Short abstract
Clinical practice guidelines (CPGs) document evidence-based information and recommendations on treatment and management of conditions. CPGs usually focus on management of a single condition; however, in many cases a patient will be at the centre of multiple health conditions (multimorbidity). Multiple CPGs need to be followed in parallel, each managing a separate condition, which often results in instructions that may interact with each other, such as conflicts in medication. Furthermore, the impetus to deliver customised care based on patient-specific information, results in the need to be able to offer guidelines in an integrated manner, identifying and managing their interactions. In recent years, CPGs have been formatted as computer-interpretable guidelines (CIGs). This enables developing CIG-driven clinical decision support systems (CDSSs), which allow the development of IT applications that contribute to the systematic and reliable management of multiple guidelines. This study focuses on understanding the use of CIG-based CDSSs, in order to manage care complexities of patients with multimorbidity. The literature between 2011 and 2017 is reviewed, which covers: (a) the challenges and barriers in the care of multimorbid patients, (b) the role of CIGs in CDSS augmented delivery of care, and (c) the approaches to alleviating care complexities of multimorbid patients. Generating integrated care plans, detecting and resolving adverse interactions between treatments and medications, dealing with temporal constraints in care steps, supporting patient-caregiver shared decision making and maintaining the continuity of care are some of the approaches that are enabled using a CIG-based CDSS.
Keywords: Clinical decision support systems, clinical practice guidelines, computer-interpretable clinical guidelines, multimorbidity, patient-centred care
Introduction
Clinical practice guidelines (CPGs)1 document care instructions used by caregivers, representing high-quality best practice, based on available evidence.2 CPGs focus on specific health conditions such as diabetes, hypertension, chronic heart failure and obesity. The Guidelines International Network (GIN), the Institute for Clinical Systems Improvement (ICSI) and the UK National Institute for Health and Care Excellence (NICE) are examples of sources of such guidelines. Some of the major benefits of CPGs include supporting clinical decision-making, improving quality of care, guiding health resource use and decreasing healthcare costs.3–5
Since early 2000, many types of guideline-driven computerised platforms, also known as, clinical decision support systems (CDSSs) have been developed to support clinicians in the delivery of care.6,7 CPGs initially need to be formatted as computer-interpretable guideline (CIG)8,9 in order to be represented and executed by computers. This involves formalising the concepts included in CPGs in an unambiguous and computer interpretable notations. Ontologies are one type of such formalism.10 The existing CIG formalisms adopt different approaches that supply computer interpretable representations, such as ontologies,10 for knowledge acquisition, clinical task management and decision-making activities, along with execution engines that can run the CIGs on computers.11 For example, approaches include decision rule models (e.g. Arden Syntax12–14), documentary models (e.g. guideline elements model (GEM)15) or process-flow models also known as task-network models (TNMs)8 (e.g. Guideline Interchange Format version 3 (GLIF3),16,17 Asbru,18 SAGE,19 EON,20 GUIDE21 and PROforma22–24).
The prevalence of multimorbidity25 (i.e. patients with multiple diseases) increases with age. More than 95% of multimorbid patients age 65 years and over.26 Of them, 60% have at least two health conditions,27 and 58% constitute 78% of all GP patient visits.28,29 Multimorbid conditions affect each other, and are closely associated with mortality, severe disability, care variations, increased health resource use and costs.30 The management of multimorbid patients is complex because the number of risk factors increases with the number of clinical conditions.31,32 To handle these patients, care plans need to be customised for each individual considering their needs and conditions (e.g. allergies, syndromes, signs) as well as social information, which incorporate a number of multi-disciplinary stakeholders, such as nurses, doctors, therapists and clinical technicians.33 Patients have varied care requirements considering aspects such as allergies, preferences and drug intolerances. Patient preferences (e.g. meal times, or individual-monitoring schedules),34 in particular, are one of the main factors affecting patient non-adherence to guidelines, which increases the risk of undesired patient outcomes.
Nonetheless, few existing CPGs refer to multimorbidity; instead they consider conditions in isolation.35 Thus, following multiple guidelines, developed in isolation, may result in conflicting or inconsistent advice for therapy. For instance, multiple drug usage (i.e. polypharmacy36) offered by multiple guidelines, can lead to adverse interactions between drugs. Therefore, coordination complexity of multiple guidelines, the medications taken and dynamic changes in patient health states, as well as maintaining patient-adherence to guidelines, are some of the main reasons why patient-centred care (PCC)2 (which aims to personalise care and treatments to the specific needs and circumstances of each patient) is needed, especially, in the case of multimorbidity. Many existing CIG-based CDSSs can generate patient-tailored recommendations31,37–45 and enhance the guideline adherence of patients over paper-based CPGs.11,46
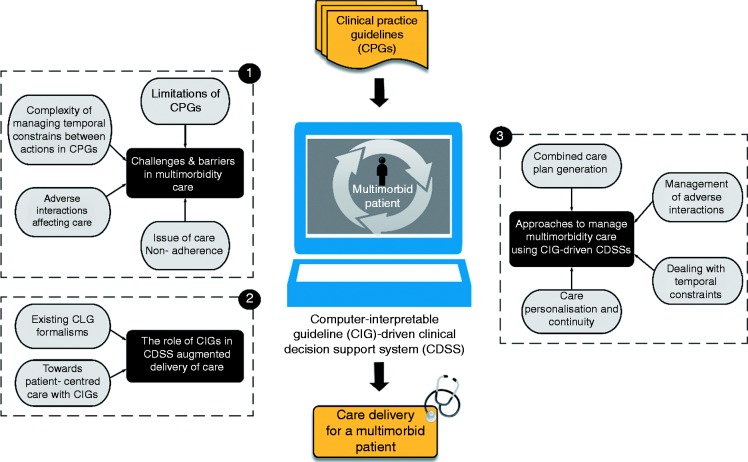
In this paper, we performed a systematic literature review on three strands that were identified as the main stages of integrating individual condition guidelines, to delivering actionable CDS recommendations to patients and caregivers (see Figure 1): (a) the major challenges and barriers in multimorbidity care; (b) the role of computer-interpretable guidelines in CDSS augmented delivery of care involving existing CIG formalisms, and the ability of CIGs to achieve a patient-centred care; and lastly (c) approaches to address the challenges of multimorbidity care using CIG-driven CDSSs.
Figure 1.
Scope of the review, under three themes.
The major challenges involve limitations of CPGs, the polypharmacy issue, conflicts occurring in patient-care flows, complexity of managing temporal constraints between actions in CPGs and non-adherences of patients to care. We reviewed a body of work proposing multimorbidity management approaches to cope with these challenges in a number of ways, i.e. studies that combine multiple guidelines and create a unified guideline in return.33,39,40,47–50 This helps to eliminate clinical task duplications and to provide health resource management. A number of works51–65 propose automatic discovery and resolution methods for dealing with adverse guideline interactions and their associated clinical knowledge constructs and some66–69 work on how to handle temporal constraints (e.g. start time, end time and duration of treatments) in multiple guidelines. These help caregivers to manage temporal interactions in guidelines and recommend safe care plans as a result. Lastly, some approaches31,37–45 aim to enhance adherence of multimorbid patients to guidelines and maintain their care continuity to improve the quality of care through using CIGs.
The scope of this review does not include CIG-based applications developed for single-disease management. However, some of the main applications are reviewed (see section ‘The role of CIGs in CDSS-augmented delivery of care’), as introductory material to provide more insights of the field to the reader.
Materials and methods
We reviewed the literature with the objective of finding the answers to the following research questions.
What are the obstacles faced in CPG implementations to supply care for multimorbid patients?
How can CIGs be used and applied at the point of care for the management of complex patients?
How can CIG-based CDSS approaches offer capability to improve the outcome of multimorbid patients, their caregivers and the treating medical centres?
To identify the relevant works, we adopted the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (www.prisma-statement.org) (Figure 2).
Figure 2.
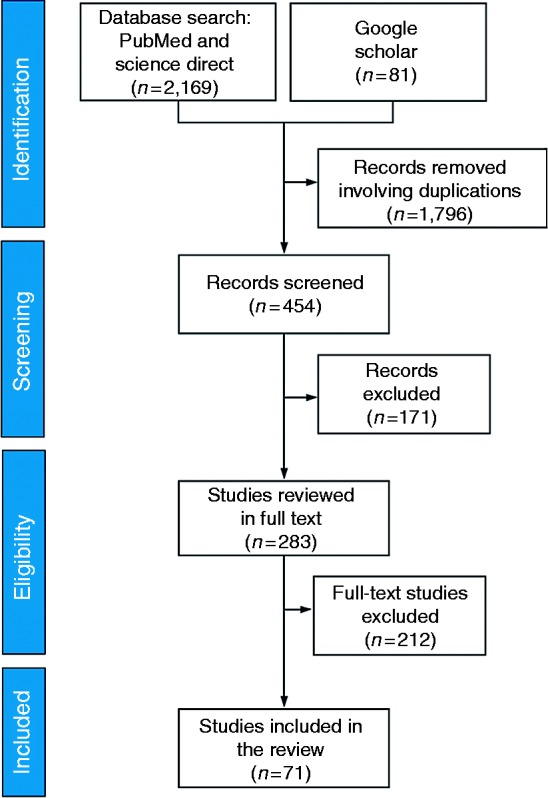
PRISMA publication search strategy along with the number of studies
Identification
We first selected and limited our search by querying Science Direct and PubMed for peer-reviewed publications on the management approaches to alleviate challenges and barriers for caring patients with many health conditions, using CIG-driven CDSSs. The search was conducted and included publications from January 2001 until 1 July 2017 by using the following terms: (‘clinical decision support systems’ OR ‘decision support systems’) OR (‘multimorbidity’ OR ‘comorbidity’) OR ‘adverse events’ OR ‘guideline adherence’ with the key terms (‘clinical guidelines’ OR ‘clinical practice guidelines’ OR ‘computer-based guidelines’ OR ‘computerised guidelines’ OR ‘computer-interpretable guidelines) OR ‘care workflows’ in title and abstract screening. Following the two-electronic database searches, we also conducted a Google Scholar (http://scholar.google.co.uk) search with the objective of potential inclusion of additional studies, again relevant to our search criteria.
In total, our electronic database searches yielded 9906 results. This number was obtained by searching the keywords that appeared in PubMed and Science Direct. In addition, 826 publication results of other sources (e.g. Google Scholar) were added, bringing the total to 10,732 publications. Afterwards, computer filtering was again applied considering the number of years and publication fields to refine subject fields related to Data Science, Medical Informatics, Engineering, Mathematics and Decision Support Systems. At this stage 8482 records were. Thus, 2169 results were obtained from the PubMed and Science Direct search and 81 results obtained from other sources (Figure 2). Afterwards, journal names were electronically filtered based on publication titles resulting in 1796 records removed for duplication.
Screening
We performed title and abstract screening by reading the remaining 454 papers. We excluded 171 publications that did not discuss computerised guidelines, computerised guidelines in the context of multi or co-morbidity care and/or its complexity (e.g. medication error, guideline non-adherence, adverse events).
Eligibility
Of the 283 remaining papers, posters, workshops and conferences papers that were also published in a peer-reviewed journal were excluded. Literature reviews were chosen as background and introductory material, but the aim of the review was to focus on primary sources and hence were excluded. Afterwards, papers that did not meet the eligibility criteria set were removed. These involved publications where no clear reference to the studied guideline was provided, unclear data collection strategy was used, or publications were of inadequate methodological quality. At this stage, 212 papers were removed in total.
