Abstract
Background
Experimental studies in animals have shown that exposure to general anaesthesia in infancy can cause loss of cells in the central nervous system and long-term impairments in neurocognitive function. Some human epidemiological studies have shown increased risk of learning disability after repeated anaesthesia exposure in early childhood. Thus, we investigated in a highly translational rhesus monkey model, whether repeated exposure in infancy to the inhalation anaesthetic sevoflurane is associated with impaired visual recognition memory during the first two yr of life.
Methods
Rhesus monkeys of both sexes were exposed to sevoflurane inhalation anaesthesia on approximately postnatal day 7 and then again 14 and 28 days later, for four h each time. Visual recognition memory was tested using the visual paired comparison task, which measures memory by assessing preference for looking at a new image over a previously-viewed image. Monkeys were tested at 6–10 months of age, again at 12–18 months of age, and again at 24–30 months of age.
Results
No memory impairment was detected at 6–10 months old, but significant impairment (reduced time looking at the novel image) was observed at 12–18 and 24–30 months old.
Conclusions
Repeated exposure of infant rhesus monkeys to sevoflurane results in visual recognition memory impairment that emerges after the first yr of life. This is consistent with epidemiological studies that show increased risk of learning disability after repeated exposure to anaesthesia in infancy/early childhood. Moreover, these deficits may emerge at later developmental stages, even when memory performance is unaffected earlier in development.
Keywords: general anaesthesia; neurotoxicity syndromes; cognitive disorder; sevoflurane; macaque, rhesus
Editor's key points.
-
•
Exposure to general anaesthesia in infant animals can cause long-term neurocognitive deficits, especially after repeated or prolonged exposures.
-
•
The effects of repeated exposure to sevoflurane anaesthesia in infancy on visual recognition memory were evaluated in a nonhuman primate model.
-
•
Three four hour exposures to sevoflurane anaesthesia in infant rhesus monkeys led to neurocognitive deficits that appeared over a year later.
The possibility that exposure to general anaesthesia in infancy or early childhood may have long-term adverse effects on neurocognitive function is of concern to anaesthetists. Anaesthetic neurotoxicity has been demonstrated in newborns of a number of species,1, 2, 3, 4, 5, 6 44, 45 although never directly observed in humans. Neurocognitive impairment after anaesthesia exposure early in life is also common in animal studies. Some human epidemiological studies have found that repeated exposure to anaesthesia early in childhood (before the age of four), is associated with increased risk of learning disability and attention deficit/hyperactivity disorder,7, 8, 9 although this observation is not universal.10 11 Moreover, single, relatively brief exposures to general anaesthesia early in childhood seem to be safe in terms of neurocognitive outcome in prospective studies, at least in terms of the endpoints and developmental stages that have been studied to date.12 13
Because repeated exposure to general anaesthesia early in development seems to be associated with greater risk of later neurocognitive difficulties, we have developed a rhesus monkey model of repeated anaesthesia exposure in infancy. This model has a number of advantages, including the ability to monitor and support physiological homeostasis during anaesthesia to a much greater degree possible than with rodents, and allows assessment of the complex cognitive behaviours that nonhuman primates can display. In this model, we exposed infant macaques to sevoflurane anaesthesia for four h on postnatal day seven, and again on days 21 and 35. We then followed the animals that live with their mothers in large social groups at the Yerkes National Primate Research Centre for two yr of socioemotional and cognitive testing starting at six months of age, five months after their last anaesthetic exposure.14
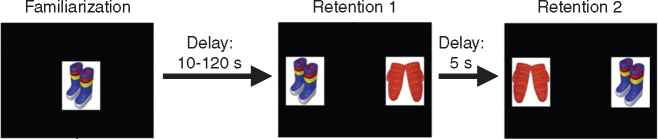
The present report describes the development of recognition memory in these subjects, which were tested at six, 12 and 24 months of age using the visual paired comparison task (VPC)15, 16, 17, 18 (Fig. 1). This task, developed to study human memory,15 16 measures incidental visual recognition memory and takes advantage of the spontaneous preference to look at novel stimuli. It requires no training, thus it is an ideal task for the study of memory in preverbal humans or non-verbal monkeys. Monkeys as young as 1 month of age will reliably spend more time looking at a novel image than the familiarized image.19 Memory is further taxed by increasing the delay between initial viewing of the familiarized object and presentation of the novel stimulus. In adult humans and monkeys, damage to structures in the medial temporal lobe yields a recognition memory impairment at long delays and may vary in the type of memory impacted (e.g. object vs spatial).20 21 Because this task is also sensitive to disruptions in memory during development as a consequence of neonatal damage to medial temporal lobe structures,17 18 22 23 our second aim was to determine whether impairment patterns followed those produced by selective damage to those structures.
