Abstract
Background
Actions of general anaesthetics on activity in the cortico-thalamic network likely contribute to loss of consciousness and disconnection from the environment. Previously, we showed that the general anaesthetic isoflurane preferentially suppresses cortically evoked synaptic responses compared with thalamically evoked synaptic responses, but how this differential sensitivity translates into changes in network activity is unclear.
Methods
We investigated isoflurane disruption of spontaneous and stimulus-induced cortical network activity using multichannel recordings in murine auditory thalamo-cortical brain slices.
Results
Under control conditions, afferent stimulation elicited short latency, presumably monosynaptically driven, spiking responses, as well as long latency network bursts that propagated horizontally through the cortex. Isoflurane (0.05–0.6 mM) suppressed spiking activity overall, but had a far greater effect on network bursts than on early spiking responses. At isoflurane concentrations >0.3 mM, network bursts were almost entirely blocked, even with increased stimulation intensity and in response to paired (thalamo-cortical + cortical layer 1) stimulation, while early spiking responses were <50% blocked. Isoflurane increased the threshold for eliciting bursts, decreased their propagation speed and prevented layer 1 afferents from facilitating burst induction by thalamo-cortical afferents.
Conclusions
Disruption of horizontal activity spread and of layer 1 facilitation of thalamo-cortical responses likely contribute to the mechanism by which suppression of cortical feedback connections disrupts sensory awareness under anaesthesia.
Key words: anaesthesia, general, anaesthetics, general, consciousness, unconsciousness, isoflurane, animals, mice, neocortex, thalamus
Editor's key points.
-
•
General anaesthetics appear to produce unconsciousness by disrupting cortical network connectivity by unclear mechanisms.
-
•
Effects of isoflurane on mono- and polysynaptic activity were investigated in mouse thalamo-cortical brain slices by multi-electrode recordings.
-
•
Isoflurane suppressed monosynaptic activity, but had a far greater effect on polysynaptic network bursts.
-
•
Disruption of network level interactions likely contributes to anaesthetic-induced loss of consciousness.
General anaesthetics produce loss of consciousness (LOC) and alter responsiveness to external stimuli by acting throughout the neural axis. While anaesthetic action on subcortical sites likely contributes to LOC,1, 2, 3 there is an emerging consensus on the importance of cortical loci. Specifically, anaesthetics disrupt cortical network connectivity,4 5 a mechanism with extensive experimental support.6, 7, 8, 9, 10, 11, 12 Recently, we showed that cortico-cortical synaptic inputs to auditory cortex are more sensitive to isoflurane than are thalamo-cortical (TC) inputs, both in vivo and in brain slices,13 further supporting anaesthetic mechanisms involving disrupted cortico-cortical signalling.
Here, we investigated the effects of isoflurane in mouse auditory TC brain slices on evoked and spontaneous neuronal responses, focusing on polysynaptic, propagating network activity (‘bursts’). Our interest in network bursts derives from the importance of the activity of ensembles, rather than of single neurones, in cortical processing.14, 15, 16 In auditory cortex, the ‘desynchronized’ state can in fact be described in terms of overlapping UP states during which ‘packets’ of spiking activity lasting ∼50–300 ms occur.17 Although the absence of neuromodulatory signals and ongoing thalamic input in brain slices precludes the expression of desynchronized activity as in vivo, the same local networks are likely engaged during these bursts,18 and their activity in the presence of anaesthetics reveals the extent to which anaesthetics disrupt these connections.
Methods
All experimental protocols conformed to American Physiological Society/National Institutes of Health guidelines and were approved by the University of Wisconsin Animal Care and Use Committee.
Slice preparation and electrophysiological recordings
Auditory TC brain slices were prepared from mice of either sex (n=24 animals; age range: p28–p92 days)13 (see Supplementary Methods for additional details). Multisite recordings were made in the auditory cortex using a multichannel electrode with 16 recording sites that spanned 1.5 mm.13 19 For most recordings the probe was oriented with the shanks parallel to the pia (‘horizontal’ orientation, in layer 5, spanning multiple columns; Fig. 1A, n=20 slices). Afferents were activated using stimulating electrodes inserted into the superior thalamic radiation, rostral to the hippocampus (TC afferents) or cortical layer 1 >500 μm caudal to the recording probe (L1 afferents). Stimuli consisted of either single pulses or short trains (4×40 Hz). For ‘paired’ stimulation, TC and L1 stimuli were presented simultaneously. Isoflurane was prepared and applied to the slice in the aqueous phase13 and concentrations confirmed after the experiment using gas phase measurements (Poet II Anesthesia Monitor, Criticare Systems, Waukesha, WI, USA).
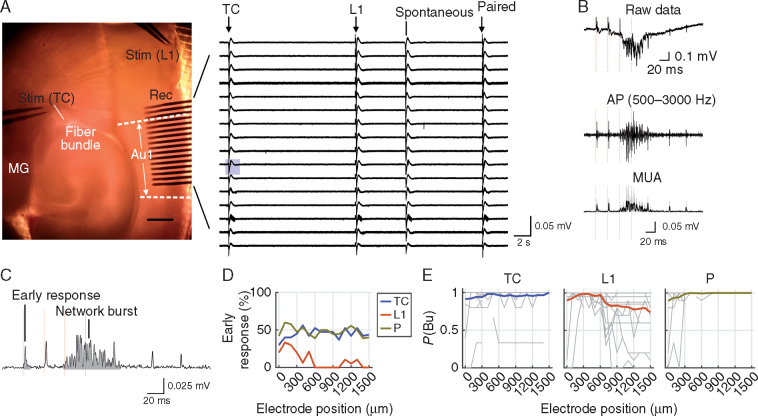
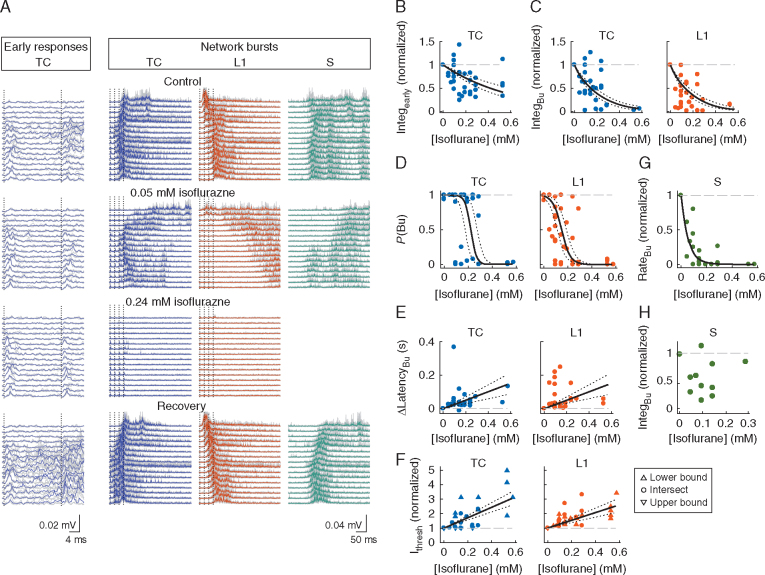
Fig 1.
Early and late responses to afferent stimulation in slices. (A) Left, arrangement of stimulation electrodes and recording array within auditory thalamo-cortical (TC) slice. MG, medial geniculate nucleus; Au1, primary auditory cortex; L1, layer 1; Stim, stimulation; Rec, recording. Right, representative raw data trace (one trial, filtered at 1–3000 Hz). Stimulation times are marked by arrows. Note the occurrence of a spontaneous network burst. (B) Exemplary response to thalamic stimulus train (red vertical lines) on an electrode marked by the blue rectangular background in A. Multi-unit action potentials (AP) were obtained by bandpass filtering the raw data traces. The AP signal was rectified and slightly smoothed with a low-pass filter (–3 dB frequency 600 Hz) to obtain the multi-unit activity (MUA) signal (bottom). (C) Same excerpt of MUA signal as shown in B with early response and network burst marked. (D) Percentage of slices with detected early responses vs electrode position for TC, L1 and paired TC–L1 stimuli (TC, L1 and P, respectively; stimulation strength=12–120 µA; slices without any response were included). Stimulation strength in each experiment was chosen such that network bursts occurred with an average probability [P(Bu)] of at least 0.95 across trials and electrodes; if this criterion was not met, the stimulation strength at which P(Bu) was maximal was chosen. Electrode position zero was closest to the cortical stimulation site. (E) Probability of network bursts vs electrode position (same letter and colour code as in D). Grey lines, individual slices; coloured lines, means across slices (n=20). Same stimulation strength as in D. Note that in the majority of slices and for the majority of recording sites P(Bu) was close to unity.