Included
The remaining 71 were included in the analysis, and publications were categorised based on the chosen three themes accordingly. Selected full text articles were evaluated for their relevance and quality. Their inclusion in this review is outlined as follows: the need of formalisation of CPGs as CIGs and their application approaches to handle patients with multimorbid clinical conditions.
Our review aims to provide more insights into the role of computerisation of guidelines in the delivery of care for multimorbid patients, by addressing the contributions and limitations of existing works in order to indicate future research directions.
Results
The studies included in the current review were published between 2001 and 2017: 77% (n=55) of records were obtained from PubMed and Science Direct, and 23% (n=16) of records were obtained from the Google Scholar search, where 13 of them were conference papers published as book chapters, and three of them published in other databases. The top four journals with the largest number of articles were the Journal of Biomedical Informatics, Journal of the American Medical Informatics Association, Artificial Intelligence in Medicine and the International Journal of Medical Informatics.
In Table 1 characteristics of the included publications, in terms of publication year and the number of publications with their percentage distributions, are given.
Table 1.
Characteristics of the included publications (n=71).
| Characteristics | Number of publications (%) |
|---|---|
| Publication year | |
| 2001–2006 | 19 (26.76) |
| 2007–2012 | 12 (16.90) |
| 2013–2017 | 40 (56.34) |
| Searches | |
| PubMed | 26 (36.62) |
| Science Direct | 29 (40.85) |
| Google Scholar | 16 (22.54) |
Of the studies 26.76% (n=19) were published between 2001 and 2006, 16.90% (n=12) of them were published between 2007 and 2012, and 56.34% (n=40) of them were published between 2013 and 2017.
Theme identification
After reading 35 full-text papers and literature reviews, published in peer-reviewed journals on the life-cycle of computerised-guideline based CDSSs and their applications, we identified our themes.
The themes of the publications reviewed are presented in Table 2. The first two themes can be considered as the building blocks of the last theme. In other words, these two themes provide introductory and essential information for the last theme.
Table 2.
The themes of the publications reviewed (n=71).
| Themes | Publications |
|---|---|
| Challenges and barriers in multimorbidity care | 30, 31, 35, 37, 40–44, 51, 54, 61, 66–68, 71, 79, 92, 97 |
| The role of CIGs in CDSS augmented Delivery of care | 8, 11-24, 31, 33, 38-42, 45,47–64, 69, 74, 91, 101–106, 108–111, 113, 114, 119, 127 |
| Management of multimorbidity care using CIG-based CDSS approaches | |
|
33, 39, 40, 47–53, 55, 56 |
|
32, 61, 66–69 |
|
51–63 |
|
31, 33, 37, 39–45,71 |
Challenges and barriers in multimorbidity care
Co-existence of multiple health conditions in an individual is steadily increasing, and aging is one of the main factors of the occurrence of these conditions.27–29 The combinations of genetic functions, lifestyle choices, environmental issues, multiple drug usage, complications of past diseases and aging, generate patients with various combinations of multimorbidity. This creates several challenges and barriers to implement care for patients with multimorbid needs. The most prominent ones are: shortcomings of CPGs; complexity of managing temporal constraints between actions in CPGs; conflicting actions affecting care; and issue of care non-adherence.
Shortcomings of CPGs to treat multimorbidity
CPGs can offer substantial benefits for the healthcare system and patients, such as helping to reduce health costs, improving consistency and quality of care.3,4 However, they are not sufficient for developing personalised therapy plans, especially for multimorbid patients.35,70 For the treatment of a patient with multiple diseases, more than two CPGs need to be implemented along with associated clinical knowledge and patient data during the patient consultation. Evaluating the patient health status individually and developing a patient-tailored care plan as a result, is not a trivial task due to several reasons.
CPGs are often in the form of texts/schemas that cause difficulties to caregivers in the interpretation of guideline contents during patient–caregiver encounters, and subsequently their implementations in care.5,38 This also causes dissemination and maintenance difficulties (e.g. updates and versioning) across healthcare organisations.37,71 When handling a patient with multimorbidity, caregivers may prefer a personalised version of a guideline.71 CIGs facilitate this care-personalisation process (see section ‘Care personalisation and continuity’), as there can be instances of a CPG tailored to the specific information of a patient (e.g. preferences, allergies, drug intolerance and social needs), which due to their computer executable nature can also be adapted to dynamic changes of the patient’s status. Versioning of guidelines plays a significant role for managing them, as defined by Grandi et al.71 with several dimensions such as valid time (i.e. the time of a guideline belongs to the state-of -the art) and transaction time (i.e. the time the guideline is applied in a computerised platform). The chosen guideline version, and its personalised version, have to be mutually temporally consistent, which is important to evaluate whether the guideline is being properly applied by caregivers.37 Evaluating patient data, as well as the vast number of clinical knowledge elements manually, is a process susceptible to medical errors.72,73
In multimorbidity care, many CPGs need to be followed in parallel.51 However, CPGs are mostly designed for the treatment of a single disease, and there is little guidance on a CPG regarding how to merge strategies and recommendations to cope with multimorbid conditions, and the needs of these complex patients.54 The simultaneous combination of multiple guidelines is also prone to adverse interactions (see section ‘Adverse interactions affecting care’). For instance, a multimorbid patient may have obesity with hypertension along with ulcer, diabetes and depression, or stroke with coronary heart failure, with Alzheimer’s and stable angina. The concurrent implementation of a clinical task – start Aspirin – to treat a duodenal ulcer (DU) in the guideline for DU, and a clinical task – stop Aspirin – to treat a transient ischemic stroke (TIS) in the guideline for TIS, can cause conflicting actions due to their different goals (see Wilk et al.51). Moreover, different guidelines may recommend different drugs that can be adversely interacted with each other. Thus, conflicting and/or inconsistent actions need to be mitigated before supplying any care and treatment to a patient. This process is referred to by Wilk et al.51 as the guideline reconciliation problem. CPGs do not involve information about conflicting clinical actions given patients’ varied health conditions.
Detecting conflicts and inconsistencies in multiple guidelines during the patient consultation is crucial, but at the same time cumbersome, and error-prone task.
Complexity of managing temporal constraints between actions in CPGs
Therapies can be one time or spread over time. Correct timing of guideline actions plays a significant role upon achieving safe therapy implementation.68 Thus, caregivers need to perform proper time management and chronological ordering of clinical activities (e.g. laboratory tests, or drug recommendations) accordingly. However, the complexity of managing care plans grows with the number of patients’ health conditions.
Caregivers need to sequence all treatment steps, arrange parallel processes and consider time constraints such as start, end and duration of treatments/signs/symptoms, and frequency of interventions to be appropriate.66 When a caregiver is not experienced or needs to manage complicated care plans, there is an obvious need for help with time constraints that should be included in the guidelines. Piovesan and Terenziani61 supply the following instance about the potential sequence related interactions in guidelines. Calcium carbonate intake leads to alkalinisation of the urine, interacting with the nalidixic acid absorption. Thus, nalidixic acid should be given after calcium carbonate intake to avoid any conflicts. Arranging drug administration sequences can make both of them beneficial for the patient, and thus perhaps not causing any health risk. We refer readers to Anselma, Piovesan and Terenziani67 for further information on temporal interactions between guidelines. In ‘Approaches to managing multimorbidity care using a CIG-based CDSS’ below, we discuss how temporal interactions and constraints can be managed in guidelines.
Adverse interactions affecting care
A patient physiology can display pharmacodynamic (i.e. the resulting effect that drugs do on the body) or pharmacokinetic (i.e. the disposition of drugs through the body) changes.75,76 Pharmacokinetics can be affected by patient-associated factors such as demographics, allergies, genetic structures, or drug intolerances that may also affect the pharmacodynamics. For instance, the issue of polypharmacy36 (the use of multiple medications by an individual) has become one of the main concerns in caring for elder patients who are fragile and have multiple health conditions.77 CPGs do not adequately address the polypharmacy issues that can be induced by multimorbid conditions.35 Some of the main outcomes of polypharmacy-related issues are inappropriate medication prescribing, poor adherence to care and adverse drug events (ADEs)78 (i.e. an injury arising from medical intervention related to a drug).79 These are significant contributors of increased health risk, hospitalisation and subsequent increased health resource use and costs.80,81 For instance, ADEs constitute more than 6% of unplanned patient attendances and are responsible for 4% of hospital bed occupancy in the UK.82 ADEs are mainly preventable and arise from inadequate drug management.83
Treatments of multimorbid conditions involve both pharmacological (e.g. drugs) and non-pharmacological (e.g. patient-education, surgery, rehabilitation, psychotherapy, etc.) activities Pharmacological activities offered by each guideline are susceptible to adverse interactions with recommendations offered by other guidelines in varied forms such as drug–drug interactions, drug–disease interactions and drug–patient interactions that can reduce the efficacy of the care or affect the life expectancy of a patient.84 The two main classifications of interactions are single-action interactions and multi-action interactions. A single action interaction appears as two guidelines have different recommendations for the same therapy. For instance, drug-dose variation may occur where two guidelines recommend different dose levels for the same drug. Multi-action interactions appear when medications recommended by different guidelines interact with each other. Drug–drug, drug–disease, drug–patient as well as drug–food interactions, can be considered as multi-action interactions, see the GuideLine INteraction Detection Architecture (GLINDA)85 project, for further information on guideline interactions. Some of the widely occurring conflicts in guideline implementations are as follows.
Drug–drug interactions occur in the case of two (or more) drugs, which (in the case of multimorbidity) may be recommended by two different guidelines. There are two main groups of drug–drug interactions:86 (a) pharmacodynamic interactions that may occur when two drugs are taken together, and their concurrent usage causes serious health outcomes, and (b) pharmacokinetic interactions that may occur when one drug affects the other drug’s efficacy. Adverse drug reactions73 are, in general, linked with pharmacokinetic drug interactions.87 Overdose of medication, if it results from multiple guideline medication recommendations, can cause serious adverse reactions with other drugs, as well as with the physiology of the patient. Drug–disease interactions occur when the intake of a drug interacts with a disease. For instance, a patient with asthma should not use non-selective beta-blocking drugs.88 Drug–patient interactions occur if a patient has allergies or intolerances for (a) prescribed drug(s), and intake(s) of this drug(s) may have an adverse effect upon the patient.
To illustrate the above, let’s consider a patient with the following chronic diseases: diabetes mellitus and hypertension. These two diseases involve different sets of clinical information (e.g. drugs to be taken, or side effects) and associated care flows. In the study of Kovalov and Bowles,54 guideline interactions of these diseases are considered. Here, the medication Nadolol offered for the care of hypertension conflicts with diabetes that causes a major drug–disease conflict; the medication Sitagliptin conflicts with a patient characteristic that causes a moderate level of patient allergy; and the use of Metformin and Acarbose medications cause a minor drug–drug conflict. Consequently, conflicting activities need to be detected and resolved before any treatment provision to maintain safe care. Interactions can be between drugs and foods that occur when drugs interact with foods or beverages such as coffee, alcohol, orange juice, grapefruit juice, etc., that destroys or worsens the effect of drugs on the body. Lastly, CPGs mostly supply information about specific time elements such as the consecutive implementation of two certain drugs. These can temporally interact in time (i.e. two drugs interact with each other if they are administered within a specific time window).