Fig 1.
Illustration of a single trial of the visual paired comparison task.
Methods
All animal procedures were approved by the Yerkes National Primate Research Centre and the Emory University Institutional Animal Care and Use Committee, and were conducted in full compliance with PHS Policy on Humane Care and Use of Laboratory Animals. Subject descriptions and anaesthetic procedures have been published elsewhere.14 Briefly, twenty newborn rhesus macaques of Indian origin (Macaca mulatta) were born in two cohorts, in the breeding colony at the Yerkes National Primate Research Centre field station. The first, six female and four male, were born in the 2012 birth season. The second cohort (four female, six male) were born the following yr. All infants were delivered vaginally without veterinary intervention in their natal group compounds. Infants were born to middle-ranking dams and were housed in large social groups of 50-100 individuals and comprising several family groups. Infants were assigned as they were born and with consideration to balancing for sex and weight to either the control group or the anaesthesia group.
Monkeys received either three anaesthetic exposures to sevoflurane (anaesthesia group), or three brief maternal separations (Control group) on or about postnatal day seven (range from day six-10), that was repeated at two-week intervals for a total of three exposure/separations between postnatal days ∼seven-35. After removal from the dam, all infants received a brief neurological exam (see14). At this point, monkeys in the anaesthetic group were mask-induced with sevoflurane (from 2 vol % to effect, maximum 8 vol % in 100% O2), intubated and catheterized for i.v. fluids. Sevoflurane was administered for 4 h, with monitoring of vital signs, depth of anaesthesia and blood gases (see Table 1 in14). After complete recovery, the infant was returned to its dam. The recovery period lasted on average 20-30 min. Subjects in the control group experienced maternal separation at the same ages, that comprised the neurological exam and a period of handling that matched in total duration the separation consciously experienced by the experimental group. Thus, on average, control infants experienced 30-40 min of maternal separation and were returned to the dam. Details of the mother-infant interactions after these separations have been published24 and did not differ between groups, indicating that the separations involved in anaesthesia exposures did not cause alterations in mother-infant bonding that might have impacted later cognitive or socioemotional behavior.
During the next four and a half months subjects were observed in their social groups and weekly accessed so as to become acclimated with the procedures for brief separations from the social group for the purposes of behavioural testing or periodic blood samples. After their emotional reactivity testing at six months14 they began formal cognitive testing on the visual paired comparison (VPC) paradigm.
All monkeys, in both control and anaesthesia groups, were exposed to anaesthetic drugs at older ages for regular health checks and when required for veterinary treatments. These drugs were not part of the initial anaesthesia/control treatment. Unlike infant rhesus monkeys, older monkeys cannot be handled safely for veterinary procedures without being sedated. For routine veterinary exams at 12, 24, and 30 months of age, all monkeys were sedated with a cocktail of 0.01 mg kg−1 dexmedetomidine (i.m.), 0.3 mg kg−1 midazolam (i.m.), and 0.01-0.03 mg kg −1 buprenorphine (i.m.). Once the monkey was safe to handle, sevoflurane (3-6 vol %, to effect) was administered by mask if required. The dexmedetomidine was antagonized with atipamezole (0.01-0.15 mg kg−1, half i.m. half i.v.) and the midazolam by flumazenil (0.02 mg kg−1, i.v.) once sevoflurane administration began. These procedures typically took five-15 min to complete. If surgical treatment was required (as for an injury), dexmedetomidine was only partially antagonized (0.03-0.05 mg kg−1 atipamezole). The intention was to avoid treatment with ketamine, propofol, or other anaesthetic agents when anaesthesia was necessary for veterinary treatment.