Data analysis
Multi-unit activity (MUA) was extracted from the raw data by bandpass filtering (500-3000 Hz), rectifying and then low-pass filtering to obtain the MUA signal used for detecting and quantifying responses to afferent stimuli (Fig. 1B and C). Induced MUA responses often consisted of two components (Fig. 1B and C): short-latency ‘early responses,’ likely reflecting monosynaptically evoked spikes, and longer latency network bursts. Spontaneous bursts were also observed and analysed as for induced bursts. MUA response magnitude was measured as the integral of the signal. We also measured the probability of burst occurrence [P(Bu)] and the latency to burst onset, from which horizontal propagation speed was derived.
Quantification of isoflurane effects
The effects of isoflurane on early and burst response magnitudes, burst latency and propagation were assayed by comparison with control values. To distinguish the effects on burst initiation, as opposed to propagation, the effects on burst properties were assessed at recording sites in the array that were closest to their initiation site (see Supplementary Methods).
In the majority of experiments, 2–4 stimulation strengths were applied, which reliably elicited network bursts in the control condition but which were at least partly rendered sub-threshold by isoflurane, particularly at higher concentrations. As a result of time constraints during an experiment, it was not always possible to test all combinations of stimulus strength and isoflurane condition, nor to anticipate the stimulation levels that would be required to elicit network activity following exposure to isoflurane. Therefore, stimulation strength was adjusted during most experiments. When possible, the responses were compared directly between isoflurane conditions. However, when stimulation strengths applied in control and isoflurane did not match, we used linear interpolation to estimate the lower and upper bounds of stimulation threshold (see Supplementary Methods).
Statistical analyses
Parameters with skewed distributions or largely different variances between groups were analysed with non-parametric statistics. For comparisons of two groups, we computed the area under the receiver-operating curve (AUROC), a non-parametric measure of effect size20 related to the Mann–Whitney U-test statistic. AUROC ranges from zero to unity, representing the probability that randomly sampled scores from the groups are different. A value of 0.5 indicates no difference, and the further its value deviates from this value, the stronger the effect. 95% confidence intervals (CI95) were computed via bootstrapping. AUROC values were complemented by P-values from Wilcoxon's signed rank test for paired data and by P-values from Kruskal–Wallis tests for several groups.
Interactions between isoflurane and response (‘early’, ‘late’) or burst (‘TC’, ‘L1’) type were analysed by fitting generalized linear mixed models (fitglme function, MATLAB, Statistics Toolbox, MathWorks, Natick, MA, USA) using maximum pseudolikelihood to allow for flexible analysis of repeated measures with unequal numbers of observations within subjects. Slice was treated as a random effect with random slopes. Link functions and distributions appropriate to the measures tested were used: log link/gamma distribution for integral, identity link/normal distribution for latency and threshold, and logit link/binomial for burst probability. Statistical significance of the interaction was determined using a χ2 test on the log-likelihood of models including vs omitting interaction terms.
Results
Afferent stimulation of TC slices elicits multiple response components
Stimulation of both TC and L1 afferents in auditory cortical slices elicits short latency monosynaptic responses (‘early’ responses) often followed by much larger, longer and more variable polysynaptic network bursts (Fig. 1).13 Early responses to TC stimuli represent monosynaptic TC synaptic responses. Early responses to L1 stimuli likely reflect a mixture of extralemniscal TC afferent responses and feedback cortico-cortical (CC) afferent responses.21 All long latency, polysynaptic network responses correspond to activity dependent on CC synaptic connections. Bursts occur spontaneously as well, and are similar to UP states in slice preparations of somatosensory and visual neocortex.19 22 23 We have shown19 that these bursts originate in layer 5. Here, we characterized their occurrence and intercolumnar propagation as well as their sensitivity to isoflurane (Fig. 1).
Early MUA responses to TC stimulation were usually observed at multiple recording sites (median of 10 sites in 17 of 20 slices). Averaged across slices, they were homogeneously distributed throughout the array (Fig. 1D). Early responses to L1 stimulation were comparatively sparse (median 2.5 sites in 10 of 20 slices), and responsive sites were mostly close to the stimulation site (Fig. 1D), likely because of the decay of cortical monosynaptic connection probability with distance. Early responses to paired stimuli were an amalgam of TC and L1 early responses (Fig. 1D; tested in 17 slices, median of nine responsive sites).
Network bursts, which had a much larger magnitude, longer duration and longer latency than early responses, were observed in all slices. When triggered by TC stimuli of sufficient intensity (TC bursts), they occurred with near unity probability across the recording array in 19/20 slices (Fig. 1E, left). In most cases, we observed a block of 4–8 adjacent electrodes with near uniform, short latencies (Fig. 2A and B), indicating a broad initiation zone, usually in the interior of the array, from which activity propagated bidirectionally. For bursts induced by L1 stimuli (L1 bursts), latency was always shortest at the electrode closest to the stimulation site, and bursts propagated horizontally in the rostro-caudal direction (Fig. 2A and B). Combined with the observation that early MUA responses to L1 stimuli were sparse (Fig. 1D), this observation suggests that L1 bursts were usually initiated in a column outside the recording site and recorded as propagating activity along the array. L1 stimuli were less effective at driving bursts throughout the recording array, even at stimulation strengths that were far supra-threshold for recording sites proximal to the stimulation site (Fig. 1E, middle). As TC bursts and bursts induced by paired stimulation (P bursts) could reliably be recorded at these same sites (Fig. 1E), we presumed that in these slices remotely generated network bursts did not propagate in a reliable manner, at least not in the rostro-caudal direction for the extent of the recording array. Therefore, in analyses below that are applied across the recording array (e.g. Figs 2, 3, Fig 5, Fig 6), recording sites with P(Bu)<0.75 even at the highest stimulation strength were excluded from analysis. As would be expected, P bursts exhibited a latency profile that shared features of both inputs (Fig. 2A and B).
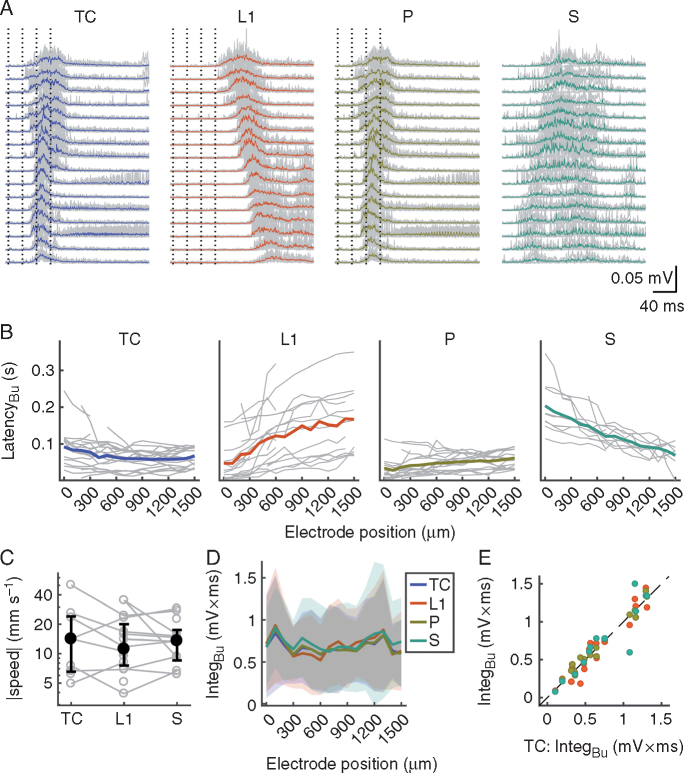
Fig 2.