Issue of care non-adherence
Patient-centred care can be defined as providing care that encompasses patient’s preferences,34 needs and values, and where patients have seized opportunities to take part in their care and treatment.2 This can be achieved through fulfilling the following fundamental aspects such as understanding patients’ feelings, and their personal context, finding common points of agreement and decisions for care management, increasing health support (e.g. increasing prevention, reducing risk and providing early detection of illnesses), and increasing the patient–caregiver relationship.89,90
Patient adherence, as defined by Christensen,93 is ‘the extent to which a person’s actions or behaviour coincides with advice or instruction from a health care provider intended to prevent, monitor, or ameliorate a disorder’. It can be affected by the patient him/herself (e.g. patient demographics, lack of information regarding disease and care), the disease (e.g. poor detection of disease symptoms), the treatment (e.g. the amount of drug intake, or existence of side effects), or the patient–caregiver partnership (e.g. patients’ preferences, doubts, beliefs and expectations shared with caregiver).94–96 Patient adherence to interventions decreases when the amount of medication use increases, because multimorbid patients need to understand and follow a significant amount of medical information regarding several medications.97 Thus, maintaining patient adherence to recommended interventions is a crucial factor in decreasing the risk of hospitalisation and in improving patient outcomes.92,96 To do so, the following three steps need to be considered. The first step is to achieve patient–caregiver agreement on a care plan, which is the product of their shared-decision making, as defined by Elwyn et al.,98 ‘where patients and caregivers make decisions together using the best available evidence by considering available care or management options with advantages and harms of each so that they can communicate with patients’ preferences and help to choose the best practice for them’.
Through shared decision-making, patient preferences (such as therapy choices or daily life preferences) can be involved in care-personalisation process, which especially enhances patients’ adherence to care plans by supplementing more insights into the treatment and thus reducing anxieties, and providing alternative options of care.99,100 The second step is to apply care based on the agreed care plan, and the last step is to pursue care according to an agreed time.95
Nonetheless, CPGs face integration difficulties in a PCC process and, they alone, are not best suited for showing adaptation to shared decision-making between patient and caregiver, patient preferences or requests while providing a care plan. To customise care for each patient, caregivers need to interpret clinical guidelines and patient’s input individually. However, this is not straightforward in the case of multi-morbidity, which involves multiple guideline interactions and integration of numerous clinical knowledge elements. Patients with many conditions who are in general are elder, fragile people, may have disabilities (e.g. cognitive impairment)30 and limited possibility to visit their caregivers. Hence, these people may need remote support (e.g. taking medication warnings or care modifications based on actual health state) to appropriately continue their care without interruptions. Several authors31,40–44 propose guideline-based computerised systems that have been used to assist caregivers (e.g. involving patient preferences in the delivery of care) to achieve patient-centred and continuous care. We review these approaches in ‘Care personalisation and continuity’ below.
In the following section, we discuss the existing CIG approaches and the life-cycle of a patient care journey with a CIG-based CDSS to address how computer support can be integrated with healthcare services, whilst offering benefit for the complexity of customising patient care plans for multi-morbid patients.
The role of CIGs in CDSS-augmented delivery of care
Decision support systems (DSSs) unify large amount of knowledge in one platform to help users in their decision-making processes. There are many types of DSSs (e.g. data, model, document, communication, or knowledge oriented) that differ based on their capabilities and scope.100 For example, knowledge-based DSSs are one of the widely used systems in clinical settings to provide clinical decision support, see for example, Zhang et al.101 and Goldstein et al.102
In the clinical context, the general purpose of CDSSs35 is ‘providing clinicians or patients with computer-generated clinical knowledge and patient-related information, intelligently filtered or presented at appropriate times, to enhance patient care’. CDSSs are designed to help caregivers for a variety of clinical issues such as data access, disease diagnosis and prognosis, treatment, monitoring and prevention. They also offer early warnings103 to caregivers on potential issues that may not be seen in time, due to the complex structure of morbidity.
Existing CIG formalisms
Guidelines are usually represented in the form of ontologies10, which are used to define knowledge to be expected by computerised systems. Ontologies have been used for knowledge representations by supplying formal and clear definitions of data in healthcare studies. Similarly, ontologies can be used to capture the knowledge in clinical guidelines, enabling the development of computerised techniques to discover, and coordinate many types of interactions between recommendations produced from guidelines, and to facilitate knowledge sharing and dissemination across professionals and institutions.
Based on the requirements for automatic application of CPGs to support caregivers in their clinical actions, many formalisms and supporting tools have been developed to make guidelines computer-interpretable, and to cope with their complexities and associated clinical knowledge constructs. Some of the well-known approaches include languages used to represent and structure information in guideline documents (e.g. guideline elements model (GEM)15); frame-based models (e.g. GASTON127); rule-based models which consider algorithms to establish information flows in guidelines such as Arden Syntax.12–14 Task-network models (TNMs)8 represent guidelines as graphical networks of tasks, defined as hierarchical graphs, in which nodes denote the actions to be executed and arcs denote the discerned relationships between them such as GLIF3,16,17 Asbru,18 SAGE,19 EON,20 GUIDE,21 Proforma,22–24 and GLARE.69,104,105
A GEM15 uses an XML (extensible mark-up language)-based knowledge model to represent heterogenous information (e.g. multiple recommendations) involved in guidelines. It supplies a standardised representation of guideline contents but not fulfil the logic of a guideline which unfolds over time. For instance, BRIDGE-Wiz application (building recommendations in a developer’s guideline editor) that built upon GEM, supports and helps the development of guidelines and the authoring of their implementable care recommendation statements.106
Arden Syntax12–14 has been developed as a rule-based language and uses medical logic modules (MLMs) (i.e. data, event, logic and action slots) maintained by a Health Level Seven (HL7) International standard (www.hl7.org) for clinical knowledge representation and execution. However, MLMs have limited capability in identifying the complicated interacting recommendations of guidelines and coping with the representation of temporal constraints, such as repetitions, starting and end time of clinical actions. To resolve these issues, TNM-driven guideline representations have been proposed that provide modelling primitives, describing the steps of CPGs and the temporal relationships between tasks.
TNM-based approaches may involve several task models like plan, action and decision. Plan denotes the collection of tasks that aims to achieve a certain objective. Action denotes the collection of tasks such as medication prescription, or tests that need to be performed during the execution of a guideline. Decision denotes the rules associated with conditions that are shaped with the patient’s health states. A significant portion of formalisms use TNMs that include patients’ states, execution states, eligibility criteria, classification schemes, goals, decisions and actions.64
GLARE69,104,105 was developed as a graph-based model, in which vertices represent actions to be executed and edges represent relations between them. GLARE uses two types of actions:74 atomic actions (e.g. work actions, pharmacological actions, decision actions, query actions and conclusions), and composite actions (plans). Atomic actions represent actions in a CIG, composite actions represent their components. GLARE represents a wide set of temporal constraints, treatment repetitions and periodicities in CIGs.104 Bottrighi and Terenziani105 introduced a recent extension of GLARE, called META-GLARE, that supports fast prototyping of clinical tasks.
Asbru18 is a task-specific, time-oriented (see ‘Discovery and resolution of adverse interactions’ below ) and intention-based language107 that developed to represent CPGs and their inter-relationships as a group of skeletal plans (i.e. possible steps in a CPG) in XML involving knowledge roles such as preferences, intentions, conditions and effects. Picard103 execution engine is designed to execute CIGs, encoded in this formalism. Asbru has been used in many projects (e.g. González-Ferrer et al.38, Peleg et al.41) and OncoCure CDSS project111 is one of that helps the oncologists in their decision-making phases for the treatment of breast cancer patients.
PROforma22–24 was designed to represent and execute medical knowledge in guidelines as a set of tasks such as decision, action, enquiry and plan, and data elements. Decisions, actions and enquiries are atomic tasks whereas plans are collections of tasks denoting the objective of the treatment. A clinical task is linked with an objective, defined in the red representation language (R2L), which is a time-oriented, control-flow representation language. Then, R2L is translated into a language based on predicate logic, called logic of R2L (LR2L). Plan can describe logical and temporal constraints. Tallis tool of Proforma22 is used to support authoring, publishing and execution of guidelines. Proforma representation was used in Health Care Services (HeCaSe2)45,108 that suggested an agent-based healthcare system for modelling CPGs and their interactions between agents (e.g. nurse, cardiologist, physician).
GLIF316,17 involves action, decision, branching and synchronization steps. The support language of GLIF3 is Resource Description Framework (RDF). Unlike Arden Syntax, GLIF3 can manage complex guidelines with many care steps.109 In Peleg et al.,110 web-based interactive clinical algorithms were developed based on this formalism for the sequencing of tasks to analyse patients with particular clinical conditions. We refer readers to Mulyar et al.11 where the workflow patterns of Asbru, GLIF and Proforma were extensively compared.
Lastly, SAGE19 uses activity graphs that define the relationships among several clinical and computational actions in terms of a workflow process model. SNOMED-CT (i.e. medical vocabulary)112 and LOINC (https://loinc.org/) are adapted in SAGE for the use of terminologies and ontologies. In SAGE, guideline ontology is represented in RDF format. Like GLIF3, SAGE is also supported by GELLO113, based on HL7 reference information model (RIM). SAGEDesktop114 is the testing tool of SAGE that can test one CIG implementation at a time.
The limitations of the existing works are mainly about guideline interoperability (e.g. merging concurrently applied more than two guidelines) and/or execution strength (e.g. adverse interaction detection and resolution).
Towards patient-centred care: The life-cycle of a patient care journey with a CIG-based CDSS
CIG-based CDSSs can provide access to, and thus inform caregivers of, updated clinical and patient data.38,91,119 They can use the information and recommendations, which are generated from the system, to discuss with their patients how to customise care for them. The main data sources of such a system may involve: (a) CPGs, and care work flows (encoded as CIGs); (b) patient data obtained from other information sources such as electronic health records (EHRs), medication sources, and health information systems (HISs); and (c) experiences from patient–caregiver encounters. Some of the objectives of CDSS applications that supplement CIG-based recommendations, are to provide remote care31,41,45 to improve the patients’ satisfaction and reduce their health risks,42 or to support caregivers in managing patients with multiple health conditions.33,39,40,47–63 The use of CIGs and their integrations into the care can be synthesized into three stages. To this day, only a limited number of works have covered all the specified stages, with some limitations (see ‘Approaches to managing multimorbidity care using a CIG-based CDSS’ below).
At the first stage, patients are usually registered to an EHR system of a medical centre. Studies have shown that the integration of EHRs with CDSSs provides considerable improvement in patient safety and economies of scale for hospitals in reducing length of stays and health costs.120,121
The second stage involves the patient consultation process, wherein treatment goals and scheduling of care steps are specified. The patient shares their concerns, doubts, beliefs, or requests about illnesses with the caregiver. CDSS can initially be used to get more information about the patient and make initial judgements about the patient’s care going forward; then, the caregiver may provide assessments about this patient to the system along with the patient’s input in order to initialise the analysis and decision-making. This information relates to the evidence-based CPGs as well as several data obtained from, for example, HISs, EHRs, medical devices, such as physiological monitoring or treatment equipment, etc. The guideline formalisation process begins with the medical staff’s clinical knowledge interpretations of CPGs; CPGs involve definitions and descriptions of clinical procedures, aims and specific objectives to achieve it with associated recommendations. Afterwards, a knowledge engineer employs one of the formalisms to represent and share the medical knowledge as CIGs using their execution engines (e.g. Picard103 for Asbru and Tallis110 for Proforma).
Further procedures can also be provided by other caregivers, independently, or can be jointly conducted by many of them (e.g. nurse, physician, diabetologist) that are not necessarily co-located with the support of a CDSS. Hence, the interactions between patients, caregivers and the system itself reflect in the produced care plan, which serves as a map of care flow. CIG-based CDSSs can also help to detect and resolve (possible) conflicts before supplying any care, to predict health risks of an existing treatment or future likelihood of a disease, and to make updates when necessary, to provide a safe care plan for this patient.
At the last stage, patients are discharged. Nevertheless, observations of daily living of patients, such as blood glucose level, blood pressure, physical activity or medication intake, could be further collected using mobile applications or web services, which can then be integrated with patient data repositories and EHR systems within a CDSS (see Peleg et al.41,42).
