Abstract
Background
Systemic inflammation and immune dysfunction has been proved to be significantly associated with cancer progression and metastasis in many cancer types, including colorectal cancer. We examined the prognostic significance of the systemic immune-inflammation index (SII) in patients with metastatic colorectal cancer (mCRC) and the relationship between the lymphocytic response to the tumor and this index.
Methods
This retrospective study evaluated 240 consecutive patients with newly diagnosed stage IV mCRC who underwent surgical resection. The SII values were calculated based on preoperative laboratory data regarding platelet, neutrophil, and lymphocyte counts. Tumor-infiltrating lymphocytes were evaluated using the surgical specimens. The overall survival and their 95% confidence interval (95% CI) were estimated by regression analyses and the Kaplan–Meier method.
Results
After a mean follow-up of 26.7 (1.1–92.4) months, 146 patients (60.8%) died. In the univariate analysis, a high SII was significantly associated with poor overall survival (P = 0.009). The multivariable analysis also confirmed that a high SII was independently associated with poor overall survival (hazard ratio: 1.462, 95% confidence interval 1.049–2.038, P = 0.025). The SII value was significantly correlated with the TILs value at the tumor’s center (P = 0.04), but not at the invasive margin (P = 0.39). When we evaluated overall survival for groupings of the tumor-infiltrating lymphocytes and SII values, we identified three distinct prognostic groups. The group with low tumor-infiltrating lymphocyte values and high SII values had the worst prognosis.
Conclusions
A high SII value independently predicts poor clinical outcomes among patients with mCRC. In addition, combining the lymphocytic response to the tumor and SII could further enhance prognostication for mCRC.
Electronic supplementary material
The online version of this article (10.1186/s12967-018-1638-9) contains supplementary material, which is available to authorized users.
Keywords: Metastatic colorectal cancer, Systemic immune-inflammation index, Immunity, Survival
Background
Colorectal cancer (CRC) is a leading cause of morbidity and mortality worldwide [1], and approximately 25% of patients with CRC have distant metastasis at the initial diagnosis [2]. In the era of cytotoxic drug combinations and molecular targeting agents, the integration of surgery and effective systemic chemotherapy has emerged as a new strategy for prolonging survival of patients with metastatic colorectal cancer (mCRC) [3–7]. However, in most cases the disease is not curable. This leads to further exploration in an effort to understand and improve treatment failure in this subset of mCRC patients.
With the success of the check-point inhibitors in a wide variety of tumor in recent years, the host immune response, notably an enhanced lymphocytic reaction, has become a recent focus of investigation [8–11]. Preexisting cytotoxic T lymphocyte cells in the tumor microenvironment can attack cancer cells by recognizing abnormally expressed neoantigens, and were required for tumor regression after immune checkpoint blockade [12]. Currently, tumor immune response with respect to lymphocytic infiltrations can be assessed in hematoxylin–eosin (H&E)-stained sections basing on the morphology characteristics of cells, which is a first pragmatic and cost-effective approach. Numerous studies confirmed the prognostic value of tumour infiltrating lymphocytes (TILs) in various types of malignancies in recent years [13–16]. Furthermore, the TILs have shown a significant prognostic power in our series of mCRC patients [17].