Properties of network bursts. (A) Excerpts of peri-stimulus MUA signals depicting thalamo-cortical (TC), L1, P and S network bursts for the experiment shown in Fig. 1A–C. Grey lines are individual trials (n=10 for TC, L1 and P), coloured lines are the means. (B) Summary across slices of latencies of TC, L1, P and S network bursts vs electrode position (grey lines, individual slices; coloured lines, means). Only caudo-rostrally propagating S bursts are shown; these were plotted with an arbitrary offset to allow for a comparison of inter-electrode latency differences with stimulated bursts. (C) Propagation speed of network bursts. Open grey circles are individual slices; black circles and error bars are the medians and inter-quartile ranges, respectively. (D) Magnitudes of bursts vs electrode position (coloured lines, means; shaded areas, standard deviations). (E) Scatter plot of L1, P and S burst magnitude (averaged across recording sites) vs TC burst magnitude (same colour code as in B and D). P, paired; S, spontaneous; IntegBu, burst integral; L1, layer 1; MUA, multi-unit activity.
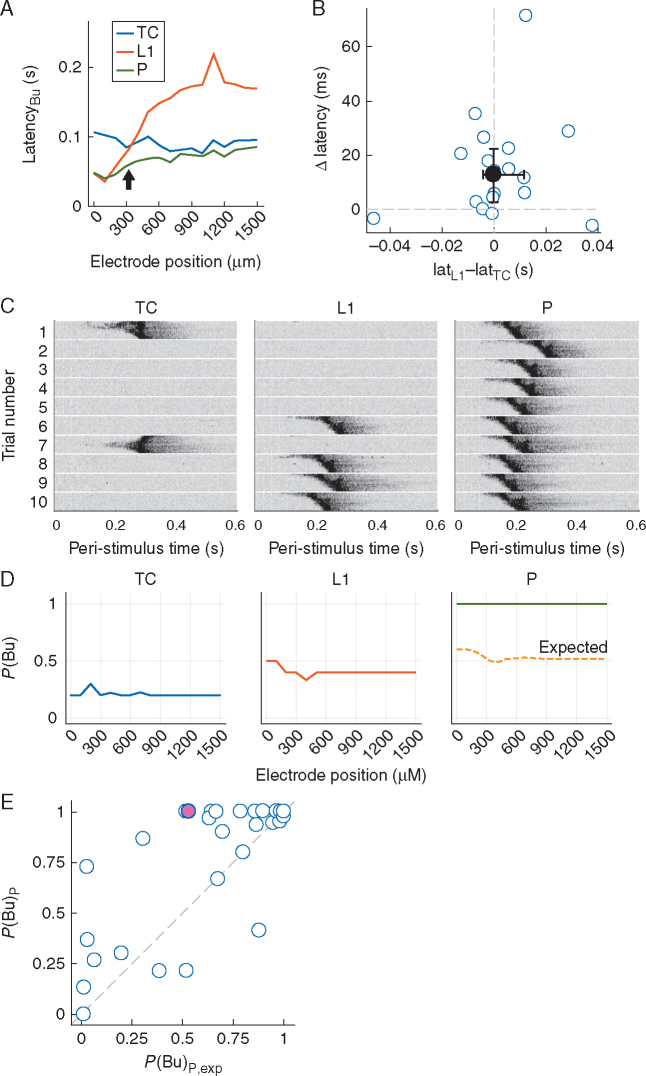
Fig 3.
Paired stimulation facilitates network burst probability. (A) Latency profiles of thalamo-cortical (TC), L1 and P network bursts in an exemplary slice showing shortened P burst latencies around the intersection of TC and L1 burst latency profiles (arrow). (B) Summary analysis of latency shortening by paired stimuli. For each slice, the recording site closest to the intersection of TC and L1 burst latency profiles, as shown exemplarily in A, was selected; the difference between L1 and TC burst latencies at that site (Lat_L1 and Lat_TC, respectively) was used as the abscissa value and the difference (latency of P bursts) – (latency of TC or L1 bursts, whichever was smaller), termed Δ latency, was used as the ordinate value. Filled circle and error bars represent the median and inter-quartile ranges, respectively. (C) Individual trials of bursts elicited by TC, L1 and paired stimuli. Signals from all 16 electrodes are depicted for each trial (top row corresponds to electrode closest to cortical stimulation site). Data were smoothed by a low-pass filter (–3dB frequency 200 Hz) for better visibility. Grey scale is identical for all plots. TC and L1 stimulation intensities were 10 and 32 µA, respectively. (D) Plots of P(Bu) vs recording site for the data shown in C. Dashed line in right-most plot is P(Bu)P,exp, the P(Bu) for paired stimuli that would be expected in the absence of any interaction between TC and L1 afferents. (E) Plot of P(Bu)Pvs P(Bu)P,exp of all experiments in data set. Each point represents P(Bu) averaged across recording sites for a specific pair of stimulation strengths. Magenta point at coordinate (0.53, 1.0) corresponds to the experiment shown in C and D. Note that the overwhelming majority of points is at or close to coordinate (1,1) because of the supra-threshold nature of most stimuli in control. P, paired; S, spontaneous; P(Bu), probability of network burst; L1, layer 1; MUA, multi-unit activity.
Fig 5.
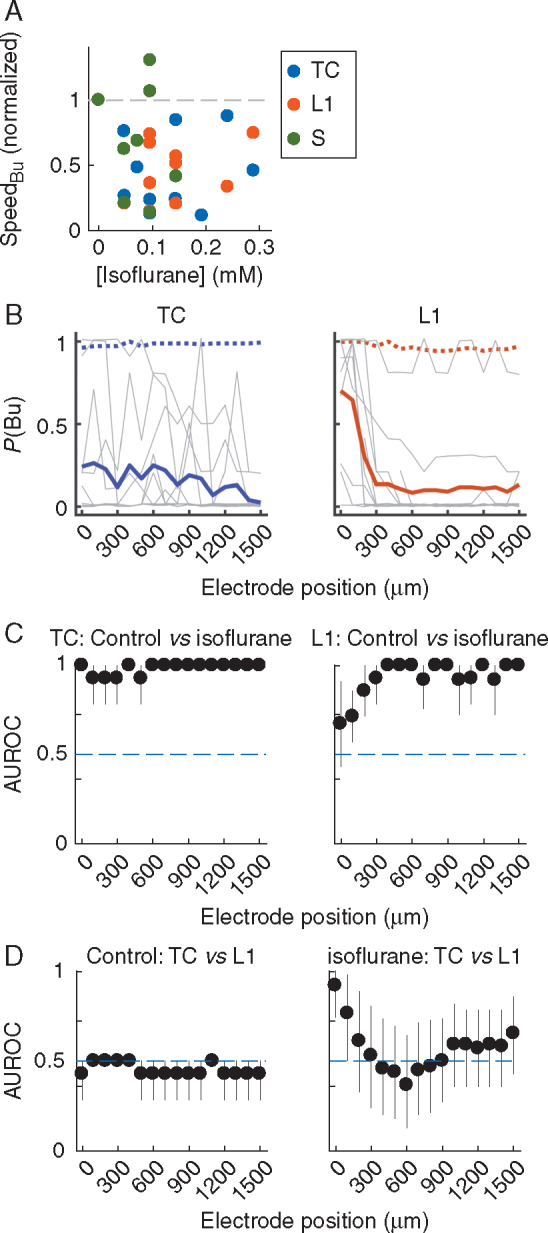
Isoflurane disrupts burst propagation. (A) Normalized burst propagation speed vs isoflurane concentration under constant stimulation strength. As a result of the strong depression of bursts by isoflurane, propagation speed could be computed for only a subset of the data (Supplementary Methods); hence, TC, L1 and S bursts were pooled. (B) Spatial profiles of P(Bu) for TC and L1 bursts in isoflurane. Each grey line is one experiment at an isoflurane concentration and stimulation strength resulting in an across recording sites average 0<P(Bu)<1.0 and a maximal between-electrodes coefficient of variation (CV) of P(Bu) (Supplementary Methods). Thick coloured traces are means; means of P(Bu) at control are plotted for comparison (dotted lines). Note that for this analysis, only sites with P(Bu)>0.75 in control were included. The isoflurane concentrations at maximal CV were [median and (inter-quartile range)] 0.24 (0.10, 0.29) mM for TC bursts and 0.14 (0.10, 0.19) for L1 bursts. (C) AUROC values and 95% CI (error bars) for the comparison of P(Bu) in control and isoflurane. Horizontal line indicates the ‘null effect’ value of 0.5. (D) AUROC values and 95% CI for the comparison of P(Bu) values for TC and L1 bursts as shown in B. TC, thalamo-cortical; L1, layer 1; S, spontaneous; P(Bu), probability of network burst; AUROC, area under the receiver-operating curve.
