Abstract
Background:
A lot of studies have shown a positive effect of transcatheter aortic valve implantation (TAVI) on left ventricular ejection fraction (LVEF).
Objectives:
We aimed to investigate the effect of TAVI on left ventricular function and correlate this phenomenon with hypertrophy degree in an early follow-up.
Materials and Methods:
Between August 2015 and July 2016, 250 consecutive patients with symptomatic severe aortic stenosis (AS) underwent TAVI in our institution. Given the aim of this analysis, only patients with an LVEF <50%, no more than moderate mitral valve regurgitation, successful valve implantation, and 1-month follow-up available were included in the study (n = 46). Patients were enrolled in a prospective database, with clinical and echocardiographic evaluations at 1 month after TAVI.
Results:
All patients had severe symptomatic AS (mean transaortic pressure gradients: 44.1 ± 13.8 mmHg and mean aortic valve area: 0.66 ± 0.19 cm2). Mean baseline LVEF was 39.3 ± 8.8%. Significant hemodynamic improvement was observed after TAVI. Mean transvalvular aortic gradient decreased significantly from 44.1 ± 13.8 mmHg to 8.9 ± 4.2 mmHg (P < 0.005). A statistically significant improvement in LVEF compared to baseline was observed in the 1st month of follow-up (39.3 ± 8.8% vs. 44.1 ± 10.1%, P < 0.019). Overall, 52.2% of patients showed an increase in LVEF, 32.6% had no change, while only 2.2% had a decrease in LVEF. Interestingly, we found a significant reverse correlation between LVEF improvement and ventricular hypertrophy measured as diastolic interventricular septum thickness (Pearson index r = −0.42). Patients showing greater improvement in LVEF were those with less than moderate hypertrophy.
Conclusions:
Patients with depressed systolic function show a consistent and early LVEF recovery after TAVI. An impaired LVEF recovery is most likely among patients with more than moderate hypertrophy, probably responsible of left ventricular fibrosis that irremediably compromises systolic function.
Keywords: Left ventricular hypertrophy, left ventricular remodeling, severe aortic stenosis, transcatheter aortic valve implantation
INTRODUCTION
Aortic stenosis (AS) is the most common primary valve disorder in the elderly, with considerable impact on morbidity and mortality. Appropriate selection of patients for aortic valve intervention is crucial, and current guidelines recommend aortic valve replacement in severe AS with symptoms or in asymptomatic patients with left ventricular ejection fraction (LVEF) <50%.[1] Transcatheter aortic valve implantation (TAVI) is an effective treatment for patients with severe AS who present a higher surgical risk or who cannot undergo surgical aortic valve replacement (SAVR).
AS has to be considered a disease of the left ventricle (LV) rather than purely affecting the aortic valve.[2] This condition causes an increase in pressure afterload and in ventricular wall stress that stimulates hypertrophy of LV myocardium.[3] Evidence shows that rising levels of hypertrophy may be maladaptive and the transition from LV hypertrophy to fibrosis results eventually in adverse effects on systolic and diastolic function.
A lot of studies have shown a positive effect of TAVI on LVEF.[4,5,6,7,8,9] Approximately one-third of patients with severe, symptomatic AS have LV systolic dysfunction.[10,11] LV recovery and associated improvements in clinical outcomes after aortic valve replacement have been demonstrated in over two-thirds of these patients.
We have aimed in our study to investigate the effect of TAVI on left ventricular function and correlate this phenomenon with hypertrophy degree in an early follow-up.
MATERIALS AND METHODS
Patient population
Between August 2015 and July 2016, 250 consecutive patients with symptomatic severe AS underwent TAVI in our institution. Given the aim of this analysis, only patients with an EF <50%, no more than moderate mitral valve regurgitation, successful valve implantation, and 1-month follow-up available were included in the study (n = 46). Pretreatment operative risk was assessed by the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) and the Society of Thoracic Surgeons (STS) score.