Inflammation has been recognized as a mechanism of immunoresistance in tumors, promoting cancer development and progression [18]. Via a complete blood count, physician can easily identify immune-inflammatory elements (neutrophils, lymphocytes and platelets), which might shed light on the inflammatory tumour microenvironment [19, 20]. Hu et al. [21] were the first to describe the systemic immune-inflammation index (SII), which is based on neutrophil, lymphocyte, and platelet counts. Subsequent research has indicated that the SII has greater prognostic value for malignant tumors than single-parameter markers such as the neutrophil-to-lymphocyte ratio (NLR), or the platelet-to-lymphocyte ratio (PLR) [22–29]. SII has been preliminarily investigated in CRC patients, Chen et al. was the first to establish the advantage prognostic value of SII than NLR and PLR in patients with CRC after radical surgery [30]. Passardi et al. and Yang et al. also confirmed the prognostic value of SII, however, SII didn’t show advantage than PLR and NLR [31, 32], and not as NLR able to predict the efficacy of bevacizumab in mCRC [32]. However, these studies [30–32] were restricted by the limited information on pathologic features and treatment regimen. In mCRC, the factors such as metastasectomy, adjuvant chemotherapy, and metastasis sites involved may confound each other in survival analysis. Thus, the independent contribution of SII to survival in the context of established prognostic factors remain to be determined in mCRC. To date the existing studies have focused on local lymphocytic reaction or systemic inflammatory responses in isolation, it is of also interest that the relationship between local immune status and the systemic environment in mCRC patients. Therefore, the present study evaluated the prognostic value of the SII in mCRC, whether the SII was correlated with TILs, and whether these factors could be combined to better predict overall survival.
Methods
Study population
This retrospective study evaluated 240 consecutive patients with newly diagnosed stage IV CRC who underwent primary tumor resection at our institution during 2009–2014. The eligibility criteria were pathologically confirmed CRC, synchronous distant metastasis at the time of the diagnosis, and available data regarding the preoperative complete blood count and follow-up results. The exclusion criteria were inflammatory bowel disease-related CRC, known hereditary CRC syndrome, preoperative chemoradiotherapy, and a history of other malignancies within the preceding 5 years. The study’s retrospective protocol was approved by the Ethics Committee of Sun Yat-Sen University Cancer Center, and all patients had provided written informed consent for their data and surgical specimens to be used for research purposes.
The preoperative complete blood count nearest to the time of the operation was used to calculate SII as (platelet count) × (the neutrophil-to-lymphocyte ratio) [21]. The median SII value was used to dichotomize the patients as having high or low SII values. The OS interval was calculated as the time from the surgery until the first instance of cancer-related death or loss to follow-up. Patients who died because of non-CRC causes were censored at the time of their death.
Tumor-infiltrating lymphocytes
Hematoxylin and eosin-stained sections from primary tumor specimens were retrieved for all patients. Counts for TILs were performed at the center of the tumor and at the invasive margin, which was defined as the interface between the tumor’s invading edge and the host stroma. The density of TILs was graded as 0 (absent), 1+ (mild), 2+ (moderate), or 3+ (marked), based on previous reports [16, 33]. For the present study, a low TILs score was defined a scores of 0–1 and a high score was defined as scores of 2–3. Where there was disagreement regarding TIL category between pathologists, joint reevaluation was performed to arrive at a consensus.
Statistical analysis
The Mann–Whitney U test was used to analyze differences in the SII distributions between the patient groups. Kaplan–Meier analyses with the log-rank test were performed to compare survival outcomes. Significant baseline characteristics were used for propensity score analysis. Multivariate analysis using a Cox proportional hazards regression model was performed based on variables with a P-value of < 0.05 from the univariate analyses. All statistical tests were two-sided and differences were considered significant at P < 0.05. All analyses were performed using IBM SPSS software (version 22.0; IBM Corp., Armonk, NY).
Results
The baseline characteristics of the 240 patients are summarized in Table 1. The median patient age was 59 years (range 18–90 years) and 157 patients (65.4%) were male. The primary tumors were located in the colon (191 patients, 79.6%) or the rectum (45 patients, 18.8%), with 179 patients having node-positive disease (74.6%) and 57 patients having poor differentiation (23.8%). Most patients (63.8%) had metastases in a single organ, although 87 patients (36.3%) had metastatic sites spread over multiple organs. Palliative resection of the primary cancer was performed for 194 patients (80.8%) and 46 of these patients also underwent metastasectomy. Postoperative chemotherapy was performed for 77.1% of these patients.
Table 1.
