Supplemental Digital Content is Available in the Text.
Keywords: Pediatric pain, Quality improvement, Lean, Needle pain, Vaccination, Procedural pain, Sucrose, Breastfeeding, Topical anesthesia, Lidocaine 4% cream, Positioning, Distraction
Abstract
Introduction:
Pain remains common, underrecognized, and undertreated in children's hospitals and pediatric clinics. Over 200,000 patients experience needle pain annually in our institution, caused by blood draws, intravenous access, vaccinations, and injections on all inpatient units, emergency departments, outpatient laboratories, and ambulatory clinics.
Objectives:
We implemented a hospital-based, system-wide initiative called the “Children's Comfort Promise,” and created a new standard of care for needle procedures that required staff to consistently offer 4 strategies: (1) topical anesthetics, (2) sucrose or breastfeeding for infants 0 to 12 months, (3) comfort positioning (including swaddling, skin-to-skin, or facilitated tucking for infants; sitting upright for children), and (4) age-appropriate distraction.
Methods:
The protocol was established system-wide in one of the largest children's hospitals in the United States using a staggered implementation approach over a 3-year period to allow for unit-specific customization and facilitation of knowledge transfer from one unit to another. All departments were required to offer all 4 strategies with appropriate education at least 95% of the time.
Results:
Comparison of baseline audits with continuous postimplementation audits revealed that wait times for services decreased, patient satisfaction increased, and staff concerns about implementation were allayed (eg, concerns about wait times and success rates of venipuncture after topical anesthesia).
Conclusion:
This is the first report of a successful system-wide protocol implementation to reduce or eliminate needle pain, including pain from vaccinations, in a children's hospital across all inpatient units, emergency departments, outpatient laboratories, and ambulatory clinics through consistent use of topical anesthesia, sucrose/breastfeeding, positioning, and distraction.
1. Introduction
According to the 2010 Declaration of Montreal, access to pain management is a fundamental human right and it is a human rights violation not to treat pain.30 Data from children's hospitals in North America and Europe reveal that pain is common, underrecognized, and undertreated.5,19,52,55,65,68,73 Needle procedures performed in childhood are a substantial source of distress. Vaccinations are the most commonly performed needle procedure in children, and pain is a common reason for vaccine hesitancy.15,35,59 It is estimated that up to 25% of adults have a fear of needles, with most fears developing in childhood.24,26 Untreated needle pain can have long-term consequences including needle phobia, preprocedural anxiety, hyperalgesia, and avoidance of health care, resulting in increased morbidity and mortality.57,58
The 2016 American Academy of Pediatrics guidelines for procedural pain in neonates concluded that newborns, especially premature infants, experience unnecessary pain during routine procedures.74 Infants admitted to neonatal intensive care units (NICUs) experienced more than ten painful procedures per day, of which the overwhelming majority were performed without analgesia.10,47 Critically ill infants may experience up to 480 painful procedures during their NICU stay.3,31 Exposure to severe pain without adequate pain management has negative long-term consequences, including increased morbidity (eg, intraventricular hemorrhage) and mortality.1,60 Exposure to pain in premature infants is associated with higher pain self-ratings during venipuncture by school age71 and poorer cognition and motor function.23
Children's Hospitals and Clinics of Minnesota is one of the largest freestanding pediatric health care systems in the United States, with 429 staffed beds on 2 campuses (50% of which are neonatal beds), 5 intensive care units, 2 emergency departments (EDs), and 26 primary and specialty clinics, providing care through more than 14,600 inpatient admissions, 25,800 surgical cases, 8700 home visits, and more than 96,400 ED and 447,000 clinic visits every year.2
Despite the evidence supporting pediatric pain management for needle procedures,9,29,57,62,64 few strategies were used and offered for children in daily practice as identified through patient, family, and staff surveys. In 2013, a prospective, single-day, cross-sectional survey and electronic medical record review of all inpatients who received medical care at our institution were conducted to estimate how well we were managing pain.19 The survey revealed that the single greatest source of pain and anxiety for our patients and families was needle procedures, such as blood draws, intravenous access, and injections. Staff surveys conducted that same year gave low priority to pain experienced during needle procedures, demonstrating a significant institution-wide gap in practice.
This report describes the implementation of a system-wide, multiyear quality improvement (QI) process to reduce needle pain using Lean methodology.21,46 The process is entitled “Children's Comfort Promise: We will do everything possible to prevent and treat pain.”14
2. Methods
2.1. Early institutional planning
To assess the current state of staff attitudes and beliefs about organizational pain management at Children's Minnesota, an online survey was administered in 2013. Results (unpublished) revealed that most responding clinical staff members were “completely satisfied” with our pain management efforts at the time, and either felt needles “were not very painful,” or felt the best we could do was “to be fast and accurate.” However, a cross-sectional patient survey conducted the same year revealed that the worst pain reported by patients was caused by needle procedures,19 highlighting the gap between staff perceptions and patient experience. Findings from the 2 surveys were presented to hospital leadership, and addressing needle pain was found to be aligned with the institution's strategic goal of improving patient experiences. Hospital leadership agreed that needle pain practices were unacceptable, and pledged commitment to support an organization-wide initiative to improve pain management practices.
Children's Minnesota used a QI approach based on The Toyota Production System (also known as Lean), with a dedicated Lean department already in existence at the time. Lean improvement systems focus on removing “waste” from work processes through observation and analysis of the current state, including front-line staff, to design a future state that effectively meets the needs of the customer.21,44,75 If waste is defined as anything that the “customer” (ie, patients, families, and clinical staff) does not value, it can be reasonably assumed that pain itself is a form of waste. This was the underlying rationale for using the organization's existing Lean-driven improvement strategy to improve pain management with respect to needle procedures. This project was declared as QI by Children's Institutional Review Board.
When a QI effort spans multiple areas of authority (eg, units, departments) and requires extensive coordination, a Lean methodology structure known as a “Value Stream” is frequently used. A value stream is a method for analyzing the current state and designing a future state that takes a service from its beginning (eg, clinician order of blood draw in an ambulatory clinic) through to the customer (eg, a child undergoes blood draw in outpatient laboratory). In this case, the “No Needless Pain Value Stream” was chartered, which involved identification of multidisciplinary core team members, leadership sponsors, scope, objectives, and metrics. A nurse (D.E.) was assigned as the Value Stream manager, supported by a Lean coach (C.W.) and physician sponsor (S.J.F.).21,75 Seeking strategic support from leadership early on (combining top-down and bottom-up approaches)28 was a key strategy, resulting in executive sponsorship from the Chief Medical Officer and the Chief Nursing Officer. The Lean Value Stream was then branded as the “Children's Comfort Promise.”14 The objective of this work, as stated in the charter, was to: “Design, test, and deploy the clinical practices and foster the culture required to eliminate all needless pain, and to minimize all moderate and severe physical pain and distress associated with, anticipated, or experienced by patients and their families through the continuum of care.”18 The first priority of this multiyear effort was to reduce or eliminate needle pain using evidence-based best practice.