In addition to the routine veterinary exams described above, 11 of the 20 monkeys (six in the anaesthesia group, five in the control group) received additional sevoflurane exposure for veterinary treatments. Of the monkeys that received additional sevoflurane, this occurred on a mean of 2.2 (range 1-6) occasions, of median duration 25 min (range 19-113). The two most extreme cases were A1f (six additional exposures of 17-70 min duration, median 37.5) and C9m (five additional exposures of 23-47 min duration, median 29); the 113 min exposure (the longest additional exposure) was in case C2f. An additional 2 monkeys in the anaesthesia group received sedative drugs alone on a single occasion for minor veterinary treatment.
VPC testing15, 16, 17, 18 was conducted using a Tobii T60/T120 eye tracker (Tobii Technology, Sweden). Six month old infants were hand-held, wrapped in a soft blanket and facing the eye tracker monitor on which visual images were displayed. Twelve and 24-month old infants sat in a custom designed plexiglas test "chair", positioned at a proper distance for the eye tracker to detect their eyes. A curtain prevented the monkey from seeing the tester, but the monkey was visible to the tester via the Tobii built-in camera. The monkey's eyes were visualized on the testing screen when the eyetracker detected them and a gaze-trail was shown over the tester's screen showing where on the screen the monkey was looking. As a precaution for accurate eyetracking and to avoid possible bias, monkey handlers always maintained their gaze downward towards the floor when holding infants. Likewise, when the older animals were seated in the chair, the handler was seated facing the monkey, with profile to the testing screen. Infants seated in testing chairs were further offered an oral syringe filled with various fruit purees that was held at the centre of the chair front so as to orient their heads in the direction of the eye tracker monitor. Fruit puree was dispensed at random intervals so as not to reward a particular gaze pattern.
For testing, the infants were accessed from their social groups and transported to the behavioural testing suite. Visual stimuli were obtained from existing testing sets used by our colleagues routinely to test infant monkeys17 18 that used photo/clip-art images of everyday junk objects (Nova Art Explosion 800,000 ClipArt). Selected images for each set were chosen to be as different as possible. As shown in Fig. 1, each testing trial consisted of a Familiarization phase, in which a single image (Sample) was presented in the centre of the screen. The image was visible while the tester timed the gaze trails crossing the image until a total of 30 s of viewing time accumulated. At this point, the image disappeared and the screen remained black for the Delay time (10, 30, 60 or 120 s). After the delay period, the first of two Retention tests began during which the Sample image and a Novel image were placed on either side of the screen for five s and the monkey was free to look at either. Both images then disappeared for another five s and then the second Retention test began in which the Sample and Novel images were again presented, but on opposite sides of the screen. Initial placement of the objects was determined pseudo randomly such that the Sample object appeared equally often on either side of the screen for the first Retention test. Each trial was separated by a 30 s inter-trial interval.
Using Tobii Studio Software, each stimulus array had predetermined regions of interest (ROIs) identified. Each ROI encompassed the image and the white background and was categorized as Sample, for familiarization stimuli, and Novel or Familiar for Retention stimuli. Because the six month old infants did not fixate or saccade in the same manner as when they were 24 months old, we recorded the duration time within each ROI, yielding a total time looking at Novel and/or Familiar stimuli. Monkeys (like humans) have an innate tendency to look at the novel stimulus, so the data were expressed as the percentage of time spent looking to the novel stimulus [(Time looking to Novel)/(Total looking time)]. Testing was repeated at 12 and 24 months of age. To keep the animals out of the group for as short a time as possible, infants were tested up to three to five days per week, with sessions lasting 30 min, comprising two to three trials per session. Testing began at six, 12, and 24 months of age and continued until at least 40 trials, 10 at each delay, had been accumulated for each subject. Because of group housing conditions testing of monkeys was intermittent (not every monkey could be accessed every weekday) and not every test session yielded trials in which monkeys accumulated 30 s of viewing time during the Familiarization phase, so testing for each time point extended for several months. Trials were excluded from analysis if monkeys showed a side bias (looking to one side of the screen only), or only looked on one retention trial, or if the tester noted undue distress or distraction in the testing notes.
