Summary
Excitatory synaptic input reaches the soma of a cortical excitatory pyramidal neuron via anatomically segregated apical and basal dendrites. In vivo, dendritic inputs are integrated during depolarized network activity, but how network activity affects apical and basal inputs is not understood. Using subcellular two-photon stimulation of Channelrhodopsin2-expressing layer 2/3 pyramidal neurons in somatosensory cortex, nucleus-specific thalamic optogenetic stimulation, and paired recordings, we show that slow, depolarized network activity amplifies small-amplitude synaptic inputs targeted to basal dendrites but reduces the amplitude of all inputs from apical dendrites and the cell soma. Intracellular pharmacology and mathematical modeling suggests that the amplification of weak basal inputs is mediated by postsynaptic voltage-gated channels. Thus, network activity dynamically reconfigures the relative somatic contribution of apical and basal inputs and could act to enhance the detectability of weak synaptic inputs.
Graphical Abstract
Highlights
-
•
In vivo subcellular optogenetic stimulation of cortical layer 2/3 pyramidal neurons
-
•
Slow network activity amplifies small-amplitude basal dendritic inputs
-
•
Apical inputs are reduced during depolarized phases of slow network activity
-
•
Basal input amplification is mediated by postsynaptic voltage-gated channels
Ferrarese et al. investigate the impact of network activity on synaptic integration in cortical L2/3 pyramidal neurons in vivo. They report a reduction of apical dendritic inputs but an amplification of small-amplitude basal inputs during depolarized phases of slow network activity. The amplification is dependent on postsynaptic voltage-gated channels.
Introduction
A defining feature of cortical pyramidal neurons is their two major classes of dendrites. Thin basal dendrites extend horizontally from the soma and a thicker apical trunk dendrite projects toward the pial surface, extending thinner oblique branches. The integration of synaptic inputs from apical and basal dendrites lies at the heart of single-cell computation (Magee, 2000, Spruston, 2008), but little is known about this process in vivo.
Recent work has suggested that synaptic inputs to basal and apical dendrites of pyramidal neurons in cortical layers 2/3 and 5 are functionally distinct. GABA-ergic inhibitory somatostatin-expressing neurons, for example, are thought to target apical dendrites while parvalbumin-expressing GABA-ergic neurons more strongly innervate somato-basal regions (Jiang et al., 2013, Markram et al., 2004). Anatomical and mapping studies suggest that different sources of excitatory input are also anatomically segregated. Apical dendrites may receive excitatory thalamic input from higher order thalamic nuclei (e.g., the posteromedial nucleus [POm]) and distant cortical regions (Meyer et al., 2010, Petreanu et al., 2009, Veinante and Deschênes, 2003), whereas basal dendrites receive input from neighboring cortical neurons (Feldmeyer et al., 2006) and sensory-driven input either directly from the primary lemniscal ventral posteromedial nucleus (VPM) (Meyer et al., 2010, Petreanu et al., 2009) or indirectly via layer 4 neurons (Feldmeyer et al., 2002). Here, we investigated whether excitatory inputs to apical and basal dendrites are treated differently during synaptic integration in single layer 2/3 pyramidal neurons in vivo.
In vivo and in vitro measurements have shown that the vast majority of unitary excitatory postsynaptic potentials (uEPSPs) reaching the soma of a pyramidal neuron via apical and basal dendrites are small in amplitude (<1 mV) (Bruno and Sakmann, 2006, Feldmeyer et al., 2006, Jouhanneau et al., 2015, Jouhanneau et al., 2018, Lefort et al., 2009, Markram et al., 1997, Song et al., 2005). Their small size is in part due to the high axial resistance of thin dendrites that impose strong cable filtering, a feature that is especially evident in the thin basal dendrites (Nevian et al., 2007, Williams and Stuart, 2002). Moreover, in vivo, cortical neurons generate action potentials and perform synaptic integration during depolarized phases of spontaneous synaptic activity (Chen et al., 2013, Cowan and Wilson, 1994, Petersen et al., 2003, Steriade et al., 1993) that could alter synaptic transmission via activation of voltage-gated ion channels, a change in the glutamatergic driving force, and an increase in background conductance. In vivo data comparing EPSPs during synaptically quiescent, hyperpolarized downstate with active, depolarized upstate phases of spontaneous activity have shown mixed results with a reduction (Bruno and Sakmann, 2006, Crochet et al., 2005), no change (Pala and Petersen, 2015), and a rescaling (Reig et al., 2015) of amplitude. The reason for these differences is unclear, but one possibility is that the modulation of synaptic input amplitude during network activity is determined by the input location.
To address this hypothesis, we used direct dendritic stimulation and paired recordings to evoke weak subthreshold inputs to apical and basal dendrites of layer 2/3 pyramidal neurons during different phases of network activity in vivo. Unexpectedly, we found that depolarized phases of slow network activity amplified weak EPSPs originating from basal dendrites while reducing the amplitude of all somatic and apical inputs. Intracellular pharmacology and modeling suggest that basal input amplification relies on postsynaptic voltage-gated channels.
Results
Mimicking Synaptic Inputs to Layer 2/3 Pyramidal Neurons with Subcellular Two-Photon Optogenetic Stimulation In Vivo
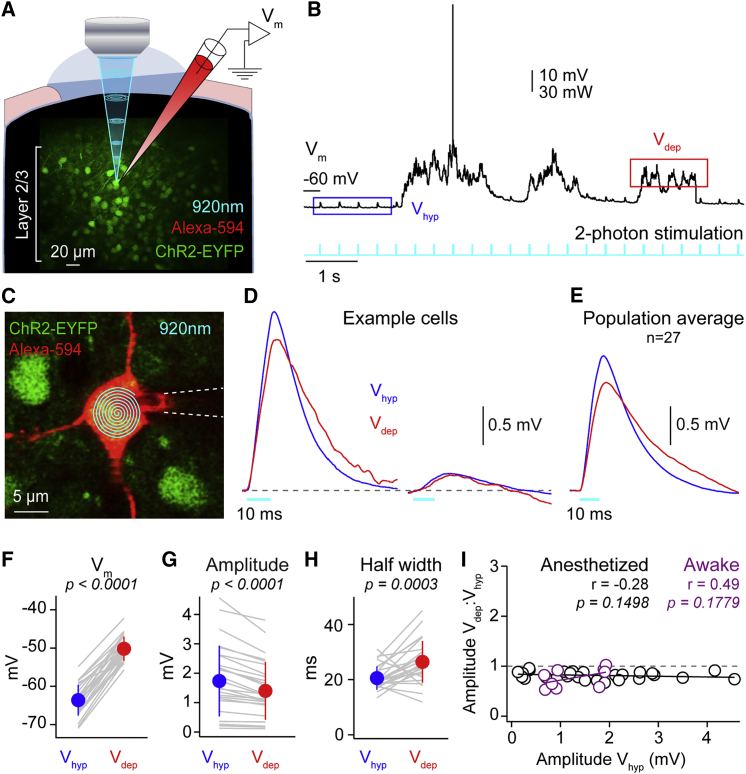
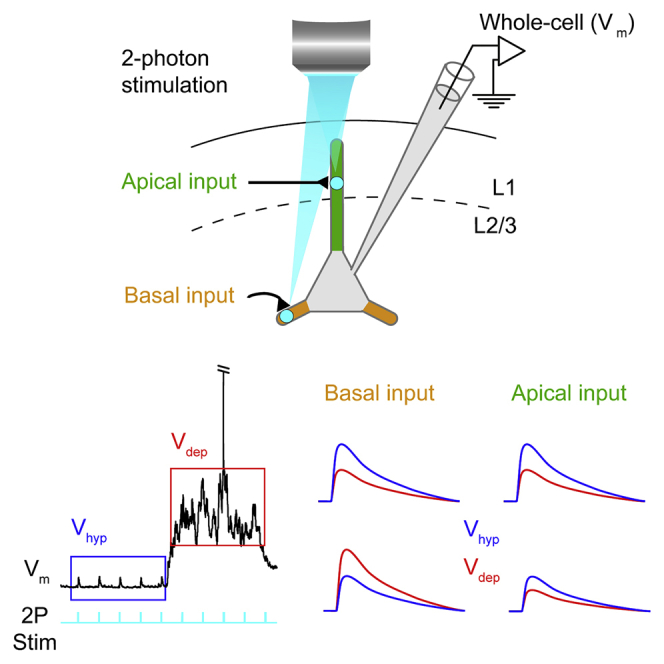
To mimic synaptic inputs from basal and apical branches within a physiologically relevant range (0.04–4.6 mV), we optically stimulated the soma or single dendritic branches of channelrhodopsin2 (ChR2)-expressing neurons in vivo and monitored the input with somatic whole-cell recordings. We expressed hChR2(T159C)-p2A-EYFP in layer 2/3 excitatory pyramidal neurons using a viral vector (AAV2/9) and the αCamKII promoter. Three to five weeks later, we performed somatic two-photon targeted whole-cell patch-clamp recordings of ChR2-EYFP-expressing neurons (Figures 1A–1C) in urethane anesthetized or awake mice during slow (<6-Hz) network activity. Visually targeted recordings were established from pyramidal neurons at a depth of 110.3 ± 22.2 μm (n = 158), using whole-cell pipettes filled with intracellular solution and Alexa Fluor 594. The mean membrane potential (Vm) in anesthetized mice was −57.96 ± 5.55 mV (n = 138) but oscillated between hyperpolarized (Vhyp) and depolarized (Vdep) phases (Figure 1B). Following establishment of the whole-cell configuration, the intracellular Alexa Fluor 594 dye was used to target two-photon optogenetic stimulation to either the soma or basal or apical oblique dendrites 17–135 μm from the soma.
Figure 1.
Response to Somatic Two-Photon Stimulation of ChR2-Expressing Layer 2/3 Pyramidal Neurons Is Reduced in Amplitude during Depolarized Phases of Slow Network Activity
(A) Schematic showing two-photon laser stimulation of ChR2-EYFP-expressing neurons.
(B) Example somatic membrane potential (Vm) recording of a layer 2/3 cortical pyramidal neuron under urethane anesthesia showing small depolarizations (optogenetic potentials [OPs]) in response to two-photon laser stimulation (cyan) during hyperpolarized (Vhyp, blue) and depolarized (Vdep, red) periods of network activity.
(C) Example in vivo image showing the path of the somatic laser stimulation (cyan).
(D) Overlaid, mean light-evoked OPs to somatic stimulation (OPsom, cyan) during Vhyp (blue) and Vdep (red) from two example neurons with different response amplitudes.
(E) Same as (C) but for population average.
(F) Somatic Vm increase as neurons transition from Vhyp to Vdep. Gray lines show data from individual cells, filled circles with error bars the mean ± SD.
(G) OPsom amplitude is significantly lower during Vdep than Vhyp.
(H) OPsom half width is significantly longer during Vdep than Vhyp.
(I) No significant correlation between the ratio Vdep:Vhyp OPsom amplitude and the log10 of Vhyp OPsom amplitude in awake (purple) and anesthetized (black). Black and purple lines are linear fits.