2.2. The 4 evidence-based best practices for reducing needle pain in children
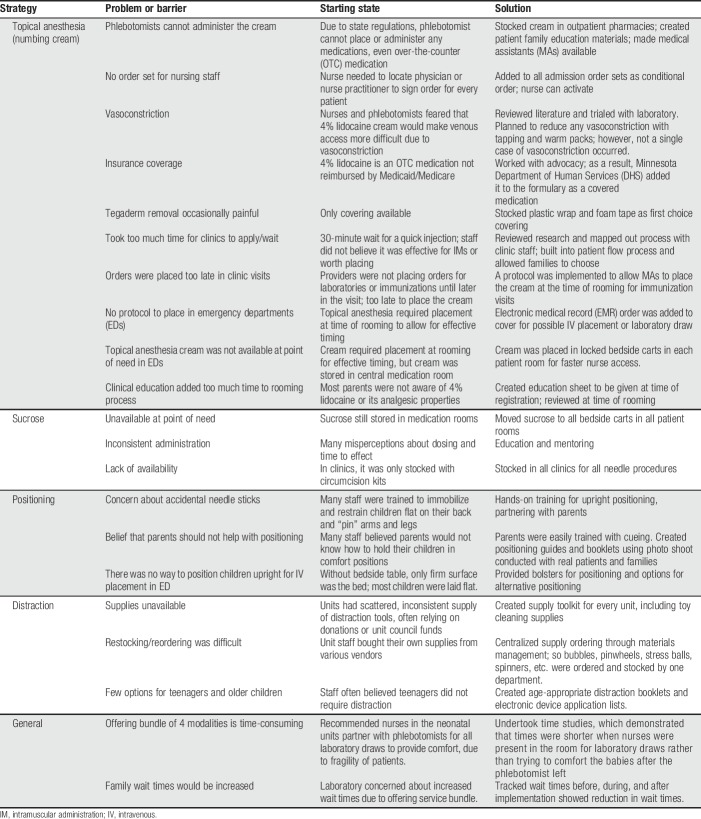
Current evidence,57,62,64 supported by guidelines from the Canadian Paediatric Society,9,29 HELPinKids,27,41,42,61 and recently brought forward by science-to-social media campaigns (“Be Sweet to Baby”12 and “It Doesn't Have to Hurt”11), strongly suggests that 4 bundled modalities should be offered for elective needle procedures to reduce or eliminate pain experienced by children. After reviewing the evidence, the core Lean Value Stream team decided to require staff to consistently offer all 4 strategies institution-wide. As directed by Lean methodology, members of the core team performed initial observations to anticipate potential barriers to offering the strategies, and brainstorm possible solutions (Table 1). Children and their parents/legal guardians had the option of declining any or all strategies, which included the following14:
(1) “Numb the skin” (for children of 36-week corrected gestational age and older). We chose to use 4% lidocaine cream63 or needle-less lidocaine application using a J-tip (sterile, single-use, disposable injector that uses pressurized gas to propel medication through the skin)38,39 as topical anesthetics.
(2) Sucrose20,54 or breastfeeding51 for infants 0 to 12 months.12
(3) Comfort positioning. Restraining children for procedures is never supportive, and creates a negative experience.33 For infants, we use swaddling, warmth, skin-to-skin contact, or facilitated tucking. For children 6 months and older, we offer sitting upright, with parents holding them on their laps or sitting nearby.
(4) Age-appropriate distraction,70 such as toys, books, blowing bubbles or pinwheels, stress balls, and using apps, videos, or games on electronic devices.
Table 1.
Examples of barriers to implementation of comfort promise strategies by strategy.
These 4 strategies were offered by front-line staff who were trained by the Value Stream manager and Lean coach. Training was accomplished using multiple modalities including a “train-the-trainer” approach, web-based training, educational videos and classes, lectures, and rounding. Strategies were presented as a bundle, with patients and families deciding what works best for them. A deferral process for children with severe needle phobia included referral to child life, psychology, and/or offering our nurse-administered nitrous gas (N2O) program to treat pain and anxiety caused by needles.17,37,77
2.3. Comfort promise implementation process
The first department to implement the new standard of care for needle procedures was the outpatient laboratory (2 locations) in early 2014, followed by 5 inpatient medical–surgical units, later that year. In 2015, both EDs, 4 neonatal areas (including 2 NICUs), 3 critical care units (2 Pediatric Intensive Care Units [PICUs], and a cardiovascular intensive care unit), 2 short-stay units, radiology, and the outpatient surgery program were implemented, and all 26 ambulatory (12 primary and 14 specialty) clinics completed implementation in 2016.
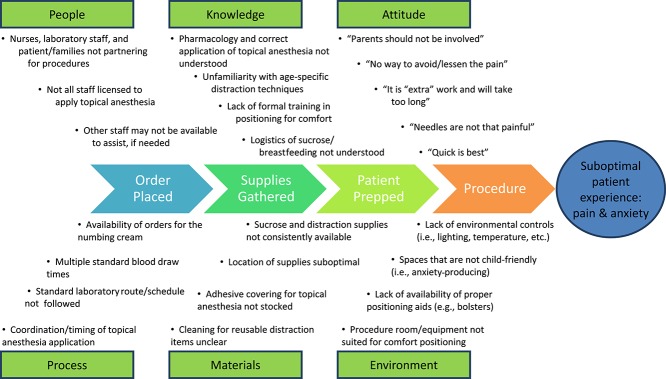
The laboratories were chosen as the pilot site because a small number of staff impact a large number of children, performing over 30,000 needle procedures per year. Lean improvement methodology prescribes direct observation of the process by front-line staff, in the normal environment. By watching the procedure repeatedly at both sites (“going to gemba”40) and hearing feedback from patients and families, the planning group identified both barriers and opportunities for improvement. See Figure 1 for the process map with initial gaps identified before implementing the Children's Comfort Promise for needle pain. Time and resource barriers were identified as primary concerns in the laboratories. To address these concerns, didactic and experiential skills-based education was offered to the laboratory staff. Resources were defined, space was revised, and a logistics plan (eg, having a standardized location for supply storage and access) was established. Ongoing mentoring and adjustments were made based on the Plan–Do–Study–Act (PDSA) model of QI, involving cycles of continuous process improvement repeated through planning (plan), implementation (do), observation (study), and making adjustments and fine-tuning the process (act) until it performs seamlessly within the established workflow and structure.36
Figure 1.