A multidisciplinary team including cardiologists, cardiothoracic surgeons, anesthesiologists, geriatricians, and interventional cardiologists evaluated all available clinical and imaging data, and a consensus decision was obtained to determine individual eligibility for TAVI. The decision to perform TAVI was conditional on the presence of severe symptomatic AS with contraindications to or high risk for SAVR, life expectancy 1 year, and anatomy suitable for intervention. All patients provided written informed consent.
Screening studies were performed in all patients before the procedure. Sizing of the transcatheter heart valve was carried out using multidetector computed tomography (MDCT) and an integration of echocardiography (transthoracic and/or transesophageal), angiography, and simultaneous aortography during balloon valvuloplasty when MDCT was not available.
Data collection and definitions
Patients were enrolled in a prospective database, with clinical and echocardiographic evaluations at 1 month after TAVI. All echocardiographic measurements were performed using a commercially available ultrasound system (Philips EPIQ 7). Transthoracic echocardiography (TTE) Doppler and two-dimensional images were obtained from parasternal long- and short-axis, apical four-chamber, and subcostal four-chamber images. TTE was reviewed to assess the pericardium, valvular anatomy and function, and cardiac function. The echocardiographic measurements included LV end-diastolic volume LVEF calculated with the Simpson's method, transvalvular pressure gradient determined by the Bernoulli formula, aortic valve area (AVA) calculated with the continuity equation (velocity-time integral method). Data were derived before and after device implantation. Measurement of the left ventricular outflow tract for calculations of AVA was performed with two-dimensional imaging in a zoomed-up parasternal long-axis view. To estimate the grade of hypertrophy, we measured LV septal and posterior wall thicknesses. All echocardiography parameters were evaluated according to the guidelines of the American Society of Echocardiography.[12]
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median with first (Q1) and third (Q3) quartiles in cases of skewed distributions. Categorical variables are described by frequencies and percentages. Differences between independent groups were tested using the Wilcoxon rank-sum test and Student's t-test for continuous variables. In cases in which the samples were paired, the Wilcoxon signed-rank test or paired Student's t-test was used. Categorical variables were compared with Chi-square test. The correlation was analyzed by Pearson method and simple linear regression with 95% confidence interval and showed as r2. All data were processed using the Statistical Package for the Social Sciences, version 20 (IBM, Armonk, NY, USA).
RESULTS
Patient characteristics
Demographic and clinical characteristics of the study population were presented in Table 1.
Table 1.
Baseline characteristics
| Clinical parameters | Overall population (n=46) |
|---|---|
| Age, years±SD | 80±3 |
| Female gender, n (%) | 19 (41.30%) |
| Hypertension, n (%) | 43 (93.4%) |
| Diabetes mellitus, n (%) | 15 (32.6%) |
| Dyslipidemia, n (%) | 28 (60.9%) |
| Prior myocardial infarction, n (%) | 10 (21.7%) |
| Prior stroke, n (%) | 1 (2.2%) |
| Prior TIA, n (%) | 1 (2.2%) |
| Prior bypass graft surgery, n (%) | 10 (21.7%) |
| Prior percutaneous coronary intervention, n (%) | 11 (23.9%) |
| NYHA class III and IV, n (%) | 38 (82.6%) |
| Logistic EuroSCORE, %±SD | 13.9±7 |
| STS score, %±SD | 5±1.7 |
BMI=Body Mass Index, TIA=Transient Ischemic Attack, NYHA=New York Heart Association, STS=Society of Thoracic Surgery
The mean age of the patients was 80 ± 3 years, and 41.3% of the patients were female. According to the New York Heart Association classification, 82.55% of the patients were in functional Class III or IV. History revealed coronary artery disease in 21.7%, hypertension in 93.4%, and diabetes mellitus in 32.6% of the patients. All patients had severe symptomatic AS (mean transaortic pressure gradients: 44.1 ± 13.8 mmHg and mean AVA: 0.66 ± 0.19 cm2). Mean baseline LVEF was 39.3 ± 8.8%. Overall, the population was at high surgical risk with a predicted 30-day mortality 13.9 ± 7% by logistic EuroSCORE and 5 ± 1.7% by STS mortality score.