Approaches to managing multimorbidity care using a CIG-based CDSS
In this section, we discuss the approaches used in the management of multimorbid patients through CIG-based clinical decision support technologies. We first review methods that use CIGs to create a combined care plan. Afterwards, we review methods to deal with temporal constraints in multiple guidelines; the issue of polypharmacy and management of adverse interactions in guideline actions and associated knowledge elements and finally, we address CIG-based approaches used for care personalisation and maintaining continuity of care.
Combined care plan generation
Over the past decades, many methodologies have been developed to cope with the unification and execution of multiple CIGs. 33,39,40,47–50 The Semantic Web based formalism, is one of the broadly applied approach that merge many CPGs by initially formalising them as CIGs. Semantic Web technologies such as the W3C web ontology language (OWL),122 is characterised by formal semantics that have been used to represent clinical knowledge in CPGs. For instance, Abidi et al.48 proposed an OWL-based CDSS to represent multiple guidelines and generated a unified knowledge model for handling comorbid patients. In the study of Jafarpour and Abidi49, multiple guidelines were merged using the merge criteria. To do so, a merging representation ontology was developed to identify the potential merge points between guidelines. OWL-driven execution engine and SWRL (semantic web rule language)123 rules were used to achieve guideline merging according to the merge criteria. The major common limitations of these works were representing and merging more than two tasks of concurrently implemented guidelines. In their later work,50 the authors extended their guideline execution approach. Initially, they used OWL1 DL (description logic)-based124 execution engine to represent clinical task transitions between executional states and rules for managing the clinical task satisfaction criterion. Afterwards, OWL2 DL-based execution engine was used that provide more functionalities (e.g. cardinality restrictions and data type expressivity) than OWL1 DL. Here, OWL2 DL supports automatic comparisons of patient values with predefined values. Lastly, an OWL2 DL + SWRL-based guideline execution engine was used that also supports mathematical calculations and iterative clinical actions. Authors emphasized that combined guideline execution approaches supply more executional performance for reasoning on complicated guideline workflow patterns like iterative clinical actions than OWL1 DL. Lack of representation and execution of temporal constraints in guidelines were the main limitations of this work.
There are also other approaches for merging guidelines such as Riaño and Collado33 that adopted a divide and conquer approach (i.e. divides the problem into sub-problems until it can be solved) to merge many treatment plans of multiple guidelines considering the severity of the patient disease. Here, three main knowledge elements were considered: decision elements which are related to the acuteness of a patient condition; action blocks denoting a set of actions such as tests to discover the severity of a condition; and table blocks denoting the treatment matrices involving treatment, patient symptom and the recommended treatment. However, concurrency relations of multiple CIG actions and how to handle parallel tasks were not discussed.
Logic-based methods (e.g. Wilk et al.51–53 and Michalowski et al.55,56), which are formal approaches to representing and reasoning the knowledge involved in CPGs, are also widely used in the literature for combining care plans of multiple guidelines. Since merged guidelines may involve duplicated clinical actions (e.g. laboratory tests, examinations, medications) and possible contradictory or inconsistent actions, these should be discovered and eliminated before initialising any care to ensure patient safety. In the subsequent sections, we address these issues and associated works.
Dealing with temporal constraints
Clinical actions defined in CPGs have to be performed according to a set of temporal constraints.68,125 These constraints can be qualitative (e.g. simultaneously, after, or before) or quantitative (e.g. days, delays, or durations such as ‘3 consecutive days’, and clinical task T1 starts ‘1 hour’ after clinical task T2) constraints between clinical actions, periodic/repeated actions or the temporal constraints which can be the part of the relations between these actions.32 To avoid the occurrence of any duplications and/or conflicts in care steps, temporal statements need to be checked for their validity when implementing guidelines.61,66 Temporal constraints are particularly important to apply correct prognosis,126 and multiple medication administrations in a certain time window. Anselma et al.67 presented an instance on how temporal knowledge about the medication Anticoagulant has an impact upon the medication action of Warfarin administration.
To date, several formalisms, such as Arden Syntax,12–14, GLIF3,16,17 EON,20 GUIDE,21 PROforma,22–24 GASTON,127 and SDA128 have been used to represent temporal constraints in guidelines and their associated clinical processes. However, Asbru18 supplies the most temporal functionality. It is a time-oriented and intention-based language that can denote temporal patterns of clinical actions (e.g. offer a specific medication) or patient states to be attained, or avoided.107 It also uses time annotations to constrain the temporal occurrences of plan elements such as in starting and finishing interval and duration interval of a clinical action.38 Intervals are stemmed from instants that denote certain time points on the time frame and bounded by the two-time instants. For instance, duration interval can be represented as a [minDur, maxDur] tuple.125 Temporal reasoning approaches have also been used to improve expressiveness of guidelines, handle inconsistencies and detect interactions occurring between CIG actions. For example, Duftschmid et al.68 proposed a simple temporal problem (STP)-driven temporal constraint propagation method based on Asbru to discover temporal inconsistencies in clinical activities. They checked the consistency of temporal scheduling constraints that was implied by the guideline’s care flow and ordering of CIG actions. The main limitation of this work was verifying temporal constraints on the execution of unordered sequential clinical tasks in guidelines. Like Asbru, GLARE can deal with complex temporal constraints in CIGs.
For instance, Anselma et al.66 proposed temporal guideline formalism based on GLARE to represent temporal constraints in clinical guidelines, and used a constraint-based temporal reasoning approach to detect inconsistencies and get minimal temporal constraints among them. Initially, non-repeated tasks of CPGs were modelled using STP,129 whose temporal constraints specify single intervals on any temporal location. STP can supply the minimal temporal constraint network that can be defined as a directed graph where vertices denote the time point of the existence of specific actions, and edges denote the temporal distances (intervals) between these actions.68 These intervals were used for the execution of guideline actions. Since repeated, periodic and composite actions cannot be handled with STP, STP-tree approach was adopted that the root of the tree consists of the action representing the whole CPG. This approach has also been adopted by Anselma et al.32 and Bottrighi et al.69 for temporal reasoning. The main contribution of this work was to enable caregivers to check the possible temporal interactions between multiple CIG actions that can happen in time. Handling disjunctive constraints (e.g. non-overlapping constraint) such as performing actions which have unordered precedence relations at different times, and assumptions made on the applications of the instances of actions, were the major limitations of this paper.
Even though several studies deal with the representation of time in CIGs and consider temporal reasoning methodologies, only a few of them have focused on multimorbidity. In their recent work, Anselma et al.67 suggested a methodology for detection and analysis of temporal interactions between the CIG actions based on the extended GLARE104,105 formalism. They coped with the CIG actions, intensions (goals) and effects (variations), and interactions (e.g. intention, variation and drug interactions) that happen in time. The authors proposed a temporal ontology which is able to represent temporal constraints in CIGs that involve temporal constraints between CIG actions, in logs that store execution times (e.g. days) of the CIG actions implemented on the certain patients, and in medical knowledge that represents temporal constraints between clinical actions, their effects and interactions. They adopted a STP-based temporal reasoning approach.32,61,66 Because of the temporal reasoning restrictions (e.g. partonomic, class-instance or hypothetical reasoning) of this approach, Anselma et al. extended it with the use of Floyd-Warshall’s algorithm to STP constraints. Thus, temporal interactions can be detected by reasoning over the ontologically-represented guideline knowledge. The major limitation of this work was the lack of practical implementation such as considering different guidelines that have varied conflicting actions.
Consequently, CIGs significantly improve the timeliness of care processes.130 Dealing with temporal constraints and performing reasoning about them, play a chief role on providing a safe treatment plan to achieve consistency and avoid adverse interaction of care actions that overlap in time.
Discovery and resolution of adverse interactions
Adverse interactions can be induced by contradicting targets of the guideline actions, the effects of CIG actions, the medication conflicts offered by different guidelines or inappropriate timing of medical processes.59 Discovery and resolution of them are imperative to generate reliable and safe combined therapies. Studies have demonstrated that CIG-driven computerised systems facilitate the elimination of medication administration errors and ADEs by recommending safe drug dose levels, arranging drug frequencies and associated durations of medications. For instance, Koutkias et al.64,65 proposed a CIG driven clinical decision support model based on GASTON127 to help identification of drug safety risks and produce alerts and recommendations for caregivers to prevent ADEs.
Besides the use of logic-based models for representing and merging knowledge elements of guidelines, they have also been used for discovering adverse interactions caused by the synchronous implementation of multiple guidelines. Wilk et al.52 proposed a constraint logic programming (CLP)-based115 model to represent guidelines and mitigate conflicting clinical actions which may occur between pairs of concurrently applied CPGs in order to provide guidance to caregivers for revising therapies in managing multimorbid patients. This work built upon their previous work which expanded with the involvement of interaction and revision operators for describing required therapy modifications, and new mitigation algorithm to identify and address adverse interactions.51 In this paper, guidelines were represented as actionable graphs (AGs) that are directed acyclic graphs (DAGs) used for representing guidelines, and involve incomplete information. Yet, there were several assumptions made regarding the model (e.g. temporal constructs of CPGs were not considered) and mitigation algorithm (e.g. iterative clinical tasks were not considered and only binary variables were used). In their latter work,55 assumptions related to the mitigation algorithm that can handle cycles and numerical measurements were relaxed, while reconciling guidelines. Medication dosage adjustment was supported.
The issues of temporal and related precedence relationships between guideline actions were addressed in Michalowski et al.56 To handle them, authors extended CLP to first-order logic (FOL) theories for developing a generalised mitigation framework. The major shortcomings of this work were the need to automate the maintenance of the precedence relationships between guideline actions, and the lack of parallel tasks and temporal characteristics. A similar adverse interaction mitigation strategy was proposed by Zhang and Zhang,57 which adopted the answer set programming (ASP) -based131 approach. Like Wilk et al51,52 and Michalowski et al.55, the authors identified conflicting actions between treatments offered by two guidelines and then used mitigation operators to modify them. Zhang and Zhang57 provided mathematical definition of the mitigation process that aimed to supply more insights into readers about how conflicting actions can be identified, addressed and modified in two concurrently applied guidelines.
In the recent work of Wilk et al,53 they addressed limitations of their previous works51,52,55 such as handling parallel tasks of multiple guidelines while generating a reconciled treatment. Besides parallel clinical tasks, temporal actions like time offset (i.e. lag between care steps) and duration in care steps were also considered. Patient-preferences were involved while generating combined care plans for multimorbid patients. To do so, authors introduced preference related revision operators to modify treatments. The major limitations of this study were the lack of complex decision nodes (i.e. more than two options) that represent real-world cases, with many decision options and lack of practical applications to prove the efficacy of their approach. A similar study, proposed by Kovalov and Bowles,54 translated clinical information into logical expressions. However, the authors mainly focused on drug conflicts offered by different guidelines and represented care pathway using pharmaceutical graphs (i.e. DAGs whose nodes denote drug administrations). Additionally, the SMT solver was used to extract the set of drugs with their levels of conflicts (e.g. safest) to be offered to caregivers. The major limitation of this work was the lack of discussion on characteristics of guidelines, such as temporal constraints and drug dose information.
While some works (e.g. Wilk et al.51–53 and Michalowski et al.55) use AGs or pharmaceutical graphs,54 some58,117,118 prefer other methods such as petri nets-based models to represent guidelines. For instance, Tan58 presented the situation calculus ontology of petri nets (SCOPE) framework for the mitigation of adverse interactions on CIG actions based on petri nets and situation calculus132 that enable users to handle iterative complex actions, represent time in guidelines, facilitate handling parallel paths and perform execution-time modifications. SCOPE aimed to generate combined therapy plans without adverse interactions. The major limitations of this work were the manual mitigation of two guidelines and not being able to adapt to execution-time modifications. There was also no evidence on the applicability and efficiency of their method when more than two guidelines are concurrently implemented.
