Abstract
Safety concerns over cell-derived pharmaceutical products being manufactured in supplements of fetal bovine serum (FBS) have ignited pleas to replace FBS. Herein, four newly marketed alternatives to FBS were compared: a xeno-free product called Cell-Ess®, a human platelet lysate marketed as GroPro®, and two mixtures of adult bovine serum varying in their proportions of neonatal growth factors, called Liporo® and FetalGro®. An endothelial cell line (C2BBe1) and a neuronal cell line (SHSY5Y) near confluency in media with 10% FBS were selectively scraped and taken through a 25-day step-wise algorithm to replace FBS, and another human endothelial cell line (HRA-19) was studied to replicate C2BBe1. Cells were stained, counted, and compared for viability, migration, and spheroids. The C2BBe1 and HRA-19 cell lines failed to proliferate in 10% Cell-Ess® but grew in 10% GroPro® or 10% FetalGro® reasonably well compared to reference 10% FBS. With SH-SY5Y, only FetalGro® approached FBS's efficacy. These were all inferior to 11 different branded lots of FBS (positive controls), but five days into switching just amongst the FBS brands, 4 of 11 supported less proliferation than reference FBS in endothelial HRA-19 (p < 0.004). Moreover, neurospheres were enriched in two branded lots of FBS and FetalGro® (each p < 0.004), neurospheres being an unwanted phenotype for any neuronal cell application. Because platelet-derived GroPro® stood out amongst the non-FBS growth supplements to allow proliferation without inducing spheroids, it seems the best (mindful that the cells still grew slower in it compared to FBS). While no perfect replacement was found amongst the alternatives to FBS, the algorithm for switching should be useful in future testing of new alternatives to FBS as the need arises to switch from FBS and expand pharmaceutical products with safety for human use.
Keywords: xeno-free, neurospheres, C2BBe1, SH-SY5Y, fetal bovine serum
Introduction
Fetal bovine serum (FBS) has long been the gold standard of mammalian cell culture supplements, used widely in cell biology, and considered to be an essential ingredient for manufacturing the new generation of ‘biologicals’ from the pharmaceutical industry: vaccines, antibodies, cellular messengers, and stem cell therapies. But, the ethics of using serum from fetal calves has always been problematic1. Also, its refractoriness to full characterization2, its instability from batch to batch3, and reports of contaminants in commercial FBS4–8 are tarnishing the gold standard of FBS. Indeed, two scientific societies in 2013 published a plea for all cell biologists to stop using FBS and switch to a substitute4 – and the plea was renewed in 20182. The United States (US) Food and Drug Administration (FDA) has responded with a perspective on the scale of use of FBS for the development of biologicals9. They showed internal surveys of new drug applications (INDs) dealing with mesenchymal stem cells, revealing that 53 of 66 studies explicitly were using FBS in manufacturing of stem cells for human clinical trials10. About this, the German online newspaper Süddeutscher Zeitung, calls FBS the ‘secret fuel’ of pharma. In a 2015 article entitled ‘The Dirty Business With Blood of Unborn Calves (translated)’, the newspaper describes pending legal action against bovine serum producers and sellers for engaging in fraudulent FBS advertising4 as well as potentially serious health concerns of bad batches of FBS tainted with viruses5,6,7 and/or Achromobacter 8.
The 3rd Workshop on FBS, Serum Alternatives, and Serum-free Media has issued a summary paper suggesting reasonable alternative growth supplements for growing human cells rather than FBS2,10,11. Human platelet lysates are already used for expanding stem cells, but problems exist how best to procure these lysates12. Some good results have been reported with six bovine newborn calf serum-based FBS alternatives, but these are still animal-based13. However, a closer examination reveals that while some human stem cells may be maintained in vitro for a while by some FBS alternatives14–19, at least two recent studies18,19 mention the lesser abilities of FBS alternatives to support expansion and differentiation as needed for pluripotent stem cells compared with FBS18,19.