Fishbone Quality Improvement Process diagram.
After successful implementation in the laboratory setting, QI efforts were expanded to all areas of the organization over a period of 24 months using baseline audit reviews, observations, and findings from the pilot to guide the Lean process. All leadership sponsors and staff members were invited to informational meetings, where the team members from the unit presented their plan for implementing the new standard. Once implementation had occurred, the PDSA cycle was engaged using results from the process audits, led by core team members.
2.4. Process and outcome measures
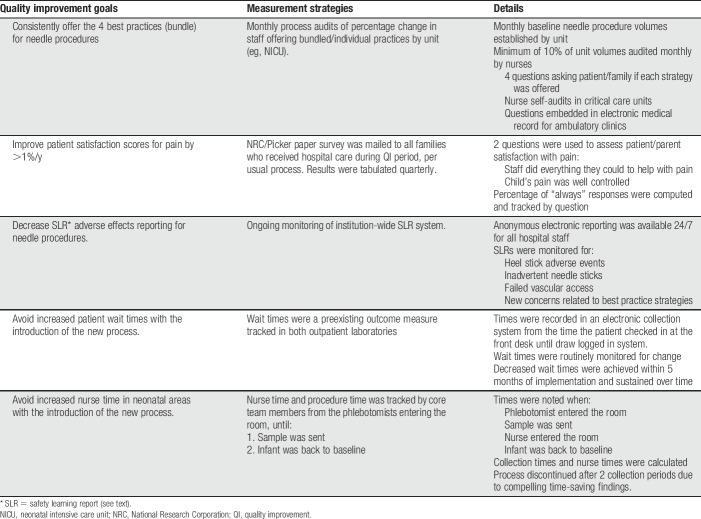
The process and outcome measures collected can be seen in Table 2 and included: patient and self-administered nurse audits to determine compliance with offering bundled services; 2 patient satisfaction questions administered through National Research Corporation (NRC)/Picker about patient/parent satisfaction with pain management to gauge improvement in patient pain experience as perceived by parents7,25,45; safety learning reports (SLRs) to measure any reduction in adverse effects; and patient wait times. Process measures were collected in all areas, tracking both baseline and postimplementation data for all 4 strategies, so that problems or barriers could be identified and resolved early on. Although it was necessary to use multiple methods to collect baseline and postimplementation data, care was taken to ensure that the data remained comparable through consistency of questions. An organization-wide target for offering the bundled services was set at 95%. The target was set high with the intent of establishing the protocol as the new standard of care for the organization.
Table 2.
Quality improvement measures and associated outcomes.
The frequency of needle procedures was gathered for each clinical area before QI implementation and minimum audit targets were set at 10% per month for process measure audits. Audit questions were administered to patients (if verbal and attending kindergarten or higher) or parents using a paper or verbal survey. Audits were brief and consisted of questions about: (1) the type of needle procedure they underwent, (2) whether or not the 4 strategies were offered, and (3) whether or not they found each of them helpful when used. Audits were scored as 100% only if all 4 strategies were offered or approved exceptions existed (eg, age criteria for sucrose).
Audits were performed by patient care managers or supervisors as part of their normal rounding each day, and interpreters were used for families who were not English-proficient. Attempts were made to visit all rooms, but interviews were only completed if patients had undergone a needle procedure in the past 24 hours. In the outpatient laboratory, a core team member performed audits 2 days per week, approaching all patients and families as they left the laboratory, until 20 surveys had been obtained per day. This audit method required modifications in the neonatal units and other critical care areas because many patients were nonverbal and parents were not present during procedures. On these units, nurses completed a written self-audit, recording type of procedure, which of the 4 strategies were offered, and noting reasons for any strategy not being offered. Approved exceptions were incorporated into the audits, including corrected gestational age and location of the needle stick. All units' audit compliance rates were monitored to ensure an adequate sampling on an ongoing basis, and results were entered and analyzed using Microsoft Excel software.
We tracked 2 NRC/Picker questions addressing patient/parent satisfaction with pain management during their encounter: How often did hospital staff do everything they could to help your child with his/her pain? and How often was your child's pain well controlled? (1 = Never to 4 = Always). We continued to track these scores throughout the Comfort Promise initiative, focusing on improvement in the percentage of patients and families who reported “Always” to both questions.
The electronic SLR system allows staff to report anonymously if any issues regarding patient care and/or safety were observed. For example, the neonatal areas had a number of SLRs each month around adverse events with heel sticks and hoped to decrease those events with the addition of improved comfort measures. Recorded time stamps were monitored at baseline and for several months after implementation in the outpatient laboratory to track wait time (noting time from patient check-in to sample logged). The NICUs also had concerns about nursing time, expressing concern about the time from initiation of the procedure (ie, laboratory staff scanning their ID band) to the baby returning to baseline (ie, not crying), and need for the nurse to be present throughout. Core team members conducted time studies in the laboratory and NICUs before and after implementation to address this concern.
2.5. Analysis plan
Microsoft Excel was used for all analyses. The percentage change in offering individual and bundled services (y/n) was computed using MS Excel as the percentage of all patients included in the patient or nurse self-administered audit periods who indicated “yes” to being offered/offering the bundled services. Monthly percentages were computed by unit for baseline, implementation, and maintenance periods. Electronic patient satisfaction scores were extracted from quarterly NRC/Picker reports for the 2 pain management satisfaction questions (see above). The percentage of patients and families who replied “always” to both questions (ie, they were very satisfied) was computed and tracked quarterly.
Electronic SLRs were reviewed on a monthly basis and the frequency and content of reports were reviewed by the Comfort Promise team quarterly. Patient wait times from check-in to laboratory draw were extracted from the laboratory collection system and descriptive statistics were run to determine the average wait times in minutes before and after Comfort Promise implementation. Nursing time in the NICU was recorded in Microsoft Excel by unit for 3 collection periods before implementation.
3. Results
The percentage of staff offering bundled services increased during the implementation period, patient and parent satisfaction with pain management improved quarterly per NRC/Picker scores, SLRs decreased, and patient wait times and nursing time decreased.