Zamborlini et al.62 is one of the significant works that extensively focuses on how to handle interactions. The authors proposed a transition-based medical recommendations model for detecting interactions called TMR4I, which is able to infer and classify interactions between multiple recommendations within multiple CIGs. They demonstrated that interactions may not only occur in CIG actions which were mainly considered as drug–drug interactions in the existing works,51,55 but also in CIG-independent interactions. In TMR4I, two types of interactions were defined as internal interactions (e.g. repetition interaction because of the same action, contradiction interaction because of the inverse transitions and/or same action, and alternative interaction because of the inverse transition) and external interactions (e.g. incompatible drugs and alternative drug interactions). Authors introduced FOL rules to detect these interactions and used external information source, DrugBank (www.drugbank.ca), for automatic detection of drug interactions. OWL122 and SPARQL133 were used for the implementation. In their latter work,63 they extended the interaction detection approach62 by enhancing its reusability of FOL rules to detect interactions and involving systematic evaluation of the interaction types. Lack of temporal constraints (e.g. duration, delay, frequency) in CIGs and temporal interactions between their actions were the major limitations of these works.
While some papers have been published on single reasoning standards, like agent-based modelling45,69,134 or logic-based modelling,51–53,55–57 a number of them focus on multiple reasoning-based standards. For instance, Piovesan and Terenziani61 extended their previous works59,60 and proposed a mixed-initiative approach based on GLARE formalism for discovering alternative ways to reconcile guidelines while handling adverse interactions on CIG actions. To do so, three reasoning methodologies were introduced. These involve a backward CIG navigation approach to extract alternative CIG care paths; temporal reasoning approach to analyse if an interaction occurs in time; and goal-based planning approach to provide caregivers a set of interaction management options (e.g. safe alternative option, dosage adjustment and effect monitoring) to follow. OWL-DL and SWRL rules were used for the implementation. Enhancing reasoning capability by recommending the most suitable option to caregivers given many options was addressed as a future work.
Care personalisation and continuity
To integrate CPGs into the patient-centred care, several CIG-based CDSSs have emerged to personalise guideline knowledge to supply patient-tailored recommendations, considering patients’ multiple health conditions, clinical history and preferences, that help to enhance patient guideline adherences.130 Some of these works also supply remote personalised care and support care continuity of patients. Remote care support helps to reduce care costs (e.g. decrease health resource use), improve the mobility and independence of the patient, as well as provide treatment for elder patients with multimorbidity that may have mental and/or physical disability.42
Isern et al.45 suggested an agent-based K4Care (knowledge-based home-care e-services for an ageing Europe) platform that supplies personalised home-care services for patients with multiple conditions. To personalise care, each patients’ health conditions and their social context were considered. State-decision-action (SDA)-based128,135 formalism was adopted which represent CPGs as diagrams with a set of variables to determine the health condition of a patient; to choose a clinical or administrative task among a set clinical or management options, to represent the clinical or administrative tasks. As the part of the K4Care project, Riaño et al.40 proposed methodologies for personalisation of patients’ conditions (e.g. clinical and social information about the patient), and intervention plans to discover clinical and social inconsistencies in the patient data. Authors represented CIGs as SDA diagrams and presented a visualisation tool to edit and unify the diagrams of all intervention plans recommended for a multimorbid patient. The major limitations of these two works were on the generation of patient-specific intervention plans where the combination of therapy plans and personalisation processes were manually performed. This may also limit the generation of alternative interventions when the number of diseases grows. In their later works,33,39 therapy plans were combined, considering the patient’s health conditions and adverse drug interactions; however, interactions can occur in many different levels of CIG actions (see Piovesan et al.50).
Likewise, Lasierra et al.31 proposed an ontology-driven home-based tele-monitoring system for complex patients. To do so, patient profile ontology was introduced that involves patients’ measurement results (e.g. weight, blood pressure, glucose, pulse), patient information and indications obtained by caregivers to manage the patient’s health condition. Caregivers can generate customised CIGs for each patient. Nonetheless, lack of distinction was made between CIG knowledge customisation level, which covers all patients with varied multimorbid conditions, and personalisation level with patients’ contextual data that’s associated with the customised guideline constructs.
In many studies, the shared-decision making process was considered as an integral part of a CDSS, that guideline-driven advice can be addressed when needed and alternative care recommendations can be obtained involving patient’s preferences and personal context (e.g. wedding, or holidays). For instance, Quaglini et al.43 proposed a methodology for integrating shared decisions into a CIG-based CDSS. This work was the part of the MobiGuide project (www.mobiguide-project.eu) that supplies guideline-based personalised care recommendations through a mobile phone interface for patients with chronic diseases. Herein shared decisions were represented using decision trees with an embedded Markov model,136 which is a stochastic model used to define a sequence of possible health states where the probability of each depends on the state reached in the prior one. Recommendations, e.g. for medication administration, were made according to these shared decisions and guidelines that are represented with Asbru formalism. Lack of generality issue of the proposed methodology (e.g. not appropriate for all patients) limits the applicability of this study. In their later work, Peleg et al.44 introduced two types of patient preferences as local preferences, and global preferences. Local preferences denote personalisation of a certain CIG action, such as arranging the blood glucose measurement alert after a specific meal time, whereas global preferences denote choosing a CIG branch among alternatives such as preferring Warfarin medication instead of Asprin. Guidelines were represented using Asbru and a graphical framework, called, GESHER.137 Lack of clinical implementation, parallel paths and methodologies for detection of interactions occurred between multiple CIGs and resolution of them were the major limitations of this work.
In their recent work,41 the authors extended their approach by proposing methods for acquiring and specifying information of parallel paths in care workflows based on CIG recommendations, and making CIGs patient-centred by customising them with patient’s personal preferences and psychosocial context. Parallel and customised CIGs were applied using Asbru, with the Picard Asbru103 execution engine to execute the model with patient data and to get patient preferences. Patient preferences were achieved through a shared decision model43 that uses decision trees to choose a CIG-based care option which best suits the patient. Patients could state their preferences captured by CIGs, used for generating personalised care plans, for example by recording meal times. In the personalisation phase, the patient’s personal contexts (e.g. vacation) or events that the caregiver may have found dangerous for the patient health (e.g. teeth bleeding) were also considered. Enhancing the patient’s perception of safety and involvement in clinical decision making were the major findings of this work. However, this work falls short in conflict detection and resolution application, creating multi-versions of CIGs,37,71 and including extensive personalisation processes (see Riaño et al.40) for patients with varied multimorbid conditions.
CIG-driven computerised systems help to achieve easy access to evidence-based information and help to improve partnership between caregivers and their patients by personalisation of care for each patient where risk factors, pros and cons of care options can be discussed together, and patient preferences can be involved in clinical decision making.98,99 Moreover, achieving care continuity through remote support such as mobile applications that facilitate patient management, help to improve quality of care and have potential to reduce health care costs by reducing complications and hospital stays in the future.42
Discussion and future work
Multimorbidity management is an increasingly relevant topic of research in the health informatics community, due to its care challenges and concerns of providing personalised therapies31,40–45 for each patient, which is the main aim of patient-centred care.2,138 Traditional CPGs face difficulties in presenting a detailed consideration of strategies and recommendations to coordinate conditions of a multimorbid patient.
For personalisation, diverse information related to all of a patient’s health conditions, clinical history, health records, as well as personal context need to be consolidated. Evaluation and amendment of many co-existing care plans, as well as coping with possible adverse interactions, make multimorbidity care much more challenging. Caregivers struggle with supplying care to patients under such complexities, without causing any treatment conflicts or making any inconsistent and/or unnecessary recommendations. When these complexities are poorly managed, they may negatively affect the duration of care and the healing process of a patient, which may result in several undesired outcomes. Moreover, eliminating care interruptions of patients after their encounters with their caregivers and sustaining their adherence to the agreed care plan are also crucial for their outcome.
CIG-driven CDSSs support caregivers in attaining these goals. They help to generate a combined care plan by merging multiple guidelines that have been encoded as CIGs. This helps to eliminate clinical task duplications and improve health resource use. They support detection of adverse and contradictory activities between guidelines. They facilitate resolution of conflicts associated with causal relationships between guideline entities (e.g. drug, disease, patient, time). CIGs, involving drug information, when integrated with appropriate algorithms can alleviate the risk of drug interactions by checking them before any therapy provision. Using CIGs, temporal constraints can be translated into a machine-readable format and monitored by CDSSs. The integration of CIGs into the patient and caregiver shared decision-making process helps to involve patient preferences in clinical decision making, plays a significant role upon the care customisation process and in maintaining patient adherence. Consideration of patient preferences in clinical decision-making phases enhances patients’ adherence to recommended interventions. Many CIG-based CDSSs supply remote support for patients, in managing their care, by suggesting evidence-based life-style recommendations involving their preferences and personal contexts. The use of CIG-based CDSSs can help to increase patients’ adherences to their health records and clinical contexts, reduce medication errors, health care costs and workloads of caregivers.
The review identified a broad range of challenges and barriers in multimorbidity care:
limitations of CPGs, complexity of managing temporal constraints in clinical procedures with concurrently applied narrative CPGs;
conflicting actions affecting therapies that can be induced by polypharmacy (e.g. ADEs);
patient characteristics and/or poor timing of clinical actions;
patients’ poor guideline adherences and care interruptions which can stem from the cognitive and/or physical disability of a multimorbid patient.
The core concepts of managing multimorbidity care through using CIG-based CDSSs are:
the unification of multiple guidelines, the mitigation and resolution of contradictory and inconsistent activities within them and of their associated knowledge elements;
the handling of temporal constraints (e.g. start/end and duration of therapies, and temporal scheduling of clinical processes) in synchronously implemented guidelines;
involving patients’ preferences and integrating their psychological context into a computerised decision support setting to achieve care personalisation for each patient;
maintaining care continuity of complex patients with remote support.
Ontology-based CDSSs, in recent years, are gaining substantial importance due to their benefits.139 However, they have still several limitations. For instance, most of the ontologies used in these systems were designed for the treatment of a single disease140,141 and provided substantially detailed ontologies that are not easily adaptable, generalizable or re-usable. Some of the published works provided general models for multimorbid disease management, yet they did not supply enough instances that can reflect real-life applications and face difficulties in merging more than two concurrently applied clinical actions together, offered by multiple guidelines. There is no single way to represent CPGs as CIGs, there are several different formalisms (e.g. process-flow models, rule-based models, etc.) with different granularities. While some of the existing works47,48,59 mainly focus on a particular issue, such as ontological representation of CPGs, merging multiple CPGs, mitigating contradictory and inconsistent activities, temporal constraint verification and more, some of these works41,53,54,58,63,67 consider combinations of these issues. Nevertheless, coping with all these complexities together is still a challenging issue in the existing literature. Especially some of the logic-based formalisms (e.g. Wilk et al.51–53, Michalowski et al.,55,56 and Zhang and Zhang57) need to prove their applicability in real-life cases due to their theoretical approaches and assumptions made. There is also a need for more research on considerations of shared decisions, patients’ preferences and their social contexts, broader automatic interaction detection and resolution methodologies involving new types of interactions such as drug-food interactions and sufficient considerations of temporal constraints under uncertainty such as delays occurring between clinical tasks. Dealing with temporal constraints for managing multimorbid patients were addressed in a few papers, yet still need more attention from researchers. Future research can cover the following open areas:
developing methodologies for merging more than two simultaneously applied clinical actions of multiple CIGs;
developing approaches for discovery and resolution of adverse interactions such as patient–guideline, patient–food, drug–food interactions;
developing models to cope with temporal constraints in multiple CIGs and associated medical processes; and
further CIG-based approaches to ensure continuity of care69 for patients with multimorbidity or patients with limited ability to frequently visit health service providers, due to their economic condition, severity of health condition and/or proximity, and who need help for care plan adjustments considering their actual health condition and personal occasions.42
This paper has made several contributions to the existing literature. To our knowledge, there is a limited number of studies that review treatment complexities and management of multimorbidity care using CIGs by providing in-depth analysis on the following themes: challenges and barriers in providing care for multimorbid patients; shortcomings of existing CPGs, and the reasons why CIGs are needed in multimorbidity care; and lastly, several approaches reviewed show how multimorbid care complexities could be handled through using CIG-driven CDSSs. The main strength of this work is to provide a greater perspective to people who aim to understand the complexities of supplying care for patients with multimorbid needs, and the role of CIGs upon their management and maintenance of patient-safety. We also made recommendations directly to readers for future research opportunities; thus, aiming to contribute to the development of future studies based on the gaps addressed in this work.