Statistical analysis
The dependent variable was percent time looking at the novel object, averaged across trials at each delay period (10, 30, 60, 120 s). Data were not collected for one control subject (C7m, see also Supplementary Information) at the 12-18 month time point because of chronic gastrointestinal illness. He was, however tested at six and 24 months. Although there were no hypotheses regarding sex differences in effect of anaesthesia exposure, we are underpowered to detect sex differences, and we have not observed sex differences in the visual paired comparison task at the ages tested in this study,17 18 exploratory repeated-measures ANOVAs with group (control, anaesthesia) and sex as between-subjects factors and delay as the within-subjects factor were initially conducted to determine whether any effects of sex were present in the data. Because no significant effects of sex were found, final analyses that examined anaesthesia effects on visual paired-comparison performance were repeated-measures ANOVAs with group as a between-subjects factor and delay as a within-subjects repeated measure. Data from individual subjects are included in Supplementary Table 1.
Because we were interested in whether effects of anaesthesia were observed at discrete developmental stages, separate ANOVAs were carried out for each time point that visual paired-comparison performance was assessed (six-10 months, 12-18 months, and 24-30 months). Huynh-Feldt correction was applied to degrees of freedom for repeated measures effects. Our goal was not to measure the differences in magnitude effects across stages, and indeed we were underpowered to detect higher-order interaction effects as a consequence. Carrying out separate analyses at distinct ages is also perhaps more relevant to human cognitive development, where the concern is typically whether deficits are reliably observed at specific time points, rather than with the relative differences in effects of anaesthesia exposure across different assessments. Because our a priori intention was to analyse each time point separately, we did not apply any adjustment for false discovery rate or multiple comparisons across the three time points. Analyses were carried out in SPSS version 23 (IBM) for Apple OS X. Main effects of anaesthetic treatment at each age indicate an overall deficit in visual recognition regardless of retention interval, and anaesthesia treatment group by delay interactions indicate a deficit in visual recognition that varied in magnitude depending on the delay between familiarization and retention. Both patterns of data are meaningful in terms of demonstrating impaired visual recognition memory.
Results
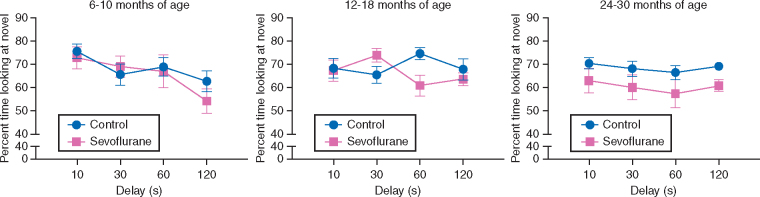
As shown in Fig. 2, no differences in VPC performance were detected between control and anaesthesia groups at testing carried out at six-10 months of age. A deficit whose magnitude varied depending on delay was observed at 12-18 months of age, and a deficit across all delays tested was observed at 24-30 months of age. At the six-10 month time point, repeated-measures ANOVA revealed a main effect of delay, F(3, 44.3) = 5.699, P = .004, but no interaction of delay and condition or main effect of condition, Fs < 1, partial eta-squared < .043. At the 12-18 month time point, repeated-measures ANOVA revealed a delay by condition interaction, F(2.94, 50.04) = 2.933, P = .043, partial eta-squared = .147, Cohen's d = .83, but no effect of delay or condition, Fs < 1, partial eta-squared < .052. At the 24-30 month time point, repeated measures ANOVA revealed a main effect of condition, F(1, 18) = 4.862, P = .041, partial eta-squared = .213, Cohen's d = 1.04, but no effects of delay or delay by condition interaction, Fs < 1, partial eta-squared < .036.
Fig 2.