Procedural data
TAVI was performed through transfemoral access and under local anesthesia with a mild systemic sedative treatment, according to patients’ need. A vascular closure device (Prostar XL, Abbott Vascular, Abbott Park, IL) was used at the end of the procedure. The prosthesis implanted was Edwards SAPIEN XT (n = 13), Medtronic CoreValve (n = 31), and Acurate neo TF Symetis (n = 2). The prosthesis was deployed retrograde over a stiff guidewire placed in LV, under fluoroscopic guidance. Device success was defined as a proper prosthesis placement with aortic mean gradient <20 mmHg, without significant (i.e., moderate/severe) aortic regurgitation, while acute procedure success was defined as an effective valve implantation procedure with no death within 24 h and no need for conversion to surgery.
Echocardiographic follow-up at 1 month
Significant hemodynamic improvement was observed after TAVI. Graphs depicting prosthesis performances at follow-up are reported in Table 2. There was no case of severe paravalvular aortic regurgitation after TAVI. Mean transvalvular aortic gradient decreased significantly from 44.1 ± 13.8 mmHg to 8.9 ± 4.2 mmHg (P < 0.005).
Table 2.
Echocardiographic Outcomes up to 1 month
| Pre TAVI (n=46) | 1-month follow-up (n=46) | |
|---|---|---|
| Left ventricular ejection fraction, %±SD | 39,3±8,8 | 44,1±10,1 |
| Peak pressure gradient, mmHg±SD | 68±20,6 | 16,6±8,2 |
| Mean pressure gradient, mmHg±SD | 44,1±13,8 | 8,9±4,2 |
| Aortic Valve Area, cm2±SD | 0,66±0,19 | 1,64±0,38 |
A statistically significant improvement in LVEF compared to baseline was observed in the 1st month of follow-up (39.3 ± 8.8% vs. 44.1 ± 10.1%, P < 0.019) [Figure 1].
Figure 1.
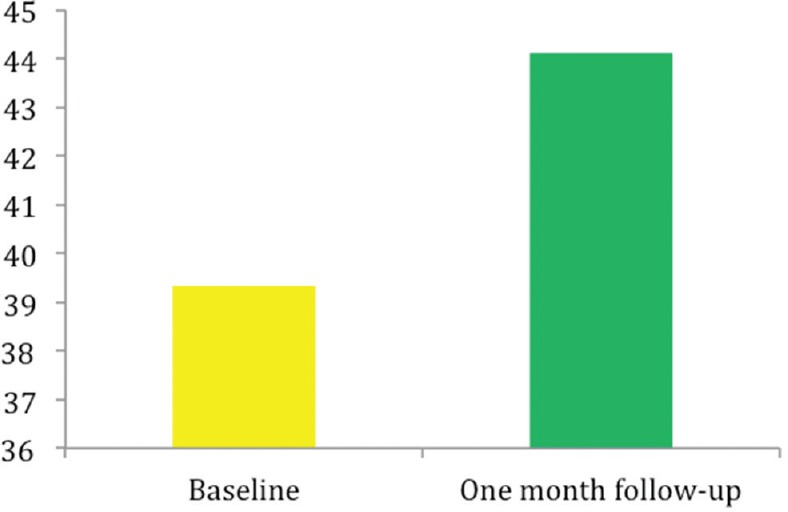
Time trends in left ventricle ejection fraction, pre and 1 month after transcatheter aortic valve implantation
Figure 2 shows LVEF variation in any single patient. Overall, 24 patients (52.2%) showed an increase in LVEF, 15 patients (32.6%) had no change, while only 1 patient (2.2%) had a decrease in LVEF.
Figure 2.