We first stimulated the soma with 10 ms, 3 Hz spiral-patterned two-photon laser stimulation (Figure 1C). This reliably triggered depolarizing optogenetic potentials (OPs) with an onset latency during Vhyp of 0.69 ± 0.22 ms, indicating a direct response to the optical stimulation, a rise time of 5.22 ± 0.93 ms, peak time of 12.54 ± 1.8 ms, half-width of 20.55 ± 4.25 ms, and decay time of 20.97 ± 9.07 ms (n = 27 cells). OPs were not present when stimulating wild-type neurons or neurons expressing EYFP, but not ChR2, and were dependent on accurate subcellular targeting (Figure S1). 10 ms two-photon laser stimuli were delivered at 3 Hz, because this was the highest frequency not susceptible to adaptation (Figures 1B and S1C–S1G). To stimulate dendrites, a small square of two-photon laser stimulation (1 μm2) was directed to individual branches (Figures 2A and S1L–S1S). Stimulation of apical and basal dendrites in Vhyp evoked an OP with similar kinetics (apical: latency 1.79 ± 0.64 ms, rise time 6.35 ± 1.97 ms, peak time 15.01 ± 3.03 ms, half width 22.32 ± 6.53 ms, decay time 27.96 ± 18.06 ms, n = 37; basal: latency 1.58 ± 0.67 ms, rise time 5.32 ± 0.83 ms, peak time 13.48 ± 2.39 ms, half width 19.67 ± 3.94 ms, decay time 23.70 ± 12.46 ms, n = 48). The OP amplitude evoked during Vhyp by apical or basal dendritic stimulation did not change with distance from the soma (Figures S2A and S2B); however, more distally evoked OPs showed longer latencies and slower kinetics (Figures S2E–S2N).
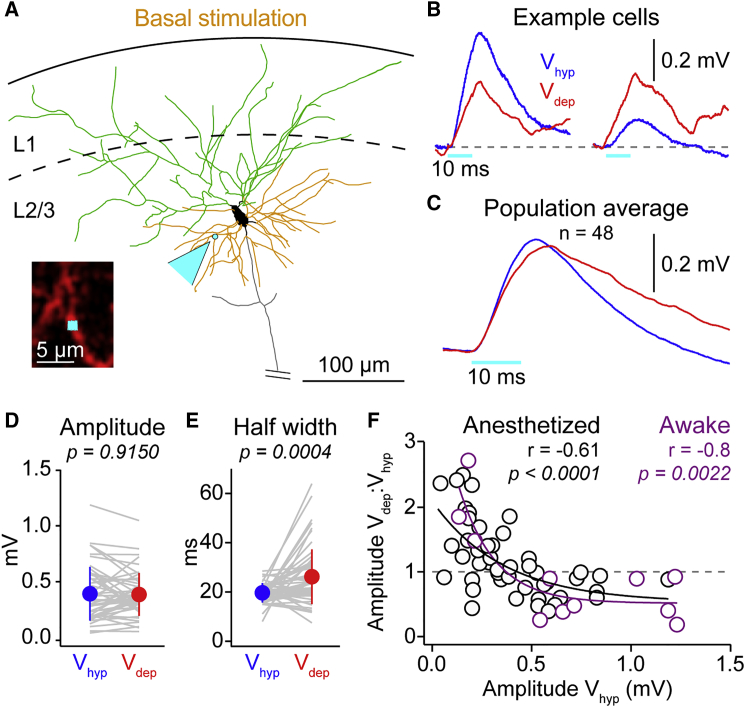
Figure 2.
Amplification of Weak Optogenetically Evoked Inputs from Basal Dendrites of Layer 2/3 Pyramidal Neurons during Depolarized Phases of Slow Network Activity
(A) Reconstruction of example layer 2/3 pyramidal neuron showing the soma (black), apical dendrites (green), and basal dendrites (orange), with the basal dendrite two-photon stimulation site highlighted by cyan arrowhead. Inset shows in vivo image of Alexa-Fluor-594-filled dendrite in red and optogenetic stimulation site in cyan.
(B) Overlaid mean OPbas from 2 example cells show a (left) decreased and (right) increased response during Vdep.
(C) Population average OPbas from Vhyp and Vdep.
(D) Amplitude of OPbas in Vdep and Vhyp is not significantly different. Gray lines show data from individual cells, filled circles with error bars the mean ± SD.
(E) OPbas half-width is significantly longer during Vdep than Vhyp.
(F) A negative correlation between the ratio of the OPbas amplitude in Vdep:Vhyp and Vhyp OPbas amplitude in awake (purple) and anesthetized (black) mice results in smaller amplitude inputs increasing and larger amplitude inputs decreasing in amplitude during Vdep. Correlations performed on the Vdep:Vhyp amplitude and log10 of the Vhyp OPbas amplitude are shown. Black and purple lines are single exponential fits.
Depolarized Network Activity Reduces the Amplitude of Somatic Inputs in Anesthetized and Awake Mice
ChR2 is a non-specific cation channel that, similar to the glutamate ligand-gated channels, has a reversal potential around 0 mV (Berndt et al., 2011). We therefore expected the amplitude of OPs to be reduced as neurons spontaneously went from Vhyp to Vdep, based on an expected amplitude reduction in Vdep compared to Vhyp proportional to (Vhyp − Vdep)/Vhyp. Indeed, somatically evoked OPs (OPsom) of all amplitudes were reduced during Vdep (Vhyp 1.74 ± 1.21 mV versus Vdep 1.4 ± 0.98 mV; n = 27; p < 0.0001; Figures 1G and 1I), likely due to the decreased driving force (see STAR Methods; Vdep OP amplitude; measured 1.4 ± 0.98 mV versus expected 1.38 ± 0.95 mV; n = 27; p = 0.1482). However, OPsom showed a significant increase in the half width (Figure 1H), which may be the result of the increase in input resistance and membrane time constant during Vdep (Figures S3A–S3D; Mateo et al., 2011, Waters and Helmchen, 2006). Whereas distinct periods of Vhyp and Vdep are hallmarks of anesthesia and slow wave sleep (Metherate and Ashe, 1993, Steriade et al., 1993), the Vm of cortical neurons in awake, resting mice also fluctuates between brief, hyperpolarized periods and a depolarized Vm (Poulet and Petersen, 2008). We also observed a reduction in OPsom amplitude as neurons went from hyperpolarized to depolarized phases of slow network activity in awake resting mice (Figure 1I).
Weak Basal Dendritic Inputs Are Amplified during Depolarized Network Activity
Excitatory synaptic inputs to pyramidal neurons are targeted to dendrites. We therefore next stimulated basal dendrites and measured responses at the soma (OPbas; Figure 2). Unexpectedly, across all recordings, OPbas amplitude was not significantly different between Vhyp to Vdep (Vhyp 0.39 ± 0.24 mV versus Vdep 0.39 ± 0.19 mV; n = 48; p = 0.9150; Figures 2C and 2D), despite the increase in Vm and the expected reduction in amplitude from the reduction in driving force during Vdep (Vdep amplitude, measured 0.39 ± 0.19 mV versus expected Vdep 0.31 ± 0.18 mV; n = 48; p = 0.0001). Like OPsom, OPbas showed a significant increase in half width during Vdep (half-width, Vhyp 19.67 ± 3.94 ms versus Vdep 26.19 ± 11.08 ms; n = 48; p = 0.0004; Figure 2E).
To examine this further, we plotted the ratio of the amplitude in Vdep to Vhyp as a function of the Vhyp amplitude (Figure 2F). This revealed that smaller amplitude basal inputs, <0.4 mV, exhibited a significant increase in amplitude in Vdep (OPbas < 0.4 mV in Vhyp; Vhyp 0.24 ± 0.10 mV versus Vdep 0.32 ± 0.14 mV; n = 30; p = 0.0002), and larger amplitude responses decreased (OPbas > 0.4 mV; Vhyp 0.64 ± 0.19 mV versus Vdep 0.50 ± 0.22 mV; n = 18; p = 0.0003; Figures 2B and 2F), resulting in a significant negative correlation. An amplitude-dependent modulation was also observed on the same basal stimulation site with different amplitude stimuli (Figure S4). To confirm that basal input amplification was present in non-anesthetized animals, we repeated stimulation in awake, resting mice (Figure S5). Analysis of OPbas amplitude during the depolarized phase of slow activity revealed a similar correlation as the anesthetized data (Figure 2F): larger amplitude basal inputs decreased, but smaller amplitude inputs increased in amplitude during Vdep.
Apical Dendritic Inputs Are Reduced during Depolarized Network Activity
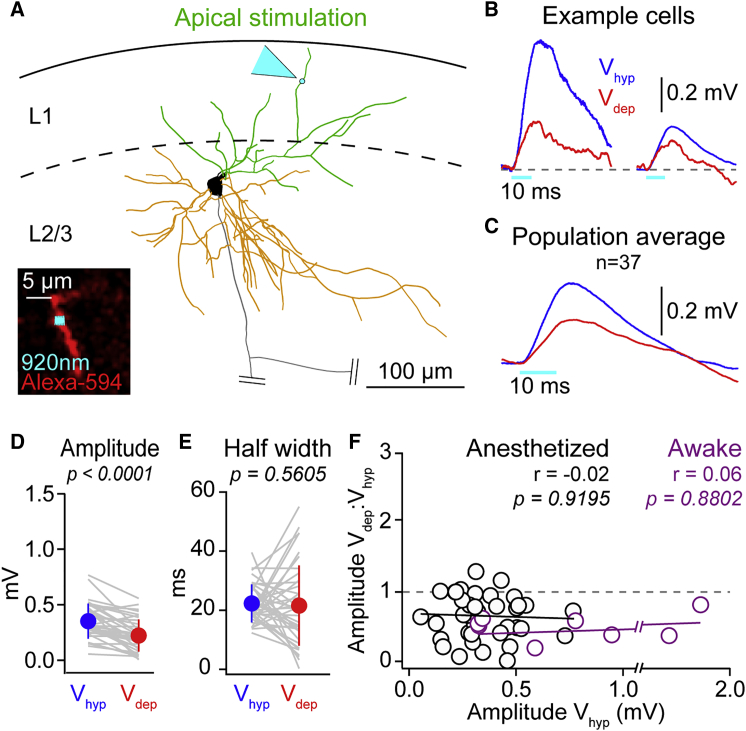
Is the amplification of weak inputs a general feature of synaptic integration in all dendritic compartments of a pyramidal neuron or specific to basal dendrites? We next stimulated apical dendrites (Figure 3) to generate OPap within the same amplitude range of OPbas. In contrast to OPbas, across the population, OPap were reduced in amplitude during Vdep (Vhyp 0.35 ± 0.16 mV versus Vdep 0.22 ± 0.14 mV; n = 37; p < 0.0001) and were significantly smaller than expected from the reduction in driving force (Vdep OP amplitude, measured 0.22 ± 0.14 mV versus expected 0.28 ± 0.13 mV; n = 37; p = 0.0224). Moreover, the OPap amplitude ratio between Vhyp:Vdep was not significantly correlated to the corresponding amplitude during Vhyp both in anesthetized and in awake animals (Figure 3F). Therefore, weak apical inputs are not amplified during depolarized network activity. Thus, the modulation of OPs by depolarized network activity is determined by the dendritic input site.
