Abstract
Background/Aims
To determine the optimal dose of LB03002, a sustained-release, once-weekly formulation of recombinant human growth hormone (rhGH), and to compare its efficacy and safety with daily rhGH in children with idiopathic short stature (ISS).
Methods
This multicenter, randomized, open-label, phase II study included GH-naïve, prepubertal children with ISS, randomized to receive daily rhGH 0.37 mg/kg/week (control, n = 16), LB03002 0.5 mg/kg/week (n = 14), or LB03002 0.7 mg/kg/week (n = 16). The primary endpoint was height velocity (HV) change at week 26.
Results
At week 26, the least square (LS) means for HV change (cm/year) with control, LB03002 0.5 mg/kg/week, and LB03002 0.7 mg/kg/week were 5.08, 3.65, and 4.38, and the LS means for the change in height standard deviation score were 0.65, 0.49, and 0.58, respectively. The lower bound of the 90% confidence interval for the difference between LB03002 0.7 mg/kg/week and the control in the LS mean for HV change (−1.72) satisfied the noninferiority margin (−1.75). Adverse events were generally mild and short-lived.
Conclusion
A once-weekly regimen of LB03002 0.7 mg/kg demonstrated noninferiority to the daily regimen of rhGH 0.37 mg/kg/week in terms of HV increments. LB03002 was well tolerated and its safety profile was comparable with that of daily rhGH.
Keywords: Sustained-release growth hormone, Idiopathic short stature, Height velocity
Introduction
With the progress in molecular biotechnology, enabling large-scale production of recombinant human growth hormone (rhGH), many studies have been conducted to evaluate the use of rhGH in conditions presenting short stature but without classical growth hormone deficiency (GHD), such as chronic renal failure, Turner syndrome, Prader-Willi syndrome, small for gestational age, and idiopathic short stature (ISS). Among these, ISS is a quite frequently encountered condition as the specific causes of short stature are determined only for a small proportion (e.g., approximately 5–20%) of children [1-3]. ISS is generally defined as the condition characterized by a height below −2.0 standard deviation score (SDS) for the corresponding age and sex, with no evidence of an underlying disorder [4, 5]. Although the use of rhGH in children with ISS remains controversial, a number of randomized controlled studies confirmed that compared with placebo or no treatment, rhGH has a positive effect on short-term growth and final adult height in children with ISS [4, 6-11].
However, most of the available rhGH products require subcutaneous injection 6 or 7 times a week for the entire treatment period, which may reduce the treatment compliance. Thus, several technologies were used in an attempt to develop longer-acting rhGH formulations [12]. LB03002 (EutropinPlus inj.) is a once-weekly sustained-release formulation of rhGH manufactured using genetically modified Saccharomyces cerevisiae and contained in sodium hyaluronate microparticles suspended in medium-chain triglycerides prior to injection [13]. The efficacy and safety of LB03002 were confirmed in children and adults with GHD [14-16]. Up to date, however, there is no evidence confirming the efficacy of the once-weekly formulation in ISS patients. The aim of this study was to investigate the efficacy and safety of LB03002, and to select its optimal dose in children with ISS. As a comparator, we used Eutropin inj., the agent sharing the same expression system with LB03002 but in the immediate-release formulation (daily rhGH), approved in South Korea for the treatment of ISS, GHD, and other indications.
Subjects, Materials, and Methods
Study Design and Principle
This was a phase II, multicenter, randomized, active-controlled (daily rhGH as control), open-label, dose-finding study (between 0.5 mg/kg/week and 0.7 mg/kg/week). The lower dose was the approved dose for GHD, considered to be a comparable dose level to that of the comparator (0.37 mg/kg/week), whereas the higher dose was the maximum dose within the safety margin provided by the animal toxicity study. All the procedures were carried out according to the Declaration of Helsinki, Good Clinical Practice, and standard operating procedures of the Sponsor. The study protocol was reviewed and approved by the Ministry of Food and Drug Safety of Korea and the institutional review board of each investigational site. Patients and their parents or legal guardians were provided with a full explanation of the purpose and nature of all study procedures, and parental written informed consent was obtained before the screening. Additionally, informed consent was sought from all the patients who were able to read and write in Korean (usually ≥7 years of age). The study was registered at ClinicalTrials.gov (identifier: NCT02042170).
Patients
Eligible patients were prepubertal children aged at least 4 years and diagnosed with ISS with bone ages (BA) less than 9 and 11 years for girls and boys, respectively. ISS was diagnosed whenever all of the following conditions were met: (i) height ≤3rd percentile for age and sex; (ii) normal GH secretion confirmed based on GH concentration ≥10 ng/mL in at least one stimulation test [17]; (iii) normal body proportion without skeletal dysplasia; and (iv) lack of pathological abnormalities that might inhibit growth. Children with GHD or other causes of short stature, including Prader-Willi syndrome, Russel-Silver syndrome, Seckel syndrome, or small for gestational age, were excluded from the study, along with the patients with some other comorbidities, such as primary hypothyroidism, adrenal insufficiency, hypogonadism, diabetes, malignancy, or closed epiphyses. In addition, children treated with sex hormones, steroids, aromatase inhibitors, thyroxine (other than administered at a stable dose for 4 weeks or more), or medications for attention deficit hyperactivity disorder were also excluded.