There are some limitations that need to be considered for future studies. We mainly considered two journal platforms, Science Direct and PubMed, and performed a Google scholar search using limited keywords. The number of scientific journal repositories and keywords can be extended involving polypharmacy, personalised care, patient centrality or precise medical terms in the abstract, and title screening to prepare a widely searched literature review. We conducted this review up to 1 July 2017, and only the main points that convey the gist of the issues were presented. Thus, these sections can be enlarged in future studies.
Conclusions
Clinical practice guidelines provide evidence-based knowledge about treatment of a patient generally with a single disease. CPGs have many benefits in care such as supporting clinical decision-making, bringing standardisation into practice, and improving the quality of care. When patients have multimorbidity, many of them need to be combined that may cause contradictory recommendations in care because of the possible adverse interactions between entities such as drug–patient, drug–drug or drug–disease, or adverse timing interactions between therapies. Therefore, treatments need to be personalised for each patient to be both safe and applicable. To make it possible, CPGs need to be integrated with the patient care workflow and patient-specific data (e.g. allergies, drug intolerances, genetics and past treatment history). Formalisation of CPGs in the form of CIGs and then adopting them in a CDSS could eliminate any limitations (e.g. passive knowledge dissemination, static design, or lack of patient data), and can increase their effectiveness in care.
In this paper, a systematic literature review is performed with the objective of providing insights into the fundamental challenges and barriers occurring in multimorbidity care, the role of CIGs in the delivery of care and the management methods to handle multimorbid patients. This work can be useful for the health informatics community, engineers, researchers, healthcare actors or data scientists, who aim to understand the treatment complexities of multimorbid patients, and how CIGs can help to manage them and become an integral part of the patient-centred care.
Contributorship
EB designed and performed the literature review and lead the writing of the paper. GD contributed to the design of the literature review, wrote parts of the paper and reviewed the manuscript. TNA contributed to the design of the literature review, wrote parts of the paper and reviewed the manuscript.
Conflict of interests
The authors did not state any conflicting interests.
Ethical approval
No ethical approval required.
Funding
EB has received a PhD scholarship from WMG, University of Warwick, relating to this work. The work of GD and TNA relating to aspects of this paper has been partially funded from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 6891810, C3-Cloud Project.
Guarantor
EB
Peer review
This manuscript was reviewed by three reviewers, the authors have elected for these individuals to remain anonymous.
References
- 1.Field MJ, Lohr KN. (eds). Clinical Practice Guidelines: Directions for a new program. Washington, DC: National Academies Press, 1990, p.50. [PubMed] [Google Scholar]
- 2.Institute of Medicine (IOM). Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: The National Academies Press, 2001, pp.39–54. [PubMed] [Google Scholar]
- 3.Field MJ, Lohr KN. (eds). Guidelines for clinical practice: From development to use. Washington, DC: National Academies Press, 1992, pp. 40–244. [PubMed] [Google Scholar]
- 4.Peleg M, Patel VL, Snow V, et al. Support for guideline development through error classification and constraint checking. In: AMIA Annual Symposium Proceedings Archive. American Medical Informatics Association. 2002, pp. 607–611. [PMC free article] [PubMed]
- 5.De Clercq P, Kaiser K, Hasman A. Computer-interpretable guideline formalisms. Stud Health Technol Inform 2008; 139: 22–43. [PMC free article] [PubMed] [Google Scholar]
- 6.Sim I, Gorman P, Greenes RA, et al. Clinical decision support systems for the practice of evidence-based medicine. J Am Med Inform Assoc 2001; 8(6): 527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genco EK, Forster JE, Flaten H, et al. Clinically inconsequential alerts: The characteristics of opioid drug alerts and their utility in preventing adverse drug events in the emergency department. Ann Emerg Med 2016; 67(2): 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peleg M, Tu S, Bury J, et al. Comparing computer-interpretable guideline models: A case-study approach. J Am Med Inform Assoc 2003; 10(1): 52–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peleg M. Computer-interpretable clinical guidelines: a methodological review. J Biomed Inform 2013; 46(4): 744–763. [DOI] [PubMed] [Google Scholar]
- 10.Studer R, Benjamins VR, Fensel D. Knowledge engineering: principles and methods. Data Knowl Eng 1998; 25(1–2): 161–197. [Google Scholar]
- 11.Mulyar N, Van der Aalst WM, Peleg M. A pattern-based analysis of clinical computer- interpretable guideline modeling languages. J Am Med Inform Assoc 2007; 14(6): 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karadimas H, Ebrahiminia V, Lepage E. User-defined functions in the Arden Syntax: An extension proposal. Artif Intell Med 2015. doi: 10.1016/j.artmed.2015.11.003. [DOI] [PubMed]
- 13.Hussain M, Afzal M, Ali T, et al. Data-driven knowledge acquisition, validation, and transformation into HL7 Arden Syntax. Artif Intell Med 2015. doi: 10.1016/j.artmed.2015.09.008. [DOI] [PubMed]
- 14.Jenders RA, Adlassnig KP, Fehre K, et al. Evolution of the Arden Syntax: Key technical issues from the standards development organization perspective. Artif Intell Med 2016. doi: 10.1016/j.artmed.2016.08.001. [DOI] [PMC free article] [PubMed]
- 15.Shiffman RN, Agrawal A, Deshpande AM, et al. An approach to guideline implementation with GEM. Stud Health Technol Inform 2001; 84(1): 271–275. [PubMed] [Google Scholar]
- 16.Boxwala AA, Peleg M, Tu S, et al. GLIF3: a representation format for sharable computer-interpretable clinical practice guidelines. J Biomed Inform 2004; 37(3): 147–161. [DOI] [PubMed] [Google Scholar]
- 17.Wang D, Peleg M, Tu SW, et al. Design and implementation of the GLIF3 guideline execution engine. J Biomed Inform 2004; 37(5): 305–318. [DOI] [PubMed] [Google Scholar]
- 18.Seyfang A, Miksch S, Marcos M. Combining diagnosis and treatment using Asbru. Int J Med Inform 2002; 68(1): 49–57. [DOI] [PubMed] [Google Scholar]
- 19.Tu SW, Campbell J, Musen MA. The SAGE guideline modeling: Motivation and methodology. Stud Health Technol Inform 2004; 101: 167–171. [PubMed] [Google Scholar]
- 20.Tu SW, Musen MA. Modeling data and knowledge in the EON guideline architecture. Stud Health Technol Inform 2001; 84(1): 280–284. [PubMed] [Google Scholar]
- 21.Ciccarese P, Caffi E, Quaglini S, et al. Architectures and tools for innovative health information systems: The Guide project. Int J Med Inform 2005; 74(7–8): 553–562. [DOI] [PubMed] [Google Scholar]
- 22.Sutton DR, Fox J. The syntax and semantics of the PROforma guideline modeling language. J Am Med Inform Assoc 2003; 10(5): 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutton DR, Taylor P, Earle K. Evaluation of PROforma as a language for implementing medical guidelines in a practical context. BMC Med Inform Decis Mak 2006; 6(1): 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grando A, Peleg M, Glasspool D. A goal-oriented framework for specifying clinical guidelines and handling medical errors. J Biomed Inform 2010; 43(2): 287–299. [DOI] [PubMed] [Google Scholar]
- 25.Salive ME. Multimorbidity in older adults. Epidemiol Rev 2013; 35(1): 75–83. [DOI] [PubMed] [Google Scholar]
- 26.Navickas R, Petric VK, Feigl AB, et al. Multimorbidity: What do we know? What should we do? J Comorbidity 2016; 6(1): 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell SP, andSaraf AA.. Epidemiology of multimorbidity in older adults with cardiovascular disease. Clin Geriatr Med 2016; 32(2): 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salisbury C, Johnson L, Purdy S, et al. Epidemiology and impact of multimorbidity in primary care: A retrospective cohort study. Br J Gen Pract 2011; 61(582): e12–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salisbury C. Multimorbidity: Redesigning health care for people who use it. The Lancet 2012; 380(9836): 7–9. [DOI] [PubMed] [Google Scholar]
- 30.Vassilaki M, Aakre JA, Cha RH, et al. Multimorbidity and risk of mild cognitive impairment. J Am Geriatr Soc 2015; 63(9): 1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lasierra N, Alesanco A, Guillén S, et al. A three-stage ontology-driven solution to provide personalised care to chronic patients at home. J Biomed Inform 2013; 46(3): 516–529. [DOI] [PubMed] [Google Scholar]
- 32.Anselma L, Bottrighi A, Giordano L, et al. A hybrid approach to the verification of computer interpretable guidelines In: Hommersom A, Lucas PJF. (eds) Foundations of Biomedical Knowledge Representation: Methods and applications. Heidelburg: Springer, 2015, pp.287–315. [Google Scholar]
- 33.Riaño D, Collado A. Model-based combination of treatments for the management of chronic comorbid patients. In: Peek N, Marín Morales R, Peleg M (eds) Artificial Intelligence in Medicine. AIME 2013. Lecture Notes in Computer Science. Vol 7885. Berlin, Heidelberg: Springer, pp. 11–16.
- 34.Montori VM, Brito JP, Murad MH. The optimal practice of evidence-based medicine: Incorporating patient preferences in practice guidelines. JAMA 2013; 310(23): 2503–2504. [DOI] [PubMed] [Google Scholar]
- 35.Sittig DF, Wright A, Osheroff JA, et al. Grand challenges in clinical decision support. J Biomed Inform 2008; 41(2): 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinman MA, Seth Landefeld C, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc 2006; 54(10): 1516–1523 [DOI] [PubMed] [Google Scholar]
- 37.Grandi F. Dynamic class hierarchy management for multi-version ontology-based personalization. J Comput Syst Sci 2016; 82(1): 69–90. [Google Scholar]
- 38.González-Ferrer A, Ten Teije A, Fdez-Olivares J, et al. Automated generation of patient-tailored electronic care pathways by translating computer-interpretable guidelines into hierarchical task networks. Artif Intell Med 2013; 57(2): 91–109. [DOI] [PubMed] [Google Scholar]
- 39.López-Vallverdú JA, Riaño D, Collado A. Rule-based combination of comorbid treatments for chronic diseases applied to hypertension, diabetes mellitus and heart failure. In: Lenz R, Miksch S, Peleg M, Reichert M, Riaño D, ten Teije A (eds) Process Support and Knowledge Representation in Health Care. ProHealth 2012, KR4HC 2012. Lecture Notes in Computer Science. Vol 7738. Berlin, Heidelberg: Springer, pp. 30–41.