Figure 3.
Optogenetic Potentials Evoked by Apical Dendrite Stimulation of Layer 2/3 Pyramidal Neurons Are Reduced in Amplitude during Depolarized Phases of Slow Network Activity
(A) Reconstruction of example layer 2/3 pyramidal neuron showing the soma (black), apical dendrites (green), and basal dendrites (orange), with the apical dendrite two-photon stimulation spot highlighted by cyan arrowhead. Inset shows in vivo image of Alexa-Fluor-594-filled dendrite in red and optogenetic stimulation site in cyan.
(B) Overlaid mean OPap from two example cells shows a reduction in amplitude as neurons go from Vhyp (blue) to Vdep (red).
(C) Population average OPap shows reduction in amplitude during Vdep.
(D) Amplitude of OPap is significantly lower in Vdep compared to Vhyp; gray lines show data from individual cells, filled circles with error bars the mean ± SD.
(E) OPap half width is not significantly different between Vhyp and Vdep.
(F) No significant correlation between the ratio of the OPap amplitude in Vdep:Vhyp and the OPap Vhyp amplitude in awake (purple) and anesthetized (black). Black and purple lines are linear fits.
Amplification of Weak Basal Dendrite Targeted Thalamic Input
The increase in weak OPbas amplitude unexpectedly counteracted the reduction in driving force associated with Vdep. To confirm whether the amplification of small-amplitude basal inputs is observed during glutamatergic synaptic transmission, we took advantage of the distinct axonal projection patterns of two thalamic nuclei that project to S1, VPM, and POm. VPM axons mostly target layer 4 neurons that subsequently project to the basal dendrites of layer 2/3 neurons (Feldmeyer et al., 2002) but also have axonal collaterals near the border of layer 4 and 2/3 that may directly contact basal dendrites of layer 2/3 neurons (Meyer et al., 2010, Petreanu et al., 2009, Viaene et al., 2011, Wimmer et al., 2010). In contrast, POm neurons project to layer 1 (Meyer et al., 2010, Wimmer et al., 2010), and mapping studies have shown that they provide short latency input to layer 2/3 neurons that are thought to be targeted to apical dendritic regions (Audette et al., 2018, Petreanu et al., 2009, Viaene et al., 2011). To activate VPM or POm neurons selectively, we infected VPM or POm with ChR2 and optically stimulated their cell bodies or cortical axons during visually targeted whole-cell recordings from layer 2/3 pyramidal neurons in vivo under anesthesia (Figure 4; Jouhanneau et al., 2014).
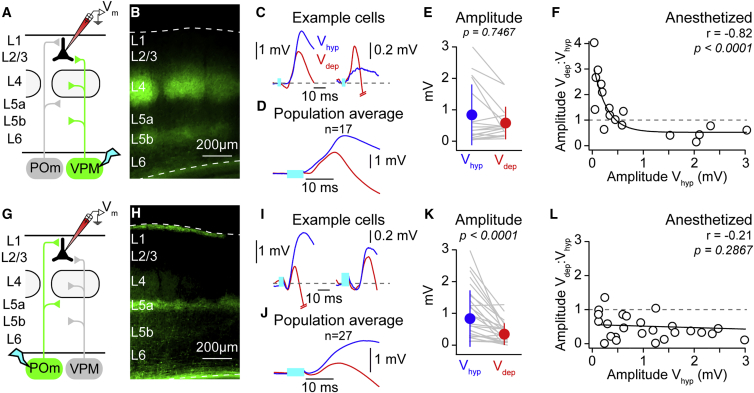
Figure 4.
Weak Glutamatergic Thalamic Inputs to Layer 2/3 Pyramidal Neurons from the Ventral Posteromedial Nucleus, but Not the Posteromedial Nucleus, Are Amplified during Depolarized Phases of Slow Network Activity
(A) Cartoon schematic showing ventral posteromedial nucleus (VPM) (green) axonal projections, a light stimulus in the thalamus (cyan), and the recording site.
(B) Example coronal slice of primary somatosensory cortex showing innervation pattern of ChR2-EYFP-expressing VPM thalamic axons; dashed white lines show pial surface and white matter.
(C) Two averaged, overlaid subthreshold responses from a cortical layer 2/3 pyramidal neuron to VPM optogenetic stimulation (cyan bar) during Vhyp (blue) and Vdep (red) states show (left) a larger amplitude example that decreases during Vdep and (right) a smaller amplitude example that increases during Vdep.
(D) As in (B) but the population average response.
(E) Across the population, there was no significant difference in the amplitude of responses to VPM stimulation in Vdep compared to Vhyp. Gray lines show data from individual cells, filled circles with error bars the mean ± SD.
(F) A significant negative correlation between log10 of the VPM-evoked responses during Vhyp and the ratio of the Vdep:Vhyp amplitude, showing the amplification of small-amplitude VPM responses during Vdep. Open circles represent mean response from a single cell; black line is a single exponential fit.
(G–L) As for (A)–(F) but for posteromedial nucleus (POm) optogenetic stimulation. Black line in (L) is a linear fit.
During Vdep, VPM and POm stimulation evoked a short latency depolarizing input and a subsequent hyperpolarization likely due to inhibition from local cortical GABA-ergic neurons. Measurement of the early VPM depolarizing response did not show an overall change in amplitude comparing Vhyp to Vdep (Vhyp 0.84 ± 0.96 mV versus Vdep 0.58 ± 0.52 mV; n = 17; p = 0.7467), whereas the early POm response was strongly reduced (Vhyp 1.48 ± 1.84 mV versus Vdep 0.57 ± 0.73 mV; n = 27; p < 0.0001). Plotting the ratio of the amplitude of the depolarizing response to VPM stimulation in Vdep:Vhyp against the Vhyp amplitude revealed a significant negative correlation (Figure 4F) similar to that observed to direct basal dendrite stimulation (Figure 2F), whereas, like direct apical stimulation (Figure 3F), POm responses showed no correlation (Figure 4L). Thus, these data show that the amplification of weak inputs is a relevant phenomenon for glutamatergic inputs and suggests that weak sensory-evoked glutamatergic input may also be amplified during depolarized network activity (Reig et al., 2015).
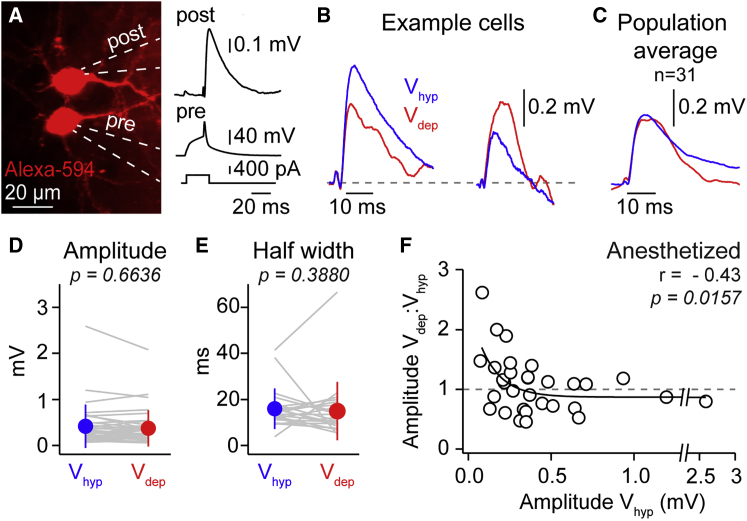
Amplification of Small-Amplitude Unitary Monosynaptic EPSPs In Vivo
Optogenetic thalamic stimulation activates a large population of presynaptic neurons that evokes network level effects. To measure whether unitary glutamatergic uEPSP also undergo weak input amplification, we performed multiple (2–4) targeted whole-cell recordings from monosynaptically connected layer 2/3 pyramidal neurons in vivo (Jouhanneau et al., 2015, Jouhanneau et al., 2018), which form the majority of their synaptic contacts on basal dendrites of neighboring excitatory neurons (Feldmeyer et al., 2006, Petreanu et al., 2009). To identify a connection and compare uEPSP amplitude between Vhyp and Vdep, we evoked single action potentials and measured the postsynaptic response (Figures 5A and 5B). Across 31 connections with a depolarizing uEPSP in Vdep (see STAR Methods), mean uEPSP amplitude and half width were not significantly different during Vdep to Vhyp (amplitude: Vhyp 0.46 ± 0.47 mV versus Vdep 0.43 ± 0.39 mV; n = 31 connections; p = 0.6636; Figure 5). Notably, however, smaller amplitude uEPSPs increased in amplitude in Vdep and larger amplitude uEPSPs decreased, resulting in a significant negative correlation between the ratio of the uEPSP amplitude in Vdep:Vhyp and the Vhyp amplitude (Figure 5F), similar to the direct basal stimulation and VPM response graphs (Figures 2F and 4F). Thus, amplification of weak inputs is a fundamental feature of the integration of monosynaptic glutamatergic inputs from neighboring layer 2/3 pyramidal neurons in vivo.
Figure 5.
Monosynaptic Glutamatergic Input Modulation by Slow Network Activity
(A) Example in vivo two-photon image of two pyramidal neurons stained with Alexa Fluor 594; recording pipettes outlined with white dashed lines; right shows test for monosynaptic connectivity from the same example pair.
(B) Two example, averaged uEPSPs with different Vhyp (blue) amplitudes; the larger uEPSP (left) is decreased in Vdep (red) whereas the smaller uEPSP is increased (right).
(C) Population-averaged, overlaid uEPSPs during Vhyp and Vdep.
(D) No change in amplitude of uEPSPs in Vdep as compared to Vhyp across the population. Gray lines show data from individual cells, filled circles with error bars the mean ± SD.
(E) No change in half width of uEPSPs during Vdep and Vhyp across the entire population.
(F) Significant correlation between log10 of the Vhyp amplitude of uEPSPs and the ratio of amplitude Vdep:Vhyp, highlighting the amplification of small-amplitude uEPSPs during Vdep. Correlations performed on the amplitude ratio Vdep:Vhyp and log10 of the Vhyp uEPSP amplitude are shown. Open circles represent mean response from a single cell; black line is a single exponential fit.