Study Procedure
Eligible patients were randomized to one of the three arms (daily rhGH 0.37 mg/kg/week, LB03002 0.5 mg/kg/week, or LB03002 0.7 mg/kg/week) in a 1: 1: 1 ratio with stratification according to the sex and age (≥7 years or < 7 years). Study visits were scheduled at the baseline, week 13, week 26, and week 30. One week after the baseline visit and at week 13, the study staff contacted the patients' parents to assure their compliance to activities set out in the consent form. Daily rhGH (control) was given 6 times per week at equally divided doses corresponding to a total weekly dose of 0.37 mg/kg, whereas the weekly rhGH (LB03002 0.5 mg/kg or 0.7 mg/kg) was administered once a week at the dose assigned to a given group. Injections were given subcutaneously by the patients' parents or legal guardians at bedtime for 26 weeks. Injection site reactions were recorded in the diary cards. In order to assess the treatment compliance, the prescribed dose and the number of vials dispensed, used, and returned were counted and recorded at the study site. The following parameters were determined at the baseline or during the screening, at week 13 and 26: height, weight, Tanner pubertal stage, thyroid function parameters, insulin-like growth factor I (IGF-I), IGF-I-binding protein 3 (IGFBP-3), complete blood count, blood chemistry, and urinalysis. Height, weight, IGF-I, and IGFBP-3 were measured again at the follow-up visit (week 30). An X-ray to determine BA and the measurement of anti-rhGH antibodies were performed both at screening and at week 26. BA was estimated using the Greulich-Pyle method by two independent pediatric endocrinologists who were blinded to the patients' demographics and assigned treatments. Blood samples for the pharmacokinetic (PK) and pharmacodynamic (PD) analyses of rhGH were taken at baseline from all patients. For patients randomized to the LB03002 groups, additional blood samples for PK and PD analyses were obtained at two time points, at 36 h and at 7 days after the last injection (26th dosing).
Assessment of Study Endpoints
The primary endpoint was the change in annualized height velocity (HV) at week 26 from baseline. HV was calculated using the following formula: HV (cm/year) = (last height measurement – height at baseline) / (number of days between the first injection and last height measurement) × 365. Standing height was measured three times using a calibrated stadiometer at each visit, and the mean values were used. To calculate the baseline HV, the baseline height and height measured at least 6 months before the baseline were used. The list of secondary endpoints included HV at week 13; height, IGF-I, IGF-I SDS, IGFBP-3, and IGFBP-3 SDS at week 13 and week 26; and height SDS for BA and chronological age (CA), BA, and ΔBA/ΔCA at week 26. The list of PK and PD parameters for LB03002 included the levels of human growth hormone (hGH), IGF-I, and IGFBP-3. Adverse events, injection site reactions, laboratory results, and anti-rhGH antibody level were considered as safety endpoints.
Assays
Serum levels of hGH, IGF-I, and IGFBP-3 were measured by chemiluminescence immunoassay using Immulite 2000 Immunoassay System (Siemens Healthcare Diagnostics Inc., Germany) at Seoul Medical Science Institute (SCL), South Korea, and anti-hGH antibodies were quantified using a radio-precipitation assay at the University of Leipzig, Germany. All assays have been validated for their limit of detection and quantification, precision, linearity, and recovery. Other laboratory parameters, such as functional parameters of the thyroid, complete blood count, blood chemistry, and urinalysis were determined at the SCL with an aid of validated assays.
Statistical Analysis
In order to select the optimal dose of LB03002, a noninferiority test for the change in HV at week 26 was carried out with the LB03002 groups against the daily rhGH group. Assuming a standard deviation (SD) of 1.7 cm/year for the change in HV and noninferiority margin of 1.75 cm/year, 12 patients per group were considered sufficient to achieve 80% power to detect the between-group difference with a one-sided significance level of 5%. The enrollment target was approximately 45 patients (15 per group) to accommodate 20% drop-out and protocol deviations. The primary endpoint was analyzed using the ANCOVA model with baseline HV covariates and treatment group, age (≥7 or < 7 years), and sex as fixed variables; two-sided 90% confidence interval (CI) for the least square (LS) mean difference was calculated, and if the lower bound of the CI was greater than −1.75, noninferiority of LB03002 to daily rhGH was to be declared. The same ANCOVA model was used for the secondary endpoints. Missing values for the primary endpoint were replaced by the last available data according to the last-observation-carried-forward approach. For the computation of height SDS, the following equation was used referencing the power in the Box-Cox transformation (L), median (M), and coefficient of variation (S) values from the growth standards for Korean children and adolescents [18]: height SDS = [power (measured height / M, L) − 1] / L × S. The SDS for IGF-I and IGFBP-3 were computed using the following equation: SDS = (measured value – mean population value) / SD of population value [19]. Treatment compliance was calculated by the following formula: (total number of vials used) / {(prescribed dose per injection × 26) / 1.2} for LB03002 groups, and (total number of vials used) / {(prescribed dose per injection × 6 × 26) / 0.8} for the comparator group. All treated patients who were randomized and received the study drug at least once were included in the safety analysis; treated patients with at least one height measurement after the baseline were included in the full analysis set (FAS), and patients without any major protocol deviations, such as treatment compliance < 80% or violation of eligibility criteria, were included in the per-protocol set (PPS), the main population for the efficacy evaluation. All statistical analyses were performed using SAS® software, version 9.2 (SAS Institute, Cary, NC, USA).
Results
Patient Disposition and Characteristics
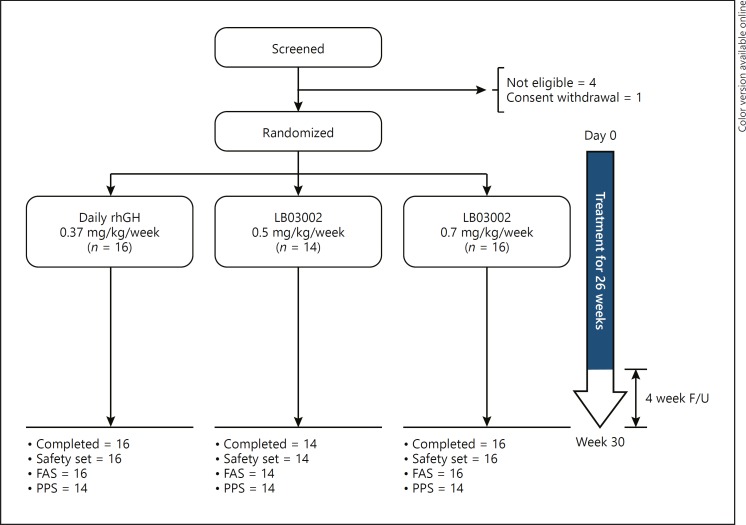
From March 2014 to March 2015, 46 out of 51 screened patients were randomized and completed the study (Fig. 1). Except for 1 patient (daily rhGH group) whose treatment compliance was 79%, all other patients reached more than 80% of treatment compliance. All randomized patients were included in the safety and FAS analyses. The PPS included 42 patients; another 4 patients were not included due to major protocol deviations, namely study drug compliance < 80% (n = 1), dosage error (n = 2), and delay in initiation of the study treatment (n = 1). All patients were naïve to rhGH treatment, born at normal gestational ages (37–41 weeks), and presented with Tanner stage I. Mean height SDS for CA in all groups was less than −2, with a range of −3.01 to −1.68, and the proportion of extremely short patients (height SDS ≤–2.25) amounted to 45% (PPS). However, HV was mostly normal for the corresponding age group; GH secretion after the provocation test was normal (≥10 ng/mL) [17], and IGF-I and IGFBP-3 levels were within the reference ranges. The treatment groups were well balanced in terms of demographic and clinical characteristics (Table 1), yet the mean BA (years) of the LB03002 0.5 mg/kg/week group was significantly lower than that of the daily rhGH group (4.72 vs. 5.93, p = 0.041) in the FAS but not in the PPS (4.72 vs. 5.6, p = 0.107).