Given these problems, one may wonder why the FBS pipeline hasn’t been refined until it is absolutely safe, reliable, and more humane source of growth supplements for cell biologists1? The International Serum Industry Association (ISIA) was founded in 2006 as a self-policing association to do exactly that. Working in cooperation with the US Department of Agriculture (USDA), one of their initial steps was establishing a ‘serum traceability certification’. We welcomed because carrying this label has allowed consumers to better be assured that their serum meets at least what were perceived then as core criteria: non-detectable levels of mycoplasma and other common bovine viruses, sub-threshold levels of serum antibodies, endotoxins, and biomarker constituents associated with inflammation, and traceability back to source herd(s). Unfortunately, the ISIA hasn’t been able to make the industry use dedicated herds and thus there continues to be rogue FBS sources of variability and contaminants4–8. The ISIA’s website (http://www.serumindustry.org) has candidly disclosed how challenging the traceability of serum really is. One recent article describes ‘FBS is available from bovine fetuses only because a proportion of female animals that are being slaughtered for meat consumption are found (often unexpectedly) to be pregnant’. In a subsequent article they add that ‘fewer and fewer US pregnant cattle suddenly came into US abattoirs 5–10 years ago’. These ISIA articles collectively revealed that starting 10 years ago US cattle ranchers began using simpler pregnancy test kits, which caused fewer pregnancies to arrive in the abattoirs, which led to an FBS shortage, which led producers to search for new herds and we now routinely therefore have marketed bottles from Central and South America, Australia, New Zealand, and mixed sources. The ‘lot numbers’ (also known as batches) can even be problematic because they derive from the pooling of sera from multiple herds over vast logistic obstacles. Not uncoincidentally, we believe, FBS prices sky-rocketed about 7 years ago to current all-time highs. Today, the worldwide FBS industry is a US$1 billion/year business4 based on annual production in excess of 500,000 liters4. The ISIA is helpful but its self-policing is complicated by a diverse industry transporting sera across multiple national borders – all to meet the insatiable demand of cell biologists for cheap FBS.
Given so many challenges in the FBS pipeline over the past 10 years, it is essential to screen the FBS brands in comparison with newer non-FBS cell culture growth supplements that are on the market. The present study compares 4 newly marketed alternatives to FBS and 11 different branded lots of FBS for efficacies to grow established human cell lines. We created a generalizable test algorithm that we can still be optimized in the need for hopefully transitioning many types of human cells from FBS to non-FBS supplements for a variety of purposes. Soliciting advice from vendors as to how to best switch cells to their alternatives to FBS, there was consensus for slow transition algorithm. At the same time, because of the known variability between different FBS lots and brands20, we also describe their testing. We examined three human cell lines daily over a 25-day transition period and employed multiple controls (i.e. the 11 different branded lots of commercial FBS were positive controls). The hypothesis was that at least one of the four newly marketed non-FBS products would perform as well as any FBS in ability to propagate human cell lines. Our findings should be of interest to anyone thinking about switching away from FBS, but especially to those developing stem cell therapies.
Materials and Methods
To acquire the growth supplements used in this study, vendors were identified online and we requested free samples. We sought sample growth supplements and FBS that had at least been ultra-filtered thrice using 0.1 micron filters to make sure they were sterile, though not going to processing steps like charcoal-filtering, exposure to irradiation, or treatment with ultraviolet light. Only two vendors expressed hesitancy to provide us with free samples unless a purchase order could be prearranged (after some discussion, we decided not to obtain those products). Two vendors sent additional lot numbers of their same brand of FBS. Another two vendors sent two bottles with the same lot number of FBS. One vendor not only sent FBS but also sent two alternatives (Rocky Mountain Biologicals (RMBI), Missoula, MT, USA). The samples arrived on dry ice except for Cell-Ess® base and Lipogro® which arrived as chilled liquids per manufacturer’s instructions. They were all within 5-year expiration dates for frozen serum.
Non-FBS Growth Supplements and Prices (Listed by Order of Arrival to the Laboratory)
Lipogro® (RMBI): a proprietary combination of adult bovine serum plus a cholesterol–lipoprotein complex from an Australian source. Labeled ‘triple-filtered, twice heat-inactivated, LPG-APG-XXX’. One bottle of lot #20120323G-R carried a list price of US$375/500 ml.
Fetalgro® (RMBI): a proprietary mixture of Lipogro® plus neonatal calf serum from the Australian source. Labeled ‘triple sterile filtered FGR-BBT-XXX’. One bottle of lot #20140930FG carried a list price of US$100/500 ml.
Cell-Ess® is a xeno-free product (Essential Pharmaceuticals, LLC, though since 2017 owned by Accord HealthCare, Durham, NC, USA), catalogue no. 25767-710-003. The mixing of Cell-Ess® base (stored at 4°C) and Cell-Ess Growth Factor Cocktail (stored at −20°C) occurred just before use as per the manufacturer’s instructions. List priced at US$410/500 ml.
GroPro® (Zenbio, Research Triangle Park, NC, USA): the SER-HPL-GROPRO brand. This human platelet lysate product was list priced at US$157/500 ml.