3.1. Percentage change in offering bundled services
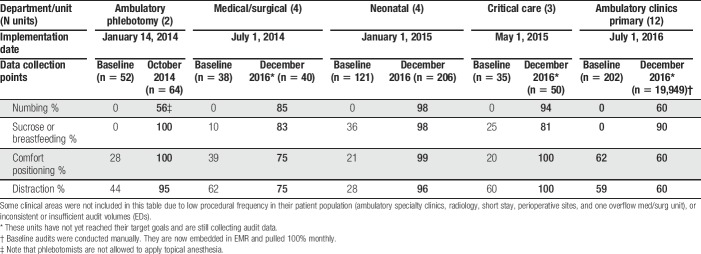
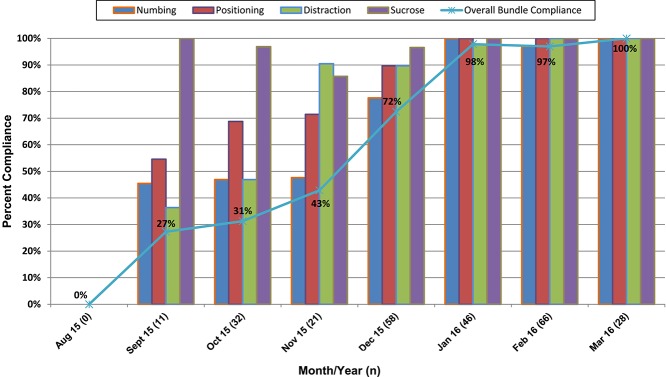
Table 3 shows how major areas of the hospital reported consistency in offering the Comfort Promise bundled services at baseline compared with current. All areas have shown improvements to date, and some areas are continuing to refine or maintain the processes that are unique to their areas (Table 3). Figure 2 shows an example of how the Minneapolis campus NICU, considered a representative neonatal unit, progressed over time in offering each of the 4 bundled services. Small gains in the initial 3 months were usual as complete dissemination of new practices to hundreds of nurses per unit took time. All units saw improvement in consistently offering all 4 strategies, with the greatest gains in administering topical anesthetics. Although baseline use of sucrose and positioning varied widely by unit, distraction techniques had been offered routinely by all units before the Value Stream, resulting in only small improvements for this modality.
Table 3.
Percentage of audits indicating best practices were offered by hospital unit, before and after comfort promise implementation.
Figure 2.
Neonatal intensive care unit compliance by bundle and each of the 4 best practices.
Initial results consistently demonstrated that patients who used the bundled strategies found them to be “very helpful”; so, this question was omitted from the surveys after the 1st year. In addition, very few patients declined any of the strategies in the inpatient setting, when education was given. Audits served a dual purpose, as education was provided in the moment if misperceptions were identified in the interview process or families had additional questions. In the outpatient setting, the numbing cream was the strategy declined most often, with the 30-minute wait time being cited as the main reason.
3.2. Patient satisfaction
National Research Corporation/Picker patient satisfaction scores45 increased from the point of implementation, and quarterly gains in satisfaction scores have been reported across all hospital units since inception of the Comfort Promise. Although scores historically show some variability from quarter to quarter (typically lower scores in third and fourth quarter), year over year comparisons by quarter saw consistent improvement. After a staggered system-wide rollout starting in 2014, the percentage of families surveyed who said hospital staff “always did everything they could to help with pain” increased from 78.3% to 85.3% (Fig. 3). Families who felt their “child's pain was always well controlled” rose from 59.6% to 72.1% (Fig. 4). Before implementation of the Comfort Promise, pain satisfaction scores had typically increased by 1% per year over the previous 10 years.
Figure 3.
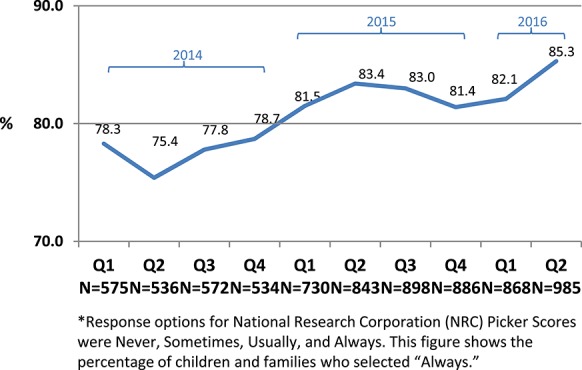
Percentage of families surveyed who said hospital staff always did everything they could to help with pain (2014–2016).
Figure 4.
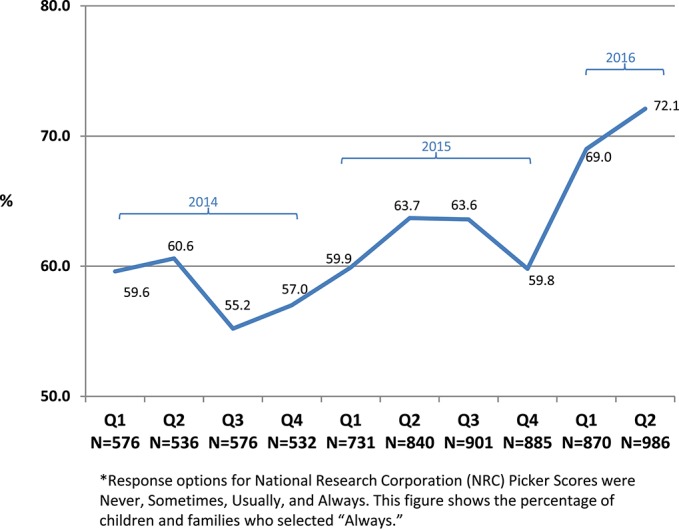
Percentage of families who felt their child's pain was always well controlled (2014–2016).
3.3. Safety learning reports and patient wait times
There was a significant reduction in electronic SLRs filed about heel sticks after implementation of the Children's Comfort Promise, procedure and nursing time decreased, and average patient wait times decreased. During observations, it was noted that nurses and phlebotomists rarely collaborated during laboratory draws, and babies experienced distress throughout the procedure as evidenced by crying, kicking, increased heart rate, oxygen desaturations, and heel bruising. Nurses raised concerns that our recommendations to be present for all laboratory draws to provide comfort to the infants during the procedure would be too time-consuming. However, time studies were undertaken and were discontinued after only 2 collection periods after implementation because there was compelling evidence that comforting babies after procedure was more time-consuming than time spent when involved in the procedure. Safety learning reports for complications with heel sticks decreased by 50% since implementation of the Comfort Promise measures, with most infants now sleeping through their laboratory draws. Time duration to undertake the needle procedures was not lengthened by implementation of the Comfort Promise. For instance, in the neonatal areas, nurses spent 40% less time in the room, and the total procedure time was decreased by 60%, whereas in the outpatient laboratory, wait and procedure times were reduced by 20% from 20 minutes to 16 minutes in 6 months and sustained over time.