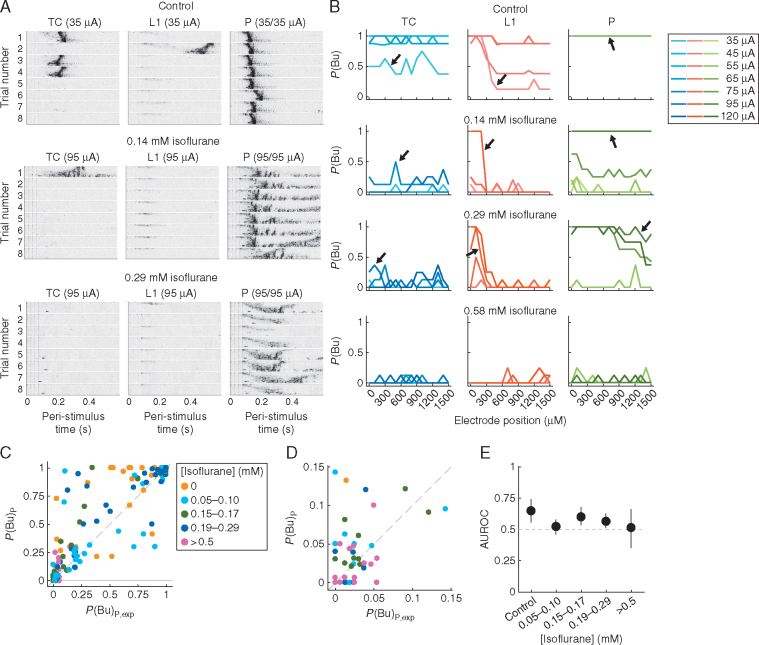
Fig 6.
Isoflurane raises thresholds for facilitation of burst probability by paired stimulation. (A) Individual trials of MUA bursts elicited by TC, L1 and paired stimuli in control conditions and at two concentrations of isoflurane (same conventions as in Fig. 3C apply). (B) Plots of P(Bu) vs recording site for TC, L1 and P bursts in control and isoflurane for the same experiment (full range of parameters shown; TC and L1 stimulation intensities were identical). Data corresponding to the example shown in A are indicated by arrows. Note that in this experiment stimulation intensities in the isoflurane conditions were increased substantially compared with control (control: 35, 45, 55, 65 µA; 0.14 mM isoflurane: 35, 45, 55, 75, 95 µA; 0.29 mM isoflurane: 55, 75, 95, 120 µA; 0.58 mM isoflurane: 55, 75, 95, 120 µA), and that bursts were most reliably elicited by paired stimuli, except at the highest isoflurane concentration. (C) Plot of P(Bu) vs P(Bu)P,exp of all experiments in the data set. Each point represents P(Bu) averaged across recording sites for a specific pair of stimulation strengths and isoflurane condition (cf. Fig. 3E). For better visibility, data were binned according to isoflurane concentration. Note facilitation of paired responses in control and at low-to-intermediate isoflurane concentrations (points in upper-left quadrant) and absence of facilitation at concentrations >0.5 mM (lower-left quadrant). (D) Zoom-in on lower-left corner of C, illustrating accumulation of points around P(Bu) close to zero at high [isoflurane]. (E) AUROC values and 95% confidence intervals [comparison of P(Bu) vs P(Bu)P,exp for each concentration bin]. TC, thalamo-cortical; L1, layer 1; P, paired; MUA, multi-unit activity; P(Bu), probability of network burst; P(Bu)P,exp, expected P(Bu) of paired stimuli; AUROC, area under the receiver-operating curve.
Network bursts also occurred spontaneously (Fig. 2A), albeit rarely (13/20 slices, median rate 0.0086 s−1), propagating in either direction. Propagation speeds of spontaneous (S) and induced bursts did not depend on their origin (Fig. 2C; Kruskal–Wallis test comparing TC, L1 and S bursts; P=0.99). All four categories of bursts (TC, L1, P and S) originated from different, spatially segregated sets of activated afferents and therefore exhibited clear differences in latency profiles and propagation. Nonetheless, they were almost identical in magnitude (Fig. 2D and E) and duration (data not shown), suggesting that once a critical number of neurones was active, internal cortical dynamics governed the extent of activity.
We investigated P bursts in detail, as coordinated stimulation of TC and L1 afferents could be a model for feedback (CC) modulation of feedforward (TC) responses. That is, in addition to interacting with TC afferents at the single-cell level, L1 afferent input might facilitate TC responses at the network level (e.g. by speeding up the generation of network bursts or, in the case of sparse inputs, by increasing the number of neurones with spiking responses to the ‘critical mass’ required to give rise to network bursts). Conversely, in case either of the afferent pathways preferentially recruited inhibitory interneurones, pairing stimuli could delay or impede burst generation. Burst latency profiles of individual slices were suggestive of the former (example in Fig. 3A): particularly at recording sites close to the intersection of TC and L1 burst latency profiles, pairing stimuli predominantly sped up burst generation, albeit to varying degrees (Fig. 3B; median Δ latency of 12.9 ms; Wilcoxon's signed rank test P=0.0016).
As the finding that paired stimulation shortened burst onset suggested a positive interaction of TC and L1 afferents, we also investigated the probability of occurrence of bursts, P(Bu). For very weak TC stimuli (i.e. those unreliable for inducing bursts), pairing with equally unreliable L1 stimuli resulted in non-linear facilitation of responses, such that paired stimulation indeed elicited bursts with high reliability (Fig. 3C). To quantify these observations, we computed the expected P(Bu) for paired stimuli under the assumption that responses to each pathway alone are independent. This quantity, termed P(Bu)P,exp, was computed by multiplying failure rates of TC and L1 stimuli (which yielded the expected failure rate of paired stimuli) and subtracting this expected failure rate from unity. For the example in Fig. 3C, independence of responses would have resulted in P(Bu)P,exp ∼0.5–0.6 (Fig. 3D, right), but the actual probability, P(Bu)P, was unity, suggesting strong facilitation. Across the whole data set, with a few exceptions, P(Bu)P was consistently higher than P(Bu)P,exp [Fig. 3E; AUROC=0.66 (0.58, 0.75); Wilcoxon's signed rank test, P=0.005, computed from n=42 pairs from the 16 slices with paired stimulation P(Bu)P,exp<1]. Thus, pairing TC and L1 stimuli predominantly facilitated burst responses.
Effects of isoflurane on evoked and spontaneous activity in the auditory cortex
Isoflurane had multiple depressant effects on both early responses and network bursts that were readily visible in the MUA data traces (Fig. 4A). We fit generalized linear mixed models (see the Methods section) and tested model fit and coefficients. In experiments in which stimulation strength was held constant throughout control and all drug applications, early responses elicited by TC stimuli declined moderately with isoflurane concentration; their magnitude was on average about 70% of the control value at 0.2 mM isoflurane [the aqueous concentration corresponding to ∼0.8%, the loss of righting reflex for mice;24 25 Fig. 4B; eβ(mM iso)=0.18 with 95% CI: (0.097, 0.34)].
Fig 4.