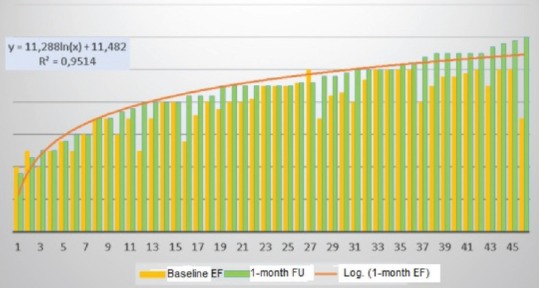
Left ventricular ejection fraction variation in any single patients
Moreover, we found a significant reverse correlation between LVEF improvement and ventricular hypertrophy measured as diastolic interventricular septum thickness (Pearson index r = −0.42) [Figure 3].
Figure 3.
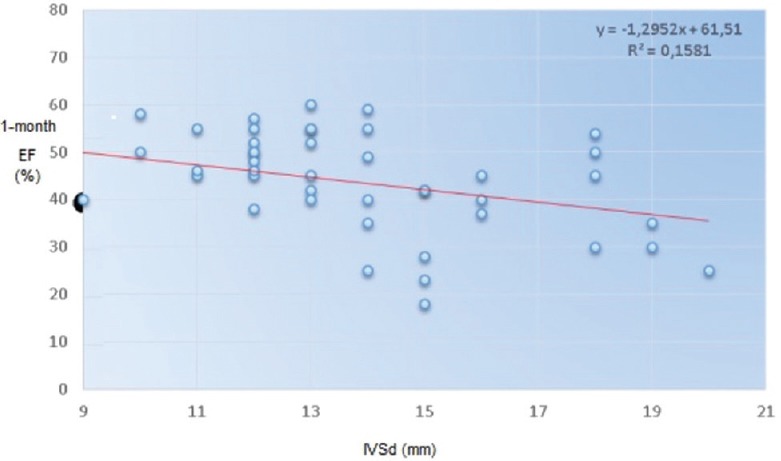
Reverse correlation between left ventricular ejection fraction at 1 month after transcatheter aortic valve implantation and ventricular hypertrophy measured as interventricular septum in diastole (interventricular septal end diastole)
This relationship is illustrated in Figure 4 where we put diastolic interventricular septum thickness (IVSd) as dependent variable and the difference between baseline EF and 1-month EF as the independent variable. The relationship shows inverse correlation between hypertrophy and EF improvement. Patients showing greater improvement in EF are those with less than moderate hypertrophy (IVSd <16 mm).
Figure 4.
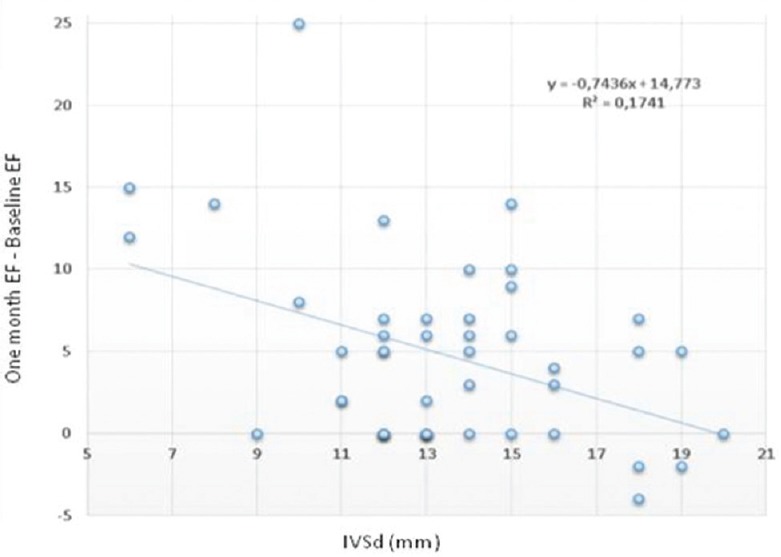
Reverse correlation between left ventricular ejection fraction improvement (1-month ejection fraction–baseline ejection fraction) and ventricular hypertrophy measured as interventricular septum in diastole
Furthermore, we noticed a better and significant improvement in EF in women than in men (regression index, r2 = 0.26 vs. 0.21) [Figure 5].