Fig. 1.
Disposition of the patients. A total of 51 children with ISS were screened; 46 were randomized; and 46 completed the study. All patients were included in the analysis. After excluding 4 patients with major protocol deviations, 42 were included in the PPS, the main analysis population for efficacy evaluation.
Table 1.
Patient demographics at baseline (PPS)
| Daily rhGH 0.37 mg/kg/week (n = 14) | LB03002 0.5 mg/kg/week (n = 14) | LB03002 0.7 mg/kg/week (n = 14) | |
|---|---|---|---|
| Age, years | 5.86 (1.29) | 5.36 (1.22) | 6.07 (1.54) |
| Male, n | 10 | 9 | 10 |
| Female, n | 6 | 5 | 6 |
| Birth weight, kg | 3.01 (0.3) | 3.11 (0.29) | 3.01 (0.28) |
| Weight, kg | 17.46 (2.42) | 16.61 (2.43) | 18.94 (6.84) |
| Mid-parental height, cm | 162.55 (7.45) | 164.44 (9.29) | 164.39 (7.44) |
| Height, cm | 105.88 (6.06) | 104.07 (6.23) | 107.47 (8.41) |
| Height SDS for CA | −2.25 (0.3) | −2.28 (0.29) | −2.28 (0.37) |
| Height SDS for CA ≤ −2.25, n | 5 | 8 | 6 |
| BA, years | 5.6 (1.53) | 4.72 (0.82) | 5.49 (1.92) |
| Height SDS for BA | −1.35 (1.28) | −0.69 (1.32) | −0.8 (1.57) |
| Height velocity, cm/year | 6.24 (1.6) | 6.11 (1.3) | 5.51 (0.94) |
| Peak GH plasma conc., ng/mL | 17.55 (10, 85.7) | 17.15 (10.6, 41) | 15.1 (10.1, 26.4) |
| IGF-I, ng/mL | 121 (51.4, 212) | 128 (50.2, 206) | 105 (56.1, 166) |
| IGF-I SDS | −0.67 (−1.39, 0.39) | −0.8 (−2.03, 0.63) | −1.3 (−2.06, 0.15) |
| IGFBP-3, ng/mL | 3,660 (2,200, 4,980) | 3,490 (2,450, 5,910) | 3,655 (2,440, 5,040) |
| IGFBP-3 SDS | 2.2 (−0.17, 5.15) | 2.47 (−0.11, 8.9) | 1.89 (0, 5.27) |
Continuous variables are presented with mean (SD) except peak GH plasma concentration, blood levels of IGF-I and IGFBP-3, and SDS of IGF-I and IGFBP-3, all of which are presented with median (min, max). Mid-parental height (MPH) (cm) was calculated using the following formula: MPH (for boys) = {paternal height + (maternal height + 13)}/2; MPH (for girls) = {(paternal height − 13) + maternal height}/2.
Efficacy Results
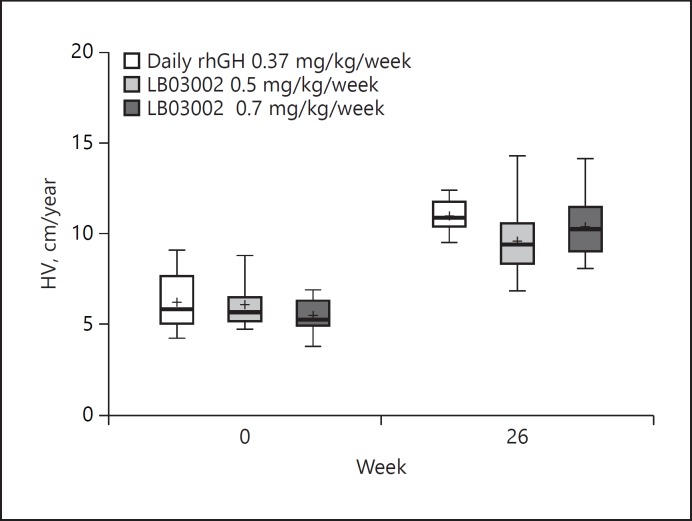
At week 26, a significant increase in HV was documented in patients from all three groups (Fig. 2). No statistically significant difference in the actual mean HV change was found between each LB03002 dose group and the daily rhGH group (control). In the HV ANCOVA, the LS mean of the change in HV at week 26 in the control, LB03002 0.5 mg/kg/week, and LB03002 0.7 mg/kg/week group was 5.08, 3.65, and 4.38, respectively. The 90% CI for the LS mean of the change in HV versus control at week 26 was −2.43 to −0.44 and −1.72 to 0.33 for LB03002 0.5 mg/kg/week and LB03002 0.7 mg/kg/week, respectively. Thus, only the LB03002 0.7 mg/kg/week group demonstrated noninferiority to control. Also, the FAS analysis yielded the same conclusion (lower bound of the 90% CI for the change in HV vs. control was −2.1 for LB03002 0.5 mg/kg/week, and −1.21 for LB03002 0.7 mg/kg/week). The ANCOVA interaction test showed that the change in HV was independent of the baseline HV, age, or sex. Consistent with the HV values, also height and height SDS for CA increased significantly in all three groups (p < 0.001), and the degree of changes at week 26 for LB03002 0.7 mg/kg/week group and control group were essentially similar (Table 2). The lower dose of LB03002 (0.5 mg/kg/week) consistently showed inferior response to the control at week 26 in terms of changes in height and height SDS in addition to the change in HV (see online suppl. Table 1; see www.karger.com/doi/10.1159/000489262 for all online suppl. material). While a post-treatment increase in BA was observed as well, it was not too advanced for CA, as indicated by the nonsignificant change in the BA advancement index (ΔBA/ΔCA) at week 26. Irrespective of the treatment, the clinical changes mentioned above coexisted with an increase in pharmacodynamic parameters, IGF-I and IGFBP-3 (Table 2). However, a significant increase from baseline was observed solely in the controls and in the LB03002 0.7 mg/kg/week group; these two groups did not differ significantly in terms of the degree of post-treatment change in IGF-I and IGFBP-3 levels.
