Abstract
Colonic perforation is a medical emergency that may be fatal if surgery cannot be performed in a timely manner. Colonic rupture in adults is caused by primary (idiopathic) and secondary factors. Although the segmental absence of muscularis propria (SAMP) is a recognized cause of secondary colonic rupture in neonates and infants, few cases have been reported in adults. Here, we present the case of a large colonic rupture caused by SAMP in a 60-year-old woman and a review of the literature. We suggest that SAMP should be included in the differential diagnosis of large perforation and/or periperforation membranous thinning of the colonic wall in adults.
KEYWORDS: Colonic perforation, Colonic rupture, Perforation of small intestine, Segmental absence of muscularis propria, Segmental muscular defect of intestine
INTRODUCTION
The segmental absence of muscularis propria (SAMP) is a rare entity that was first described in 1967 in neonates who presented with intestinal obstruction [1]. Since then, several reports about infants and neonates with various clinical manifestations such as intestinal obstruction, volvulus, intussusception, and perforation have been published worldwide [2]. The first adult case of SAMP manifesting as colonic perforation was published in 1997 [3] and several have been reported since then [4,5,6,7,8,9,10].
Physicians may be unfamiliar with SAMP because there are few cases and no reported specific clinical presentations. Until now, the etiology of adult SAMP has not been elucidated. Here, we report another case of adult SAMP and review the literature. We suggest that, despite its rarity, SAMP should be considered a possibility in the differential diagnosis of spontaneous, nontraumatic colonic rupture in adults.
CASE REPORT
A 60-year-old woman with hepatitis B and schizophrenia visited our outpatient department regularly. In the past, she underwent left nephrectomy for unknown reasons. On November 8, 2016, she was sent to our emergency department. The physical examination showed low abdominal tenderness with rebounding pain and hypoactive bowel sounds. The laboratory data revealed leukocytosis (white blood cell: 9.65 × 103/μL) with a slight predominance of neutrophils (neutrophils: 74.7%). Abdominal sonography showed ileus and stool impaction. Abdominal computed tomography revealed sigmoid colon perforation and pneumoperitoneum [Figure 1]. Under the clinical impression of a sigmoid colon perforation, the Hartmann procedure was performed. Intraoperatively, a perforated sigmoid colon was found, with fecal material throughout the abdominal cavity. The perforated sigmoid colon was resected. The patient received an uneventful course of treatment and was discharged 1 month after the operation. During the 6-month follow-up, her general condition was good.
Figure 1.
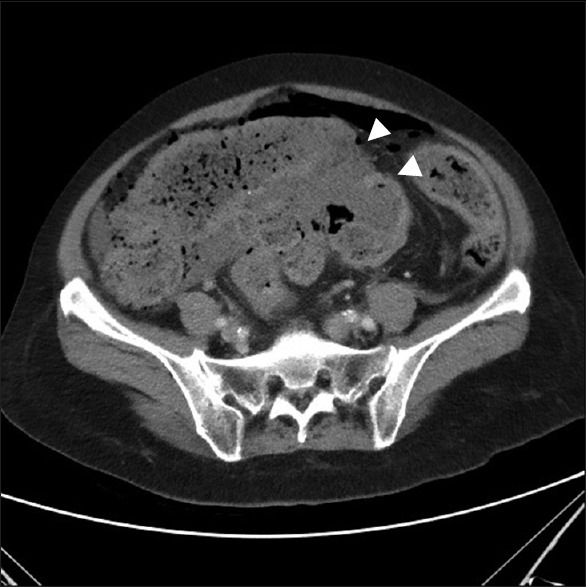
Abdominal computed tomography showed sigmoid colon perforation (arrowhead) with pneumoperitoneum and stool impaction
Grossly, the resected colon measured 24.2 cm in length and included a 4.1 cm × 2.5 cm perforation. The serosa was coated with red-brownish to yellowish fecal material. On sectioning, no polyp, diverticulum, or tumor was identified. However, thinning of the colonic wall was noted around the colonic perforation [Figure 2].