Baseline characteristics
| N = 240 | |
|---|---|
| Age (years) | |
| < 65 | 179 (74.6) |
| ≥ 65 | 61 (25.4) |
| Sex, no. (%) | |
| Male | 157 (65.4) |
| Female | 83 (34.6) |
| Histological grade (%) | |
| Mod/well differentiated | 156 (65.0) |
| Poorly differentiated | 57 (23.8) |
| Other or missing | 27 (11.3) |
| Primary site (%) | |
| Colon | 191 (79.6) |
| Rectum | 45 (18.8) |
| Others | 4 (1.7) |
| T-stage (depth of invasion) (%) | |
| T1–3 | 144 (60.0) |
| T4 | 93 (38.8) |
| Tx | 3 (1.3) |
| N-stage (lymphatic invasion) (%) | |
| N0 | 58 (24.2) |
| N+ | 179 (74.6) |
| Nx | 3 (1.3) |
| MMR status (%) | |
| dMMR | 12 (5.0) |
| pMMR | 228 (95.0) |
| Metastasectomy (%) | |
| + | 46 (19.2) |
| − | 194 (80.8) |
| No. of metastatic organs (%) | |
| Single | 153 (63.8) |
| Multiple | 87 (36.3) |
| Adjuvant chemotherapy (%) | |
| Negative | 55 (22.9) |
| Positive | 185 (77.1) |
| Metastatic sites (%) | |
| Liver | 148 (61.7) |
| Extrahepatic disease | 92 (38.3) |
MMP mismatch repair, dMMR mismatch repair-deficient, pMMR mismatch repair-proficient
The median SII value was 649.45. Table 2 shows that a high SII value was significantly associated with multiple metastatic sites (P = 0.017) and marginally associated with a primary tumor in the rectum (P = 0.071). No other associations were observed between the SII and the other clinicopathological factors. Relative to patients with a low SII value, patients with a high SII experienced significantly shorter OS (hazard ratio [HR]: 1.548, 95% confidence interval [CI] 1.116–2.146; log-rank P = 0.008, Fig. 1). In the univariate analyses, survival was associated with age, primary site, T-stage, lymph node status, number of metastatic organs, metastasectomy, and adjuvant therapy (Table 3). To eliminate inherent biases, significant factors (age, primary site, T-stage, lymph node status, number of metastatic organs, metastasectomy, and adjuvant therapy) were also used for propensity score analysis (Additional file 1: Table S1). As expected, the raw and normalized results were consistent (P = 0.022; Additional file 2: Figure S1). In the multivariate analysis, which was adjusted for those risk factors, a high SII still independently predicted poor OS (HR: 1.462, 95% CI 1.049–2.038; log-rank P = 0.025).
Table 2.
Associations between clinicopathologic variables and SII
| Characteristics | Mean | s.e.m. | Median | P-value |
|---|---|---|---|---|
| Age (years) | ||||
| < 65 | 857.17 | 50.70 | 637.76 | 0.36 |
| ≥ 65 | 866.76 | 81.21 | 697.67 | |
| Gender | ||||
| Male | 898.28 | 56.40 | 650.76 | 0.35 |
| Female | 786.47 | 63.49 | 637.76 | |
| Histologic grade | ||||
| Mod/well differentiated | 862.53 | 49.40 | 699.31 | 0.27 |
| Poorly differentiated | 818.38 | 87.84 | 585.0 | |
| Primary site | ||||
| Colon | 884.17 | 47.35 | 697.67 | 0.07 |
| Rectum | 737.66 | 99.95 | 595.71 | |
| T-stage | ||||
| T1–3 | 897.49 | 60.33 | 675.11 | 0.55 |
| T4 | 784.97 | 58.11 | 613.79 | |
| LN status | ||||
| pN0 | 933.26 | 94.50 | 694.81 | 0.28 |
| pN+ | 827.44 | 48.37 | 637.94 | |
| MMR status | ||||
| dMMR | 897.14 | 195.05 | 747.13 | 0.71 |
| pMMR | 857.63 | 44.17 | 648.99 | |
| Metastatic sites | ||||
| Liver | 877.09 | 53.67 | 668.48 | 0.43 |
| Extrahepatic disease | 831.49 | 71.88 | 639.15 | |
| No. of metastatic organs | ||||
| Single | 797.31 | 51.82 | 607.71 | 0.02 |
| Multiple | 969.17 | 74.88 | 775.77 | |
SII systemic immune-inflammation index, LN lymph node
Fig. 1.