4. Discussion
This is the first report of a successful system-wide implementation of a protocol to reduce or eliminate needle pain, including pain from vaccinations, in a children's hospital for all inpatient units, EDs, outpatient laboratories, and ambulatory clinics by offering a bundle of topical anesthesia, sucrose/breastfeeding, positioning, and distraction. An estimated 200,000 children now benefit annually from the Comfort Promise initiative to reduce and eliminate pain caused by elective blood draws, intravenous access, and injections. Wait times decreased and patient satisfaction increased between 2014 and 2016.
Organizational culture has been identified as key to changing pain management practices.6,8,32,67 Published studies indicate that making pain management an organizational priority can improve practices. Quality improvement pain studies to date are promising,49 although generally small scale with change not always being evaluated over a sustained period.16,36,43,53,69 This structured initiative was successful both due to staff and leadership support, which included a letter signed by the Children's Minnesota Chief Executive Officer, Chief Operating Officer, Chief Nursing Officer, and Chief Medical Officer stating that, as an institution, we will offer the bundled services, including topical anesthesia, to all patients and that we will not hold children down for elective needle procedures. The decision of the organization to supply 4% lidocaine in all service areas (removing the burden for families) was critical to the success of the process. This was achieved through early efforts to work with the Minnesota Department of Human Services to secure reimbursement for 4% lidocaine as an essential over-the-counter pain medication. Approval was secured in April 2014. Despite its availability, the 30-minute wait time for the cream was still a deterrent to its use but concerns were reduced after time studies that showed a decrease in wait times and time needed to address adverse reactions to blood draws. Work has been ongoing in the ambulatory setting to educate families about placing cream properly before their visit and to further improvements in workflow to accommodate early placement of the cream.
A framework for implementation was essential because education and policy alone are often insufficient.69 Supporting and encouraging multidisciplinary staff members who created processes and embraced the Comfort Promise was important, as was putting institutional resources behind the change initiative. This included mobilizing a full-time clinical resource team (nurse, child life, and lean staff member) supported by a physician champion. Because change happens through influence rather than by command,22 the main aim of the Comfort Promise team was to establish trust and ensure engagement of the front-line staff, to build a culture that would foster and sustain meaningful change across roles and responsibilities.72 Culture shift takes time and patience. Although over 75% of the children were offered or received the bundled services in nearly all areas within 2 months of rollout, it took 9 months for the first inpatient unit to consistently offer all 4 best practice strategies for 95% of needle procedures. This was a relatively short period, considering the fact that it took our institution 4 years to increase adherence to hand-washing policies from 50% to over 94%.
The rollout of the “Comfort Promise” was associated with increases in patient satisfaction (Figs. 3 and 4). Although we cannot demonstrate causation, there were no other pain-directed, system-wide initiatives implemented in the period. Reasons for a temporary decrease in Q4 2015 may include regression to the mean, expected statistical variation, or uncontrolled covariates (such as possible longer waits in the ED and/or decreased staff willingness to offer the bundled modalities during a busy winter 2015/2016 season).
Resistance toward implementation at the individual staff and unit level presented challenges during the rollout process. We found that the key to overcoming this resistance was providing necessary resources, support, and training to staff: “The new way had to be easier than the old way.” When we could demonstrate that wait times went down instead of up (as often anticipated by staff); that topical anesthetics did not decrease the chance of venous cannulation (there was not a single report of venous constriction impeding cannulation, confirming published data38,48); and—most importantly—that the 4 strategies provided an immediate benefit to patients (eg, fewer tears; more calm and cooperative children), the Comfort Promise was embraced by nearly all staff. The Lean Value Stream process involves regular process audits, implementation of knowledge translation strategies,76 development of educational and outreach materials (see supplemental figure for an example, available at http://links.lww.com/PR9/A24), and utilization of PDSA cycles. After implementing the Children's Comfort Promise for needles institution-wide, we tied leadership performance improvement bonuses to successful achievement of target goals. The new care standard was integrated into all organizational policies, the electronic medical record, and new staff orientation, making nonadherence a performance issue.
4.1. Barriers
Table 1 shows examples of implementation barriers that were experienced as they relate to each of the 4 best practices. Because the Comfort Promise was implemented in different locations at different time points, barriers were more likely to be averted in locations with later implementation through careful application of lessons learned. Acceptance of the numbing cream in ambulatory clinics by parents is an area of continued focus, with further education targeting correct application before the visit and additional workflow improvements made during the visit.
4.2. Limitations
One limitation during the rollout of the Lean Value Stream was the reliance on process audits with different collection methods. This created variability and challenges in obtaining data quickly enough to provide feedback to the staff and make process adjustments. Accuracy of self-audits by nursing staff was also a limiting factor, although efforts were made to verify those results through observations by core team members and spot audits from laboratory personnel, which were closely aligned. Recent changes to the electronic medical record allow clinicians performing the needle procedure to review monthly posted audit results, with the Comfort Promise core team continuing to monitor and perform PDSA cycles in clinical areas not achieving their goals. In addition, pain satisfaction scores do not necessarily correlate with effective pain management;34 however, they do correlate with perceived pain relief and participation in treatment.50
4.3. Conclusion and implications
Analgesic treatment is mandatory for children undergoing painful procedures, and avoidable suffering is unacceptable, even for the so-called minor interventions.4,13 Findings from this institution-wide QI project targeting pain associated with needle procedures, along with similar findings at other institutions, suggest that QI strategies coupled with knowledge translation strategies are key components of successful pediatric pain management strategies at the institutional level.56,76 The Children's Comfort Promise has become our institution's new standard of care for needle procedures. It has drawn institution-wide, interdisciplinary attention, resulting in increased awareness of the importance of optimal pain management for all patients and families. This in turn will be an important catalyst in the development and rollout of future local and national interventions aimed at minimizing various sources of pain.
The 4 bundled modalities for needle pain prevention and treatment (ie, consistent use of topical anesthesia, sucrose/breastfeeding, positioning, and distraction) are not a proprietary aspect of our “Children's Comfort Promise: We promise to do everything to prevent and treat pain,” and pediatric institutions are encouraged to replicate our process and use the bundled modalities as they see fit. Strategies from the current Comfort Promise are being taught and implemented in 2017 to 2018 at 4 children's hospitals in North America through a grant from The MAYDAY Fund, with the aim of developing and refining the process, tools, and educational materials needed to replicate these efforts throughout the pediatric community, and establishing a new standard of care for needle procedures.66
Disclosures
None of the authors have any financial or other conflicts of interest to report.