Fig. 2.
Annualized height velocity (PPS). Before initiating rhGH treatment, median HV (cm/year) across three groups was less than 6 cm/year. A 6-month treatment resulted in significant increases in HV (p < 0.001), with median changes (cm/year) of 4.85, 3.1, and 4.69 in the daily rhGH, LB03002 0.5 mg/kg/week, and LB03002 0.7 mg/kg/week groups, respectively. The top and bottom of the box represent the 25th and 75th percentiles, the horizontal line inside the box represents the median, the symbol (+) represents mean, and the whiskers represent 5th and 95th percentile.
Table 2.
Changes from baseline in efficacy endpoints (PPS)
| Daily rhGH 0.37 mg/kg/week (n = 14) | LB03002 0.5 mg/kg/week (n = 14) | LB03002 0.7 mg/kg/week (n = 14) | |
|---|---|---|---|
| ΔHeight, cm | |||
| Week 13 | 2.77 (2.49, 3.05) | 2.64 (2.37, 2.91) | 2.54 (2.25, 2.82) |
| Week 26 | 5.53 (5.14, 5.92) | 4.81 (4.43, 5.18) | 5.19 (4.8, 5.59) |
| ΔHeight velocity, cm/year | |||
| Week 13 | 4.99 (3.92, 6.07) | 4.62 (3.53, 5.71) | 4.12 (3, 5.24) |
| Week 26 | 5.08 (4.35, 5.81) | 3.65 (2.91, 4.39) | 4.38 (3.63, 5.14) |
| ΔHeight SDS for CA | |||
| Week 26 | 0.65 (0.57, 0.74) | 0.49 (0.4, 0.58) | 0.58 (0.5, 0.67) |
| ΔHeight SDS for BA | |||
| Week 26 | 0.51 (0.32, 0.69) | 0.45 (0.27, 0.64) | 0.32 (0.14, 0.5) |
| ΔBA, years | |||
| Week 26 | 0.62 (0.48, 0.76) | 0.55 (0.42, 0.69) | 0.73 (0.6, 0.87) |
| ΔBA/ΔCA | |||
| Week 26 | 0.02 (0, 0.04) | 0 (−0.02, 0.02) | 0.03 (0.01, 0.05) |
| ΔIGF-I, ng/mL | |||
| Week 13 | 73.6 (40.43, 106.76) | 24.89 (−7.92, 57.7) | 50.79 (19.17, 82.4) |
| Week 26 | 92.3 (40.06, 144.55) | 28.3 (−23.39, 79.99) | 73.8 (23.99, 123.6) |
| ΔIGF-I SDS | |||
| Week 13 | 0.95 (0.43, 1.46) | 0.35 (−0.16, 0.87) | 0.87 (0.35, 1.4) |
| Week 26 | 1.3 (0.52, 2.08) | 0.41 (−0.36, 1.19) | 1.12 (0.33, 1.91) |
| ΔIGFBP-3, ng/mL | |||
| Week 13 | 681.03 (324.66, 1037.41) | 149.87 (−214.75, 514.49) | 379.84 (26.14, 733.53) |
| Week 26 | 806.84 (453.95, 1159.73) | 276.97 (−84.09, 638.03) | 622.96 (272.72, 973.2) |
| ΔIGFBP-3 SDS | |||
| Week 13 | 1.34 (0.55, 2.13) | 0.32 (−0.49, 1.12) | 0.87 (0.08, 1.65) |
| Week 26 | 1.69 (0.93, 2.46) | 0.62 (−0.15, 1.4) | 1.31 (0.55, 2.06) |
Data are changes from baseline at each time point, presented as LS mean (90% CI).
PK/PD Results
Baseline blood concentrations of hGH in both LB03002 groups were similar, with individual values ranging from 0.05 to 18.5 ng/mL. When blood samples were collected at 36 h after the 26th dosing, both groups showed a significant increase in the hGH level. This parameter increased in a dose-dependent manner, with a median (min, max) hGH level (ng/mL) equal to 15.15 (1.16, 38.8) and 40.55 (7.42, 83) for the 0.5 mg/kg/week and 0.7 mg/kg/week group, respectively (p < 0.001). At 7 days after the 26th dosing, blood concentrations of hGH in nearly half of the patients were lower than 5 ng/mL, and no significant between-group differences were found in hGH levels (p = 0.467). Also, IGF-I and IGFBP-3 concentrations at 36 h after the 26th dosing were significantly higher than at the baseline (p < 0.001), without statistically significant between-group differences in either parameter (see online suppl. Table 2).