Neonatal sevoflurane exposure had a delayed impact on memory performance as measured by the VPC task. Performance was analysed separately at each of three age ranges, therefore the effects of sevoflurane exposure across the four delays (pink lines) vs controls (blue lines) are presented in the three panels: six-10 months, 12-18 months, and 24-30 months (see text). Anaesthesia exposed monkeys were unimpaired when tested at six-10 months (left panel). However they displayed a delay-dependent deficit at 12-18 months (centre panel). In contrast, at 24-30 months of age, monkeys that were exposed to sevoflurane three times in the first month of life showed a delay-independent impairment with respect to control subjects. Control subjects shown in blue; anaesthesia exposed subjects shown in pink. Error bars indicate the SEM.
Discussion
Multiple exposures to sevoflurane over the first month of life resulted in a delayed, mild cognitive impairment that was not evident until the second yr of life, corresponding roughly to the age of three to six yr old in humans. There was no reliable difference between the anaesthesia and control groups tested at six-10 months of age. These findings seem contrary to expectation, given our previous report of altered emotional behaviour in these same animals at six months of age.14 However, similar delayed memory deficits have been shown in longitudinal studies in monkeys with neonatal temporal lobe damage and in children diagnosed with developmental amnesia.25 In rhesus monkeys, damage to the hippocampal formation17 sustained in the second week of life impacts memory performance on VPC, but only at later ages. In these studies, young monkeys were tested on VPC that revealed no effect of hippocampal lesions at one and a half or six months of life, however when these same animals were tested at 18 or 48 months of age (preadolescence or adulthood, respectively) their performance fell to chance at the longer delays. Thus, extensive damage sustained in infancy to a structure important for memory in adulthood did not impact memory behaviour at the time of damage. This is likely as a result of the prolonged postnatal maturation of the hippocampal formation in primates.
Similar findings have been reported in humans with developmental amnesia in which perinatal hypoxic events damaged the hippocampus. The effects of this damage were not observed until much later in childhood when those functions normally develop.26 In those patients, semantic memory was relatively spared, whereas episodic memory was severely impaired.27, 28, 29 More pertinent to the present study, patients with developmental amnesia were impaired on the VPC task when tested as adults.23 Thus, disturbances in emotional behaviour after repeated anaesthesia exposure in infancy, may manifest earlier than impairments in memory. Notably, both impairments in memory and disturbances in emotional behaviour, marked by increased anxiety, manifest after early hippocampal damage in monkeys.17 18 30 This pattern is consistent with an early anaesthesia-induced neurobehavioural deficit being caused by hippocampal dysfunction, although considerable additional work remains to be done to validate this hypothesis. In this regard, a report that recollection processes in recognition memory were specifically vulnerable to early anaesthesia exposure in both rats and humans31 is notable. Accurately reporting that an object has been seen before (recognition memory) may be supported by recollection of the specific prior event, or by a general feeling of familiarity for the object.32 The recollective aspect of recognition memory has been linked to hippocampal function.33 Although these processes are not dissociated in the VPC procedure used in our study, the finding of specific impairment in recollection processes31 also points to hippocampal dysfunction as being central to the phenotype of cognitive impairments after early exposure to general anaesthesia.