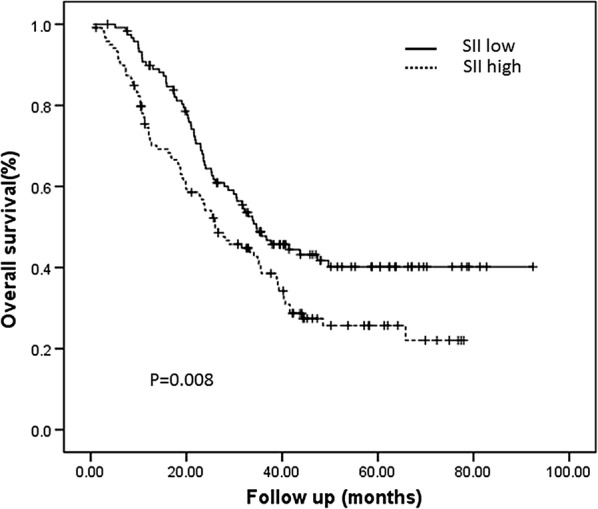
Prognostic value of the systemic immune-inflammation index. Estimated Kaplan–Meier curves for overall survival grouped according to systemic immune-inflammation index (SII) level; in all patients with mCRC (n = 240), hazard ratio = 1.548 95% CI 1.116–2.146, P = 0.008; low SII < 649.45; high SII ≥ 649.45
Table 3.
Association of SII with prognosis (overall survival) in the whole study population
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years) | ||||
| < 65 | – | 0.003 | – | 0.018 |
| ≥ 65 | 1.699 (1.197–2.410) | 1.540 (1.077–2.202) | ||
| Gender | ||||
| Male | – | 0.484 | ||
| Female | 1.128 (0.805–1.579) | |||
| Histologic grade | ||||
| Mod/well differentiated | – | 0.245 | ||
| Poorly differentiated | 1.147 (0.910–1.446) | |||
| Primary site | ||||
| Colon | – | 0.025 | – | 0.078 |
| Rectum | 0.634 (0.426–0.944) | 0.685 (0.450–1.043) | ||
| T-stage | ||||
| T1–3 | – | 0.005 | – | 0.002 |
| T4 | 1.579 (1.149–2.169) | 1.669 (1.203–2.316) | ||
| LN status | ||||
| pN0 | – | 0.006 | – | 0.028 |
| pN+ | 1.762 (1.178–2.635) | 1.571 (1.049–2.352) | ||
| MMR status | ||||
| dMMR | – | 0.054 | ||
| pMMR | 2.415 (0.986–5.916) | |||
| Adjuvant chemotherapy | ||||
| Negative | – | 0.015 | – | 0.011 |
| Positive | 0.625 (0.429–0.912) | 0.602 (0.406–0.892) | ||
| Metastasectomy | ||||
| − | – | < 0.001 | – | 0.004 |
| + | 0.380 (0.231–0.624) | 0.479 (0.289–0.795) | ||
| Metastatic sites | ||||
| Liver | – | 0.705 | ||
| Extrahepatic disease | 0.937 (0.670–1.312) | |||
| No. of metastatic organs | ||||
| Single | – | 0.008 | – | 0.010 |
| Multiple | 1.350 (1.080–1.688) | 1.361 (1.076–1.721) | ||
| SII | ||||
| Low | – | 0.009 | – | 0.025 |
| High | 1.548 (1.116–2.146) | 1.462 (1.049–2.038) | ||
SII systemic immune-inflammation index, CI confidence interval, HR hazard ratio, LN lymph node
The relationship between the SII and TILs values is shown in Table 4. A low TILs value in the tumor’s center was associated with a high pre-operative SII value (P = 0.041). No significant association was observed between the SII value and the TILs value at the invasive margin.