Figure 5.
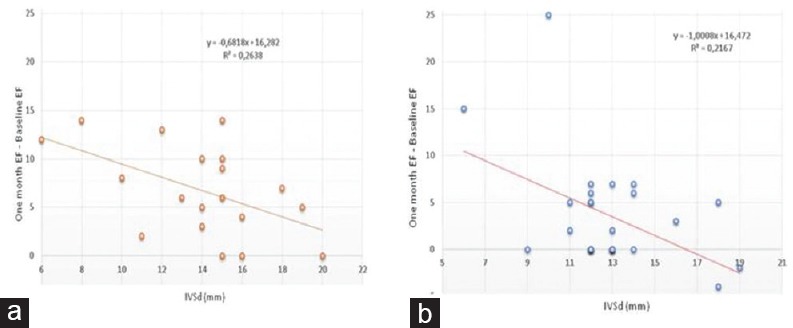
Reverse correlation between left ventricular ejection fraction improvement (1-month ejection fraction–baseline ejection fraction) and ventricular hypertrophy measured as interventricular septum in diastole in women (a) and in men (b)
DISCUSSION
AS has to be considered a disease of LV rather than purely affecting the aortic valve. First, the result of the increase in pressure afterload and ventricular wall stress is responsible of hypertrophy of the left ventricular myocardium. Second, AS is often associated to depressed LVEF as a result of afterload mismatch or intrinsic contractile dysfunction.
Although patients with depressed LVEF have a high risk for postoperative death, successful aortic valve replacement induces a recovery of left ventricular function in most patients. There is still a paucity of data on correlation of hypertrophy and LVEF recovery after TAVI.
This single-center analysis adds considerably to the current knowledge about LV systolic function recovery after TAVI with the following observations: (i) an important improvement of aortic valve function with a decrease of mean gradient and an increase of valve area, (ii) a significant increase in LVEF after TAVI at early follow-up, and (iii) earlier LVEF recovery in patients with less than moderate LV hypertrophy.
LVEF improvement after TAVI has been investigated in literature.[9,13,4] A previous study conducted in our institution has already demonstrated that favorable outcomes after successful TAVI are associated with sustained clinical and functional cardiovascular benefits up to 4 year follow-up.[14] Barbanti et al. confirmed these results in a larger sample up to 5-year follow-up.[15] Kamperidis et al. demonstrated the improvement of LV systolic function after TAVI after 6 months and 1 year.[7] Other studies have investigated LV recovery at 5 ± 3 months[8] and at 3-month follow-up.[9]
Data concerning surgical prosthesis show a sustained LVEF recovery only 6 months after surgical interventions, probably because the extracorporeal circulation is responsible of inflammatory effects that delay the recovery of systolic function. On the other hands, TAVI allows an early LVEF recovery because the ventricle is not negatively affected by the intervention.
The general finding of a rapid recovery after TAVI is consistent with the findings from the Placement of Aortic Transcatheter Valve (PARTNER) trials.[5,6] In PARTNER A, ≈50% of patients demonstrated early LVEF recovery (≥10% absolute increase in EF at 30-day follow-up after SAPIEN TAVR). The mean EF between 30 days and 1 year after CoreValve TAVR is predominantly unchanged in the early LVEF recovery group (46.6 ± 11.6% vs. 49.0 ± 11.5%, P = NS). As was also seen in the PARTNER A trial, the CoreValve group without early LVEF recovery showed an increase in EF over the 1-year follow-up period: 31.5 ± 6.9% versus 43.3 ± 12.3% (P < 0.001). These congruent findings suggest that for the minority of patients without early recovery in the 1st day after TAVR, hemodynamic improvement may be still possible during the intermediate clinical course.