Isoflurane suppresses stimulated and spontaneous bursts. (A) Excerpts of MUA data traces illustrating the effects of isoflurane on stimulated early responses (left block of traces; TC stimulation only) and network bursts (right block). Grey traces are three representative trials; coloured thick traces are averages. Note the resilience of early responses to isoflurane, in contrast to network bursts, which were completely suppressed at the higher isoflurane concentration and reappeared upon washout of the anaesthetic. Stimulation strength was 60/70 µA for TC and L1 stimuli, respectively. (B) Magnitude of early response to TC stimulation, normalized to control, vs isoflurane concentration. Data in this panel and panels C–E are from a subset of experiments with constant stimulation intensity across control and isoflurane conditions (n=15 experiments with responses at 1–3 tested concentrations of isoflurane each). Each point is the average of two recording sites showing the strongest early responses. In panels B–F, black curves are generalized linear mixed model fits to the data (see the Methods section); dotted curves are 95% confidence intervals. Dashed grey lines are at the normalized control value of the parameter in question. (C) Plots of TC and L1 burst magnitudes vs isoflurane concentration. In all panels depicting burst parameters, each point is the average of four representative recording sites per slice at the burst initiation zone. (D) Plots of P(Bu) vs isoflurane concentration for TC and L1 bursts. (E) Burst latency difference (latency at control – latency at isoflurane) vs isoflurane concentration. (F) Threshold stimulation intensity for burst generation (Ithresh), normalized to control, vs isoflurane concentration. Ithresh was defined as the current required for eliciting bursts with P(Bu)≥0.75. Upward- and downward-pointing triangles represent estimated lower and upper bounds of Ithresh, respectively (see Supplementary Methods). (G) Frequency of occurrence of spontaneous network bursts, normalized to control, vs isoflurane concentration (n=14 slices with spontaneous bursts during control). Black curve is a monoexponential fit to the data [τ with 95% CI: 0.056 (0.039, 0.073)]. (H) Normalized magnitude of spontaneous bursts. Spontaneous bursts were almost completely suppressed at isoflurane concentrations >0.2 mM; as a result of the resulting sparsity of magnitude values, no fit was applied (data from five slices). P(Bu), probability of network burst; TC, thalamo-cortical; L1, layer 1; MUA, multi-unit activity; Integearly, integral of early response.
In contrast to its modest suppressive effect on early spiking responses, isoflurane profoundly suppressed burst responses. For TC stimuli, network bursts were more sensitive to isoflurane than early responses [Fig. 4C, left; eβ(mM iso)=0.0055 with 95% CI: (0.0023, 0.013); likelihood ratio test: χ2(1)=6.48, P=0.011]. The effect on magnitude of network bursts did not differ significantly between TC and L1 bursts (Fig. 4C; likelihood of model without interaction term for burst type was greater than full model). The probability of occurrence of network bursts decreased steeply with isoflurane concentration, and the effect differed for TC vs L1 bursts [Fig. 4D; TC bursts, odds ratio 0.0083 (0.0022, 0.0313) per 0.1 mM isoflurane; L1 bursts, odds ratio 0.0642 (0.0375, 0.1097)]. L1 bursts were less sensitive to a unit increase in isoflurane [likelihood ratio test: χ2(1)=4495.1, P<0.00001], but were more fragile [odds ratio 0.0048 (0.0003, 0.0822)], resulting in a decrease in L1 burst probability at low isoflurane concentrations. These observations are consistent with a model in which L1 bursts arise from a near-threshold level of activation, as we expect if L1 bursts propagate from outside the array, while TC bursts arise from more widespread activation within the array (Supplementary Fig. S1). Isoflurane delayed burst onset [Fig. 4E; slopes of fitted lines are 18.6 (5.95, 31.3) and 35.0 (19.6, 50.5) ms per 0.1 mM isoflurane, respectively] but the effect was not significantly different on TC vs L1 bursts [χ2(1)=2.97, P=0.085]. A complementary finding was that stimuli of higher intensity were required to elicit bursts in the presence of isoflurane [Fig. 4F shows normalized stimulation intensities required for P(Bu)≥0.75; see Supplementary Methods]. Thus, thresholds for induction of bursts were raised by 1.5–2-fold at surgical doses [i.e. 1 median alveolar concentration (MAC); 0.3 mM] of isoflurane, but were not significantly different between TC and L1 bursts, χ2(1)=3.28, P=0.070. Finally, isoflurane also greatly diminished the rate of spontaneous bursts (Fig. 4G) and depressed their magnitude (Fig. 4H; see panel A for a raw data example).
In addition to the suppressive effects on burst initiation illustrated in Fig. 4, both speed and spatial extent of horizontal propagation were profoundly altered by isoflurane (Fig 4, Fig 5). Propagation speed was slowed, with apparently little dependence on isoflurane concentration (Fig. 5A; median reduction=54% across all concentrations; because of the sparsity of bursts in the presence of isoflurane, we pooled TC, L1 and S bursts). (The absence of concentration dependence for the effect of isoflurane on propagation speed likely arose because of a necessary selection bias: to compute propagation speed, we require bursts to occur over a wide extent of the electrode array, and thus at high concentrations of isoflurane we may be analysing only the strongest induced bursts.) Moreover, isoflurane limited the spatial range of L1 bursts, which often faded beyond the first few recording sites closest to the stimulation electrode [Fig. 5B–D; note that here we are only considering recording sites for which P(Bu)>0.75 in control conditions, unlike the similar panel in Fig. 1E]. By contrast, TC bursts did not show a comparable pattern of fading, consistent with the notion of a wide burst initiation zone.
Next, we investigated how isoflurane affected P bursts. As in control conditions (Fig. 3), paired stimulation in moderate concentrations of isoflurane elicited network bursts with high reliability for stimuli that were by themselves less effective (Fig. 6). In the example shown in Fig. 6A, TC and L1 stimuli were barely able to induce bursts on their own (note that in isoflurane stimulation strength had to be increased substantially to induce burst responses). However, paired stimulation reliably generated bursts (with altered dynamics) across the recording array in both isoflurane conditions. Thus, the non-linear facilitation observed under control conditions with paired stimulation of TC and L1 afferents was still evident at moderate concentrations of isoflurane. Once stimulation intensities had been adjusted to account for threshold shifts, burst responses could be ‘rescued’ by pairing TC and L1 stimulation. At higher isoflurane concentrations (>1 MAC), it was virtually impossible to induce bursting responses even with strong paired stimulation (Fig. 6B–E).
Discussion
Spiking responses to TC and layer 1 afferent stimulation
Stimulation of TC afferents evoked short latency spikes on multiple electrodes in the recording array, consistent with the activation of synaptic terminals across a wide swath of auditory cortex.21 Based on observed latencies and comparison with our previous work,19 we conclude that the vast majority of this activity is monosynaptically driven. We cannot exclude a small contribution from antidromically activated cells, estimated previously at around 3% of detected early spikes,19 and from polysynaptic activity. However, we note that the magnitude of spiking activity detected at these short latencies was vastly smaller than that detected during network bursts, as described.19 26, 27, 28
Layer 1 CC afferents are classically described as modulatory, rather than ‘driving’ inputs,29 30 consistent with the paucity of early spiking observed here. However, spiking activity driven by CC afferents in superficial layers has been observed.31 32 Indeed, L1 stimuli could induce propagating bursts largely indistinguishable from TC-induced activity (Fig. 2D and E). This suggests that L1 stimuli were effective at monosynaptically driving spiking activity, but at sites close to the stimulating electrode and not sampled by the recording electrode array. Thus, the most parsimonious explanation of both the sparsity and spatial gradient of early L1 responses is the decay of cortical monosynaptic connection probability with distance.33, 34, 35
TC and L1 afferents triggered network bursts with low threshold and high probability. The dual time course of responses in our slice experiments [i.e. a short latency, well-timed direct response to afferent input followed by long latency, variable and labile components reflecting intracortical activity (bursts)] is paralleled by the multiphasic sensory responses observed in vivo. For example, auditory evoked responses observed in clinical settings are composed of early and late components, the latter exhibiting a state dependence with useful diagnostic implications.36 The bursts we observed here are similar to brief UP states or network events observed in vivo under non-rapid eye movement sleep and anaesthesia,37 38 and are thought to represent fragments of the desynchronized state associated with active engagement with the environment.14 39 The observation that this type of bistable network activity exists under waking conditions as well38 40, 41, 42 lends further motivation to study this activity in slices. These bursts reflect entry into an activated state by the cortical network, and might enhance signal propagation through the cortical hierarchy.15 19 23 Wave-like horizontal spread of induced network activity has been reported previously,22 28 38 43 likely contributing to information sharing between cortical networks and modulating cortical sensory responses coinciding with the arrival of propagating activity.43, 44, 45 Anaesthetic suppression of network bursts is of interest for understanding their role in sensory processing and the mechanisms of general anaesthesia.