Basal Input Amplification Is Mediated by Postsynaptic Voltage-Gated Channels
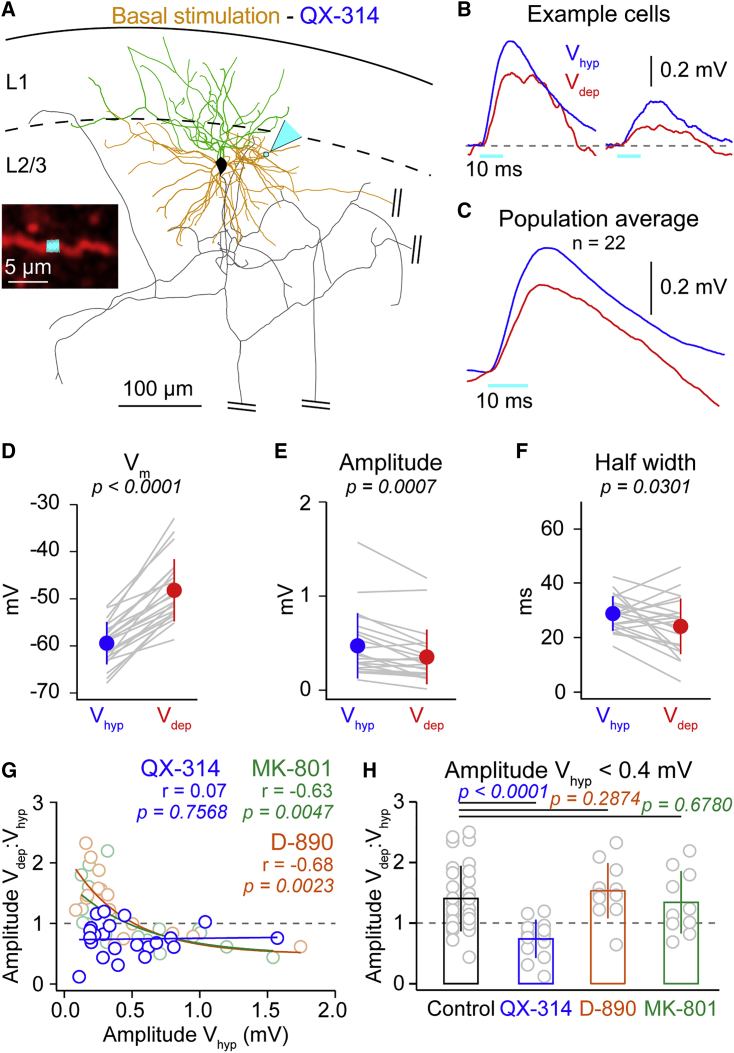
We next returned to basal dendrite optogenetic stimulation to address possible cell-intrinsic, postsynaptic mechanisms underlying the amplification of weak basal inputs (Figure 6A). Cortical slice experiments have shown that uEPSP amplitudes can be modulated by varying the postsynaptic Vm (Deisz et al., 1991, González-Burgos and Barrionuevo, 2001, Markram et al., 1997, Stuart and Sakmann, 1995), suggesting that voltage-gated channels might be important in weak input amplification. We used intracellular antagonists to block different types of voltage-gated channels without affecting local network activity (1 mM MK-801 to block NMDA, 200 μM D-890 to block voltage-dependent Ca2+ channels, and 1 mM QX-314 to block voltage-dependent Na+ channels and, to a minor extent, K+ channels). Before stimulation, we waited 10 min for the dendrite to be visible and for the drugs to perfuse. During intracellular application of MK-801, D-890, and QX-314, neurons maintained a normal resting Vm and spontaneous subthreshold network activity. Action potential firing, however, was completely absent in QX-314 recordings, due to the block of Na+ channels.
Figure 6.
Amplification of Weak Basal Inputs Is Blocked by Intracellular Application of QX-314
(A) Biocytin reconstruction of example cell from basal dendrite optogenetic stimulation experiment, showing the apical (green) and basal (orange) dendrite, the axon (gray, truncated), and the optogenetic stimulation spot (cyan arrow). Inset shows in vivo image of Alexa-Fluor-594-filled dendrite in red and optogenetic stimulation site in cyan.
(B) Both large- and small-amplitude mean example OPbas show a reduction in amplitude from Vhyp (blue) to Vdep (red) during whole-cell recordings with 1 mM QX-314 in intracellular solution.
(C) Population mean OPbas during intracellular QX-314 application is reduced in Vdep.
(D) Vm increase as neurons transition from Vhyp to Vdep during experiments using intracellular QX-314. Gray lines show data from individual cells, filled circles with error bars the mean ± SD.
(E) A significant reduction of OPbas amplitude in Vdep compared to Vhyp.
(F) OPbas half width is significantly smaller in Vdep in comparison with Vhyp.
(G) No correlation between state modulation of OPbas amplitude and the log10 of Vhyp OP amplitude during QX-314 application (blue); significant correlation during MK-801 (light green) and D-890 (light orange) application. Open circles represent mean response from a single cell, blue line shows linear fit, and green and orange lines single exponential fit.
(H) The ratios of the Vdep:Vhyp amplitude for small-amplitude OPbas (<0.4 mV) are significantly different during intracellular QX-314 application, but not during MK-801 or D-890. Gray open circles show data from single cells; bars show mean ± SD.
One possible mechanism underlying the amplification could be that NMDA channels, primed by glutamate release during Vdep, are activated by the depolarization of the OP. However, the amplification of weak OPbas was unaffected by the blocking of NMDA channels with MK-801 (Figure 6G). Likewise, blocking of voltage-gated Ca2+ channels by D-890 also did not alter basal input amplification (Figure 6G). Inclusion of QX-314 into the intracellular solution, however, had a strong and robust effect. In contrast to control data and recordings with MK-801 or D-890 in the pipette, small-amplitude OPbas were reduced in amplitude during Vdep with QX-314 in the pipette (OPbas < 0.4 mV QX-314 Vhyp 0.25 ± 0.08 mV versus OPbas < 0.4 mV QX-314 Vdep 0.19 ± 0.09 mV; n = 13; p = 0.0171; Figures 6A–6E). Moreover, in contrast to the increase in half width observed in wild-type OPbas (Figure 1H), QX-314 reduced OPbas half width during Vdep (Figure 6F). This could be linked to decreased input resistance as neurons transition from Vhyp to Vdep in QX-314-treated neurons (Figures S3E–S3G; Remme and Rinzel, 2011, Waters and Helmchen, 2006). Plotting the ratio of the amplitude of the OPbas response in Vdep:Vhyp against the OPbas Vhyp amplitude during QX-314 application showed no significant correlation (Figure 6G). Thus, only QX-314 blocked the boosting of small OPbas during network activity (Figure 6H). Together, our data suggest that postsynaptic voltage-gated channels are required for the amplification of small-amplitude basal inputs during depolarized network activity in vivo.
Modeling a Postsynaptic Voltage-Gated Channel-Dependent Mechanism
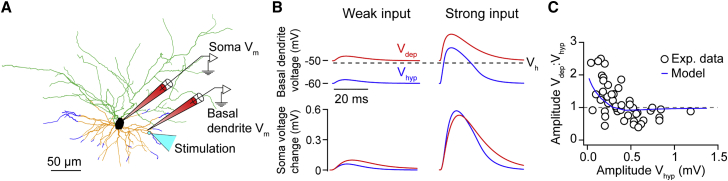
If basal input amplification is achieved via a postsynaptic voltage-gated ion channel (VGC), what are the activation, kinetics, and anatomical distribution requirements of channels that could underlie this effect? We developed a compartmental model of a reconstructed layer 2/3 pyramidal neuron (Figure 7A; see STAR Methods) to address these questions. Based on the results of pharmacological blocking, we hypothesized the involvement of an amplifying current, i.e., a voltage-gated current that amplifies voltage changes in a certain subthreshold voltage regime (see Remme and Rinzel, 2011). Typical examples of such currents are the persistent Na+ current, a low-threshold activated Ca2+ current, or NMDA receptor currents. Assuming that the putative VGC activates in a voltage range between Vhyp and Vdep (−60 to −50 mV), but not far below, the current can account for the amplification of weak basal inputs in the following way (Figure 7B): at Vhyp, weak input to a basal dendrite (blue traces, left column) is not able to significantly activate the channel and leads to a small response at the soma (bottom panel). Strong input (blue traces, right column), on the other hand, activates the current during Vhyp, leading to much larger responses at the soma. At Vdep (red traces), both weak and strong inputs activate the voltage-gated current in the basal dendrites, leading to a proportionally larger response to weak inputs.
Figure 7.
Model Analysis Identifies a Potential Mechanism Underlying the Amplification of Weak Basal Inputs based on Voltage-Dependent Currents
(A) Layer 2/3 pyramidal cell model; location of the simulated input is indicated by a cyan circle and arrow in the basal dendrites (orange); location of the simulated Vm recordings is indicated by two electrodes on the soma and on a basal dendrite close to the input stimulation; distal segments of the basal dendrites express a VGC (dark blue).
(B) Voltage response to input in basal dendrites (basal dendrite electrode in A) recorded at the location of the stimulus (top) and at the soma (bottom; see soma electrode in A). Responses are shown to weak (left) and strong (right) input in both Vdep (red curves) and Vhyp (blue traces). Black dotted line in top panels marks the half-activation voltage, Vh, of the current (see STAR Methods and Figure S6B). Dotted line in top panels marks the half-activation voltage of the current (see Figure S6B). Soma voltage change with respect to holding voltage (−60 mV for Vhyp or −50 mV for Vdep) is shown.
(C) Ratio of somatic amplitudes in Vdep versus Vhyp shown as a function of the Vdep amplitudes for basal input. Both model results (blue curve) and experimental data (open circles) are shown.
We quantitatively modeled current properties that might be necessary to account for our results (Figure 7C) by varying the voltage dependence and kinetics of the hypothetical current, as well as its density and distribution across the cell over physiologically plausible ranges (see STAR Methods). For each parameter combination, we simulated basal input during Vhyp and Vdep, recorded the somatic voltage response, and compared the response amplitude to the experimental observations (Figure 7C). The data were well fit by a group of parameter settings (Figures 7 and S6) that all shared the following features: the current was activated in a voltage range above Vhyp (>−60 mV), it activated faster than the membrane time constant, and the channels were distributed across the distal basal dendrites (>70 μm from the soma; see blue dendritic branches in Figure 7A), ensuring that the active current only affects basal inputs and not the somatic inputs (Zhuchkova et al., 2013). Together with the pharmacology, this model provides support for a postsynaptic VGC mechanism to underlie the amplification of basal input and suggests suitable kinetics and subcellular distributions.
Discussion
Here, we compared the integration of excitatory synaptic inputs in apical versus basal dendrites of layer 2/3 primary somatosensory cortex pyramidal neurons in vivo. Because layer 2/3 pyramidal neurons fire sparsely, often with single action potentials (Barth and Poulet, 2012), we examined the postsynaptic responses to single inputs. We show that weak inputs from basal dendrites are amplified whereas inputs of all amplitudes from apical dendrites are attenuated during slow depolarized network activity. This was true not only of direct optogenetically evoked responses but also of thalamic and monosynaptic cortical glutamatergic inputs. Amplification of weak basal inputs could be blocked with an intracellular voltage-dependent ion channel antagonist, and compartmental modeling identified a plausible voltage-dependent channel mechanism. Together, our findings highlight an unexpected dendritic region specificity in the impact of depolarized network activity on synaptic integration in vivo.