Acknowledgements
The authors thank all Children's Minnesota employees, the Children's Minnesota Youth Advisory Council, Family Advisory Council, members of the Comfort Promise Core Advisory Team (Maura Fitzgerald, Barbara Symalla, Jeri Kayser, Melissa Haun, Julie Yang, and Mary Hendricks), Alison Kolste for her thorough review of this manuscript, and our executive sponsors, Roxanne Fernandes and Phil Kibort. The authors appreciate their continued support, time, and dedication to making the Children's Comfort Promise initiative a sustainable success.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A24.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
References
- [1].Anand KJ, Barton BA, McIntosh N, Lagercrantz H, Pelausa E, Young TE, Vasa R. Analgesia and sedation in preterm neonates who require ventilatory support: results from the NOPAIN trial. Neonatal Outcome and Prolonged Analgesia in Neonates. Arch Pediatr Adolesc Med 1999;153:331–8. [DOI] [PubMed] [Google Scholar]
- [2].Annual report 2016 Children's Minnesota, 2016. Available at: https://www.childrensmn.org/downloads/2017/06/2016-childrens-mn-annual-report.pdf. Accessed July 1, 2018. [Google Scholar]
- [3].Barker DP, Rutter N. Exposure to invasive procedures in neonatal intensive care unit admissions. Arch Dis Child Fetal Neonatal Ed 1995;72:F47–F48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bellieni CV, Johnston CC. Analgesia, nil or placebo to babies, in trials that test new analgesic treatments for procedural pain. Acta Paediatr 2016;105:129–36. [DOI] [PubMed] [Google Scholar]
- [5].Birnie KA, Chambers CT, Fernandez CV, Forgeron PA, Latimer MA, McGrath PJ, Cummings EA, Finley GA. Hospitalized children continue to report undertreated and preventable pain. Pain Res Manag 2014;19:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Botti M, Bucknall T, Manias E. The problem of postoperative pain: issues for future research. Int J Nurs Pract 2004;10:257–63. [DOI] [PubMed] [Google Scholar]
- [7].Bovier PA, Charvet A, Cleopas A, Vogt N, Perneger TV. Self-reported management of pain in hospitalized patients: link between process and outcome. Am J Med 2004;117:569–74. [DOI] [PubMed] [Google Scholar]
- [8].Bucknall T, Manias E, Botti M. Acute pain management: implications of scientific evidence for nursing practice in the postoperative context. Int J Nurs Pract 2001;7:266–73. [DOI] [PubMed] [Google Scholar]
- [9].Canadian Paediatric Society. Reduce the pain of vaccination in babies. Ottowa, Canada: Canadian Paediatric Society, 2014. http://www.caringforkids.cps.ca/uploads/handout_images/3p_babiesto1yr_e.pdf. [Google Scholar]
- [10].Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, Saizou C, Lapillonne A, Granier M, Durand P, Lenclen R, Coursol A, Hubert P, de Saint Blanquat L, Boelle PY, Annequin D, Cimerman P, Anand KJ, Breart G. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA 2008;300:60–70. [DOI] [PubMed] [Google Scholar]
- [11].Centre for Pediatric Pain Research. It doesn't have to hurt. Halifax, Nova Scotia, Canada: Centre for Pediatric Pain Research, 2016. http://itdoesnthavetohurt.ca. [Google Scholar]
- [12].CHEO's Be Sweet to Babies Research Team and the University of Ottawa's School of Nursing. Be sweet to babies. Ottowa, Ontario, Canada: Children's Hospital of Eastern Ontario, 2014. http://www.cheo.on.ca/en/BeSweet2Babies. [Google Scholar]
- [13].Children's Healthcare Australasia. Children and young people's rights in healthcare services charter. Available at: http://www.awch.org.au/pdfs/Charter-Children-Young%20People-Healthcare-Au-version-FINAL-210911b-web.pdf. Accessed July 1, 2018.
- [14].Children's Hospitals and Clinics of Minnesota. The Comfort Promise. Minneapolis, MN:Children's Hospitals and Clinics of Minnesota, 2018. Available at: www.childrensMN.org/ComfortPromise. [Google Scholar]
- [15].Edwards KM, Hackell JM; Committee on Infectious Diseases, The Committee on Practice and Ambulatory Medicine. Countering vaccine hesitancy. Pediatrics 2016;138:e20162146. [DOI] [PubMed] [Google Scholar]
- [16].Ellis JA, McCleary L, Blouin R, Dube K, Rowley B, MacNeil M, Cooke C. Implementing best practice pain management in a pediatric hospital. J Spec Pediatr Nurs 2007;12:264–77. [DOI] [PubMed] [Google Scholar]
- [17].Friedrichsdorf SJ. Nitrous gas analgesia and sedation for lumbar punctures in children: has the time for practice change come? Pediatr Blood Cancer 2017;64:e26625. [DOI] [PubMed] [Google Scholar]
- [18].Friedrichsdorf SJ, Eull D, CA W. Children's Comfort Promise: how can we do everything possible to prevent and treat pain in children using quality improvement strategies? (Commentary). Pediatr Pain Lett 2016;18:26–30. [Google Scholar]
- [19].Friedrichsdorf SJ, Postier A, Eull D, Weidner C, Foster L, Gilbert M, Campbell F. Pain outcomes in a US Children's hospital: a prospective cross-sectional survey. Hosp Pediatr 2015;5:18–26. [DOI] [PubMed] [Google Scholar]
- [20].Gao H, Gao H, Xu G, Li M, Du S, Li F, Zhang H, Wang D. Efficacy and safety of repeated oral sucrose for repeated procedural pain in neonates: a systematic review. Int J Nurs Stud 2016;62:118–25. [DOI] [PubMed] [Google Scholar]
- [21].Graban M. Lean hospitals: improving quality, patient safety, and employee engagement. New York, NY: CRC Press, 2016. [Google Scholar]
- [22].Grenny J, Patterson K, Maxfield D, McMillan R, Switzler A. Influencer: the new science of leading change. New York, NY: McGraw-Hill Education Books, 2013. [Google Scholar]
- [23].Grunau RE, Whitfield MF, Petrie-Thomas J, Synnes AR, Cepeda IL, Keidar A, Rogers M, Mackay M, Hubber-Richard P, Johannesen D. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. PAIN 2009;143:138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Guideline statement: management of procedure-related pain in children and adolescents. J Paediatr Child Health 2006;42(suppl 1):S1–S29. [DOI] [PubMed] [Google Scholar]
- [25].Haller G, Agoritsas T, Luthy C, Piguet V, Griesser AC, Perneger T. Collaborative quality improvement to manage pain in acute care hospitals. Pain Med 2011;12:138–47. [DOI] [PubMed] [Google Scholar]
- [26].Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995;41:169–75. [PubMed] [Google Scholar]
- [27].Help Eliminate Pain in Kids & Adults, 2018. Available at: http://phm.utoronto.ca/helpinkids/index.html. Accessed July 1, 2018.