Our results are also consistent with preclinical studies of paediatric anaesthetic exposure. We elected to study monkeys exposed to sevoflurane three times to determine the outcome of a relatively extensive anaesthesia exposure in the absence of any concurrent disease or pathology. Although our data do not address the question of whether repeated exposure is more detrimental than a single exposure, other studies in rats34 and monkeys35 have reported more severe impacts of repeated isoflurane exposure than single exposure on cognition, and motor and emotional behavior, respectively. Cynomolgus monkeys exposed once to sevoflurane for five h on PND six were unimpaired on reward-based tests of learning and memory given at seven-10 months of age.36 Synaptic loss in the hippocampus is more extensive in adult rats exposed to sevoflurane repeatedly as neonates compared with rats exposed once.37 46
Data in humans on this point are mixed. The initial epidemiological study that inspired our investigations8 reported that children that received multiple anaesthetic exposures before the age of four yr were at greater risk of learning disability during school yr (until the age of 19), but those exposed to anaesthesia only once were at no greater risk (see also9). Two recent studies that have determined impact of single vs repeated anaesthesia on the Early Development Inventory, a questionnaire filled out by kindergarten teachers about school readiness, found no greater risk of repeated relative to single exposures,10 11 and the impact of any anaesthesia exposure was small. This may relate to the age of assessment (kindergarten as opposed to later schooling) and to the instrument used; more extensive formal neuropsychological tests may detect effects of early anaesthesia exposure that are not reflected in measures of academic achievement.38 Some studies have detected adverse neurocognitive outcomes in children after single exposures to anaesthesia,31 38, 39, 40 but recent prospective studies have not,12 13 even when children were assessed with detailed neuropsychological tests at age eight-15 yr.13 Although this is reassuring in terms of demonstrating safety of relatively brief single surgical procedures carried out in children under general anaesthesia, one cannot conclude that repeated or prolonged exposures are similarly benign.41 42
Strengths in our model include the ability to have our subjects reared with their mothers and in large social groups, similar to human children. Another is the use of a memory test based on spontaneous behaviour, allowing cognitive assessment without the "confound" of training which may allow monkeys exposed to anaesthesia to develop alternative strategies to support good performance. Because rule learning can take months at these young ages, the use of this task also allowed us to assess memory at a young age. A main limitation to the study is the sample size. Although including both sexes may be viewed as a strength, and we have a large group size for animal research, we are underpowered to detect effects of sex, or interactions of sex with anaesthesia exposure. We only used a single task and stimulus modality, mainly because of limitations of time to accumulate enough testing trials across all delays within relatively short testing periods. However testing with perceptually more ambiguous stimuli (for example, black and white instead of color images) may have been useful to detect more subtle deficits.43 Also, monkeys were exposed to additional sedative and anaesthetic drugs after their exposure in infancy, and thus it is a logical possibility that some deficits are attributable to later drug exposure. However, both groups (anaesthesia and control) experienced these later exposures, and they were usually brief in duration and occurred later in development than the initial exposures. Thus, we do not think they contribute to the neurocognitive deficits seen in the group of monkeys exposed to sevoflurane in early infancy. Furthermore, deficits in emotional reactivity emerged before these later exposures, indicating that the phenotype of early repeated sevoflurane exposure in monkeys does not require subsequent drug exposure to emerge.14
To conclude, impaired neurocognitive function consequent to early anaesthesia exposure can emerge later in development, even when function is intact at earlier time points. We are currently testing these subjects on rule-based tests of memory and executive function to further elucidate the extent and duration of these deficits. Our finding of a delayed-onset, mild cognitive impairment after early sevoflurane exposure in monkeys suggests that future clinical studies may consider the age at evaluation as an important factor in determining the long-term safety of anaesthetics in children.
Authors' contributions
Study design/planning: M.C.A., M.G.B.
Study conduct: M.C.A., K.L.M., M.G.B.
Data analysis: M.C.A., M.G.B.
Writing paper: M.C.A., M.G.B.
Revising paper: all authors
Acknowledgments
We thank Tracy Davis, B.A., Jordan Johnson, B.S., and John Murnan M.S. for their assistance with collecting behavioural data. We would also like to thank the veterinary, colony management, and animal care staff at the Yerkes National Primate Research Centre Field Station for their support for this project.
Declaration of interest
None declared.
Funding
This work was supported by the National Institutes of Health (NIH)/National Institute of Child Health and Development (grant R01-HD068388). Yerkes National Primate Research Centre is supported by NIH/Office of the Director P51-OD011132, formerly NIH/National Centre for Research Resources P51-RR000165.
Handling editor: Hugh C. Hemmings Jr
Footnotes
Supplementary material is available at British Journal of Anaesthesia online.