Two-Photon Subcellular Optogenetic Stimulation as a Tool for Studying Synaptic Integration In Vivo
Synaptic integration in vivo involves the processing of spatially separated dendritic inputs during depolarized network activity. Whereas the location of active dendritic inputs can be now identified with functional imaging (Chen et al., 2013, Jia et al., 2010), the integration of subthreshold inputs with network activity in vivo has typically been studied without identification of the input site using sensory (Chadderton et al., 2014, Crochet et al., 2011, Longordo et al., 2013, Reig et al., 2015, Sachdev et al., 2004), electrical (Reig et al., 2015, Sachdev et al., 2004), or optogenetic stimulation (Mateo et al., 2011, Pala and Petersen, 2015, Pala and Petersen, 2018) or simultaneous recordings (Bruno and Sakmann, 2006, Crochet et al., 2005, Jouhanneau et al., 2018). Two-photon glutamate uncaging allows location-specific control of synaptic inputs and has been used in silenced networks in vivo (Noguchi et al., 2011), but its use in active networks is limited because the caged compound can act as an antagonist of GABA transmission (Maier et al., 2005). Channelrhodopsin2 can be expressed in genetically defined cell types, thus avoiding non-specific activation of inhibitory inputs, and can be rapidly activated by two-photon light stimulation (Packer et al., 2012, Prakash et al., 2012). Similar to measurements of simulated dendritic input in cortical slice experiments, the latency and time course of evoked OPs are correlated with the distance of the input site from the soma. Within 135 μm from the soma, we did not observe a correlation of OP amplitude with distance resembling prior cortical slice experiments using simultaneous somatic and basal dendritic recordings (Nevian et al., 2007). Although the rise time of an OP is slightly slower than a glutamatergic uEPSP, future experiments could use ChR2 variants with faster kinetics. These data, alongside the similarities between the modulation of OPs and monosynaptic glutamatergic inputs (Figure 5) by network activity, support the use of this method to further investigate synaptic integration in vivo under different behavioral and cortical states.
Cortical Depolarized Network Activity Amplifies Weak Inputs to Basal Dendrites
Spontaneous network activity dominates the membrane potential of cortical neurons and has been observed in direct dendritic recordings in vivo (Waters and Helmchen, 2004), but its impact on synaptic integration is still debated. A central result of our study is that network activity reweights apical and basal inputs separately, suppressing apical but enhancing weak basal inputs (Figures 2 and 3). Such an amplification is also present for glutamatergic inputs from VPM and neighboring pyramidal neurons (Figures 4 and 5), two sources of synaptic inputs thought to target basal dendrites of layer 2/3 pyramidal neurons (Feldmeyer et al., 2006, Meyer et al., 2010, Petreanu et al., 2009). At first glance, this result appears counterintuitive. The increase in EPSP amplitude goes against the reduction in driving force during Vdep and the increased membrane conductance. However, an increase of OPbas amplitude at more depolarized potentials resembles the voltage dependency of evoked and dendritically simulated EPSPs in cortical slice experiments (Andreasen and Lambert, 1999, Deisz et al., 1991, González-Burgos and Barrionuevo, 2001, Markram et al., 1997, Stuart and Sakmann, 1995). Moreover, the broadening of OPbas half width during Vdep goes together with the increase in input resistance observed in Vdep (Figures 2 and S3; Mateo et al., 2011, Waters and Helmchen, 2006).
To examine whether postsynaptic voltage-dependent ion channels were involved in basal input amplification without affecting network activity required intracellular antagonists. Our experiments show that basal input amplification could be blocked by application of the VGC blocker QX-314. A modeling approach suggested that the putative channel should be localized in distal basal dendrites, activate close to Vdep (at around −50 mV), and be activated faster than the membrane time constant. The hypothesized activation function of the putative current ensures that, at hyperpolarized potentials, strong basal dendritic inputs are required for channel opening and the resulting amplification, and weak inputs do not suffice. In contrast, at depolarized potentials, both weak and strong basal inputs are amplified by the current. As a consequence, response amplitudes to weak and strong inputs differ strongly in the hyperpolarized state, and the difference is much reduced in the depolarized state.
Reduction in Apical and Somatic Responses during Depolarized Network Activity
As predicted in models and observed in cortical slices during conductance injection (Bernander et al., 1991, Destexhe and Paré, 1999, Destexhe et al., 2003, Williams, 2004), the somatic impact of somatic and apical dendritic inputs is reduced during network activity with the apical responses reduced more than expected based on the change in driving force. So, alongside the increase in conductance, what mechanisms could reduce apical inputs during depolarized activity? Somatostatin-expressing GABA-ergic inhibitory interneurons are thought to contact apical dendritic regions of pyramidal neurons (Jiang et al., 2013); hence, one hypothesis could be that apical dendrite targeting inhibitory interneurons shunt apically evoked uEPSPs as they propagate to the soma. If this were the case, significant differences in the impact of apical inputs on the somatic voltage during periods of movement should occur, as somatostatin-expressing neurons are known to be strongly modulated by behavioral state (Gentet et al., 2012, Muñoz et al., 2017). Testing this prediction will require rapid manipulation of somatostatin-expressing neurons activity during apical dendritic stimulation.
Functional Impact on Sensory Processing and Synaptic Integration
Cortical network activity is known to have a fundamental impact on cortical sensory processing (Chance et al., 2002, Petersen et al., 2003, Sachdev et al., 2004, Shu et al., 2003). Our thalamic optogenetic stimulation data predict that the cortical synaptic response to weak somatosensory stimuli, going via VPM to the cortex, would be amplified and may help in the perceptual detection of weak tactile inputs. In support of this proposal, a recent study found a comparable amplification of weak subthreshold inputs during low-intensity acoustic stimulation in depolarized states (Reig et al., 2015). Reig et al. (2015) concluded that the effect was likely the result of a combination of an increase in postsynaptic membrane conductance and in the presynaptic recruitment of additional inhibitory inputs during Vdep. We suggest that postsynaptic voltage-dependent channels also play a major role in boosting the cortical representation of weak sensory inputs during depolarized network activity.
Conclusions and Future Work
Axo-dendritic synaptic connections from local layer 2/3 cortical excitatory neurons are mostly formed on basal dendrites (Feldmeyer et al., 2006, Petreanu et al., 2009), whereas inputs from distant cortical neurons and higher order thalamic nuclei terminate in cortical layer 1, likely targeting apical dendrites (Meyer et al., 2010, Petreanu et al., 2009, Veinante and Deschênes, 2003, Wimmer et al., 2010). Thus, slow cortical network activity appears to dynamically alter the relative contribution of distinct synaptic information to the soma of pyramidal neurons.
Our findings suggest that, during slow cortical activity in resting animals, bottom-up, sensory, and local input dominates the somatic response. Recent work has observed an increase in EPSP amplitude to cortical GABA-ergic interneurons during movement (Pala and Petersen, 2018), and one possibility is that higher order and top-down apical inputs to pyramidal neurons may play a more dominant role in somatic integration and spike generation during desynchronized cortical activity. Future work must therefore now assess the relative impact of apical and basal inputs in attentive and behaving mice.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| AAV2/9 pAAV-αCaMKII-hChR2(T159C)-p2A-EYFP | Charité Vector Core | VCA-43a |
| AAV2/9 pAAV-αCaMKII-hChR2(E123T/T159C)-p2A-EYFP | Charité Vector Core | BA-150a |
| pLenti-Synapsin-hChR2(H134R)-EYFP | Charité Vector Core | BLV-679 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| NaCl | Sigma-Aldrich | S7653 |
| KCl | Sigma-Aldrich | P9333 |
| HEPES | Sigma-Aldrich | H3375 |
| MgCl2∗6H2O | Sigma-Aldrich | M2670 |
| CaCl2∗2H2O | Sigma-Aldrich | C5080 |
| NaOH | Sigma-Aldrich | S8045 |
| Potassium D-gluconate | Sigma-Aldrich | G4500 |
| KCl | Sigma-Aldrich | P9333 |
| Adenosine 5′-triphosphate magnesium salt | Sigma-Aldrich | A9187 |
| Phosphocreatine disodium salt hydrate | Sigma-Aldrich | P7936 |
| Guanosine 5′-triphosphate sodium salt hydrate | Sigma-Aldrich | G8877 |
| HEPES | Sigma-Aldrich | H3375 |
| KOH | Sigma-Aldrich | P5958 |
| Biocytin | Tocris | 3349 |
| QX-314 bromide | Tocris | 1014 |
| (+)-MK-801 maleate | Tocris | 0924 |
| D-890 | Abcam | ab120333 |
| Alexa Fluor 594 | ThermoFisher | A10438 |
| Urethane | Sigma-Aldrich | U2500 |
| Metamizol | Zentiva | 416485 |
| Isoflurane | Cp-pharma | 1214 |
| Ketamine 10% | WDT | 9089.01.00 |
| Rompun 2% Xylazin | Bayer | KP0CTJS |
| Vectastain Elite ABC-Peroxidase kit | Biozol | VEC-PK-6100 |
| Denture acrylic | Heraeus | 64707963 |
| Agarose, Type III-A | Sigma-Aldrich | A9793 |
| Mowiol | Sigma-Aldrich | 81381 |
| Roti-Histofix 4% (PFA) | Roth | P087.4 |
| Sodium phosphate monobasic dihydrate | Sigma-Aldrich | 71505 |
| Sodium phosphate monobasic monohydrate | Sigma-Aldrich | S9638 |
| Sodium phosphate dibasic dihydrate | Sigma-Aldrich | 71643 |
| Experimental Models: Organisms/Strains | ||
| Mouse-C57BL/6J | FEM Charité | C57BL/6J |
| Mouse-Nex-cre | Klaus Nave | Nex-cre |
| Mouse-Ai9 | The Jackson Lab | 007909 |
| Mouse-GAD67-GFP | Yuchio Yanagawa | GAD67-GFP |
| Mouse-fosGFP | The Jackson Lab | 014135 |
| Software and Algorithms | ||
| IGORpro 6 | Wavemetrics | https://www.wavemetrics.com |
| MATLAB | MathWorks | https://www.mathworks.com |
| NEURON | NEURON | https://neuron.yale.edu/neuron |
| Neurolucida | Microbrightfield | https://www.mbfbioscience.com/neurolucida |
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, James F.A. Poulet (james.poulet@mdc-berlin.de).
Experimental Model and Subject Details
All experimental procedures were approved by the Berlin animal ethic committee (LAGeSo) and carried out in accordance with European animal welfare law. P18-52 C57BL/6J mice of both sexes were used for dendritic stimulation experiments. For thalamic stimulation experiments, C57BL/6J (FEM Charité) and fosGFP (The Jackson Lab, Stock No 014135) mice of both sexes were used. For monosynaptic connectivity NEX-cre (Goebbels et al., 2006) x Ai9 (The Jackson Lab, Stock No 007909) mice, fosGFP mice (The Jackson Lab, Stock No 014135), and GAD-67 (Tamamaki et al., 2003) mice of both sexes were used.
