolated hepatocytes from male and female rats. Toxicol. Lett 181:81–6. [DOI] [PubMed] [Google Scholar]
- 164.Yang CH, Glover KP, Han X. 2010. Characterization of cellular uptake of perfluorooctanoate via organic anion-transporting polypeptide 1A2, organic anion transporter 4, and urate transporter 1 for their potential roles in mediating human renal reabsorption of perfluorocarboxylates. Toxicol. Sci 117:294–302. [DOI] [PubMed] [Google Scholar]
- 165.Jones PD, Hu W, De Coen W, Newsted JL, Giesy JP. 2003. Binding of perfluorinated fatty acids to serum proteins. Environ. Toxicol. Chem 22:2639–49. [DOI] [PubMed] [Google Scholar]
- 166.Arnot JA, Gobas FA. 2004. A food web bioaccumulation model for organic chemicals in aquatic ecosystems. Environ. Toxicol. Chem 23:2343–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.