- [28].Hurst K. Top-down and bottom-up quality management [editorial]. Int J Health Care Qual Assur 2010;23:629–30. [DOI] [PubMed] [Google Scholar]
- [29].Immunize Canada. Reduce the pain of vaccination in kids and teens. Ottowa, Ontario, Canada: Immunize Canada, 2014. http://www.immunize.ca/uploads/pain/3p_kidsandteens_e.pdf. [Google Scholar]
- [30].International Association for the Study of Pain (IASP). Declaration of Montréal, 2010. Available at: http://www.iasp-pain.org/DeclarationofMontreal?navItemNumber=582. Accessed July 1, 2018.
- [31].Johnston CC, Collinge JM, Henderson SJ, Anand KJ. A cross-sectional survey of pain and pharmacological analgesia in Canadian neonatal intensive care units. Clin J Pain 1997;13:308–12. [DOI] [PubMed] [Google Scholar]
- [32].Jordan-Marsh M, Hubbard J, Watson R, Deon Hall R, Miller P, Mohan O. The social ecology of changing pain management: do I have to cry? J Pediatr Nurs 2004;19:193–203. [DOI] [PubMed] [Google Scholar]
- [33].Karlson K, Darcy L, Enskär K. The use of restraint is never supportive (poster). Nordic Society of Pediatric Hematology/Oncology (NOPHO) 34th Annual meeting 2016 and 11th Biannual Meeting of Nordic Society of Pediatric Oncology Nurses (NOBOS); May 27–31, 2016. Reykjavik, Iceland.
- [34].Kelly AM. Patient satisfaction with pain management does not correlate with initial or discharge VAS pain score, verbal pain rating at discharge, or change in VAS score in the Emergency Department. J Emerg Med 2000;19:113–16. [DOI] [PubMed] [Google Scholar]
- [35].Kennedy A, Basket M, Sheedy K. Vaccine attitudes, concerns, and information sources reported by parents of young children: results from the 2009 HealthStyles survey. Pediatrics 2011;127(suppl 1):S92–S99. [DOI] [PubMed] [Google Scholar]
- [36].Langley GL, Moen R, Nolan KM, Nolan TW, Norman CL, Provost LP. The improvement guide: a practical approach to enhancing organizational performance. San Francisco: Jossey-Bass Publishers, 2009. [Google Scholar]
- [37].Livingston M, Lawell M, McAllister N. Successful use of nitrous oxide during lumbar punctures: a call for nitrous oxide in pediatric oncology clinics. Pediatr Blood Cancer 2017;64:e26610. [DOI] [PubMed] [Google Scholar]
- [38].Lunoe MM, Drendel AL, Brousseau DC. The use of the needle-free jet injection system with buffered lidocaine device does not change intravenous placement success in children in the emergency department. Acad Emerg Med 2015;22:447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lunoe MM, Drendel AL, Levas MN, Weisman SJ, Dasgupta M, Hoffmann RG, Brousseau DC. A randomized clinical trial of jet-injected lidocaine to reduce venipuncture pain for young children. Ann Emerg Med 2015;66:466–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McClam Liebengood S, Cooper M, Nagy P. Going to the gemba: identifying opportunities for improvement in radiology. J Am Coll Radiol 2013;10:977–9. [DOI] [PubMed] [Google Scholar]
- [41].McMurtry CM, Pillai Riddell R, Taddio A, Racine N, Asmundson GJ, Noel M, Chambers CT, Shah V; HELPinKids&Adults Team. Far from “Just a Poke”: common painful needle procedures and the development of needle fear. Clin J Pain 2015;31(10 suppl):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].McMurtry CM, Taddio A, Noel M, Antony MM, Chambers CT, Asmundson GJ, Pillai Riddell R, Shah V, MacDonald NE, Rogers J, Bucci LM, Mousmanis P, Lang E, Halperin S, Bowles S, Halpert C, Ipp M, Rieder MJ, Robson K, Uleryk E, Votta Bleeker E, Dubey V, Hanrahan A, Lockett D, Scott J. Exposure-based interventions for the management of individuals with high levels of needle fear across the lifespan: a clinical practice guideline and call for further research. Cogn Behav Ther 2016;45:217–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Megens JH, Van Der Werff DB, Knape JT. Quality improvement: implementation of a pain management policy in a university pediatric hospital. Paediatr Anaesth 2008;18:620–7. [DOI] [PubMed] [Google Scholar]
- [44].Moraros J, Lemstra M, Nwankwo C. Lean interventions in healthcare: do they actually work? A systematic literature review. Int J Qual Health Care 2016;28:150–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].National Research Cooperation. Picker patient experience questionnaire. Lincoln, NE: NRC Health, 2016. http://www.nationalresearch.com. [Google Scholar]
- [46].Poksinska B. The current state of Lean implementation in health care: literature review. Qual Manag Health Care 2010;19:319–29. [DOI] [PubMed] [Google Scholar]
- [47].Roofthooft DW, Simons SH, Anand KJ, Tibboel D, van Dijk M. Eight years later, are we still hurting newborn infants? Neonatology 2014;105:218–26. [DOI] [PubMed] [Google Scholar]
- [48].Schreiber S, Ronfani L, Chiaffoni GP, Matarazzo L, Minute M, Panontin E, Poropat F, Germani C, Barbi E. Does EMLA cream application interfere with the success of venipuncture or venous cannulation? A prospective multicenter observational study. Eur J Pediatr 2013;172:265–8. [DOI] [PubMed] [Google Scholar]
- [49].Schurman JV, Deacy AD, Johnson RJ, Parker J, Williams K, Wallace D, Connelly M, Anson L, Mroczka K. Using quality improvement methods to increase use of pain prevention strategies for childhood vaccination. World J Clin Pediatr 2017;6:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schwenkglenks M, Gerbershagen HJ, Taylor RS, Pogatzki-Zahn E, Komann M, Rothaug J, Volk T, Yahiaoui-Doktor M, Zaslansky R, Brill S, Ullrich K, Gordon DB, Meissner W. Correlates of satisfaction with pain treatment in the acute postoperative period: results from the international PAIN OUT registry. PAIN 2014;155:1401–11. [DOI] [PubMed] [Google Scholar]
- [51].Shah PS, Herbozo C, Aliwalas LL, Shah VS. Breastfeeding or breast milk for procedural pain in neonates. Cochrane Database Syst Rev 2012;12:CD004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shomaker K, Dutton S, Mark M. Pain prevalence and treatment patterns in a US children's hospital. Hosp Pediatr 2015;5:363–70. [DOI] [PubMed] [Google Scholar]