Figure 2.
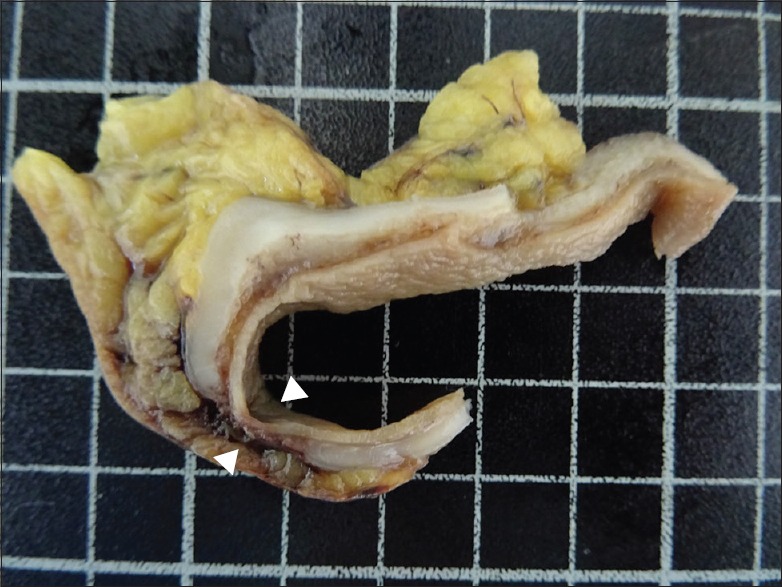
Gross findings in the colonic cut section showed an abrupt absence of muscularis propria (arrowhead) with sparing mucosa, submucosa, and serosa
Microscopically, the colonic wall around the perforation lacked a full-thickness muscularis propria and had a tapered off and blunt-end appearance but no necrosis, no significant inflammatory cell infiltration, no granulation tissue formation [Figure 3a and b], and no residual Auerbach's plexus (Meissner's plexus) in the region of the muscular defect. However, the mucosa, muscularis mucosae, and the submucosal and serosal layers were normally preserved [Figure 3c]. Near the perforation, there was focal ulceration with sloughing epithelium and necrotic debris in the mucosa; no thickening or disruption of fiber arrangement in the muscularis mucosae; vascular congestion, edematous connective tissue, mild inflammation, and adipose tissue replacement in the colonic wall [Figure 3d]; and necrotic debris, aggregated neutrophils, hemorrhage, congestion, and peritonitis at the perforation site. No sign of diverticulum, malignancy, vasculitis, or ischemic colitis was observed.
Figure 3.
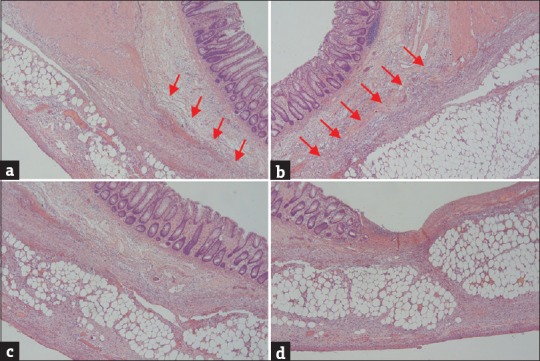
(a and b) The affected colon showed the full-thickness absence of muscularis propria (arrow) with a blunt-end appearance in the absence of necrosis, significant inflammatory cell infiltrates, or granulation tissue. (c) The mucosa, muscularis mucosae, submucosal layers, and serosal layers were normal in the absence of muscularis propria. (d) The mucosa showed focal ulceration with sloughing epithelium, necrotic debris, and aggregated neutrophils. The muscularis mucosae was normally preserved in the area of the muscular defect. The submucosal and subserosal layers revealed vascular congestion, mild inflammation, and adipose tissue replacement in the colonic wall (H and E, ×40)
DISCUSSION
Colonic rupture requires immediate surgical intervention. Most patients have underlying colonic diseases that progress to secondary colonic rupture, such as malignancy (lymphoma and metastatic carcinoma) and infection (tuberculosis and cytomegalovirus infection). However, some cases can be idiopathic. In the absence of specific pathologic findings, this case was regarded as primary idiopathic colonic rupture with remarkable microscopic features of SAMP.