- 40.Riaño D, Real F, López-Vallverdú JA, et al. An ontology-based personalization of health-care knowledge to support clinical decisions for chronically ill patients. J Biomed Inform 2012; 45(3): 429–446. [DOI] [PubMed] [Google Scholar]
- 41.Peleg M, Shahar Y, Quaglini S, et al. MobiGuide: a personalized and patient-centric decision-support system and its evaluation in the atrial fibrillation and gestational diabetes domains. User Model-adapt Interact 2017; 27(2):159–213. [Google Scholar]
- 42.Peleg M, Shahar Y, Quaglini S, et al. Assessment of a personalized and distributed patient guidance system. Int J Med Inform 2017; 101:108–130. [DOI] [PubMed] [Google Scholar]
- 43.Quaglini S, Shahar Y, Peleg M, et al. Supporting shared decision making within the MobiGuide project. In AMIA Annual Symposium Proceedings, 2013; 1175–1184. [PMC free article] [PubMed]
- 44.Peleg M, Shahar Y, Quaglini S. Making healthcare more accessible, better, faster, and cheaper: the MobiGuide Project. European J ePractice 2014; 20: 5–20. [Google Scholar]
- 45.Isern D, Moreno A, Sánchez D, et al. Agent-based execution of personalised home care treatments. Appl Intell 2011; 34(2): 155–180. [Google Scholar]
- 46.Bonacin R, Pruski C, Da Silveira M. Architecture and services for formalising and evaluating care actions from computer-interpretable guidelines. Int J Med Eng Inform 2013; 5(3): 253–268. [Google Scholar]
- 47.Abidi SR, Abidi SS. Towards the merging of multiple clinical protocols and guidelines via ontology-driven modeling. In: Combi C, Shahar Y, Abu-Hanna A (eds) Artificial Intelligence in Medicine. AIME 2009. Lecture Notes in Computer Science. Vol 5651 Berlin, Heidelberg: Springer, pp. 81–85.
- 48.Abidi S, Cox J, Shepherd M, et al. Using OWL ontologies for clinical guidelines based comorbid decision support. In: 45th Hawaii International Conference on System Sciences, Maui, HI: IEEE, 2012, pp. 3030–3038.
- 49.Jafarpour B, Abidi SS. Merging disease-specific clinical guidelines to handle comorbidities in a clinical decision support setting. In: Peek N., MarÍn Morales R., Peleg M. (eds) Artificial Intelligence in Medicine. AIME 2013. Lecture Notes in Computer Science. Vol 7885, Berlin, Heidelberg: Springer, pp. 28–32.
- 50.Jafarpour B, Abidi SR, Abidi SS. Exploiting semantic web technologies to develop OWL-based clinical practice guideline execution engines. IEEE J Biomed Health Inform. 2016; 20(1): 388–398. [DOI] [PubMed] [Google Scholar]
- 51.Wilk S, Michalowski M, Michalowski W, et al. Reconciling pairs of concurrently used clinical practice guidelines using constraint logic programming. In AMIA Annu Symp Proc 2011. (Vol. 2011, p. 944–953). [PMC free article] [PubMed]
- 52.Wilk S, Michalowski W, Michalowski M, et al. Mitigation of adverse interactions in pairs of clinical practice guidelines using constraint logic programming. J Biomed Inform 2013; 46(2): 341–353. [DOI] [PubMed] [Google Scholar]
- 53.Wilk S, Michalowski M, Michalowski W, et al. Comprehensive mitigation framework for concurrent application of multiple clinical practice guidelines. J Biomed Inform 2017; 66: 52–71 [DOI] [PubMed] [Google Scholar]
- 54.Kovalov A, Bowles JKF (2016) Avoiding Medication Conflicts for Patients with Multimorbidities. In: Ábrahám E., Huisman M. (eds) Integrated Formal Methods. IFM 2016. Lecture Notes in Computer Science, vol 9681. Springer, Cham.
- 55.Michalowski M, Wilk S, Michalowski W, Lin D, Farion K, Mohapatra S. Using Constraint Logic Programming to Implement Iterative Actions and Numerical Measures during Mitigation of Concurrently Applied Clinical Practice Guidelines. In: Peek N., Marín Morales R., Peleg M. (eds) Artificial Intelligence in Medicine. AIME 2013. Lecture Notes in Computer Science. Vol 7885. Springer, Berlin, Heidelberg, 2013.
- 56.Michalowski M, Wilk S, Tan X, et al. First-order logic theory for manipulating clinical practice guidelines applied to comorbid patients: a case study. InAMIA Annual Symposium Proceedings 2014 (Vol. 2014, p. 892). American Medical Informatics Association. [PMC free article] [PubMed]
- 57.Zhang Y and Zhang Z. Preliminary Result on Finding Treatments for Patients with Comorbidity. In: Miksch S., Riaño D., ten Teije A. (eds) Knowledge Representation for Health Care. KR4HC 2014. Lecture Notes in Computer Science. Vol 8903. Springer, Cham, 2014.
- 58.Tan X. Towards a formal representation of clinical practice guidelines for the treatment of comorbid patients. In BIBM, Shanghai, China: IEEE, 2013, pp. 578–583.
- 59.Piovesan L, Molino G, Terenziani P. An ontological knowledge and multiple abstraction level decision support system in healthcare. Decis Anal 2014; 1(1): 1–8 [Google Scholar]
- 60.Piovesan L, Molino G and Terenziani P. Supporting Multi-level User-driven Detection of Guideline Interactions.In Proceedings of the International Conference on Health Informatics - Volume 1: HEALTHINF, (BIOSTEC 2015), 2015. pp. 413–422. ISBN 978-989-758-068-0.
- 61.Piovesan L, Terenziani PA. Mixed-Initiative Approach to the Conciliation of Clinical Guidelines for Comorbid Patients. In: Riaño D, Lenz R, Miksch S, Peleg M, Reichert M, and ten Teije A (eds) Knowledge Representation for Health Care. AIME 2015. Lecture Notes in Computer Science. Vol 9485. Springer, Cham, 2015.
- 62.Zamborlini V, Hoekstra R, Da Silveira M, et al. Inferring recommendation interactions in clinical guidelines: Case studies on multimorbidity. Semantic Web 2016; 7(4): 421–446. [Google Scholar]
- 63.Zamborlini V, Da Silveira M, Pruski C, et al. Analyzing interactions on combining multiple clinical guidelines. Artif Intell Med 2017; 81:78–93. [DOI] [PubMed] [Google Scholar]
- 64.Koutkias V, Kilintzis V, Stalidis G, et al. Knowledge engineering for adverse drug event prevention: On the design and development of a uniform, contextualized and sustainable knowledge-based framework. J Biomed Inform 2012; 45(3): 495–506. [DOI] [PubMed] [Google Scholar]
- 65.Koutkias VG, McNair P, Kilintzis V, et al. From adverse drug event detection to prevention. Method Inform Med 2014; 53(06): 482–492. [DOI] [PubMed] [Google Scholar]
- 66.Anselma L, Terenziani P, Montani S, et al. Towards a comprehensive treatment of repetitions, periodicity and temporal constraints in clinical guidelines. Artif Intell Med 2006; 38(2): 171–195. [DOI] [PubMed] [Google Scholar]
- 67.Anselma L, Piovesan L, Terenziani P. Temporal detection and analysis of guideline interactions. Artif Intell Med 2017; 76: 40–62. [DOI] [PubMed] [Google Scholar]
- 68.Duftschmid G, Miksch S, Gall W. Verification of temporal scheduling constraints in clinical practice guidelines. Artif Intell Med 2002; 25(2): 93–121 [DOI] [PubMed] [Google Scholar]
- 69.Bottrighi A, Molino G, Montani S, et al. Supporting a distributed execution of clinical guidelines. Comput Methods Programs Biomed 2013; 112(1): 200–210. [DOI] [PubMed] [Google Scholar]
- 70.Kilsdonk E, Peute LW, Riezebos RJ, et al. Uncovering healthcare practitioners’ information processing using the think-aloud method: From paper-based guideline to clinical decision support system. Int J Med Inform 2016; 86: 10–19. [DOI] [PubMed] [Google Scholar]
- 71.Grandi F, Mandreoli F, Martoglia R. Efficient management of multi-version clinical guidelines. J Biomed Inform 2012; 45(6): 1120–1136 [DOI] [PubMed] [Google Scholar]
- 72.Breuker C, Abraham O, di Trapanie L, et al. Patients with diabetes are at high risk of serious medication errors at hospital: Interest of clinical pharmacist intervention to improve healthcare. Eur J Intern Med 2017; 38: 38–45. [DOI] [PubMed] [Google Scholar]
- 73.Aronson JK. Medication errors: Definitions and classification. Br J Clin Pharmacol 2009; 67(6): 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Terenziani P, Molino G, Torchio M. A modular approach for representing and executing clinical guidelines. Artif Intell Med 2001; 23(3): 249–276. [DOI] [PubMed] [Google Scholar]
- 75.Kuhlmann J, Mu¨ck W. Clinical-pharmacological strategies to assess drug interaction potential during drug development. Drug Saf 2001; 24(10): 715–725. [DOI] [PubMed] [Google Scholar]
- 76.Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: Basic principles and practical applications. Br J Clin Pharmacol 2004; 57(1): 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dagli RJ, Sharma A. Polypharmacy: A global risk factor for elderly people. J Int Oral Health 2014; 6(6): i–ii. [PMC free article] [PubMed] [Google Scholar]
- 78.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA 1995; 274(1): 29–34. [PubMed] [Google Scholar]
- 79.Ghibelli S, Marengoni A, Djade CD, et al. Prevention of inappropriate prescribing in hospitalized older patients using a computerized prescription support system (INTERcheck®). Drugs Aging 2013; 30(10): 821–828. [DOI] [PubMed] [Google Scholar]
- 80.Morimoto T, Gandhi TK, Seger AC, et al. Adverse drug events and medication errors: Detection and classification methods. Qual Saf Health Care 2004; 13(4): 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harpaz R, DuMouchel W, Shah NH, et al. Novel data– mining methodologies for adverse drug event discovery and analysis. Clin Pharmacol Ther 2012; 91(6): 1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18820 patients. BMJ 2004; 329(7456): 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akçura MT, Ozdemir ZD. Drug prescription behaviour and decision support systems. Decis Support Syst 2014; 57: 395–405. [Google Scholar]
- 84.Van Roon EN, Flikweert S, Le Comte M, et al. Clinical relevance of drug-drug interactions. Drug Safety 2005; 28(12): 1131–1139. [DOI] [PubMed] [Google Scholar]
- 85.Stanford University. GLINDA: GuideLine INteraction Detection Architecture. http://glinda-project.stanford.edu/ (accessed by 14 April 2017).
- 86.Corrie K, Hardman JG. Mechanisms of drug interactions: Pharmacodynamics and pharmacokinetics. Anaesth Intensive Care 2017; 18(7): 331–334. [Google Scholar]
- 87.Yeh ML, Chang YJ, Wang PY, et al. Physicians’ responses to computerized drug-drug interaction alerts for outpatients. Comput Methods Programs Biomed 2013; 111(1): 17–25. [DOI] [PubMed] [Google Scholar]
- 88.Tan Y, Hu Y, Liu X, et al. Improving drug safety: From adverse drug reaction knowledge discovery to clinical implementation. Methods 2016; 110: 14–25. [DOI] [PubMed] [Google Scholar]
- 89.Stewart M. Towards a global definition of patient centred care: The patient should be the judge of patient centred care. Br Med J 2001; 322(7284): 444–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Little P, Everitt H, Williamson I, et al. Preferences of patients for patient centred approach to consultation in primary care: Observational study. BMJ 2001; 322(7284): 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sacchi L, Parimbelli E, Panzarasa S, et al. Combining Decision Support System-Generated Recommendations with Interactive Guideline Visualization for Better Informed Decisions. In: Holmes J, Bellazzi R, Sacchi L, and Peek N. (eds) Artificial Intelligence in Medicine. AIME 2015. Lecture Notes in Computer Science. Vol 9105. Springer, Cham, 2015.