Method Details
Surgery and intrinsic optical imaging
Mice were anesthetized with 1%–2% isoflurane in O2, then dental cement and glue were used to implant a lightweight metal post and recording chamber over primary somatosensory cortex. 30 minutes prior to surgery mice were administered a subcutaneous injection of metamizole (200 mg/kg). During anesthesia, a rectal probe and heating pad were used to maintain mouse core body temperature at 37°C. After surgery, mice were placed on a heating pad at 37°C until their recovery was complete. For 24 hours after surgery, metamizole was added to drinking water (200 mg/ml). In their home cages, mice had access to food and water ad libitum and were checked and weighed daily. Primary somatosensory whisker or forepaw cortex were identified with intrinsic optical imaging or stereotactic coordinates of the C2 whisker or forepaw, respectively. All anesthetized recordings were made under 1.5 g/kg urethane anesthesia. For awake experiments mice were head-restrained and paw-tethered as previously described (Milenkovic et al., 2014, Zhao et al., 2016). A force-feedback sensing arm (Aurora Scientific, Dual-Mode Lever Arm systems 300-C) was placed on the ventral surface of the tethered forepaw to monitor paw movement and allow identification of quiet, resting periods associated with slow cortical activity.
Virus injections
P8-12 mice were anesthetized using i.p. injections of a ketamine (120 mg/kg) and xylazine (10 mg/kg) mix and placed in a stereotactic frame (Angle Two, Leica). Stereotactic coordinates were determined and a craniotomy was performed by drilling over the somatosensory barrel cortex (1.3 mm posterior and 3 mm lateral to Bregma) or the forepaw cortex (0.2 anterior and 2 mm lateral to Bregma). Next, a glass injection pipette (10 μm diameter tip) containing the viral vector solution was connected to an oil piston pressure injection system (MO-10; Narishige) and inserted into layer 2/3 (100-300 μm from pial surface) through the intact dura.
Cortical neurons were infected with channelrhodopsin-2 (ChR2) using an adeno-associated viral vector (AAV2/9) containing pAAV-αCaMKII-hChR2(T159C)-p2A-EYFP or pAAV-αCaMKII-hChR2(E123T/T159C)-p2A-EYFP (Berndt et al., 2011). 500-1000 nL of virus were injected slowly at 50 nl/min. The injection pipette was removed slowly, the brain covered with petroleum jelly (Vaseline), and the skin resealed. Mice were left in their home cage for 21-40 days while waiting for ChR2-EYFP expression. To infect the ventral posteromedial nucleus (VPM) and the posteromedial nucleus (POm) of the thalamus, a lentivirus encoding ChR2-EYFP (pLenti-Synapsin-hChR2(H134R)-EYFP; Addgene 20945) was injected in P9-12 mice (Jouhanneau et al., 2014). The procedure was similar to that for cortical infection except that the craniotomy was performed at 1.8 mm posterior and 1.75 mm lateral to Bregma (VPM) or at 1.8 mm posterior and 1.25 mm lateral to Bregma (POm). An injection pipette was inserted to a vertical depth of 3.45 mm (VPM) or at 2.8 (POm). At that point, 500-600 nL of viral solution were injected slowly at a rate of 50 to 100 nL per minute. Mice were left for 2 weeks while waiting for ChR2 expression after which a second craniotomy was made over the hemisphere contralateral to the recording (1.8 mm posterior; 2 mm lateral) for the insertion of an optical fiber (200 μm diameter; Thorlabs) coupled to a 450–480 nm blue light source (473 nm DPSS Laser System; LabSpec) into the somatosensory thalamus. To optogenetically activate VPM or POm neurons, a 3 ms light pulse (∼40 mW) was delivered at 0.25 Hz. In some experiments, VPM or POm projections were directly activated by blue light pulses (3 ms, ∼40 mW) delivered to the surface of the brain that lay over the recording site. Histological sections from every mouse were used to confirm the thalamic infection site and the distinctive cortical axonal projection for VPM (L5b and L4) and POm (L5a and L1).
Two-photon targeted whole-cell patch clamp recordings
To access the brain for electrophysiological recordings, the skull was covered with Ringer’s solution (in mM): 135 NaCl, 5 KCl, 5 HEPES, 1.8 CaCl2, 1 MgCl2 and a small craniotomy (∼1 mm diameter) was made over primary somatosensory cortex to expose the brain and the dura was carefully removed with a needle. For two-photon optogenetic stimulation experiments a drop of 1.8% agarose in Ringer’s solution was placed on top of the craniotomy to stabilize the brain. A Femto2D in vivo two-photon laser-scanning microscope (Femtonics) was used to visualize cells at 920 nm, for EYFP identification or 820 nm, for Alex Fluor 594 dye (Thermo Fisher) identification with a Chameleon Ultra II (Coherent) Ti-sapphire pulsed laser light source via a 40x 0.8 NA water immersion objective (Olympus). Two high-sensitivity photomultipliers (PMT) were used to detect fluorescent signals. Imaging was controlled with MES software (Femtonics) running in MATLAB (MathWorks). Whole-cell patch clamp recordings were made with 2 mm borosilicate glass electrodes (Hilgenberg) with a resistance of 5-7 MΩ. Recording pipettes were filled with intracellular solution containing, in mM: 135 potassium D-gluconate, 4 KCl, 10 HEPES, 10 phosphocreatine, 4 MgATP, 0.3 Na3GTP (adjusted to pH 7.3 with KOH), 2mg/ml biocytin for anatomical reconstructions and Alexa Fluor® 594 dye (Thermo Fisher). In a subset of experiments, 1 mM QX-314 bromide (Tocris), or 1 mM MK-801 maleate (Tocris), or 200 μM D-890 (Abcam) were added. Recordings were made using an Axon MultiClamp 700B amplifier (Molecular Devices) in current clamp mode with an Ag/AgCl ground electrode in the recording chamber. Using motorized micromanipulators (Luigs & Neumann) the pipettes were inserted into the brain under visual control at an angle of 34° applying positive pressure of 130-180 mbar. While lowering pipettes into the tissue until about 120 μm depth, pressure was gradually reduced to 50-80 mbar. Cells were approached at low positive pressure (30 mBar) and contact with a neuron was identified by live two-photon images and the resistance changes were visualized on an oscilloscope (Tektronix TDS2024C). Upon contact, negative pressure was applied to form a gigaseal and subsequently break in and enter whole-cell recording configuration. To reduce the level of optical stimulation of ChR2-expressing neurons during the visualization of the EYFP signal, a few, low-power (∼5 mW) raster scan images were collected at 920 nm then, once a neuron was identified as expressing EYFP, we used 820 nm light to target the dark shadows of cell somata against the background of the intracellular fluorescent Alexa Fluor 594 dye. Recordings were filtered at 10 kHz and digitized at 20 kHz via an ITC18 (Heka) analog-to-digital converter connected to a PC under the control of IGORpro (Wavemetrics). The membrane potential was not corrected for the liquid junction potential.
For monosynaptic connectivity experiments, up to 4 recording pipettes were inserted into the brain and 2 to 4 pyramidal neurons were targeted as previously described (Jouhanneau et al., 2015, Jouhanneau et al., 2018). To evoke single action potentials, square current pulses (10-20 ms, 100-400 pA) were injected into each cell at a rate of 1 or 0.5 Hz. Z stack images (2 μm/slice) were made after the termination of the recordings to confirm cell identity.
Subcellular two-photon optogenetic stimulation
Two-photon optogenetic stimulation was performed with the imaging laser source (at 920 nm wavelength) opened for 10 ms to deliver 10-25 mW (measured below objective). Somatic stimulation was performed with a spiral scan line (diameter: 8 μm, thread pitch: 0.45 μm). The spiral scan line was scanned two times with constant speed (19 μm/ms) during this stimulation epoch.
The cell was filled with red Alexa Fluor 594 during whole-cell recordings and the dendrites were imaged at 820 nm. At the beginning of each recording, at least 30 somatic stimuli were applied and the amplitude of an average Vhyp response was evaluated online as a measure of the neuronal responsiveness to light; the power of further subcellular stimulations could then be tuned accordingly. Next, dendritic stimulations were targeted to thin apical or basal dendrites using the red Alexa signal in the dendrites for in vivo guidance. Dendrites were selected with no neighboring dendrites in the same optical plane (not closer than ∼15 µm). Apical dendrites were identified by following the branching of the apical dendritic trunk emerging from the top of the pyramidal cell body and moving toward the pial surface. In contrast, basal dendrites were identified by following the branching of laterally emerging dendrites moving around the soma focal plane. We then used a zigzag scan line (side length: 1 μm, displacement: 0.1 μm) to activate individual dendritic regions at the same speed as somatic stimulations, resulting in 10 epochs in 10 ms. Cells were stimulated 250 times in one trial at 3 Hz; following each trial, the stimulation positions were checked and readjusted if necessary. 3 to 6 trials were performed per dendrite. Optical stimulation was controlled using MES software (Femtonics) running in MATLAB (MathWorks).
Histology
Mice were deeply anesthetized by i.p. injection of urethane and transcardially perfused with 4% paraformaldehyde (PFA). The brain was fixed in 4% PFA overnight and stored in phosphate buffer. A Leica VT1000 S vibrating microtome was used to make 100 μm thick coronal or tangential slices that were subsequently stained for cytochrome oxidase and biocytin with a standard ABC kit (Vectastain) with DAB enhancement. Slices were mounted in Mowiol and stored at 4°C before stained neurons were reconstructed using NeuroLucida software (MicroBrightField). Any putative GABA-ergic inhibitory interneurons were excluded from the dataset.
Electrophysiological inclusion parameters
Recorded neurons were included in the dataset only when they met specific parameters related to the health of the neuron and quality of the patch. If the average Vhyp Vm was above −50 mV, the cell was excluded. At the beginning of each recording, a firing pattern was assessed by injecting 0.5 s steps of current (−200, −100, +50, +100, +150, +200, +250 and +300 pA). Neurons which did not respond with action potentials (APs) to the +300-pA stimulus or whose APs reached peak amplitudes below −10 mV were excluded. Only recordings with an access resistance below 60 MΩ were included in the dataset.
Compartmental model
Numerical simulations for Figure 7 used a compartmental model of one of the reconstructed layer 2/3 pyramidal neurons. The soma contours created with the Neurolucida software were replaced by a series of cylinders with the same total membrane surface area. The model used intracellular resistivity Ri = 200 Ω cm and membrane capacitance Cm = 1 μF/cm2. Dendrites were discretized into compartments with a length of ≤ 0.1 times the frequency-dependent length constant at 100 Hz.
A leak conductance gleak was distributed uniformly across soma and dendrites. Active properties consisted of a non-inactivating voltage-gated amplifying current: where we set the reversal potential EVGC to a strongly depolarized value of 50 mV. The gating variable n evolved according to . The activation function was characterized by its half activation voltage Vh and the reciprocal slope parameter k. The activation time constant of the current was considered voltage-independent. The peak conductance density of the amplifying current was a parameter that was used for basal dendrite compartments further than xb μm from the soma and for apical compartments further than xa μm from the soma, otherwise it was set to 0.
Simulations were performed to constrain the seven undetermined parameters, which were independently varied over physiologically plausible ranges: gleak (0.08-0.4 mS/cm2), (0.005-0.15 mS/cm2), xb (0-160 μm), xa (0-300 μm), Vh (−57 - −39 mV), k (0.5-5 mV), (0.1-10 ms). An optogenetic stimulus was simulated as a local conductance change in a basal compartment ∼70 μm from the soma (see Figure 7A) or at the soma itself. The conductance time course was described by an alpha function: , where the time constant ms was fit to the experimental data in which the soma was directly stimulated and recorded. The membrane current generated by the optogenetic stimulus was with reversal potential mV.