SAMP was first reported by Emanuel et al. in 1967 as a new entity that caused intestinal obstruction [1]. Since then, SAMP has been increasingly reported worldwide, mostly in neonates and infants with predisposing risk factors such as prematurity, low birth weight, and comorbidities associated with prematurity [2]. Darcha et al. in 1997 reported the first case of SAMP in an adult [3]. To our knowledge, only 15 adult cases of SAMP (including the present case) have been reported in the literature [3,4,5,6,7,8,9,10].
The clinical features of these 15 cases are shown in Table 1. Patients ranged in age from 28 to 68 years, with a slight female predominance (female:male = 9:6). The clinical manifestations were perforation (13 cases, 87%) and distention (2 cases, 13%). The affected site was the large intestine in 9 cases (60%) and small intestine in 6 cases (40%). Its size ranged from pinhole to 4.1 cm at the greatest dimension. The clinical outcomes of most cases were good, except for 2 patients who died of pulmonary edema and renal failure at 3 and 21 days postoperatively, respectively.
Table 1.
Clinical findings of the segmental absence of muscularis propria in adults
| Case | Authors/year | Patient | Location and gross findings | Perforation size (cm) | Follow-up | |
|---|---|---|---|---|---|---|
| Age | Sex | |||||
| 1 | Darcha et al. [3], 1997 | 64 | Female | Sigmoid colon: Perforation | Unknown | Unknown |
| 2 | Tawfik et al. [4], 1998 | 34 | Male | Jejunum: Distended and adhesions | No perforation | Survive |
| 3 | Aldalati et al. [5], 2009 | Middle age | Male | Jejunum: Dilated and redundant with multiple wide-neck diverticula | No perforation | Survive |
| 4 | Procházka et al. [6], 2010 | 28 | Female | Ascending colon: 2 perforations | Unknown | Survive |
| 5-11 | Tamai et al. [7], 2013 | 44-89 Mean: 63.3 Median: 61 |
Female: 4 Male: 3 |
Jejunum (2): Perforation Ileum (1): Perforation Ascending colon (1): Perforation Sigmoid colon (3): Perforation |
Pinhole-sized to approximately 3 cm | No recurrence: 5 Died: 2* Died of other disease: 1 |
| 12 | Nandedkar et al. [8], 2015 | 48 | Male | Small intestine: Perforation | 1.0 | Survive |
| 13 | Rewhorn et al. [9], 2015 | 68 | Female | Distal sigmoid colon: Perforation | Unknown | Survive |
| 14 | Nawar and Sawyer [10], 2016 | 64 | Female | Descending colon: Perforation | 2.7 | Survive |
| 15 | Our case 2016 | 60 | Female | Sigmoid colon: Perforation | 4.1 | Survive |
*Died of pulmonary edema and renal failure at 3 and 21 days after the operation
The clinical manifestations of SAMP are apparently different in infants and adults. The involved site is predominantly the small intestine in infants and the colon, especially the sigmoid colon (6 of 9 colons, 67%), in adults. The most common clinical manifestations of SAMP are intestinal obstruction, necrotizing enterocolitis, volvulus, intussusception, and perforation in infants and perforation (87%) and segmental distended colon in adults.
Current SAMP pathogenesis hypotheses apply to neonates and infants because of the predominance of cases in infants. In most cases, SAMP is either congenital or acquired. Hypotheses include (1) abnormal embryogenesis leading to incomplete or discontinuous myogenesis; (2) small intestinal atresia and congenital absence of intestinal musculature due to vascular insufficiency in utero; and (3) ischemic events that postnatally injure both the mucosa and muscularis propria.