Safety Results
During the 30-week study period, 7 (44%), 9 (64%), and 10 (63%) patients from the control, LB03002 0.5 mg/kg/week, and LB03002 0.7 mg/kg/week groups experienced 12, 28, and 23 adverse events, respectively (Table 3). The adverse events were mostly mild except 1 severe and 3 moderate events reported in the LB03002 0.5 mg/kg/week group. Eight adverse events were related with the rhGH treatment, mostly with LB03002 0.5 mg/kg/week (n = 7) and LB03002 0.7 mg/kg/week (n = 1): injection site pruritus (n = 1), acute tonsillitis (n = 1), bronchitis (n = 2), acute otitis media (n = 1), upper respiratory tract infection (n = 1), atopic dermatitis (n = 1), and rash (n = 1) as preferred terms. Two serious adverse events (meningitis and thermal burn) were reported with LB03002 0.5 mg/kg/week; however, neither had any causal relationship with the treatment.
Table 3.
List of adverse events
| Preferred term | Daily rhGH 0.37 mg/kg/week (n = 16) | LB03002 0.5 mg/kg/week (n = 14) | LB03002 0.7 mg/kg/week (n = 16) |
|---|---|---|---|
| Ear pain | 0 (0) | 1 (1) | 0 (0) |
| Dyspepsia | 0 (0) | 0 (0) | 1 (1) |
| Enteritis | 0 (0) | 1 (1) | 2 (2) |
| Vomiting | 0 (0) | 1 (1) | 0 (0) |
| Injection site pruritus | 0 (0) | 1 (1) | 1 (1) |
| Acute tonsillitis | 0 (0) | 1 (1) | 1 (2) |
| Bronchitis | 0 (0) | 2 (4) | 0 (0) |
| Folliculitis | 0 (0) | 1 (1) | 0 (0) |
| Furuncle | 1 (1) | 0 (0) | 0 (0) |
| Hordeolum | 1 (1) | 0 (0) | 1 (1) |
| Laryngitis | 1 (1) | 0 (0) | 0 (0) |
| Meningitis | 0 (0) | 1 (1)a | 0 (0) |
| Nasopharyngitis | 3 (3) | 1 (2) | 2 (5) |
| Otitis externa | 0 (0) | 0 (0) | 1 (1) |
| Acute otitis media | 0 (0) | 1 (1) | 0 (0) |
| Pharyngitis | 1 (1) | 2 (2) | 0 (0) |
| Tonsillitis | 1 (1) | 1 (1) | 0 (0) |
| Tracheitis | 0 (0) | 0 (0) | 1 (1) |
| Upper respiratory tract infection | 1 (1) | 3 (6) | 1 (2) |
| Varicella | 0 (0) | 0 (0) | 1 (1) |
| Foot fracture | 1 (1) | 0 (0) | 0 (0) |
| Laceration | 0 (0) | 1 (1)a | 0 (0) |
| Thermal burn | 0 (0) | 1 (1)b | 0 (0) |
| Arthralgia | 0 (0) | 0 (0) | 1 (1) |
| Headache | 0 (0) | 0 (0) | 1 (4) |
| Balanoposthitis | 0 (0) | 1 (1) | 0 (0) |
| Allergic rhinitis | 1 (1) | 0 (0) | 0 (0) |
| Atopic dermatitis | 0 (0) | 1 (1)a | 1 (1) |
| Rash | 1 (1) | 1 (1) | 0 (0) |
Numbers before parentheses represent the number of patients experiencing each adverse event, whereas those in the parentheses represent the total number of each event.
Moderate in intensity.
Severe in intensity; the rest of the events were mild in intensity.
Regarding injection site reactions (warmth, erythema, and swelling), swelling occurred most frequently, and the patients receiving LB03002 experienced local reactions more frequently than those administered daily rhGH (Table 4). However, the intensity was generally tolerable, and the reactions were mostly resolved within 6 days (Table 4). The severity of pain, marked on the 150-mm visual analogue scale (VAS), did not exceed half of the VAS length and showed a decreasing tendency with repeated injections.
Table 4.
Number of patients with injection site reactions by maximal intensity
| Daily rhGH 0.37 mg/kg/week (n = 16) | LB03002 0.5 mg/kg/week (n = 14) | LB03002 0.7 mg/kg/week (n = 16) | |
|---|---|---|---|
| Maximum intensity | |||
| Warmth | |||
| None | 12 | 5 | 5 |
| Mild | 4 | 8 | 9 |
| Moderate | 0 | 1 | 2 |
| Erythema | |||
| None | 12 | 2 | 1 |
| Mild (<5 mm) | 3 | 5 | 4 |
| Moderate (≥5 mm, <20 mm) | 0 | 5 | 7 |
| Severe (≥20 mm) | 1 | 2 | 4 |
| Swelling | |||
| None | 9 | 1 | 0 |
| Mild (<3 mm) | 6 | 2 | 4 |
| Moderate (≥3 mm, <20 mm) | 1 | 6 | 5 |
| Severe (≥20 mm) | 0 | 5 | 7 |
|
Duration (after 1st dosing) Warmth | |||
| 0~3 day | 2 | 4 | 4 |
| 4~6 day | 1 | 1 | 1 |
| Over 7 day | 0 | 0 | 0 |
| Erythema | |||
| 0~3 day | 1 | 5 | 6 |
| 4~6 day | 1 | 3 | 5 |
| Over 7 day | 1 | 0 | 0 |
| Swelling | |||
| 0~3 day | 1 | 5 | 10 |
| 4~6 day | 1 | 6 | 3 |
| Over 7 day | 0 | 0 | 0 |
The intensity of each reaction is based on the maximum intensity experienced by each patient anytime during the whole study period. The information about reaction duration is based on the diary records collected during the first week of dosing.