FBS Branded Lots and Prices (Listed by Order of Arrival to the Laboratory)
Premium Select Brand FBS (Atlanta Biologicals, Atlanta, GA, USA; ATL BIOL FBS): labeled ‘triple-filtered, USA Cat. #S11550’. The two bottles of lot #H1212 carried a list price of US$421/500 ml.
F4135 Brand FBS (Sigma-Aldrich, St. Louis, MO, USA): labeled ‘US origin BK197 heat-inactivated’. This was purchased by a colleague (and gifted to us – see acknowledgements). The list price at the time was US$565/500 ml.
Triple-Filtered Brand FBS (RMBI): labeled ‘USA, FBS-BBT-XXX’. One bottle lot #20150428FS carried a list price of US$269/500 ml.
Premium Brand FBS (Corning, NY, USA; obtained from Thermo Fisher Scientific, New York, NY, USA): labeled ‘USA, #35-015-CF’. One bottle lot #35015125 carried a list price of US$405/500 ml.
BenchMark Brand FBS (Gemini Bio, Woodland, CA, USA): labeled ‘100106 from mixed USDA-approved sources in the USA, Canada, and Mexico’. One bottle lot #050A86E carried a list price of US$539/500 ml.
Foundation Brand FBS (Gemini Bio): labeled ‘900108 from unspecified USDA-approved sources’. One bottle lot #050A80E carried a list price of US$469/500 ml.
Premium Select Brand FBS (Biowest, Riverside, MO, USA): labeled ‘USDA-approved Costa Rican origin’. Two bottles, lots #S1620 and #316S14, carried a list price of US$510/500 ml.
ACC816 Brand FBS (Access Biologicals, Vista, CA, USA): labeled ‘USA’. Lot #A15003 (one bottle) and #A15006 (another bottle) each carried a list price of US$285/500 ml.
Qualified Brand FBS (Gibco, obtained from Thermo Fisher Scientific): labeled ‘USA #26140087’. One bottle lot #1715928 carried a list price of US$571/500 ml.
Premium Brand FBS (Seradigm, Radnor, PA, USA; a VWR Life Science Company): labeled ‘USA’. Lot #014B15 (one bottle) and #190B14 (one bottle) carried a list price of US$574/500 ml.
Triple-Filtered Brand FBS (American Type Culture Collection (ATCC®), Manassas, VA, USA): labeled ‘USA #30-2020’. One bottle, lot #62818223, was purchased by a colleague (gift of HB). List price was US$568/500 ml.
Complete Media
Complete media was made beginning with 50 ml basal Dulbecco’s modified eagle medium: nutrient mixture F-12 (DMEM/F12) Ham’s media (1:1) in 15 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and sodium bicarbonate, and 2.5 mM L-glutamine (HyClone Thermo, Waltham, MA, USA: Cat #SH30023.01), to which was added 0.5 ml of a sterile mixture of penicillin /streptomycin (55 IU/ml each from ATCC® #30-2300), to which was added the FBS of choice, or test growth supplements described above to achieve a dilution of 10% (v/v).
Cell Lines
The SH-SY5Y was purchased from ATCC® (no. CRL-266). It has history going back to a human neuroblastoma and repeated selections until this subline, expressing the neuronal phenotype21,22. Much of the literature on SH-SY5Y cells has focused on induced dopaminergic status23, but to achieve such a phenotype requires sequential co-treatments with retinoic acid plus phorbol esters like 12-O-tetradecanoylphorbol-13-acetate23, which makes the cells stop dividing, and we didn’t do that. Our SH-SY5Y cells existed mostly as stellate dividing cells with very few if any neurospheres in the reference FBS23. Moreover, this monocytic phenotype is important because of its dependence on FBS24. When the percentage of FBS in the media is less than 10% (v/v), or the complete media is not refed often enough, the SH-SY5Y monocytes reliably convert to almost all neurospheres25. Neurospheres are organized clumps of cells in the G0 stage of the cell cycle that eventually detach from the substrate, demarking a chemo-therapeutically resistant phenotype that in most studies of transplantation is unwanted25. However, because neurospheres have been shown to be reversible (FBS added back will restore the cultures to monocytes24), we could expect that neurospheres in any given cultures would reveal a weaker growth supplement. To this we added second tester cell line, C2BBe1 (from ATCC® no. CRL-2102) which was chosen because its differentiation in culture is also known to depend on adequate (10%) FBS26. C2BBe1 cells are a clonal variant of the Caco-2 cell line – more homogeneous and forming cobblestone-like lawns that exhibit apical/basal polarity, tight junctions, and molecular transporters consistent with the transport of molecules across the small intestine27. The third cell line was chosen because it is a similar human gut endothelial model cell line, in the hope of replicating the findings with C2BBe1. It is HRA19a1.1α2F (HRA-19; Sigma-Aldrich, no. 09071517). At low seeding density, HRA-19 appears identical to C2BBe1, but as their density increases the HRA-19 cells produce floating clumps similar to neurospheres, called tumorspheres28. Thus, we set out to study all three cell lines under different types of growth supplements.