Early responses are modestly suppressed by isoflurane
Isoflurane reduced the magnitude of short latency TC responses, by 41% at 0.24 mM isoflurane. Although there is evidence that anaesthetics suppress spontaneous cortical spiking activity,46, 47, 48, 49 few studies compared evoked spiking responses in the absence or presence of anaesthetic.48 50 In these studies, barbiturate anaesthesia at surgical doses reduced responses to acoustic stimuli by ∼50–80%, a larger effect than observed here with isoflurane. Accumulated effects along the ascending sensory pathway might account for these differences. For example, isoflurane directly suppresses thalamic relay cells,51 an effect bypassed here with direct TC fibre stimulation. By contrast, the isoflurane effect on short latency TC spike responses was substantially larger than the 16% block of TC synaptic responses at 0.24 mM isoflurane.13 The difference between synaptic vs spiking response sensitivity likely reflects the non-linear sensitivity of spiking to small changes in sub-threshold membrane potential signals.52 Although the effects of isoflurane on short latency spiking responses were modest, we note that there is an amplification of the effect at the TC synapse, contributing to the reduced activation of cortical networks by synaptically driven spiking activity.
Suppression of network activity by isoflurane
We found more dramatic suppression by isoflurane of spiking within induced network bursts. At doses comparable with surgical anaesthesia in vivo (>0.3 mM), network bursts were almost entirely eliminated even at the highest stimulus intensities. Thus, isoflurane effectively decouples the cortical network from inputs that typically drive an activated state. These observations are consistent with current models of LOC under anaesthesia in which suppression of intracortical connections plays a prominent role.4 53 The difference in sensitivity of L1 and TC burst probability (Fig. 4D) likely reflects differential effects on burst propagation compared with burst initiation.
Previous studies have shown that general anaesthetics synchronize network activity at moderate concentrations and disrupt this activity at higher concentrations.54, 55, 56 Consistent with these findings, we observed complete suppression of induced network bursts at the highest concentrations of isoflurane tested. At concentrations corresponding to sub-hypnotic and just-hypnotic doses in vivo, the effects of isoflurane on network burst probability and magnitude were variable. Indeed, the distribution of P(Bu) across slices in response to TC stimulation in the presence of isoflurane was bimodal, with some slices exhibiting little effect and some exhibiting near-complete suppression. We have yet to identify the source of this variability, but it could relate to small differences in network structure57 or complement of isoflurane-sensitive loci in the network that are amplified during the non-linear coupling between afferent stimulation and network activation. This variability might relate to increased trial-by-trial variability observed for population responses at intermediate doses of anaesthetic in vivo,58 and which we have postulated disrupts sensory processing even at sub-hypnotic doses.59
Isoflurane suppressed spontaneous network activity and increased the latency and reduced the spatial spread and propagation speed of stimulus-induced network activity. Our recapitulation in the absence of brainstem sleep/wake nuclei and the hypothalamus of previously reported effects on network activity in vivo suggests a prominent role for cortical actions of anaesthetics. Indeed, all of these effects are consistent with increased network activation thresholds. Mechanistically, the increased threshold for inducing network bursts and their slowed propagation are likely secondary to multiple effects at the molecular and cellular level that combine to dampen network excitability,60, 61, 62, 63 including increased spike thresholds in cortical and thalamic cells,51 64 65 decreased glutamate release66, 67, 68 and enhanced phasic and tonic gamma-aminobutyric acid type A (γABAA) receptor-mediated inhibition.46 69, 70, 71 Although isoflurane can increase excitability of single cells via blockade of the hyperpolarization-activated current Ih,72 and cause suppression of inhibition in vivo and paradoxical excitation in cortical networks,73, 74, 75 it is clear that net effects on excitability observed here were suppressive and accumulated across multiple synapses.
Non-linear, network-level interactions between TC and L1 afferents could contribute to feedback (i.e. CC) modulation of sensory afferent responses. Integration of these information streams is proposed to underlie predictive coding,76 with CC afferents altering responses in sensory cortex at the single-cell77, 78, 79, 80 and circuit levels.23 81 82 Luczak and colleagues14 proposed that top-down control of auditory cortical responses occurs via changes in the probability of network events (‘packets’) corresponding to brief UP states. Consistent with our observations that TC and L1 bursts are similar in duration and magnitude, afferent input to a cortical column can trigger network activity, irrespective of its origin, and sub-threshold inputs from a variety of sources can sum to generate network bursts, analogous to synaptic inputs to a single cell summing non-linearly to trigger a spike.15
Disruption of these network-level interactions likely contributes to LOC under anaesthesia.4 53 The effect of isoflurane on these interactions appeared to be largely determined by the increased activation threshold observed for each afferent pathway alone. Increasing stimulation intensity was able to compensate for increased thresholds and restore facilitative interactions, failing only at high isoflurane concentrations. Thus, it appears that isoflurane does not interfere with these interactions per se, it just makes the network harder to activate. This raises the possibility that the effect of isoflurane is dominated by the suppression of glutamate release at CC synapses,68 rather than the effects on the integrative process at the single-cell level. In this scenario, increasing stimulation strength under isoflurane compensates for reduced synaptic release, and the postsynaptic cell is still capable of integrating these inputs to generate spiking activity and contribute to burst activity. Future experiments aimed at understanding the synaptic and cellular mechanisms of the effects of isoflurane on network bursts and network-level interactions between afferent pathways in sensory processing will shed additional light on mechanisms of anaesthesia and the neural basis of sensory awareness.
Authors’ contributions
Study design, data collection, manuscript preparation: A.R.
Contributed equally to this paper: H.H., A.R.
Data analysis, manuscript preparation: B.M.K.
Data collection, manuscript preparation: C.A.M.
Study design, data analysis, manuscript preparation: M.I.B., H.H.
Declaration of interest
The authors declare no competing financial interests.
Funding
This work was supported by the National Institutes of Health (R01 GM109086 and R01 GM116916 to M.I.B.), the International Anesthesia Research Society (Mentored Research Award grant to A.R.), and the Department of Anesthesiology, School of Medicine and Public Health, University of Wisconsin, Madison, WI, USA.
Acknowledgements
The authors thank Sean Grady (UW Department of Anesthesiology) for technical support on this project.
Handling editor: Hugh C. Hemmings Jr
Footnotes
Supplementary material is available at British Journal of Anaesthesia online.