Recent studies have evaluated the effect of TAVI on reduced LVEF patients. Dauerman et al.[4] studied 156 patients from the CoreValve Extreme and high-risk trials with LVEF ≤40% at baseline who had 30-day LVEF data. Approximately two-thirds of patients showed a ≥10% increase in EF in the first 48 h after TAVR. Patients were more likely to have early LVEF recovery if they had a mean gradient of >40 mmHg and less baseline myocardial damage (as evidenced by previous MI).
This study wants to demonstrate, in particular, that patients with depressed LVEF before TAVI show a consistent (39.3% vs. 44.1%) and early LVEF recovery, just 1 month after the procedure.
Factors associated with a missed improvement were not directly analyzed in this study, but we supposed and verified a correlation with a severe and maladaptive hypertrophy in patients. In these patients, probably, the transition from left ventricular hypertrophy to fibrosis has resulted eventually in adverse effects on systolic and diastolic function.
Indeed, AS has to be considered a disease of the LV rather than purely affecting the aortic valve.[2] The excessive pressure overload associated to AS is responsible of shear stress, increase in pressure afterload and in ventricular wall stress, and finally of LV concentric hypertrophy.[3] Myocytes enlarge and wall thickness increases to restore wall stress and preserve left ventricular function. Patients display a broad spectrum of hypertrophic response. Evidence shows that rising levels of hypertrophy may be maladaptive. The landmark Framingham study has linked increasing hypertrophy with the progression of heart failure,[16] and left ventricular hypertrophy is now considered a marker of adverse prognosis across a number of cardiac conditions.[17] This hypertrophic process evolves in areas of fibrosis that occurs as a form of scarring after myocyte death and injury.
The transition from left ventricular hypertrophy to fibrosis results eventually in adverse effects on systolic and diastolic function, affecting LV compliance. In this analysis, indeed, growing state of hypertrophy (IVSd >16) has been associated to a lower recovery of systolic function, probably because at that point, hypertrophy become a pathologic entity.
Finally, comparing correlation between LV recovery in women and in men, we see that women have showed a better improvement. The reason of this discovery has to be better investigated in a larger study.
Study limitations
The present analysis has some limitations: first, the relatively small sample size. Second, the grade of hypertrophy was estimated with LV septal and posterior wall thicknesses, instead of mass calculation, as more properly indicated in the recent recommendations for cardiac chamber quantification.[18] Third, the assessment of left ventricular function by means of LVEF measurement can be not adequate at all, and more detailed evaluation of myocardial mechanics, myocardial remodeling, and flow is required to understand the pathology and assess the likely benefit to be gained from TAVI. Strain tissue echocardiography can provide diagnostic and prognostic information, and an integrated multimodality approach to evaluation can help identify those patients likely to benefit from TAVI when the clinical scenario is not clear-cut.