Table 4.
Associations between inflammation markers and immune cell density in the tumor microenvironment
| Lymphocytes | N (%) | |||
|---|---|---|---|---|
| Mean | s.e.m. | Median | P-value | |
| Central region | 0.04 | |||
| Low grade | 913.41 | 55.12 | 697.66 | |
| High grade | 749.95 | 65.37 | 592.43 | |
| Invasive margin | 0.39 | |||
| Low grade | 862.19 | 45.49 | 649.45 | |
| High grade | 845.45 | 126.12 | 655.72 | |
SII systemic immune-inflammation index
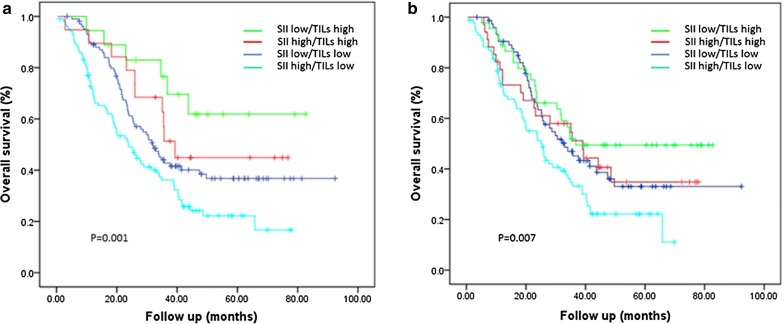
Survival outcomes were also evaluated according to combinations of the TILs and SII values, which identified three prognostic groups. Patients with a high TILs value at the invasive margin and a low SII value had the most favorable prognosis, while patients with a low TILs value at the invasive margin and a high SII value had the poorest prognosis (HR: 0.578, 95% CI 0.438–0.763; P < 0.001). Patients with either low SII and low TILs values, or with high SII and high TILs values, had similar intermediate prognoses (Fig. 2a). A similar trend was observed when we evaluated the combination of the SII value and TILs value in the tumor’s center (HR: 0.668, 95% CI 0.528–0.846; P = 0.001, Fig. 2b).
Fig. 2.
The combined prognostic role of the systemic immune-inflammation index and tumor-infiltrating lymphocytes. Estimated Kaplan–Meier curves for overall survival grouped according systemic immune-inflammation index (SII) and tumor-infiltrating lymphocytes (TILs) category. a Patients grouped according to TILs level at the invasive margin and SII; The 5-year overall survival were: low SII and high TILs: 60%; high SII and high TILs: 44%; low SII and low TILs: 37%; high SII and low TILs: 20%. b Patients grouped according to TILs level in the tumor’s central region and SII; low SII and high TILs: 49%; high SII and high TILs: 33%; low SII and low TILs: 33%; high SII and low TILs: 21%
Discussion
To the best of our knowledge, this is the first study to evaluate the clinical relevance of the SII among patients with stage IV CRC and also the first study to evaluate the relationship between the SII and the lymphocytic response to the tumor based on the TILs value. Our results indicate that the preoperative SII value predicted prognosis among our patients, and that the SII value was significantly correlated with the TILs value in the tumor’s center, but not at the invasive margin. Moreover, the combination of the SII and TILs values provided a useful tool for predicting mCRC survival outcomes. These findings suggest that immune and inflammation processes play significant roles in the progression of mCRC.