When looking at the individual subject's IGF-I SDS, 10 and 11 subjects in the LB03002 0.5 mg/kg/week and LB03002 0.7 mg/kg/week group, respectively, had IGF-I greater than 2 SDS at 36 h after the last injection. However, the nonphysiological level of IGF-I was mostly normalized at 7 days after the last injection (see online suppl. Fig. 1). No laboratory abnormalities were assessed to be clinically relevant. None of the patients tested positive for anti-hGH antibodies at the baseline, whereas at week 26, anti-hGH antibodies were detected in 3 and 6 patients from the LB03002 0.5 mg/kg/week and 0.7 mg/kg/week group, respectively. The subgroup analysis demonstrated that the presence of anti-hGH antibodies did not affect the change in HV at week 26 or blood hGH concentration at 36 h after the 26th dosing (data not shown).
Discussion
In this open-label, randomized study targeting Korean children diagnosed with ISS, LB03002 0.7 mg/kg/week was noninferior to daily rhGH administered 6 times per week at the total dose of 0.37 mg/kg/week. Both products were well tolerated without significant adverse reactions.
Despite the use of thin needles and alternative delivery routes, daily administration of rhGH may be inconvenient. Owing to the long duration of the therapy, this may negatively affect patient's or caregiver's adherence to the treatment schedules. Indeed, one previous study demonstrated a significant difference in the mean treatment duration between good compliance and moderate-poor compliance groups (2.78 vs. 3.78 years, p = 0.001) [20]. Moreover, available evidence shows that poor adherence to the rhGH regimen is quite frequent and may negatively affect the treatment outcome [21-24]. Even though there is no evidence to prove a better adherence with long-acting rhGH than with daily rhGH, reducing the dosing frequency to once a week, theoretically, can be considered a benefit to patients and their caregivers, if proved to be at least as safe and effective as daily rhGH. Based on this premise, we conducted a randomized study in children with ISS to select the optimal dose of LB03002; the safety and efficacy of this agent, administered at 0.5 or 0.7 mg/kg/week, were compared with respective characteristics of daily rhGH given at a labeled dose for ISS patients (0.37 mg/kg/week). The first dosage regimen of LB03002 (0.5 mg/kg/week) is the recommended dose of this product for pediatric GHD patients. For the second regimen, a higher dosage (0.7 mg/kg/week) than the one approved for GHD was to be tested based on the evidence that ISS patients show worse growth response to rhGH therapy than patients with severe GHD [10]. High-dose rhGH therapy (0.7 mg/kg/week, daily) had previously been demonstrated to be effective and safe in GH-deficient adolescents who were most growth-retarded at the onset of puberty [25]. Finally, the PK/PD profiles of LB03002 administered at either 0.5 or 0.7 mg/kg/week were shown to be suitable for a long-term treatment of children with GHD [16, 26]. Although the PK/PD profiles of LB03002 in children with ISS were out of the scope of the present study, sparse data on the dynamics of hGH, IGF-I, and IGFBP-3 levels imply that they may be similar to those documented previously in GHD patients. The increase in hGH, IGF-I, and IGFBP-3 observed at 36 h after the last dose may imply that the patients were adequately exposed to LB03002, though a physiologically high level of IGF-I may be of concern. Further, the return of hGH, IGF-I, and IGFBP-3 to their baseline levels at 7 days after the last dose may suggest that repeated administration of LB03002 did not result in accumulation thereof.
In terms of the primary efficacy evaluation, administration of either LB03002 (irrespective of the dose) or the comparator resulted in a significant increase in HV in ISS patients (p < 0.0001). The changes in HV documented in all three groups (3.65–5.08 cm/year) were comparable with the assumed effect size (3.99 cm/year) and the 1-year change observed in other studies [8, 27, 28]. Also, the height SDS gain (LS mean ranging from 0.49 to 0.65) observed during the 26-week study period was within the expected range, owing a 0–0.7 SDS gain over 1 year documented in a systematic review analyzing the effects of rhGH on short-term growth in children with ISS [4]. However, LB03002, administered at 0.5 mg/kg/week, failed to prove noninferiority to the control in terms of the HV change. The first plausible explanation is that the LB03002 dose (0.5 mg/kg/week) might not have been high enough to improve the HV of children with ISS. In the previous phase III trial of Korean GHD patients [15], HV achieved after administration of LB03002 at 0.5 mg/kg/week was not inferior to that obtained with daily rhGH 0.21 mg/kg/week. This implies that a higher dose (0.7 mg/kg/week) of LB03002 should be considered for children with ISS. The other potential explanation is that the number of patients per group was too small to balance individual variability inherent to the heterogenous genetic makeup of the ISS population [29].
Overall, the adverse event rate was higher in the LB03002 groups than in the controls; however, the frequency of adverse drug reactions (ADRs) was low (4 patients in the LB03002 groups had a total of 8 ADRs), and most ADRs (7/8) were classified as mild. Also, injection site reactions were more common in the LB03002 groups, but usually resolved within 3–6 days since the onset, and the injection site pain caused by LB03002 waned within 2–3 days. The severity of pain in all three groups tended to decrease with repeated dosing, which implies that the patients tolerated well either the daily or the weekly regimen. Moreover, high treatment compliance (≥80%) across all three groups suggests that the intensity of local reactions, among them pain, was not clinically significant and did not prevent the patients from following the treatment regimen. Therefore, the safety profiles of LB03002 at both doses administered to children with ISS for 26 weeks were assessed to be acceptable and comparable with that of the control. Meanwhile, favorable long-term (2–3 years) safety data of LB03002 can be referenced both from the phase II/III [26] and phase III [16] studies conducted in GHD children.
Some cautions should be taken while interpreting the results of this study. First, the annualized HV data were based on the height measurements taken at 6-month intervals. Considering the potential seasonal variance of individual growth, the 1-year period would be ideal; however, 6 months were considered to be a minimum necessary period for this type of study. Second, the safety profile of LB03002 in ISS patients was determined on the basis of a relatively short follow-up; thus, the safety of this agent needs to be verified in a longer time perspective, considering that rhGH treatment is typically administered for several years. Third, it should be remembered that diagnostic criteria for ISS vary from country to country; some authors recommend more stringent cutoff values for height SDS than those applied in this study (height SDS < −2.25 or even < −3.0) [30]. Finally, prescription of rhGH to otherwise healthy children, such as ISS patients, should be preceded by a systematic and critical evaluation of multiple factors, e.g., social well-being, functional impact, and economic burden, rather than solely a height gain [31, 32]. All these factors need to be considered in future studies of LB03002.