Supplementary Data
References
- 1.Devor M, Zalkind V. Reversible analgesia, atonia, and loss of consciousness on bilateral intracerebral microinjection of pentobarbital. Pain. 2001;94:101–112. doi: 10.1016/S0304-3959(01)00345-1. [DOI] [PubMed] [Google Scholar]
- 2.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:979–984. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 3.Baker R, Gent TC, Yang Q. Altered activity in the central medial thalamus precedes changes in the neocortex during transitions into both sleep and propofol anesthesia. J Neurosci. 2014;34:13326–13335. doi: 10.1523/JNEUROSCI.1519-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mashour GA. Cognitive unbinding: a neuroscientific paradigm of general anesthesia and related states of unconsciousness. Neurosci Biobehav Rev. 2013;37:2751–2759. doi: 10.1016/j.neubiorev.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrarelli F, Massimini M, Sarasso S. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc Natl Acad Sci USA. 2010;107:2681–2686. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrouff J, Perlbarg V, Boly M. Brain functional integration decreases during propofol-induced loss of consciousness. Neuroimage. 2011;57:198–205. doi: 10.1016/j.neuroimage.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Peltier SJ, Kerssens C, Hamann SB, Sebel PS, Byas-Smith M, Hu X. Functional connectivity changes with concentration of sevoflurane anesthesia. Neuroreport. 2005;16:285–288. doi: 10.1097/00001756-200502280-00017. [DOI] [PubMed] [Google Scholar]
- 9.Fagerholm ED, Scott G, Shew WL. Cortical entropy, mutual information and scale-free dynamics in waking mice. Cerebral Cortex. 2016;26:3945–3952. doi: 10.1093/cercor/bhw200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imas OA, Ropella KM, Ward BD, Wood JD, Hudetz AG. Volatile anesthetics disrupt frontal-posterior recurrent information transfer at gamma frequencies in rat. NeurosciLett. 2005;387:145–150. doi: 10.1016/j.neulet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Boly M, Moran R, Murphy M. Connectivity changes underlying spectral EEG changes during propofol-induced loss of consciousness. J Neurosci. 2012;32:7082–7090. doi: 10.1523/JNEUROSCI.3769-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee U, Ku S, Noh G, Baek S, Choi B, Mashour GA. Disruption of frontal-parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology. 2013;118:1264–1275. doi: 10.1097/ALN.0b013e31829103f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raz A, Grady SM, Krause BM, Uhlrich DJ, Manning KA, Banks MI. Preferential effect of isoflurane on top-down versus bottom-up pathways in sensory cortex. Front Syst Neurosci. 2014;8:191. doi: 10.3389/fnsys.2014.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luczak A, McNaughton BL, Harris KD. Packet-based communication in the cortex. Nat Rev Neurosci. 2015;16:745–755. doi: 10.1038/nrn4026. [DOI] [PubMed] [Google Scholar]
- 15.Yuste R. From the neuron doctrine to neural networks. Nat Rev Neurosci. 2015;16:487–497. doi: 10.1038/nrn3962. [DOI] [PubMed] [Google Scholar]
- 16.Diesmann M, Gewaltig MO, Aertsen A. Stable propagation of synchronous spiking in cortical neural networks. Nature. 1999;402:529–533. doi: 10.1038/990101. [DOI] [PubMed] [Google Scholar]
- 17.Luczak A, Bartho P, Harris KD. Gating of sensory input by spontaneous cortical activity. JNeurosci. 2013;33:1684–1695. doi: 10.1523/JNEUROSCI.2928-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neske GT. The slow oscillation in cortical and thalamic networks: mechanisms and functions. Front Neural Circuits. 2016;9:88. doi: 10.3389/fncir.2015.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krause BM, Raz A, Uhlrich DJ, Smith PH, Banks MI. Spiking in auditory cortex following thalamic stimulation is dominated by cortical network activity. Front Syst Neurosci. 2014;8:170. doi: 10.3389/fnsys.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown CD, Davis HT. Receiver operating characteristics curves and related decision measures: a tutorial. Chemometr Intell Lab Syst. 2006;80:24–38. [Google Scholar]
- 21.Cruikshank SJ, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol. 2002;87:361–384. doi: 10.1152/jn.00549.2001. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- 23.Rigas P, Castro-Alamancos MA. Impact of persistent cortical activity (up states) on intracortical and thalamocortical synaptic inputs. J Neurophysiol. 2009;102:119–131. doi: 10.1152/jn.00126.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franks NP, Lieb WR. Selective actions of volatile general anaesthetics at molecular and cellular levels. Br J Anaesth. 1993;71:65–76. doi: 10.1093/bja/71.1.65. [DOI] [PubMed] [Google Scholar]
- 25.Lazarenko RM, Willcox SC, Shu S. Motoneuronal TASK channels contribute to immobilizing effects of inhalational general anesthetics. J Neurosci. 2010;30:7691–7704. doi: 10.1523/JNEUROSCI.1655-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beierlein M, Fall CP, Rinzel J, Yuste R. Thalamocortical bursts trigger recurrent activity in neocortical networks: layer 4 as a frequency-dependent gate. J Neurosci. 2002;22:9885–9894. doi: 10.1523/JNEUROSCI.22-22-09885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gil Z, Amitai Y. Adult thalamocortical transmission involves both NMDA and non-NMDA receptors. J Neurophysiol. 1996;76:2547–2554. doi: 10.1152/jn.1996.76.4.2547. [DOI] [PubMed] [Google Scholar]
- 28.Wester JC, Contreras D. Columnar interactions determine horizontal propagation of recurrent network activity in neocortex. J Neurosci. 2012;32:5454–5471. doi: 10.1523/JNEUROSCI.5006-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crick F, Koch C. Constraints on cortical and thalamic projections: the no-strong-loops hypothesis. Nature. 1998;391:245–250. doi: 10.1038/34584. [DOI] [PubMed] [Google Scholar]
- 30.Sherman SM, Guillery RW. Distinct functions for direct and transthalamic corticocortical connections. J Neurophysiol. 2011;106:1068–1077. doi: 10.1152/jn.00429.2011. [DOI] [PubMed] [Google Scholar]
- 31.Covic EN, Sherman SM. Synaptic properties of connections between the primary and secondary auditory cortices in mice. Cereb Cortex. 2011;21:2425–2441. doi: 10.1093/cercor/bhr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mignard M, Malpeli JG. Paths of information flow through visual cortex. Science. 1991;251:1249–1251. doi: 10.1126/science.1848727. [DOI] [PubMed] [Google Scholar]
- 33.Lohmann H, Rorig B. Long-range horizontal connections between supragranular pyramidal cells in the extrastriate visual cortex of the rat. J Comp Neurol. 1994;344:543–558. doi: 10.1002/cne.903440405. [DOI] [PubMed] [Google Scholar]
- 34.Ichinose T, Murakoshi T. Electrophysiological elucidation of pathways of intrinsic horizontal connections in rat visual cortex. Neuroscience. 1996;73:25–37. doi: 10.1016/0306-4522(96)00026-7. [DOI] [PubMed] [Google Scholar]
- 35.Butovas S, Schwarz C. Spatiotemporal effects of microstimulation in rat neocortex: a parametric study using multielectrode recordings. J Neurophysiol. 2003;90:3024–3039. doi: 10.1152/jn.00245.2003. [DOI] [PubMed] [Google Scholar]
- 36.Nourski KV, Banks MI, Steinschneider M. Electrocorticographic delineation of human auditory cortical fields based on effects of propofol anesthesia. Neuroimage. 2017;152:78–93. doi: 10.1016/j.neuroimage.2017.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 38.Sakata S, Harris KD. Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron. 2009;64:404–418. doi: 10.1016/j.neuron.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Destexhe A, Hughes SW, Rudolph M, Crunelli V. Are corticothalamic ′up′ states fragments of wakefulness? Trends Neurosci. 2007;30:334–342. doi: 10.1016/j.tins.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petersen CCH, Hahn TTG, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci USA. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poulet JF, Fernandez LM, Crochet S, Petersen CC. Thalamic control of cortical states. Nat Neurosci. 2012;15:370–372. doi: 10.1038/nn.3035. [DOI] [PubMed] [Google Scholar]
- 42.McGinley MJ, David SV, McCormick DA. Cortical membrane potential signature of optimal states for sensory signal detection. Neuron. 2015;87:179–192. doi: 10.1016/j.neuron.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato TK, Nauhaus I, Carandini M. Traveling waves in visual cortex. Neuron. 2012;75:218–229. doi: 10.1016/j.neuron.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 44.Roland PE, Hanazawa A, Undeman C. Cortical feedback depolarization waves: a mechanism of top-down influence on early visual areas. Proc Natl Acad Sci USA. 2006;103:12586–12591. doi: 10.1073/pnas.0604925103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilad A, Meirovithz E, Slovin H. Population responses to contour integration: early encoding of discrete elements and late perceptual grouping. Neuron. 2013;78:389–402. doi: 10.1016/j.neuron.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Hentschke H, Schwarz C, Antkowiak B. Neocortex is the major target of sedative concentrations of volatile anaesthetics: strong depression of firing rates and increase of GABAA receptor-mediated inhibition. Eur J Neurosci. 2005;21:93–102. doi: 10.1111/j.1460-9568.2004.03843.x. [DOI] [PubMed] [Google Scholar]