Elevated levels of inflammatory markers are often associated with more advanced disease, which may be related to a greater tumor burden and/or on-going chronic inflammatory processes [34]. The present study revealed that the SII value was significantly associated with multiple metastatic sites, which agrees with the findings for germ-cell tumors and suggests that systemic inflammation may reflect a tumor’s invasive characteristics [35]. However, the SII value was not associated with other clinicopathological factors, such as T stage and lymph node status. Our further investigation did not find any association between the SII value and the TILs value at the invasive margin, but we observed significant correlation between SII and the presence of TILs in the tumor microenvironment, suggesting the interactions between pro-inflammatory environment and antitumor immunity intratumorally. Thus, systemic inflammation as expressed by SII may be linked to both the tumor and the tumor microenvironment, although the related mechanisms remain incompletely understood.
There is increasing evidence that inflammatory markers can help predict clinical outcomes for various cancers [26, 27, 36–39]. The present study is the first to evaluate the prognostic value of the SII in mCRC and confirmed that high SII values were associated with significantly poorer OS. The prognostic value of this index is likely related to the function of peripheral neutrophils, lymphocytes, and platelets, which are used to determine the SII values. In this context, recent evidence suggests that neutrophils and platelets can promote cancer cell proliferation, invasion, immune evasion, and metastasis via multiple mechanisms [40, 41]. Lymphopenia is especially common in advanced cancer, as observed in the present study, and reflects an inefficient immune system that may produce a favorable microenvironment for the spread of tumor cells [42].
Immune mechanisms have been associated with tumor progression [18]. TILs in the primary tumor predicts prognosis in mCRC were also previously reported in our works [17]. Recognizing the significant role of inflammation and the immune system in the antitumor response and cancer development, it is of interest to define whether systemic inflammation and lymphocytic response could be combined to better predict survival outcomes. We evaluated various combinations of the SII and TILs values, which revealed three prognostic groups. The first group had high TILs and low SII values and experienced favorable prognosis. The second group had low TILs and high SII values and experienced unfavorable prognosis. The third group had either high or low values for both TILs and SII, and experienced similar intermediate prognosis. Thus, it appears that the combination of SII and TILs provides additional prognostic value among cases of mCRC. We speculate that a TILs-related excellent prognosis might be counteracted in a pro-inflammatory environment, although further research is needed to evaluate this possibility and elucidate the underlying mechanism.
Our study has several limitations. First, the retrospective nature limits the availability of blood count information at uniform time points before the operation. Second, the TILs density in full-face stained sections is a relatively crude marker for the antitumor immune response, as it does not differentiate between specific subpopulations of immune cells. Nevertheless, this measure is simple, readily available, and does not require additional immunohistochemical staining, which makes it both practical and cost-effective for clinical use.
Conclusions
This is the first report to demonstrate that the SII has prognostic value among patients with mCRC, and that the combination of the SII and TILs values provided added prognostic value in this setting. Properly designed prospective studies are needed to further explore these interesting findings.
Additional files
Additional file 1: Table S1. Associations between significant factors and SII after use of PS weighting.
Additional file 2: Figure S1. Prognostic value of the systemic immune-inflammation index after use of PS weighting. Estimated Kaplan–Meier curves for overall survival grouped according to systemic immune-inflammation index (SII) level after use of PS weighting. The log-rank test was used to compare the curves.
Authors’ contributions
QKX: data acquisition and analysis, writing. PC, PS, WZH, CJ, PFK, SSL, HTC, YZY, DW: data acquisition. WMH: methodology. LY: review and editing. LPX: conceptualization, funding acquisition, data acquisition, review and editing. All authors read and approved the final manuscript.
Acknowledgements
The authors acknowledge the assistance of their colleagues at the State Key Laboratory of Oncology in South China and the Department of Pathology, Sun Yat-Sen University Cancer Center.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (https://www.researchdata.org.cn), with the approval RDD number as RDDA2018000841.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study’s retrospective protocol was approved by the Ethics Committee of Sun Yat-Sen University Cancer Center, and all patients had provided written informed consent for their data and surgical specimens to be used for research purposes.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (81272641).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- SII
systemic immune-inflammation index
- mCRC
metastatic colorectal cancer
- TILs
tumour infiltrating lymphocytes
- HR
hazard ratio
- CI
confidence interval
Contributor Information
Qian-Kun Xie, Email: xieqk@sysucc.org.cn.