In conclusion, the once-weekly regimen of LB03002 0.7 mg/kg demonstrated noninferiority to the daily-divided regimen of rhGH 0.37 mg/kg/week in terms of HV increments. A 26-week treatment contributed to a significant increase in auxological variables (HV, height gain, and height SDS) and concomitant changes in PD markers. Both doses of LB03002 were well tolerated and their safety profiles were comparable with that of daily rhGH. A confirmatory efficacy study needs to be undertaken with LB03002 0.7 mg/kg/week in children with ISS.
Disclosure Statement
This study was sponsored by LG Life Sciences, Ltd., which is now merged with LG Chem, Ltd., Korea. S.H. is employed by LG Chem, Ltd. J.S.H., K.-H.L., H.-W.Y., B.-K.S., C.H.S., H.-S.H., H.-S.K., and C.H.S. are the Steering Committee members of LGS (LG Growth Study). Otherwise, the authors have no competing interest to declare that could be perceived as prejudicing the impartiality of the research reported.
Supplementary Material
Supplementary data
Acknowledgement
We thank the patients and their caregivers; Suin Lee of LG Chem, Ltd., for her support in clinical operations; and Hye-Ryon Kim and Szymon Brużewicz (SciencePro) for editorial assistance in the preparation of the manuscript.
References
- 1.Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah Growth Study: growth standards and the prevalence of growth hormone deficiency. J Pediatr. 1994 Jul;125((1)):29–35. doi: 10.1016/s0022-3476(94)70117-2. [DOI] [PubMed] [Google Scholar]
- 2.Voss LD, Mulligan J, Betts PR, Wilkin TJ. Poor growth in school entrants as an index of organic disease: the Wessex growth study. BMJ. 1992 Dec;305((6866)):1400–2. doi: 10.1136/bmj.305.6866.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wit JM, Clayton PE, Rogol AD, Savage MO, Saenger PH, Cohen P. Idiopathic short stature: definition, epidemiology, and diagnostic evaluation. Growth Horm IGF Res. 2008 Apr;18((2)):89–110. doi: 10.1016/j.ghir.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Bryant J, Baxter L, Cave CB, Milne R. Recombinant growth hormone for idiopathic short stature in children and adolescents. Cochrane Database Syst Rev. 2007 Jul;((3)):CD004440. doi: 10.1002/14651858.CD004440.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Ranke MB. Towards a consensus on the definition of idiopathic short stature. Horm Res. 1996;45(Suppl 2):64–6. doi: 10.1159/000184851. [DOI] [PubMed] [Google Scholar]
- 6.Şıklar Z, Kocaay P, Çamtosun E, İsakoca M, Hacıhamdioğlu B, Savaş Erdeve Ş, et al. The Effect of Recombinant Growth Hormone Treatment in Children with Idiopathic Short Stature and Low Insulin-Like Growth Factor-1 Levels. J Clin Res Pediatr Endocrinol. 2015 Dec;7((4)):301–6. doi: 10.4274/jcrpe.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leschek EW, Rose SR, Yanovski JA, Troendle JF, Quigley CA, Chipman JJ, et al. National Institute of Child Health and Human Development-Eli Lilly & Co. Growth Hormone Collaborative Group Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004 Jul;89((7)):3140–8. doi: 10.1210/jc.2003-031457. [DOI] [PubMed] [Google Scholar]
- 8.Kemp SF, Kuntze J, Attie KM, Maneatis T, Butler S, Frane J, et al. Efficacy and safety results of long-term growth hormone treatment of idiopathic short stature. J Clin Endocrinol Metab. 2005 Sep;90((9)):5247–53. doi: 10.1210/jc.2004-2513. [DOI] [PubMed] [Google Scholar]
- 9.Cohen P, Rogol AD, Weng W, Kappelgaard AM, Rosenfeld RG, Germak J, American Norditropin Study Group Efficacy of IGF-based growth hormone (GH) dosing in nonGH-deficient (nonGHD) short stature children with low IGF-I is not related to basal IGF-I levels. Clin Endocrinol (Oxf) 2013 Mar;78((3)):405–14. doi: 10.1111/cen.12014. [DOI] [PubMed] [Google Scholar]
- 10.Cohen P, Germak J, Rogol AD, Weng W, Kappelgaard AM, Rosenfeld RG, American Norditropin Study Group Variable degree of growth hormone (GH) and insulin-like growth factor (IGF) sensitivity in children with idiopathic short stature compared with GH-deficient patients: evidence from an IGF-based dosing study of short children. J Clin Endocrinol Metab. 2010 May;95((5)):2089–98. doi: 10.1210/jc.2009-2139. [DOI] [PubMed] [Google Scholar]
- 11.Albertsson-Wikland K, Aronson AS, Gustafsson J, Hagenäs L, Ivarsson SA, Jonsson B, et al. Dose-dependent effect of growth hormone on final height in children with short stature without growth hormone deficiency. J Clin Endocrinol Metab. 2008 Nov;93((11)):4342–50. doi: 10.1210/jc.2008-0707. [DOI] [PubMed] [Google Scholar]
- 12.Saenger PH, Mejia-Corletto J. Long-Acting Growth Hormone: an Update. Endocr Dev. 2016;30:79–97. doi: 10.1159/000439333. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Kim CW. Development and Characterization of Sodium Hyaluronate Microparticle-Based Sustained Release Formulation of Recombinant Human Growth Hormone Prepared by Spray-Drying. J Pharm Sci. 2016 Feb;105((2)):613–22. doi: 10.1016/j.xphs.2015.11.046. [DOI] [PubMed] [Google Scholar]
- 14.Biller BM, Ji HJ, Ahn H, Savoy C, Siepl EC, Popovic V, et al. Effects of once-weekly sustained-release growth hormone: a double-blind, placebo-controlled study in adult growth hormone deficiency. J Clin Endocrinol Metab. 2011 Jun;96((6)):1718–26. doi: 10.1210/jc.2010-2819. [DOI] [PubMed] [Google Scholar]