- 47.Hudetz AG, Vizuete JA, Imas OA. Desflurane selectively suppresses long-latency cortical neuronal response to flash in the rat. Anesthesiology. 2009;111:231–239. doi: 10.1097/ALN.0b013e3181ab671e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaese BH, Ostwald J. Anesthesia changes frequency tuning of neurons in the rat primary auditory cortex. J Neurophysiol. 2001;86:1062–1066. doi: 10.1152/jn.2001.86.2.1062. [DOI] [PubMed] [Google Scholar]
- 49.Zurita P, Villa AEP, Deribaupierre Y, Deribaupierre F, Rouiller EM. Changes of single-unit activity in the cats auditory thalamus and cortex associated to different anesthetic conditions. Neurosci Res. 1994;19:303–316. doi: 10.1016/0168-0102(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 50.Ter Mikaelian M, Sanes DH, Semple MN. Transformation of temporal properties between auditory midbrain and cortex in the awake mongolian gerbil. J Neurosci. 2007;27:6091–6102. doi: 10.1523/JNEUROSCI.4848-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ries CR, Puil E. Mechanism of anesthesia revealed by shunting actions of isoflurane on thalamocortical neurons. J Neurophysiol. 1999;81:1795–1801. doi: 10.1152/jn.1999.81.4.1795. [DOI] [PubMed] [Google Scholar]
- 52.Crochet S, Poulet JF, Kremer Y, Petersen CC. Synaptic mechanisms underlying sparse coding of active touch. Neuron. 2011;69:1160–1175. doi: 10.1016/j.neuron.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 53.Mashour GA. Top-down mechanisms of anesthetic-induced unconsciousness. Front Syst Neurosci. 2014;8:115. doi: 10.3389/fnsys.2014.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imas OA, Ropella KM, Ward BD, Wood JD, Hudetz AG. Volatile anesthetics enhance flash-induced gamma oscillations in rat visual cortex. Anesthesiology. 2005;102:937–947. doi: 10.1097/00000542-200505000-00012. [DOI] [PubMed] [Google Scholar]
- 55.Lissek T, Obenhaus HA, Ditzel DAW. General anesthetic conditions induce network synchrony and disrupt sensory processing in the cortex. Front Cell Neurosci. 2016;10:64. doi: 10.3389/fncel.2016.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lukatch HS, Kiddoo CE, MacIver MB. Anesthetic-induced burst suppression EEG activity requires glutamate-mediated excitatory synaptic transmission. Cereb Cortex. 2005;15:1322–1331. doi: 10.1093/cercor/bhi015. [DOI] [PubMed] [Google Scholar]
- 57.Barttfeld P, Uhrig L, Sitt JD, Sigman M, Jarraya B, Dehaene S. Signature of consciousness in the dynamics of resting-state brain activity. Proc Natl Acad Sci USA. 2015;112:887–892. doi: 10.1073/pnas.1418031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kisley MA, Gerstein GL. Trial-to-trial variability and state-dependent modulation of auditory- evoked responses in cortex. J Neurosci. 1999;19:10451–10460. doi: 10.1523/JNEUROSCI.19-23-10451.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banks MI. Anesthetic modulation of auditory perception: linking cellular, circuit and behavioral effects. In: Hudetz AG, Pearce RA, editors. Suppressing the Mind: Anesthetic Modulation of Memory and Consciousness. Humana Press; New York: 2010. pp. 81–97. [Google Scholar]
- 60.Richards CD. Actions of general anaesthetics on synaptic transmission in the CNS. Br J Anaesth. 1983;55:201–207. doi: 10.1093/bja/55.3.201. [DOI] [PubMed] [Google Scholar]
- 61.MacIver MB, Roth SH. Inhalation anaesthetics exhibit pathway-specific and differential actions on hippocampal synaptic responses in vitro. Br J Anaesth. 1988;60:680–691. doi: 10.1093/bja/60.6.680. [DOI] [PubMed] [Google Scholar]
- 62.Richards CD. Anaesthetic modulation of synaptic transmission in the mammalian CNS. Br J Anaesth. 2002;89:79–90. doi: 10.1093/bja/aef162. [DOI] [PubMed] [Google Scholar]
- 63.Pocock G, Richards CD. Excitatory and inhibitory synaptic mechanisms in anaesthesia. Br J Anaesth. 1993;71:134–147. doi: 10.1093/bja/71.1.134. [DOI] [PubMed] [Google Scholar]
- 64.Fujiwara N, Higashi H, Nishi S, Shimoji K, Sugita S, Yoshimura M. Changes in spontaneous firing patterns of rat hippocampal neurones induced by volatile anaesthetics. J Physiol. 1988;402:155–175. doi: 10.1113/jphysiol.1988.sp017198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.el-Beheiry H, Puil E. Anaesthetic depression of excitatory synaptic transmission in neocortex. Exp Brain Res. 1989;77:87–93. doi: 10.1007/BF00250570. [DOI] [PubMed] [Google Scholar]
- 66.MacIver MB, Mikulec AA, Amagasu SM, Monroe FA. Volatile anesthetics depress glutamate transmission via presynaptic actions. Anesthesiology. 1996;85:823–834. doi: 10.1097/00000542-199610000-00018. [DOI] [PubMed] [Google Scholar]
- 67.Wu XS, Sun JY, Evers AS, Crowder M, Wu LG. Isoflurane inhibits transmitter release and the presynaptic action potential. Anesthesiology. 2004;100:663–670. doi: 10.1097/00000542-200403000-00029. [DOI] [PubMed] [Google Scholar]
- 68.Westphalen RI, Desai KM, Hemmings HC., Jr. Presynaptic inhibition of the release of multiple major central nervous system neurotransmitter types by the inhaled anaesthetic isoflurane. Br J Anaesth. 2013;110:592–599. doi: 10.1093/bja/aes448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caraiscos VB, Newell JG, You T. Selective enhancement of tonic GABAergic inhibition in murine hippocampal neurons by low concentrations of the volatile anesthetic isoflurane. J Neurosci. 2004;24:8454–8458. doi: 10.1523/JNEUROSCI.2063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jenkins A, Greenblatt EP, Faulkner HJ. Evidence for a common binding cavity for three general anesthetics within the GABAA receptor. J Neurosci. 2001;21:RC136. doi: 10.1523/JNEUROSCI.21-06-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Banks MI, Pearce RA. Dual actions of volatile anesthetics on GABA(A) IPSCs: dissociation of blocking and prolonging effects. Anesthesiology. 1999;90:120–134. doi: 10.1097/00000542-199901000-00018. [DOI] [PubMed] [Google Scholar]
- 72.Chen X, Shu S, Kennedy DP, Willcox SC, Bayliss DA. Subunit-specific effects of isoflurane on neuronal Ih in HCN1 knockout mice. J Neurophysiol. 2009;101:129–140. doi: 10.1152/jn.01352.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCarthy MM, Brown EN, Kopell N. Potential network mechanisms mediating electroencephalographic beta rhythm changes during propofol-induced paradoxical excitation. J Neurosci. 2008;28:13488. doi: 10.1523/JNEUROSCI.3536-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haider B, Häusser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature. 2013;493:97–100. doi: 10.1038/nature11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferron JF, Kroeger D, Chever O, Amzica F. Cortical inhibition during burst suppression induced with isoflurane anesthesia. J Neurosci. 2009;29:9850–9860. doi: 10.1523/JNEUROSCI.5176-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bastos AM, Usrey WM, Adams RA, Mangun GR, Fries P, Friston KJ. Canonical microcircuits for predictive coding. Neuron. 2012;76:695–711. doi: 10.1016/j.neuron.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Banks MI, Uhlrich DJ, Smith PH, Krause BM, Manning KA. Descending projections from extrastriate visual cortex modulate responses of cells in primary auditory cortex. Cereb Cortex. 2011;21:2620–2638. doi: 10.1093/cercor/bhr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shlosberg D, Patrick SL, Buskila Y, Amitai Y. Inhibitory effect of mouse neocortex layer I on the underlying cellular network. Eur J Neurosci. 2003;18:2751–2759. doi: 10.1111/j.1460-9568.2003.03016.x. [DOI] [PubMed] [Google Scholar]
- 79.Zhang S, Xu M, Kamigaki T. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science. 2014;345:660–665. doi: 10.1126/science.1254126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gomez-Laberge C, Smolyanskaya A, Nassi JJ, Kreiman G, Born RT. Bottom-up and top-down input augment the variability of cortical neurons. Neuron. 2016;91:540–547. doi: 10.1016/j.neuron.2016.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reig R, Sanchez-Vives MV. Synaptic transmission and plasticity in an active cortical network. PLoS One. 2007;2:e670e. doi: 10.1371/journal.pone.0000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy BA. disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci. 2013;16:1662–1670. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.