For each parameter combination, the conductance stimulus was applied during Vhyp where the uniform holding potential was −60 mV and during Vdep with holding potential −50 mV. The peak conductance of the optogenetic stimulus was varied over a range to obtain somatic depolarizations of up to 1.5 mV for the basal input (see Figures 2D and 2F) and up to 4.2 mV for the somatic input (see Figures 1G and 1I). The ratio of the somatic voltage amplitudes in Vdep to Vhyp was computed and the sum squared error with the experimental observations was computed for basal and somatic stimuli combined in order to find parameter sets that account for the amplification of the basal but not the somatic inputs. Simulations and analysis were carried out in NEURON (Hines and Carnevale, 1997) and MATLAB (the MathWorks, Inc.).
Quantification and Statistical Analysis
Datasets
Subcellular ChR2 stimulation results included data from primary whisker and primary forepaw somatosensory cortex. As we observed identical findings in both regions, the datasets were combined. All experiments using awake mice were made from primary somatosensory forepaw cortex. A subset of the VPM and POm stimulation dataset was already published (Jouhanneau et al., 2014); however, the comparison between Vdep and Vhyp response was not previously reported. Likewise, a subset of monosynaptic connections used in the analysis shown in Figure 5 was included in previous analyses (Jouhanneau et al., 2015, Jouhanneau et al., 2018), however, the comparison of uEPSP amplitude during Vhyp versus Vdep was not previously reported.
Selection of Vhyp/Vdep
Subcellular OP, thalamic and single AP evoked responses were separated into responses during depolarized (Vdep) or hyperpolarized (Vhyp) phases based on the prestimulus Vm. Typically, a histogram of the Vm was generated and the point equidistant from the two normally distributed curves over Vhyp and Vdep states was taken as a reference to split the states. Trials falling into a ± 2 to ± 5 mV window from the divide, or those sweeps with a standard deviation > 1.5 mV (as measured between two windows −50 to −1 ms and +50 to +100 ms), were considered to be in transition states and removed from further analysis. In cases without clear bimodal distributions of the Vm, and in awake data, Vhyp and Vdep thresholds were defined at a set distance from the most hyperpolarized value in the sweep. All data were visually inspected to confirm the automatic sorting. Layer 2/3 neurons fire extremely sparsely, but those segments with spontaneously occurring APs were removed from further analysis.
Amplitude measurement of subthreshold responses
The amplitudes of optogenetic potentials (OPs), VPM responses and uEPSPs were measured from the averaged response. The amplitude of the response (signal) was measured as the difference between the average Vm ± 0.5 ms around the peak response and the 1 ms average of the Vm baseline (−1 to −2 ms before stimulus onset). Noise was calculated by randomly selecting a time point before the onset of the stimulus and measuring the Vm difference between the 1 ms average around each time point and the amplitude of the response. The signal to noise ratio was calculated by measuring the variance of response amplitude and the background Vm variance −30 to −10 ms prior to the stimulus onset on each individual trial. Next the mean variance was calculated and the response variance (signal) was divided by the background variance (noise). Any monosynaptic connectivity data with a hyperpolarizing response to the presynaptic spike, suggesting inhibitory neuron activation, were removed from the dataset. The latency was defined as the crossing point of two linear fits: the first from −15 ms to −5 ms prior to the presynaptic spike (for monosynaptic connectivity data) or onset of the laser pulse (for optogenetic stimulation data), the second between time points corresponding to 20 to 80% of the peak Vm response amplitude. In addition, we calculated the half width of the OPs as the difference in time between 50% of the rising phase and 50% of the decay phase of the evoked response. The expected OP amplitude value in Vdep was calculated using the change in pre-stimulus Vm and assuming a reversal potential of 0 mV for OPs.
Input resistance
−100 pA, 80 ms current pulses were injected via the recording pipette at 5.55 Hz. The Vm responses to the current pulses were then split into Vdep and Vhyp states, as discussed above, and averaged. Access resistance was subtracted offline using an exponential fit of the Vm from a 2 ms period after the start of current injection (Zhao et al., 2016). The difference in Vm between the baseline and the time point at which the fit crossed the onset time of current injection was taken as the access resistance. The input resistance was calculated from the difference in Vm between the current injection response corrected for access resistance and the prestimulus Vm. Tau was calculated from the exponential fit of the relaxation phase of the Vm from 2 ms after the end of the hyperpolarizing pulse.
In vivo data statistics
Custom written scripts in IGORpro (Wavemetrics) and MATLAB (MathWorks) were used to analyze all data. Correlations between Vhyp amplitude and the ratio of Vdep: Vhyp response amplitude are calculated on the log10 of the Vhyp amplitude with Pearson’s linear correlation in IGORpro. Correlations between ratio of Vdep: Vhyp response amplitude and stimulation site distance from the soma were calculated using Pearson’s linear correlation. The mean number of stimuli delivered in Vdep were: Soma anesthetized 110 ± 102, soma awake 60 ± 31, basal anesthetized 192 ± 105, basal awake 114 ± 52, apical anesthetized 219 ± 140, apical awake 106 ± 75, VPM 83 ± 60, POm 90 ± 74, uEPSP 69 ± 27, basal QX-314 154 ± 93, basal MK-801 226 ± 129, basal D-890 272 ± 150. For statistical analysis, we used two-tailed non-parametric tests. Paired data were tested using the Wilcoxon signed rank test and unpaired data with the Wilcoxon rank sum test unless otherwise stated. Data in results and on figures show mean ± standard deviation (SD) unless otherwise stated.
Acknowledgments
We thank Janett König for technical help and Alison Barth, Evgeny Bobrov, and Philipp Schnepel for comments on an earlier version of the manuscript. This work was funded by the European Research Council (ERC-2010-StG-260590 and ERC-2015-CoG-682422; J.F.A.P.), the Deutsche Forschungsgemeinschaft (DFG) (Exc 257 NeuroCure, DFG-FOR-1341-BaCoFun, and DFG-FOR-2143-Interneuron; J.F.A.P.), the Thyssen Foundation, the European Union’s FP7 Programme (3x3Dimaging 323945; J.F.A.P.), the Helmholtz Association (J.F.A.P.), and the Bundesministerium für Bildung und Forschung (01GQ0901 and 01GQ1403; S.S.). B.R. was funded by the European Research Council (ERC 682426), and B.R. and K.G. by the Hungarian National Research, Development and Innovation Office (KFI_16-1-2016-0177, GINOP_2.1.1-15-2016-00979, VKSZ_14-1-2015-0155, and NAP-2.0/VIII/2), and the European Union’s Horizon (712821) and FP7 (ICT-2011-C 323945) programs. J.K. was funded by the Humboldt-Universität zu Berlin in the framework of the Excellence Initiative of the BMBF and DFG (Emmy-Noether KR 4062/4–1).
Author Contributions
Conceptualization, L.F., J.-S.J., and J.F.A.P.; Methodology, L.F., B.R., G.K., and M.W.H.R.; Investigation, L.F., J.-S.J., and M.W.H.R.; Formal Analysis, L.F., J.-S.J., M.W.H.R., and J.K.; Writing – Original Draft, J.F.A.P.; Writing – Review & Editing, L.F., J.-S.J., M.W.H.R., S.S., and J.F.A.P.; Resources, S.S. and J.F.A.P.; Visualization, L.F., J.-S.J., M.W.H.R., and J.F.A.P.; Supervision, S.S. and J.F.A.P.; Funding Acquisition, B.R., G.K., J.K., S.S., and J.F.A.P.
Declaration of Interests
G.K. and B.R. are founders of Femtonics Kft. B.R. is a member of its scientific advisory board.
Published: September 25, 2018
Footnotes
Supplemental Information includes six figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.08.088.
Supplemental Information
References
- Andreasen M., Lambert J.D. Somatic amplification of distally generated subthreshold EPSPs in rat hippocampal pyramidal neurones. J. Physiol. 1999;519:85–100. doi: 10.1111/j.1469-7793.1999.0085o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audette N.J., Urban-Ciecko J., Matsushita M., Barth A.L. POm thalamocortical input drives layer-specific microcircuits in somatosensory cortex. Cereb. Cortex. 2018;28:1312–1328. doi: 10.1093/cercor/bhx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth A.L., Poulet J.F.A. Experimental evidence for sparse firing in the neocortex. Trends Neurosci. 2012;35:345–355. doi: 10.1016/j.tins.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Bernander O., Douglas R.J., Martin K.A., Koch C. Synaptic background activity influences spatiotemporal integration in single pyramidal cells. Proc. Natl. Acad. Sci. USA. 1991;88:11569–11573. doi: 10.1073/pnas.88.24.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A., Schoenenberger P., Mattis J., Tye K.M., Deisseroth K., Hegemann P., Oertner T.G. High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proc. Natl. Acad. Sci. USA. 2011;108:7595–7600. doi: 10.1073/pnas.1017210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno R.M., Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science. 2006;312:1622–1627. doi: 10.1126/science.1124593. [DOI] [PubMed] [Google Scholar]
- Chadderton P., Schaefer A.T., Williams S.R., Margrie T.W. Sensory-evoked synaptic integration in cerebellar and cerebral cortical neurons. Nat. Rev. Neurosci. 2014;15:71–83. doi: 10.1038/nrn3648. [DOI] [PubMed] [Google Scholar]
- Chance F.S., Abbott L.F., Reyes A.D. Gain modulation from background synaptic input. Neuron. 2002;35:773–782. doi: 10.1016/s0896-6273(02)00820-6. [DOI] [PubMed] [Google Scholar]
- Chen X., Rochefort N.L., Sakmann B., Konnerth A. Reactivation of the same synapses during spontaneous up states and sensory stimuli. Cell Rep. 2013;4:31–39. doi: 10.1016/j.celrep.2013.05.042. [DOI] [PubMed] [Google Scholar]
- Cowan R.L., Wilson C.J. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J. Neurophysiol. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- Crochet S., Chauvette S., Boucetta S., Timofeev I. Modulation of synaptic transmission in neocortex by network activities. Eur. J. Neurosci. 2005;21:1030–1044. doi: 10.1111/j.1460-9568.2005.03932.x. [DOI] [PubMed] [Google Scholar]
- Crochet S., Poulet J.F.A., Kremer Y., Petersen C.C.H. Synaptic mechanisms underlying sparse coding of active touch. Neuron. 2011;69:1160–1175. doi: 10.1016/j.neuron.2011.02.022. [DOI] [PubMed] [Google Scholar]
- Deisz R.A., Fortin G., Zieglgänsberger W. Voltage dependence of excitatory postsynaptic potentials of rat neocortical neurons. J. Neurophysiol. 1991;65:371–382. doi: 10.1152/jn.1991.65.2.371. [DOI] [PubMed] [Google Scholar]
- Destexhe A., Paré D. Impact of network activity on the integrative properties of neocortical pyramidal neurons in vivo. J. Neurophysiol. 1999;81:1531–1547. doi: 10.1152/jn.1999.81.4.1531. [DOI] [PubMed] [Google Scholar]