Algorithm for Switching From FBS
Each cell line was on day-4 after plating when the cells were near confluency in complete media supplemented with 10% FBS. They were unsynchronized when trypsinized and plated into six-well plates (MilliporeSigma, Burlington, MA, USA). It should be noted that although trypsin/ ethylenediaminetetraacetic acid (EDTA; SigmaAldrich) was used for seeding the wells, it was not used subsequently in the screening algorithm. By avoiding trypsin/EDTA, we avoided media × trypsinization confounding interactions. These were seeded at 2.0 × 105 cells/well in 4 ml of fresh media (still in the reference FBS) and allowed another 5 days to expand in a humidified 37°C CO2 incubator. Ultimately, three wells in each six-well plate were experimental wells and three wells were control wells. The wells were scraped down the middle using a sterile scraper to produce precise 14 mm open swaths (Thermo Fisher Scientific, no. 179707). These were precisely scraped using a template taped on the underside of each well. Another advantage to scraping over trypsinizing was the ability to monitor both the cell’s divisions and migration – see below. The wells were washed (5 ml of sterile phosphate-buffered saline (PBS)/well) and the media replaced with the test media (different types of FBS or growth supplements). A 25-day period followed with media changes every 5 days (see algorithm Table 2). There were some re-scrapings, as before with floaters re-removed, during this process and the attached cells were re-refed with step-wise progressions towards complete replacement of FBS (Table 2). After the last media change, the cells were in pure 10% new supplement and allowed to grow another 5 days in the incubator before the final assessment.
Algorithm with Different Brands of FBS
The 11 brands of positive control FBS were compared slightly differently in that the changeover to new media occurred immediately rather than over a step-wise transition period. After scraping the zones in the wells, the cells were immediately given the new complete media with different lots of FBS (10%). They went into the humidified CO2 incubator for daily monitoring with a final assessment occurring 6 days later. Otherwise, the comparisons were identical to the studies above when comparing between the non-FBS supplements.
Assessments
Cell migration was assessed under phase contrast microscopy by daily scoring numbers of cells in the middle zones of scraped wells. The time course differed between the cell lines because the SH-SY5Ys moved inward rapidly, almost completely after 1 day in the FBS, while the migration of the endothelial cell lines was slower and actually could not be traced to a cell migration mechanism. That is, the C2BBe1 and HRA-19 cells detach floaters which landed randomly in locations and in some cases established new islands of cells inside the scraped zones. This ‘floating-first’ mechanism took a longer time than the direct migration seen with SH-SY5Ys, and therefore the scoring of endothelial cell migration was done at the end on day-6 of test media. A blinded rater was taught and scored ‘0.1’ for when almost no cells made it to the scraped zones (zeros were never seen), and ‘10.0’ was the upper level of migration where so many cells filled-in the zones that there was no discernable difference between scraped zones and unscraped zones.
Numbers of neurospheres (SH-SY5Y) and/or tumorspheres (C2BBe1 and HRA-19) per well were counted at 5 days after changing to new growth media. The counting was done using low magnification (100×) micrographs, moving between six prescribed overlapping fields of view in each well.
Cell counts were made with an automated hemocytometer by cellometry (Nexelcom, Lawrence, MA, USA). The technique not only counts cells but separates them into viable/dead numbers and measures cell diameter in microns. Moreover, the samples were separated into floating versus attached phenotypes. ‘Floaters’ is a term to include singlet floating cells as well as the cells derived by trituration from floating neurospheres and/or tumorspheres. In addition to the floaters, the attached cells were separately washed in PBS, trypsinized, resuspended, pelleted (125 × g for 5 min at room temperature) and dispersed for staining slides and cellometry. To assess cell viability, they were stained with trypan blue for 2 min. Our main outcome measures were numbers of cells per well, average cell diameters, and average percent viabilities. When the phrase ‘total cells’ is used it means we added together the floater cell counts plus the attached cell counts.