CONCLUSIONS
Patients with depressed systolic function show a consistent and early LVEF recovery after TAVI. An impaired LVEF recovery is most likely among patients with more than moderate hypertrophy, probably responsible of LV fibrosis that irremediably compromises systolic function.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS); Vahanian A. Alfieri O, Andreotti F, Antunes MJ, et al. Guidelines on the management of valvular heart disease (version 2012) Eur Heart J. 2012;33:2451–96. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 2.Badiani S, van Zalen J, Treibel TA, Bhattacharyya S, Moon JC, Lloyd G, et al. Aortic stenosis, a left ventricular disease: Insights from advanced imaging. Curr Cardiol Rep. 2016;18:80. doi: 10.1007/s11886-016-0753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carabello BA. The relationship of left ventricular geometry and hypertrophy to left ventricular function in valvular heart disease. J Heart Valve Dis. 1995;4(Suppl 2):S132–8. [PubMed] [Google Scholar]
- 4.Dauerman HL, Reardon MJ, Popma JJ, Little SH, Cavalcante JL, Adams DH, et al. Early recovery of left ventricular systolic function after corevalve transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2016:9. doi: 10.1161/CIRCINTERVENTIONS.115.003425. pii: e003425. [DOI] [PubMed] [Google Scholar]
- 5.Elmariah S, Palacios IF, McAndrew T, Hueter I, Inglessis I, Baker JN, et al. Outcomes of transcatheter and surgical aortic valve replacement in high-risk patients with aortic stenosis and left ventricular dysfunction: Results from the Placement of Aortic Transcatheter Valves (PARTNER) trial (cohort A) Circ Cardiovasc Interv. 2013;6:604–14. doi: 10.1161/CIRCINTERVENTIONS.113.000650. [DOI] [PubMed] [Google Scholar]
- 6.Passeri JJ, Elmariah S, Xu K, Inglessis I, Baker JN, Alu M, et al. Transcatheter aortic valve replacement and standard therapy in inoperable patients with aortic stenosis and low EF. Heart. 2015;101:463–71. doi: 10.1136/heartjnl-2014-306737. [DOI] [PubMed] [Google Scholar]
- 7.Kamperidis V, Joyce E, Debonnaire P, Katsanos S, van Rosendael PJ, van der Kley F, et al. Left ventricular functional recovery and remodeling in low-flow low-gradient severe aortic stenosis after transcatheter aortic valve implantation. J Am Soc Echocardiogr. 2014;27:817–25. doi: 10.1016/j.echo.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Poulin F, Carasso S, Horlick EM, Rakowski H, Lim KD, Finn H, et al. Recovery of left ventricular mechanics after transcatheter aortic valve implantation: Effects of baseline ventricular function and postprocedural aortic regurgitation. J Am Soc Echocardiogr. 2014;27:1133–42. doi: 10.1016/j.echo.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Giannini C, Petronio AS, Talini E, De Carlo M, Guarracino F, Grazia M, et al. Early and late improvement of global and regional left ventricular function after transcatheter aortic valve implantation in patients with severe aortic stenosis: An echocardiographic study. Am J Cardiovasc Dis. 2011;1:264–73. [PMC free article] [PubMed] [Google Scholar]
- 10.Elhmidi Y, Bleiziffer S, Deutsch MA, Krane M, Mazzitelli D, Lange R, et al. Transcatheter aortic valve implantation in patients with LV dysfunction: Impact on mortality and predictors of LV function recovery. J Invasive Cardiol. 2014;26:132–8. [PubMed] [Google Scholar]
- 11.Halkos ME, Chen EP, Sarin EL, Kilgo P, Thourani VH, Lattouf OM, et al. Aortic valve replacement for aortic stenosis in patients with left ventricular dysfunction. Ann Thorac Surg. 2009;88:746–51. doi: 10.1016/j.athoracsur.2009.05.078. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–643. doi: 10.1161/CIR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 13.Bauer F, Coutant V, Bernard M, Stepowski D, Tron C, Cribier A, et al. Patients with severe aortic stenosis and reduced ejection fraction: Earlier recovery of left ventricular systolic function after transcatheter aortic valve implantation compared with surgical valve replacement. Echocardiography. 2013;30:865–70. doi: 10.1111/echo.12171. [DOI] [PubMed] [Google Scholar]
- 14.Gulino S, Barbanti M, Deste W, Immè S, Aruta P, Bottari V, et al. Four-year durability of clinical and haemodynamic outcomes of transcatheter aortic valve implantation with the self-expanding corevalve. EuroIntervention. 2016;12:e1031–8. doi: 10.4244/EIJY15M10_08. [DOI] [PubMed] [Google Scholar]
- 15.Barbanti M, Petronio AS, Ettori F, Latib A, Bedogni F, De Marco F, et al. 5-year outcomes after transcatheter aortic valve implantation with corevalve prosthesis. JACC Cardiovasc Interv. 2015;8:1084–91. doi: 10.1016/j.jcin.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 17.Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ, et al. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778–85. doi: 10.1056/NEJM200006153422403. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–3.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]