SUPPLEMENTARY MATERIAL
References
- 1.Brambrink AM, Back SA, Riddle A. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol. 2012;72:525–535. doi: 10.1002/ana.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brambrink AM, Evers AS, Avidan MS. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brambrink AM, Evers AS, Avidan MS. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116:372–384. doi: 10.1097/ALN.0b013e318242b2cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jevtovic-Todorovic V, Hartman RE, Izumi Y. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikizad H, Yon JH, Carter LB, Jevtovic-Todorovic V. Early exposure to general anesthesia causes significant neuronal deletion in the developing rat brain. Ann N Y Acad Sci. 2007;1122:69–82. doi: 10.1196/annals.1403.005. [DOI] [PubMed] [Google Scholar]
- 6.Cattano D, Young C, Straiko MMW, Olney JW. Subanesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesth Analg. 2008;106:1712–1714. doi: 10.1213/ane.0b013e318172ba0a. [DOI] [PubMed] [Google Scholar]
- 7.Sprung J, Flick RP, Katusic SK. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87:120–129. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilder RT, Flick RP, Sprung J. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flick RP, Katusic SK, Colligan RC. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–e1061. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham MR, Brownell M, Chateau DG, Dragan RD, Burchill C, Fransoo RR. Neurodevelopmental assessment in kindergarten in children exposed to general anesthesia before the age of 4 Years: a retrospective matched cohort study. Anesthesiology. 2016;125:667–677. doi: 10.1097/ALN.0000000000001245. [DOI] [PubMed] [Google Scholar]
- 11.O'Leary JD, Janus M, Duku E. A population-based study evaluating the association between surgery in early life and child development at primary school entry. Anesthesiology. 2016;125:272–279. doi: 10.1097/ALN.0000000000001200. [DOI] [PubMed] [Google Scholar]
- 12.Davidson AJ, Disma N, de Graaff JC. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239–250. doi: 10.1016/S0140-6736(15)00608-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun LS, Li G, Miller TLK. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312–2320. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raper J, Alvarado MC, Murphy KL, Baxter MG. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123:1084–1092. doi: 10.1097/ALN.0000000000000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fantz RL. Visual experience in infants: Decreased attention to familiar patterns relative to novel ones. Science. 1964;146:668–670. doi: 10.1126/science.146.3644.668. [DOI] [PubMed] [Google Scholar]
- 16.Fagan JF. Memory in the infant. J Exp Child Psychol. 1970;9:217–226. doi: 10.1016/0022-0965(70)90087-1. [DOI] [PubMed] [Google Scholar]
- 17.Zeamer A, Heuer E, Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. J Neurosci. 2010;30:9157–9165. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeamer A, Richardson RL, Weiss AR, Bachevalier J. The development of object recognition memory in rhesus macaques with neonatal lesions of the perirhinal cortex. Dev Cogn Neurosci. 2015;11:31–41. doi: 10.1016/j.dcn.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachevalier J, Brickson M, Hagger C. Limbic-dependent recognition memory in monkeys develops early in infancy. Neuroreport. 1993;4:77–80. doi: 10.1097/00001756-199301000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J Neurosci. 2004;24:2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarado MC, Bachevalier J. Comparison of the effects of damage to the perirhinal and parahippocampal cortex on transverse patterning and location memory in rhesus macaques. J Neurosci. 2005;25:1599–1609. doi: 10.1523/JNEUROSCI.4457-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pascalis O, Bachevalier J. Neonatal aspiration lesions of the hippocampal formation impair visual recognition memory when assessed by paired-comparison task but not by delayed nonmatching-to-sample task. Hippocampus. 1999;9:609–616. doi: 10.1002/(SICI)1098-1063(1999)9:6<609::AID-HIPO1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 23.Munoz M, Chadwick M, Perez-Hernandez E, Vargha-Khadem F, Mishkin M. Novelty preference in patients with developmental amnesia. Hippocampus. 2011;21:1268–1276. doi: 10.1002/hipo.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raper J, Bush A, Murphy KL, Baxter MG, Alvarado MC. Multiple sevoflurane exposures in infant monkeys do not impact the mother-infant bond. Neurotoxicol Teratol. 2016;54:46–51. doi: 10.1016/j.ntt.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachevalier J, Vargha-Khadem F. The primate hippocampus: ontogeny, early insult and memory. Curr Opin Neurobiol. 2005;15:168–174. doi: 10.1016/j.conb.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Gadian DG, Aicardi J, Watkins KE, Porter DA, Mishkin M, Vargha-Khadem F. Developmental amnesia associated with early hypoxic-ischaemic injury. Brain. 2000;123:499–507. doi: 10.1093/brain/123.3.499. [DOI] [PubMed] [Google Scholar]