Ping Chen, Email: chenping@sysucc.org.cn.
Wan-Ming Hu, Email: huwm@sysucc.org.cn.
Peng Sun, Email: sunpeng1@sysucc.org.cn.
Wen-Zhuo He, Email: hewzh@sysucc.org.cn.
Chang Jiang, Email: jiangchang@sysucc.org.cn.
Peng-Fei Kong, Email: kongpf@sysucc.org.cn.
Shou-Sheng Liu, Email: liushsh@sysucc.org.cn.
Hai-Tian Chen, Email: seth_chen2017@163.com.
Yuan-Zhong Yang, Email: yangyuanzh@sysucc.org.cn.
Dan Wang, Email: wangdan@sysucc.org.cn.
Lin Yang, Phone: +8620-87343107, Email: yanglin@sysucc.org.cn.
Liang-Ping Xia, Phone: +8620-87343107, Email: xialp@sysucc.org.cn.
References
- 1.Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 3.Scheer MG, Sloots CE, van der Wilt GJ, et al. Management of patients with asymptomatic colorectal cancer and synchronous irresectable metastases. Ann Oncol. 2008;19(11):1829–1835. doi: 10.1093/annonc/mdn398. [DOI] [PubMed] [Google Scholar]
- 4.Kim YW, Kim IY. The role of surgery for asymptomatic primary tumors in unresectable stage IV colorectal cancer. Ann Coloproctol. 2013;29(2):44–54. doi: 10.3393/ac.2013.29.2.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarantino I, Warschkow R, Worni M, et al. Prognostic relevance of palliative primary tumor removal in 37,793 metastatic colorectal cancer patients: a population-based, propensity score-adjusted trend analysis. Ann Surg. 2015;262(1):112–120. doi: 10.1097/SLA.0000000000000860. [DOI] [PubMed] [Google Scholar]
- 6.He WZ, Rong YM, Jiang C, et al. Palliative primary tumor resection provides survival benefits for the patients with metastatic colorectal cancer and low circulating levels of dehydrogenase and carcinoembryonic antigen. Chin J Cancer. 2016;35(1):58. doi: 10.1186/s40880-016-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Rooijen KL, Shi Q, Goey KKH, et al. Prognostic value of primary tumour resection in synchronous metastatic colorectal cancer: individual patient data analysis of first-line randomised trials from the ARCAD database. Eur J Cancer. 2018;91:99–106. doi: 10.1016/j.ejca.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 11.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 12.Hamid O, Schmidt H, Nissan A, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldan V, Griffiths R, Hawkins RE, et al. Efficient and reproducible generation of tumour-infiltrating lymphocytes for renal cell carcinoma. Br J Cancer. 2015;112(9):1510–1518. doi: 10.1038/bjc.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang BW, Seo AN, Yoon S, et al. Prognostic value of tumor-infiltrating lymphocytes in Epstein–Barr virus-associated gastric cancer. Ann Oncol. 2016;27(3):494–501. doi: 10.1093/annonc/mdv610. [DOI] [PubMed] [Google Scholar]
- 15.Taylor RC, Patel A, Panageas KS, et al. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. 2007;25(7):869–875. doi: 10.1200/JCO.2006.08.9755. [DOI] [PubMed] [Google Scholar]
- 16.Klintrup K, Makinen JM, Kauppila S, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005;41(17):2645–2654. doi: 10.1016/j.ejca.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Xie QK, He WZ, Hu WM, et al. Tumor-infiltrating lymphocyte as a prognostic biomarker in stage IV colorectal cancer should take into account the metastatic status and operation modality. Cancer Manag Res. 2018;10:1367–1375. doi: 10.2147/CMAR.S162147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 20.Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14(6):e218–e228. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 21.Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 22.Hong X, Cui B, Wang M, et al. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med. 2015;236(4):297–304. doi: 10.1620/tjem.236.297. [DOI] [PubMed] [Google Scholar]
- 23.Wang K, Diao F, Ye Z, et al. Prognostic value of systemic immune-inflammation index in patients with gastric cancer. Chin J Cancer. 2017;36(1):75. doi: 10.1186/s40880-017-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aziz MH, Sideras K, Aziz NA, et al. The systemic-immune-inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels: a retrospective multicenter cohort study. Ann Surg. 2018 doi: 10.1097/SLA.0000000000002660. [DOI] [PubMed] [Google Scholar]