Long-Term Growth Studies
Longer-term comparisons were performed on the six growth supplements that showed the best results in the short-term studies above; that is, the six that were statistically better in one measure or the other from the others, yet statistically identical to each other after the aforementioned 5–6-day comparisons. They were ATCC® FBS, Gibco FBS, Biowest FBS, ATL BIOL FBS, Seradigm FBS and RMBI FetalGro®. The C2BBe1 and SH-SY5Y cell lines were taken directly from the liquid nitrogen tank and thawed in different complete media with different brands of supplements (10%). Media was changed 3 days later and then every 7 days using traditional trypsin/EDTA dissociation methods to passage the cells this time. Following each passage, cellometry was performed as above. Cells were re-passaged (1:4 dilutions in media weekly).
Statistical Analysis
The categorical data (supplement type X cell line) were subjected to one-way analysis of variance (ANOVA) followed by post-hoc analyses to compare each experimental group with its unique control group when the experiment was run (GraphPad Prizm, La Jolla, CA, USA). In the post-hoc analyses, two-tailed Student’s t tests were set for significance level of p < 0.004, according to Tukey’s multiple comparisons correction.
Results
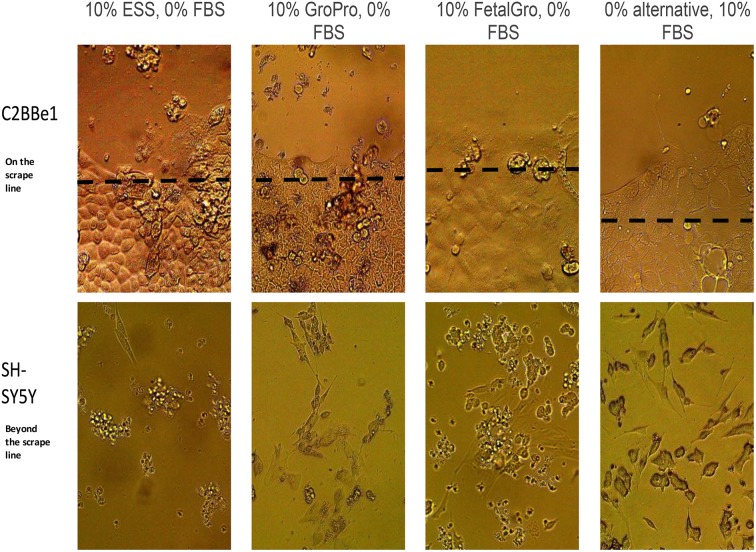
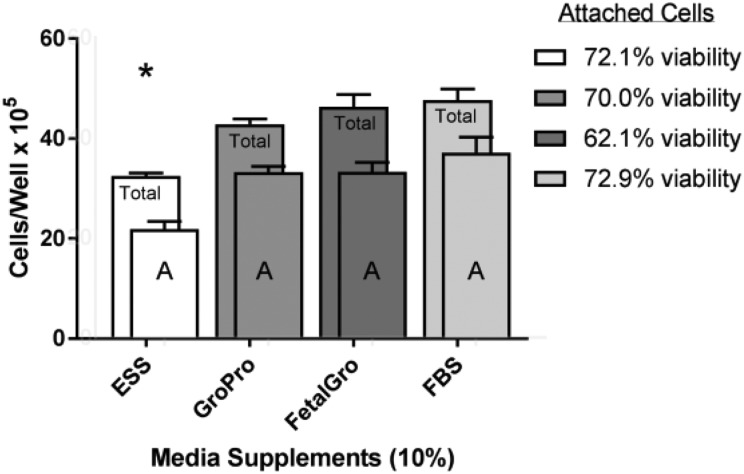
C2BBe1 cells were a primary target of interest when scraped and transitioned gradually over 25 days into media containing different types of non-FBS supplements. In the unscraped regions of the wells the C2BBe1 cells appeared to be typical endothelial cells regardless of which non-FBS supplement was used (Fig. 1 upper panels). Beyond the scrape boundaries, however, the few C2BBe1 cells that gradually moved inward when growing in a non-FBS alternative tended to look unhealthy and loosely attached to the plate. In Cell-Ess®, the line where the cells had existed post-scraping retreated towards the edges of the well, and no cells moved inward (Fig. 1 upper row). To back-up these observations, Fig. 2 also displays the counts of C2BBe1 cells at the end of the study on day-25 when the experimental cells existed in 10% non-FBS media for 5 days. At this point, the cell counts in GroPro® and FetalGro® were almost as good as with 10% FBS; statistically identical post-study for the three supplements: 10% GroPro® = 10% FetalGro® = 10% FBS (Fig. 2). However, C2BBe1 numbers in 10% Cell-Ess® were very low, suggesting that the cells had not proliferated at all during the 25-day transition (Fig. 2). It is also noteworthy that the percent viability measured by trypan blue exclusion was constant at 70% for all media – even with Cell-Ess®. Since this level of viability is considered normal for this cell line (Fig. 2 insert), it appears that despite the cells not proliferating as much as in FBS, at least no toxicity seems to have occurred with any of the non-FBS supplements. No difference was found between the supplements in terms of cell diameter or number of tumorspheres (data not shown because they were not significantly different). We conclude therefore that the only statistical deficits with C2BBe1 cells came when they were grown in 10% Cell-Ess®, and these were due to less proliferation and backward migration rather than due to toxicity (Figs. 1 upper row and 2).