- 27.Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- 28.Vargha-Khadem F, Salmond CH, Watkins KE, Friston KJ, Gadian DG, Mishkin M. Developmental amnesia: effect of age at injury. Proc Natl Acad Sci USA. 2003;100:10055–10060. doi: 10.1073/pnas.1233756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baddeley A, Vargha-Khadem F, Mishkin M. Preserved recognition in a case of developmental amnesia: implications for the acquisition of semantic memory? J Cogn Neurosci. 2001;13:357–369. doi: 10.1162/08989290151137403. [DOI] [PubMed] [Google Scholar]
- 30.Raper J, Wilson M, Sanchez M, Payne C, Bachevalier J. Increased anxiety-like behaviors, but blunted cortisol stress response after neonatal hippocampal lesions in monkeys. Psychoneuroendocrinology. 2017;76:57–66. doi: 10.1016/j.psyneuen.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stratmann G, Lee J, Sall JW. Effect of general anesthesia in infancy on long-term recognition memory in humans and rats. Neuropsychopharmacology. 2014;39:2275–2287. doi: 10.1038/npp.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yonelinas AP, Aly M, Wang W-C, Koen JD. Recollection and familiarity: examining controversial assumptions and new directions. Hippocampus. 2010;20:1178–1194. doi: 10.1002/hipo.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy KL, Baxter MG. Long-term effects of neonatal single or multiple isoflurane exposures on spatial memory in rats. Front Neurol. 2013;4:87. doi: 10.3389/fneur.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coleman K, Robertson ND, Dissen GA. Isoflurane anesthesia has long-term consequences on motor and behavioral development in infant rhesus macaques. Anesthesiology. 2017;126:74–84. doi: 10.1097/ALN.0000000000001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou L, Wang Z, Zhou H. Neonatal exposure to sevoflurane may not cause learning and memory deficits and behavioral abnormality in the childhood of Cynomolgus monkeys. Sci Rep. 2015;5:11145. doi: 10.1038/srep11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amrock LG, Starner ML, Murphy KL, Baxter MG. Long-term effects of single or multiple neonatal sevoflurane exposures on rat hippocampal ultrastructure. Anesthesiology. 2015;122:87–95. doi: 10.1097/ALN.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 38.Ing CH, Dimaggio CJ, Malacova E. Comparative analysis of outcome measures used in examining neurodevelopmental effects of early childhood anesthesia exposure. Anesthesiology. 2014;120:1319–1332. doi: 10.1097/ALN.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 39.Ing C, DiMaggio C, Whitehouse A. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–e485. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 40.Dimaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–1151. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warner DO, Flick RP. Anaesthetics, infants, and neurodevelopment: case closed? Lancet. 2016;387:202–204. doi: 10.1016/S0140-6736(15)00669-8. [DOI] [PubMed] [Google Scholar]
- 42.Baxter MG, Alvarado MC. Monkey in the middle: translational studies of pediatric anesthetic exposure. Anesthesiology. 2017;126:6–8. doi: 10.1097/ALN.0000000000001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeamer A, Meunier M, Bachevalier J. Stimulus similarity and encoding time influence incidental recognition memory in adult monkeys with selective hippocampal lesions. Learn Mem. 2011;18:170–180. doi: 10.1101/lm.2076811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boscolo A, Ori C, Bennett J, Wiltgen B, Jevtovic-Todorovic V. Mitochondrial protectant pramipexole prevents sex-specific long-term cognitive impairment from early anaesthesia exposure in rats. Br J Anaesth. 2013;110:i47–i52. doi: 10.1093/bja/aet073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Creeley C, Dikranian K, Dissen G, Martin L, Olney J, Brambrink A. Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth. 2013;110:i29–i38. doi: 10.1093/bja/aet173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang MQ, Ji MH, Zhao QS. Neurobehavioural abnormalities induced by repeated exposure of neonatal rats to sevoflurane can be aggravated by social isolation and enrichment deprivation initiated after exposure to the anaesthetic. Br J Anaesth. 2015;115:752–760. doi: 10.1093/bja/aev339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.