- 92.Sacchi L, Rubrichi S, Rognoni C, et al. From decision to shared-decision: Introducing patients’ preferences into clinical decision analysis. Artif Intell Med 2015; 65(1): 19–28. [DOI] [PubMed] [Google Scholar]
- 93.Christensen AJ. Patient Adherence to Medical Treatment Regimens: Bridging the gap between behavioral science and biomedicine. New Haven, CT: Yale University Press, 2004, p.3. [Google Scholar]
- 94.Baiardini I, Braido F, Bonini M, et al. Why do doctors and patients not follow guidelines? Curr Opin Allergy Clin Immunol 2009; 9(3): 228–233. [DOI] [PubMed] [Google Scholar]
- 95.Feldman SR, Vrijens B, Gieler U, et al. Treatment adherence intervention studies in dermatology and guidance on how to support adherence. Am J Clin Dermatol 2017; 18(2): 253–271. [DOI] [PubMed] [Google Scholar]
- 96.Murad MH. Clinical practice guidelines: a primer on development and dissemination. Mayo Clin Proc 2017; 92(3): 423–433. [DOI] [PubMed] [Google Scholar]
- 97.Fredericksen RJ, Gibbons L, Brown S, et al. Medication understanding among patients living with multiple chronic conditions: Implications for patient-reported measures of adherence. Res Social Adm Pharm 2018;14(6): 540–544. [DOI] [PMC free article] [PubMed]
- 98.Elwyn G, Laitner S, Coulter A, et al. Implementing shared decision making in the NHS. BMJ 2010; 341: c5146. [DOI] [PubMed] [Google Scholar]
- 99.Patton LL. At the interface of medicine and dentistry: Shared decision-making using decision aids and clinical decision support tools. Oral Surg Oral Med Oral Pathol 2017; 123(2): 147–149. [DOI] [PubMed] [Google Scholar]
- 100.Kaplan RM, Frosch DL. Decision making in medicine and health care. Annu. Rev. Clin. Psychol 2005; 1: 525–556. [DOI] [PubMed] [Google Scholar]
- 101.Zhang YF, Gou L, Tian Y, et al. Design and development of a sharable clinical decision support system based on a semantic web service framework. J Med Syst 2016; 40(5): 118. [DOI] [PubMed] [Google Scholar]
- 102.Goldstein MK, Hoffman BB, Coleman RW, et al. Patient safety in guideline-based decision support for hypertension management: ATHENA DSS. J Am Med Inform Assoc 2002; 9(6): S11–6. [PMC free article] [PubMed] [Google Scholar]
- 103.Shalom E, Shahar Y, Lunenfeld E. An architecture for a continuous, user-driven, and data-driven application of clinical guidelines and its evaluation. J Biomed Inform 2016; 59: 130–148. [DOI] [PubMed] [Google Scholar]
- 104.Terenziani P, Montani S, Bottrighi A, et al. The GLARE approach to clinical guidelines: main features. Stud Health Technol Inform 2004; 101: 162–166. [PubMed] [Google Scholar]
- 105.Bottrighi A, Terenziani P. META-GLARE: A meta-system for defining your own computer interpretable guideline system-Architecture and acquisition. Artif Intell Med 2016; 72: 22–41. [DOI] [PubMed] [Google Scholar]
- 106.Shiffman RN, Michel G, Rosenfeld RM, et al. Building better guidelines with BRIDGE-Wiz: development and evaluation of a software assistant to promote clarity, transparency, and implementability. J Am Med Inform Assoc 2011; 19(1): 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shahar Y, Miksch S, Johnson P. The Asgaard project: a task-specific framework for the application and critiquing of time-oriented clinical guidelines. Artif Intell Med 1998; 14(1–2): 29–51. [DOI] [PubMed] [Google Scholar]
- 108.Isern D, Sánchez D, Moreno A. Ontology-driven execution of clinical guidelines. Comput Methods Programs Biomed 2012; 107(2): 122–139. [DOI] [PubMed] [Google Scholar]
- 109.Peleg M, Boxwala AA, Bernstam E, et al. Sharable representation of clinical guidelines in GLIF: relationship to the Arden Syntax. J Biomed Inform. 2001; 34(3): 170–181. [DOI] [PubMed] [Google Scholar]
- 110.Peleg M, Fox J, Patkar V, et al. A computer-interpretable version of the AACE, AME, ETA medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract 2014; 20(4): 352–359. [DOI] [PubMed] [Google Scholar]
- 111.Eccher C, Seyfang A, Ferro A. Implementation and evaluation of an Asbru-based decision support system for adjuvant treatment in breast cancer. Comput Methods Programs Biomed 2014; 117(2): 308–321. [DOI] [PubMed] [Google Scholar]
- 112.Donnelly K. SNOMED-CT: The advanced terminology and coding system for eHealth. Stud Health Technol Inform 2006; 121: 279. [PubMed] [Google Scholar]
- 113.Sordo M, Boxwala AA, Ogunyemi O, et al. Description and status update on GELLO: a proposed standardized object-oriented expression language for clinical decision support. Stud Health Technol Inform 2004; 107: 164–168. [PubMed]
- 114.Berg D, Ram P, Glasgow J, et al. SAGEDesktop: an environment for testing clinical practice guidelines. In Conf Proc IEEE Eng Med Biol Soc 2004: 2, pp. 3217–3220. [DOI] [PubMed]
- 115.Jaffar J, Maher MJ. Constraint logic programming: A survey. J Logic Prog 1994; 19: 503–581. [Google Scholar]
- 116.Rautenberg W. A Concise Introduction to Mathematical Logic. New York, NY: Springer; 2006. [Google Scholar]
- 117.Peleg M, Tu SW, Manindroo A, et al. Modeling and analyzing biomedical processes using workflow/Petri Net models and tools. Stud Health Technol Inform 2004; 107(1): 74–78. [PubMed] [Google Scholar]
- 118.Beccuti M, Bottrighi A, Franceschinis G, et al. Modeling clinical guidelines through Petri Nets. In: Combi C, Shahar Y and Abu-Hanna A (eds) Artificial Intelligence in Medicine. AIME 2009. Lecture Notes in Computer Science. Vol 5651, Springer, Berlin, Heidelberg, pp 61–70.
- 119.González-Ferrer A, Peleg M. Understanding requirements of clinical data standards for developing interoperable knowledge-based DSS: a case study. Comput Stand Inter 2015; 42: 125–136. [Google Scholar]
- 120.O’Reilly D, Holbrook A, Blackhouse G, et al. Cost effectiveness of a shared computerized decision support system for diabetes linked to electronic medical records. J Am Med Inform Assoc 2011; 19(3): 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bologva EV, Prokusheva DI, Krikunov AV, et al. Human-computer interaction in electronic medical records: From the perspectives of physicians and data scientists. Procedia Comput Sci 2016; 100: 915–920. [Google Scholar]
- 122.Grau BC, Horrocks I, Motik B, et al. OWL 2: The next step for OWL. Web Semant 2008; 6(4): 309–322. [Google Scholar]
- 123.W3C. SWRL: A semantic web rule language combining OWL and RuleML, https://www.w3.org/Submission/SWRL/ (2004, accessed by 15 April 2017).
- 124.Motik B, Sattler U, Studer R. Query answering for OWL-DL with rules. J Web Semant 2005; 3(1): 41–60. [Google Scholar]
- 125.Iannaccone M, Esposito M. Formal specification of temporal constraints in clinical practice guidelines In: Skulimowski A and Kacprzyk J (eds) Knowledge, Information and Creativity Support Systems: Recent Trends, Advances and Solutions. Advances in Intelligent Systems and Computing. Vol 364, Springer, Cham, pp. 373–386. [Google Scholar]
- 126.Gospodarowicz M, O’Sullivan B. Patient management scenario: A framework for clinical decision and prognosis. Semin Oncol 2003; 21(1): 8–12. [DOI] [PubMed] [Google Scholar]
- 127.De Clercq PA, Hasman A, Blom JA, et al. Design and implementation of a framework to support the development of clinical guidelines. Int J Med Inform 2001; 64(2–3): 285–318. [DOI] [PubMed] [Google Scholar]
- 128.Riaño D. The SDA model: A set theory approach. International Symposium on Computer-Based Medical Systems (CBMS'07), Maribor, Slovenia: IEEE, pp. 563–568.
- 129.Dechter R, Meiri I, Pearl J. Temporal constraints network. Artif Intell 1991; 49: 61–95. [Google Scholar]
- 130.Rood E, Bosman RJ, Van Der Spoel JI, et al. Use of a computerized guideline for glucose regulation in the intensive care unit improved both guideline adherence and glucose regulation. J AmMed Inform Assoc 2005; 12(2): 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eiter T, Ianni G, Schindlauer R, et al. A uniform integration of higher-order reasoning and external evaluations in answer-set programming. In: Proceedings of the 19th International Joint Conference on Artificial Intelligence (IJCAI-05}. Professional Book, pp. 90–96
- 132.Reiter R. Knowledge in action: Logical foundations for specifying and implementing dynamical systems. Cambridge, MA: MIT Press, 2001. [Google Scholar]
- 133.W3C. SPARQL Query language for RDF, https://www.w3.org/TR/rdf-sparql-query/ (2008, accessed by 15 April 2017).
- 134.Sánchez-Garzón I, Fdez-Olivares J, Onaindía E, et al. Treatment Plans of Comorbid Patients. In: Peek N, Marín Morales R, Peleg M (eds) Artificial Intelligence in Medicine. AIME 2013. Lecture Notes in Computer Science. Vol 7885. Berlin, Heidelberg: Springer, pp. 23–27.
- 135.Bohada JA, Riaño D, López-Vallverdú JA. Automatic generation of clinical algorithms within the state-decision-action model. Expert Syst Appl 2012; 39(12): 10709–10721. [Google Scholar]
- 136.Schaefer AJ, Bailey MD, Shechter SM, et al. Modeling medical treatment using markov decision processes. In: Brandeau M, Sainfort F and Pierskalla WP (eds) Operations Research and Health Care. International Series in Operations Research & Management Science. Boston, MA: Springer, pp. 593–612.
- 137.Hatsek A, Shahar Y, Taieb-Maimon M, et al. A scalable architecture for incremental specification and maintenance of procedural and declarative clinical decision-support knowledge. Open Med Informat J 2010; 4: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Elwyn G, Dehlendorf C, Epstein RM, et al. Shared decision making and motivational interviewing: Achieving patient-centred care across the spectrum of health care problems. Ann Fam Med 2014; 12(3): 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fox J, Glasspool D, Patkar V, et al. Delivering clinical decision support services: There is nothing as practical as a good theory. J Biomed Inform 2010; 43(5): 831–843. [DOI] [PubMed] [Google Scholar]
- 140.Malhotra A, Younesi E, Gündel M, et al. ADO: A disease ontology representing the domain knowledge specific to Alzheimer's disease. Alzheimers Dement (AMST) 2014; 10(2), 238–246. [DOI] [PubMed] [Google Scholar]
- 141.Waqialla M, Razzak MI. An Ontology-based framework aiming to support cardiac rehabilitation program. Procedia Comput Sci 2016; 96, 23–32. [Google Scholar]