- 15.Hwang JS, Lee HS, Chung WY, Han HS, Jin DK, Kim HS, et al. Efficacy and safety of LB03002, a once-weekly sustained-release human GH for 12-month treatment in Korean children with GH deficiency. Eur J Endocrinol. 2013 Jul;169((2)):179–85. doi: 10.1530/EJE-13-0148. [DOI] [PubMed] [Google Scholar]
- 16.Khadilkar V, Radjuk KA, Bolshova E, Khadgawat R, El Kholy M, Desai M, et al. 24-month use of once-weekly GH, LB03002, in prepubertal children with GH deficiency. J Clin Endocrinol Metab. 2014 Jan;99((1)):126–32. doi: 10.1210/jc.2013-2502. [DOI] [PubMed] [Google Scholar]
- 17.Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, et al. 2007 ISS Consensus Workshop participants Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008 Nov;93((11)):4210–7. doi: 10.1210/jc.2008-0509. [DOI] [PubMed] [Google Scholar]
- 18.Korea Center for Disease Control and Prevention The Korean Pediatric Society, The Committee for the Development of Growth Standard for Korean Children and Adolescents: Korean children and adolescents growth standard (commentary for the development of 2007 growth chart) [Government report online] http://www.cdc.go.kr/CDC/cms/cmsFileDownload.jsp?fid=28&cid=1235&fieldName=attachGrp&index=4 (accessed 8 Apr 2015)
- 19.Hyun SE, Lee BC, Suh BK, Chung SC, Ko CW, Kim HS, et al. Reference values for serum levels of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in Korean children and adolescents. Clin Biochem. 2012 Jan;45((1-2)):16–21. doi: 10.1016/j.clinbiochem.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 20.De Pedro S, Murillo M, Salinas I, Granada ML, Martinez M, Puig-Domingo M, et al. Variability in adherence to rhGH treatment: socioeconomic causes and effect on children's growth. Growth Horm IGF Res. 2016 Feb;26:32–5. doi: 10.1016/j.ghir.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Lanes R. Long-term outcome of growth hormone therapy in children and adolescents. Treat Endocrinol. 2004;3((1)):53–66. doi: 10.2165/00024677-200403010-00006. [DOI] [PubMed] [Google Scholar]
- 22.Cutfield WS, Derraik JG, Gunn AJ, Reid K, Delany T, Robinson E, et al. Non-compliance with growth hormone treatment in children is common and impairs linear growth. PLoS One. 2011 Jan;6((1)):e16223. doi: 10.1371/journal.pone.0016223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapoor RR, Burke SA, Sparrow SE, Hughes IA, Dunger DB, Ong KK, et al. Monitoring of concordance in growth hormone therapy. Arch Dis Child. 2008 Feb;93((2)):147–8. doi: 10.1136/adc.2006.114249. [DOI] [PubMed] [Google Scholar]
- 24.Desrosiers P, O'Brien F, Blethen S. Patient outcomes in the GHMonitor: the effect of delivery device on compliance and growth. Pediatr Endocrinol Rev. 2005 Feb;2(Suppl 3):327–31. [PubMed] [Google Scholar]
- 25.Mauras N, Attie KM, Reiter EO, Saenger P, Baptista J. High dose recombinant human growth hormone (GH) treatment of GH-deficient patients in puberty increases near-final height: a randomized, multicenter trial. Genentech, Inc., Cooperative Study Group. J Clin Endocrinol Metab. 2000 Oct;85((10)):3653–60. doi: 10.1210/jcem.85.10.6906. [DOI] [PubMed] [Google Scholar]
- 26.Péter F, Bidlingmaier M, Savoy C, Ji HJ, Saenger PH. Three-year efficacy and safety of LB03002, a once-weekly sustained-release growth hormone (GH) preparation, in prepubertal children with GH deficiency (GHD) J Clin Endocrinol Metab. 2012 Feb;97((2)):400–7. doi: 10.1210/jc.2011-2234. [DOI] [PubMed] [Google Scholar]
- 27.Genentech Collaborative Study Group Idiopathic short stature: results of a one-year controlled study of human growth hormone treatment. J Pediatr. 1989 Nov;115((5 Pt 1)):713–9. doi: 10.1016/s0022-3476(89)80647-x. [DOI] [PubMed] [Google Scholar]
- 28.Soliman AT, abdul Khadir MM. Growth parameters and predictors of growth in short children with and without growth hormone (GH) deficiency treated with human GH: a randomized controlled study. J Trop Pediatr. 1996 Oct;42((5)):281–6. doi: 10.1093/tropej/42.5.281. [DOI] [PubMed] [Google Scholar]
- 29.Dauber A, Rosenfeld RG, Hirschhorn JN. Genetic evaluation of short stature. J Clin Endocrinol Metab. 2014 Sep;99((9)):3080–92. doi: 10.1210/jc.2014-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, et al. Drug and Therapeutics Committee and Ethics Committee of the Pediatric Endocrine Society Guidelines for Growth Hormone and Insulin-Like Growth Factor-I Treatment in Children and Adolescents: Growth Hormone Deficiency, Idiopathic Short Stature, and Primary Insulin-Like Growth Factor-I Deficiency. Horm Res Paediatr. 2016;86((6)):361–97. doi: 10.1159/000452150. [DOI] [PubMed] [Google Scholar]
- 31.Allen DB. Growth Promotion Ethics and the Challenge to Resist Cosmetic Endocrinology. Horm Res Paediatr. 2017;87((3)):145–52. doi: 10.1159/000458526. [DOI] [PubMed] [Google Scholar]
- 32.Arnhold IJ, Sandberg DE. Additional Considerations to the Ethics of Growth Promotion and Challenges to Human Growth Hormone (hGH)-for-Height Therapy. Horm Res Paediatr. 2017;88((3-4)):305–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data