- 25.Fankhauser CD, Sander S, Roth L, et al. Systemic inflammatory markers have independent prognostic value in patients with metastatic testicular germ cell tumours undergoing first-line chemotherapy. Br J Cancer. 2018;118(6):825–830. doi: 10.1038/bjc.2017.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He WZ, Yin CX, Guo GF, et al. Initial neutrophil lymphocyte ratio is superior to platelet lymphocyte ratio as an adverse prognostic and predictive factor in metastatic colorectal cancer. Med Oncol. 2013;30(1):439. doi: 10.1007/s12032-012-0439-x. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, He WZ, Kong PF, et al. Clinical baseline and prognostic difference of platelet lymphocyte ratio (PLR) in right-sided and let-sided colon cancers. BMC Cancer. 2017;17(1):873. doi: 10.1186/s12885-017-3862-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lolli C, Basso U, Derosa L, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Oncotarget. 2016;7(34):54564–54571. doi: 10.18632/oncotarget.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z, Zhang J, Lu Y, et al. Aspartate aminotransferase–lymphocyte ratio index and systemic immune-inflammation index predict overall survival in HBV-related hepatocellular carcinoma patients after transcatheter arterial chemoembolization. Oncotarget. 2015;6(40):43090–43098. doi: 10.18632/oncotarget.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23(34):6261–6272. doi: 10.3748/wjg.v23.i34.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Xu H, Guo X, et al. Pretreatment inflammatory indexes as prognostic predictors for survival in colorectal cancer patients receiving neoadjuvant chemoradiotherapy. Sci Rep. 2018;8(1):3044. doi: 10.1038/s41598-018-21093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Passardi A, Scarpi E, Cavanna L, et al. Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget. 2016;7(22):33210–33219. doi: 10.18632/oncotarget.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ropponen KM, Eskelinen MJ, Lipponen PK, et al. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182(3):318–324. doi: 10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 34.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 35.Chovanec M, Cierna Z, Miskovska V, et al. Systemic immune-inflammation index in germ-cell tumours. Br J Cancer. 2018;118(6):831–838. doi: 10.1038/bjc.2017.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 37.Sarraf KM, Belcher E, Raevsky E, et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137(2):425–428. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 38.Ishizuka M, Nagata H, Takagi K, et al. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer. 2013;109(2):401–407. doi: 10.1038/bjc.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhindi B, Hermanns T, Wei Y, et al. Identification of the best complete blood count-based predictors for bladder cancer outcomes in patients undergoing radical cystectomy. Br J Cancer. 2016;114(2):207–212. doi: 10.1038/bjc.2015.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang W, Ferrara N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol Res. 2016;4(2):83–91. doi: 10.1158/2326-6066.CIR-15-0313. [DOI] [PubMed] [Google Scholar]
- 42.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Associations between significant factors and SII after use of PS weighting.
Additional file 2: Figure S1. Prognostic value of the systemic immune-inflammation index after use of PS weighting. Estimated Kaplan–Meier curves for overall survival grouped according to systemic immune-inflammation index (SII) level after use of PS weighting. The log-rank test was used to compare the curves.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (https://www.researchdata.org.cn), with the approval RDD number as RDDA2018000841.