- [53].Simons J, MacDonald LM. Changing practice: implementing validated paediatric pain assessment tools. J Child Health Care 2006;10:160–76. [DOI] [PubMed] [Google Scholar]
- [54].Stevens B, Yamada J, Ohlsson A, Haliburton S, Shorkey AS. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev 2016;7:CD001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Stevens BJ, Harrison D, Rashotte J, Yamada J, Abbott LK, Coburn G, Stinson J, Le May S. Pain assessment and intensity in hospitalized children in Canada. J Pain 2012;13:857–65. [DOI] [PubMed] [Google Scholar]
- [56].Stevens BJ, Yamada J, Estabrooks CA, Stinson J, Campbell F, Scott SD, Cummings G; CIHR Team in Children's Pain. Pain in hospitalized children: effect of a multidimensional knowledge translation strategy on pain process and clinical outcomes. PAIN 2014;155:60–8. [DOI] [PubMed] [Google Scholar]
- [57].Taddio A, Appleton M, Bortolussi R, Chambers C, Dubey V, Halperin S, Hanrahan A, Ipp M, Lockett D, MacDonald N, Midmer D, Mousmanis P, Palda V, Pielak K, Riddell RP, Rieder M, Scott J, Shah V. Reducing the pain of childhood vaccination: an evidence-based clinical practice guideline. CMAJ 2010;182:E843–E855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Taddio A, Chambers CT, Halperin SA, Ipp M, Lockett D, Rieder MJ, Shah V. Inadequate pain management during routine childhood immunizations: the nerve of it. Clin Ther 2009;31(suppl 2):S152–S167. [DOI] [PubMed] [Google Scholar]
- [59].Taddio A, Ipp M, Thivakaran S, Jamal A, Parikh C, Smart S, Sovran J, Stephens D, Katz J. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine 2012;30:4807–12. [DOI] [PubMed] [Google Scholar]
- [60].Taddio A, Katz J, Ilersich AL, Koren G. Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet 1997;349:599–603. [DOI] [PubMed] [Google Scholar]
- [61].Taddio A, McMurtry CM, Shah V, Riddell RP, Chambers CT, Noel M, MacDonald NE, Rogers J, Bucci LM, Mousmanis P, Lang E, Halperin SA, Bowles S, Halpert C, Ipp M, Asmundson GJ, Rieder MJ, Robson K, Uleryk E, Antony MM, Dubey V, Hanrahan A, Lockett D, Scott J, Votta Bleeker E; HELPinKids&Adults. Reducing pain during vaccine injections: clinical practice guideline. CMAJ 2015;187:975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Taddio A, Parikh C, Yoon EW, Sgro M, Singh H, Habtom E, Ilersich AF, Pillai Riddell R, Shah V. Impact of parent-directed education on parental use of pain treatments during routine infant vaccinations: a cluster randomized trial. PAIN 2015;156:185–91. [DOI] [PubMed] [Google Scholar]
- [63].Taddio A, Pillai Riddell R, Ipp M, Moss S, Baker S, Tolkin J, Malini D, Feerasta S, Govan P, Fletcher E, Wong H, McNair C, Mithal P, Stephens D. Relative effectiveness of additive pain interventions during vaccination in infants. CMAJ 2016;190:e227–e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Taddio A, Shah V, McMurtry CM, MacDonald NE, Ipp M, Riddell RP, Noel M, Chambers CT; HELPinKids&Adults Team. Procedural and physical interventions for vaccine injections: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin J Pain 2015;31(10 suppl):S20–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Taylor EM, Boyer K, Campbell FA. Pain in hospitalized children: a prospective cross-sectional survey of pain prevalence, intensity, assessment and management in a Canadian pediatric teaching hospital. Pain Res Manag 2008;13:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].The Mayday Fund. Grants. New York, NY: The MAYDAY Fund, 2017. http://www.maydayfund.org/grants. [Google Scholar]
- [67].Treadwell MJ, Franck LS, Vichinsky E. Using quality improvement strategies to enhance pediatric pain assessment. Int J Qual Health Care 2002;14:39–47. [DOI] [PubMed] [Google Scholar]
- [68].Twycross A, Collis S. How well is acute pain in children managed? A snapshot in one English hospital. Pain Manag Nurs 2013;14:e204–e215. [DOI] [PubMed] [Google Scholar]
- [69].Twycross A, Dowden SJ. 2010 Do organizational quality improvement strategies improve pain management? Pediatric Pain Letter Special Interest Group on Pain in Childhood 2010;12:7–10. [Google Scholar]
- [70].Uman LS, Birnie KA, Noel M, Parker JA, Chambers CT, McGrath PJ, Kisely SR. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev 2013:CD005179. [DOI] [PubMed] [Google Scholar]
- [71].Valeri BO, Ranger M, Chau CM, Cepeda IL, Synnes A, Linhares MB, Grunau RE. Neonatal invasive procedures predict pain intensity at school age in children born very preterm. Clin J Pain 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].VitalSmarts. Children's Minnesota: using the influencer model to create a new standard of pediatric care. Provo, UT: VitalSmarts, 2018. YouTube. https://youtu.be/ZXKaLdMNDlc. [Google Scholar]
- [73].Walther-Larsen S, Pedersen MT, Friis SM, Aagaard GB, Romsing J, Jeppesen EM, Friedrichsdorf SJ. Pain prevalence in hospitalized children: a prospective cross-sectional survey in four Danish university hospitals. Acta Anaesthesiol Scand 2017;61:328–37. [DOI] [PubMed] [Google Scholar]
- [74].Watterberg KL, Cummings JJ, Benitz WE, Eichenwald EC, Poindexter BB, Stewart DL, Aucott SW, Goldsmith JP, Puopolo KM, Wang KS, Tobias JD, Agarwal R, Anderson CT, Hardy CA, Honkanen A, Rehman MA, Bannister CF. Prevention and management of procedural pain in the neonate: an update. Pediatrics 2016;137:e20154271. [DOI] [PubMed] [Google Scholar]
- [75].Womack JP, Jone JT. Lean thinking: Banish waste and create wealth in your corporation. United Kingdom: Simon and Schuster, 2003. [Google Scholar]
- [76].Zhu LM, Stinson J, Palozzi L, Weingarten K, Hogan ME, Duong S, Carbajal R, Campbell FA, Taddio A. Improvements in pain outcomes in a Canadian pediatric teaching hospital following implementation of a multifaceted knowledge translation initiative. Pain Res Manag 2012;17:173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zier JL, Liu M. Safety of high-concentration nitrous oxide by nasal mask for pediatric procedural sedation: experience with 7802 cases. Pediatr Emerg Care 2011;27:1107–12. [DOI] [PubMed] [Google Scholar]