Tawfik et al. proposed that SAMP can remain asymptomatic until adulthood. Secondary factors such as previous surgery or ischemic events secondary to cardiovascular disease may aggravate the condition and trigger colonic rupture in otherwise asymptomatic adults [4,5]. However, ischemia cannot explain SAMP in adults who have no vascular disease, and additional etiologies must be further elucidated.
In this case, no evidence indicated recent ischemic change, and thus, we could not exclude congenital causes. We suggest that our patient may have had SAMP since childhood, and colonic rupture may have been precipitated by increased intracolonic pressure due to stool impaction or ileus secondary to previous surgery. Our patient took regular medication for schizophrenia and had had left nephrectomy.
CONCLUSION
The increasing number of case reports in recent decades indicates that the incidence of SAMP may be underestimated. We suggest that SAMP should be included in the differential diagnosis of perforation and/or periperforation membranous thinning of the colonic wall in adults.
Declaration of patient consent
The authors certify that the appropriate signed consent form was obtained from the patient. The patient gave her consent to publication of her images and other clinical information. The patient understood that her name would not be published, that all due effort would be made to conceal her identity, and that, despite such effort, anonymity could not be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Emanuel B, Gault J, Sanson J. Neonatal intestinal obstruction due to absence of intestinal musculature: A new entity. J Pediatr Surg. 1967;2:332–5. doi: 10.1016/s0022-3468(67)80213-6. [DOI] [PubMed] [Google Scholar]
- 2.Stephens D, Arensman R, Pillai S, Alagiozian-Angelova V. Congenital absence of intestinal smooth muscle: A case report and review of the literature. J Pediatr Surg. 2009;44:2211–5. doi: 10.1016/j.jpedsurg.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Darcha C, Orliaguet T, Levrel O, Pezet D, Lointier P, Chipponi J, et al. Segmental absence of colonic muscularis propria. Report of a case in an adult. Ann Pathol. 1997;17:31–3. [PubMed] [Google Scholar]
- 4.Tawfik O, Newell B, Lee KR. Segmental absence of intestinal musculature in an adult. Dig Dis Sci. 1998;43:397–9. doi: 10.1023/a:1018879011103. [DOI] [PubMed] [Google Scholar]
- 5.Aldalati O, Phelan C, Ibrahim H. Segmental absence of intestinal musculature (SAIM): A case report in an adult. BMJ Case Rep 2009. 2009 doi: 10.1136/bcr.01.2009.1425. pii: bcr01.2009.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Procházka V, Svoboda T, Soucek O, Kala Z. Segmental absence of the muscularis propria layer in the colonic wall – A rare cause of colonic perforation during pregnancy. Rozhl Chir. 2010;89:679–81. [PubMed] [Google Scholar]
- 7.Tamai M, Satoh M, Tsujimoto A. Segmental muscular defects of the intestine: A possible cause of spontaneous perforation of the bowel in adults. Hum Pathol. 2013;44:2643–50. doi: 10.1016/j.humpath.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Nandedkar SS, Malukani K, Patidar E, Nayak R. Segmental absence of intestinal musculature: A rare case report. Int J Appl Basic Med Res. 2015;5:222–4. doi: 10.4103/2229-516X.165378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rewhorn M, Oliphant R, Jackson A, Keltie R, Going J, Finn P, et al. Perforation of the sigmoid colon secondary to segmental absence of the intestinal musculature (SAIM) in an adult. Int J Colorectal Dis. 2015;30:143–4. doi: 10.1007/s00384-014-1957-0. [DOI] [PubMed] [Google Scholar]
- 10.Nawar NA, Sawyer PR. Segmental absence of intestinal musculature in a 64-year-old female: Case report and literature review. Am J Case Rep. 2016;17:749–54. doi: 10.12659/AJCR.900013. [DOI] [PMC free article] [PubMed] [Google Scholar]