- Destexhe A., Rudolph M., Paré D. The high-conductance state of neocortical neurons in vivo. Nat. Rev. Neurosci. 2003;4:739–751. doi: 10.1038/nrn1198. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D., Lübke J., Silver R.A., Sakmann B. Synaptic connections between layer 4 spiny neurone-layer 2/3 pyramidal cell pairs in juvenile rat barrel cortex: physiology and anatomy of interlaminar signalling within a cortical column. J. Physiol. 2002;538:803–822. doi: 10.1113/jphysiol.2001.012959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D., Lübke J., Sakmann B. Efficacy and connectivity of intracolumnar pairs of layer 2/3 pyramidal cells in the barrel cortex of juvenile rats. J. Physiol. 2006;575:583–602. doi: 10.1113/jphysiol.2006.105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet L.J., Kremer Y., Taniguchi H., Huang Z.J., Staiger J.F., Petersen C.C.H. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat. Neurosci. 2012;15:607–612. doi: 10.1038/nn.3051. [DOI] [PubMed] [Google Scholar]
- Goebbels S., Bormuth I., Bode U., Hermanson O., Schwab M.H., Nave K.-A. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis. 2006;44:611–621. doi: 10.1002/dvg.20256. [DOI] [PubMed] [Google Scholar]
- González-Burgos G., Barrionuevo G. Voltage-gated sodium channels shape subthreshold EPSPs in layer 5 pyramidal neurons from rat prefrontal cortex. J. Neurophysiol. 2001;86:1671–1684. doi: 10.1152/jn.2001.86.4.1671. [DOI] [PubMed] [Google Scholar]
- Hines M.L., Carnevale N.T. The NEURON simulation environment. Neural Comput. 1997;9:1179–1209. doi: 10.1162/neco.1997.9.6.1179. [DOI] [PubMed] [Google Scholar]
- Jia H., Rochefort N.L., Chen X., Konnerth A. Dendritic organization of sensory input to cortical neurons in vivo. Nature. 2010;464:1307–1312. doi: 10.1038/nature08947. [DOI] [PubMed] [Google Scholar]
- Jiang X., Wang G., Lee A.J., Stornetta R.L., Zhu J.J. The organization of two new cortical interneuronal circuits. Nat. Neurosci. 2013;16:210–218. doi: 10.1038/nn.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouhanneau J.-S., Ferrarese L., Estebanez L., Audette N.J., Brecht M., Barth A.L., Poulet J.F.A. Cortical fosGFP expression reveals broad receptive field excitatory neurons targeted by POm. Neuron. 2014;84:1065–1078. doi: 10.1016/j.neuron.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Jouhanneau J.-S., Kremkow J., Dorrn A.L., Poulet J.F.A. In vivo monosynaptic excitatory transmission between layer 2 cortical pyramidal neurons. Cell Rep. 2015;13:2098–2106. doi: 10.1016/j.celrep.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouhanneau J.-S., Kremkow J., Poulet J.F.A. Single synaptic inputs drive high-precision action potentials in parvalbumin expressing GABA-ergic cortical neurons in vivo. Nat. Commun. 2018;9:1540–1550. doi: 10.1038/s41467-018-03995-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefort S., Tomm C., Floyd Sarria J.-C., Petersen C.C.H. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron. 2009;61:301–316. doi: 10.1016/j.neuron.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Longordo F., To M.-S., Ikeda K., Stuart G.J. Sublinear integration underlies binocular processing in primary visual cortex. Nat. Neurosci. 2013;16:714–723. doi: 10.1038/nn.3394. [DOI] [PubMed] [Google Scholar]
- Magee J.C. Dendritic integration of excitatory synaptic input. Nat. Rev. Neurosci. 2000;1:181–190. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- Maier W., Corrie J.E.T., Papageorgiou G., Laube B., Grewer C. Comparative analysis of inhibitory effects of caged ligands for the NMDA receptor. J. Neurosci. Methods. 2005;142:1–9. doi: 10.1016/j.jneumeth.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Markram H., Lübke J., Frotscher M., Roth A., Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J. Physiol. 1997;500:409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H., Toledo-Rodriguez M., Wang Y., Gupta A., Silberberg G., Wu C. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Mateo C., Avermann M., Gentet L.J., Zhang F., Deisseroth K., Petersen C.C.H. In vivo optogenetic stimulation of neocortical excitatory neurons drives brain-state-dependent inhibition. Curr. Biol. 2011;21:1593–1602. doi: 10.1016/j.cub.2011.08.028. [DOI] [PubMed] [Google Scholar]
- Metherate R., Ashe J.H. Ionic flux contributions to neocortical slow waves and nucleus basalis-mediated activation: whole-cell recordings in vivo. J. Neurosci. 1993;13:5312–5323. doi: 10.1523/JNEUROSCI.13-12-05312.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H.S., Wimmer V.C., Hemberger M., Bruno R.M., de Kock C.P.J., Frick A., Sakmann B., Helmstaedter M. Cell type-specific thalamic innervation in a column of rat vibrissal cortex. Cereb. Cortex. 2010;20:2287–2303. doi: 10.1093/cercor/bhq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic N., Zhao W.-J., Walcher J., Albert T., Siemens J., Lewin G.R., Poulet J.F.A. A somatosensory circuit for cooling perception in mice. Nat. Neurosci. 2014;17:1560–1566. doi: 10.1038/nn.3828. [DOI] [PubMed] [Google Scholar]
- Muñoz W., Tremblay R., Levenstein D., Rudy B. Layer-specific modulation of neocortical dendritic inhibition during active wakefulness. Science. 2017;355:954–959. doi: 10.1126/science.aag2599. [DOI] [PubMed] [Google Scholar]
- Nevian T., Larkum M.E., Polsky A., Schiller J. Properties of basal dendrites of layer 5 pyramidal neurons: a direct patch-clamp recording study. Nat. Neurosci. 2007;10:206–214. doi: 10.1038/nn1826. [DOI] [PubMed] [Google Scholar]
- Noguchi J., Nagaoka A., Watanabe S., Ellis-Davies G.C.R., Kitamura K., Kano M., Matsuzaki M., Kasai H. In vivo two-photon uncaging of glutamate revealing the structure-function relationships of dendritic spines in the neocortex of adult mice. J. Physiol. 2011;589:2447–2457. doi: 10.1113/jphysiol.2011.207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer A.M., Peterka D.S., Hirtz J.J., Prakash R., Deisseroth K., Yuste R. Two-photon optogenetics of dendritic spines and neural circuits. Nat. Methods. 2012;9:1202–1205. doi: 10.1038/nmeth.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pala A., Petersen C.C.H. In vivo measurement of cell-type-specific synaptic connectivity and synaptic transmission in layer 2/3 mouse barrel cortex. Neuron. 2015;85:68–75. doi: 10.1016/j.neuron.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pala A., Petersen C.C. State-dependent cell-type-specific membrane potential dynamics and unitary synaptic inputs in awake mice. eLife. 2018;7:350. doi: 10.7554/eLife.35869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C.C.H., Hahn T.T.G., Mehta M., Grinvald A., Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc. Natl. Acad. Sci. USA. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L., Mao T., Sternson S.M., Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet J.F.A., Petersen C.C.H. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- Prakash R., Yizhar O., Grewe B., Ramakrishnan C., Wang N., Goshen I., Packer A.M., Peterka D.S., Yuste R., Schnitzer M.J., Deisseroth K. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat. Methods. 2012;9:1171–1179. doi: 10.1038/nmeth.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig R., Zerlaut Y., Vergara R., Destexhe A., Sanchez-Vives M.V. Gain modulation of synaptic inputs by network state in auditory cortex in vivo. J. Neurosci. 2015;35:2689–2702. doi: 10.1523/JNEUROSCI.2004-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remme M.W.H., Rinzel J. Role of active dendritic conductances in subthreshold input integration. J. Comput. Neurosci. 2011;31:13–30. doi: 10.1007/s10827-010-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev R.N.S., Ebner F.F., Wilson C.J. Effect of subthreshold up and down states on the whisker-evoked response in somatosensory cortex. J. Neurophysiol. 2004;92:3511–3521. doi: 10.1152/jn.00347.2004. [DOI] [PubMed] [Google Scholar]
- Shu Y., Hasenstaub A., Badoual M., Bal T., McCormick D.A. Barrages of synaptic activity control the gain and sensitivity of cortical neurons. J. Neurosci. 2003;23:10388–10401. doi: 10.1523/JNEUROSCI.23-32-10388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Sjöström P.J., Reigl M., Nelson S., Chklovskii D.B. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 2005;3:e68. doi: 10.1371/journal.pbio.0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat. Rev. Neurosci. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Steriade M., Nuñez A., Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J. Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G., Sakmann B. Amplification of EPSPs by axosomatic sodium channels in neocortical pyramidal neurons. Neuron. 1995;15:1065–1076. doi: 10.1016/0896-6273(95)90095-0. [DOI] [PubMed] [Google Scholar]
- Tamamaki N., Yanagawa Y., Tomioka R., Miyazaki J., Obata K., Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J. Comp. Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Veinante P., Deschênes M. Single-cell study of motor cortex projections to the barrel field in rats. J. Comp. Neurol. 2003;464:98–103. doi: 10.1002/cne.10769. [DOI] [PubMed] [Google Scholar]
- Viaene A.N., Petrof I., Sherman S.M. Properties of the thalamic projection from the posterior medial nucleus to primary and secondary somatosensory cortices in the mouse. Proc. Natl. Acad. Sci. USA. 2011;108:18156–18161. doi: 10.1073/pnas.1114828108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J., Helmchen F. Boosting of action potential backpropagation by neocortical network activity in vivo. J. Neurosci. 2004;24:11127–11136. doi: 10.1523/JNEUROSCI.2933-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J., Helmchen F. Background synaptic activity is sparse in neocortex. J. Neurosci. 2006;26:8267–8277. doi: 10.1523/JNEUROSCI.2152-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S.R. Spatial compartmentalization and functional impact of conductance in pyramidal neurons. Nat. Neurosci. 2004;7:961–967. doi: 10.1038/nn1305. [DOI] [PubMed] [Google Scholar]
- Williams S.R., Stuart G.J. Dependence of EPSP efficacy on synapse location in neocortical pyramidal neurons. Science. 2002;295:1907–1910. doi: 10.1126/science.1067903. [DOI] [PubMed] [Google Scholar]
- Wimmer V.C., Bruno R.M., de Kock C.P.J., Kuner T., Sakmann B. Dimensions of a projection column and architecture of VPM and POm axons in rat vibrissal cortex. Cereb. Cortex. 2010;20:2265–2276. doi: 10.1093/cercor/bhq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W.-J., Kremkow J., Poulet J.F.A. Translaminar cortical membrane potential synchrony in behaving mice. Cell Rep. 2016;15:2387–2399. doi: 10.1016/j.celrep.2016.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuchkova E., Remme M.W.H., Schreiber S. Somatic versus dendritic resonance: differential filtering of inputs through non-uniform distributions of active conductances. PLoS ONE. 2013;8:e78908. doi: 10.1371/journal.pone.0078908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.