Fig. 1.
Visual appearance of cells in Non-FBS versus FBS media. Cells were observed under phase contrast microscopy (750×) at the end of 25 days (last 5 days) in different media supplements. See Table 1 for an explanation of scrape line and progression of media changes.
FBS: fetal bovine serum.
Fig. 2.
Comparison of growth of C2BBe1 cells in non-FBS supplements versus FBS. This was done after the last step in the progression, after the cells had been scraped in swaths and allowed to proliferate for 5 more days in their respective media (Table 1). Cells were stained and counted by cellometry at the end of these 5 days in their media. ‘Total’ refers to the sum of the portions of: attached cells (A) plus floater cells (not shown). Values represent the mean ± SEM. An asterisk indicates the cells in Cell-Ess® yielded statistically lower values than in FBS. None of the other supplements yielded statistical differences from FBS regardless of total cells or attached (A) cells (Seradigm brand).
FBS: fetal bovine serum; SEM: standard error of the mean.
Table 1.
Step-Wise Transition to Non-FBS Media of Cell Lines.
| Experimental day | Treatment of cells | Type of media replacement |
|---|---|---|
| 0a | Scraped zones, discarded floating cells with media (in 10% FBS), washed & replaced media | 10% Non-FBS + 10% FBS |
| 5 | Scraped zones, discarded floating cells, washed & replaced media | 10% Non-FBS + 2% FBS |
| 10 | Discarded floating cells, washed & replaced media | 10% Non-FBS + 1% FBS |
| 15 | Discarded floating cells, washed & replaced media | 10% Non-FBS + 0.5% FBS |
| 20 | Scraped zones, counted floating cells, washed & replaced media | 10% Non-FBS |
| 25 | Counted floating cells, harvested only the attached cells, stained and counted | 10% Non-FBS |
aPrior to starting (i.e. prior to Day-0), logarithmically growing 75 cm2 flasks of each cell line (SH-SY5Y and C2BBe1) had been harvested using trypsin/EDTA, and plated into untreated 12-well plates at 2.0 × 105 cells/well in complete media containing 10% FBS. These were returned to the incubator and proliferated 5 days at 37°C in the 5% CO2 incubator. At that time, the cells appeared near confluence, identical from well to well.
EDTA: ethylenediaminetetraacetic acid; FBS: fetal bovine serum.
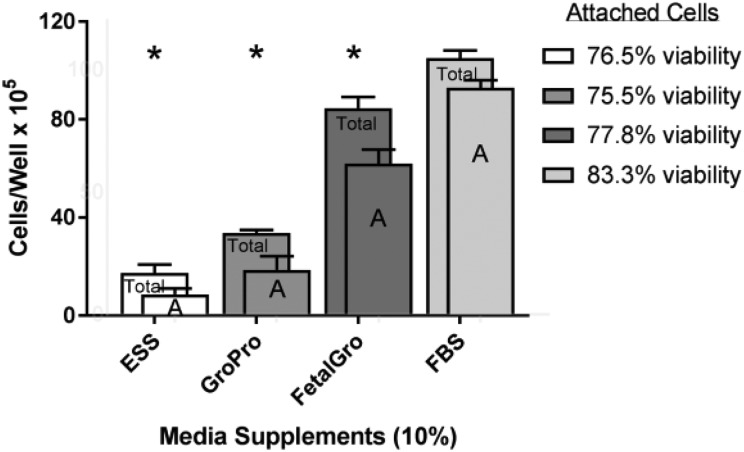
The situation was different with SH-SY5Y cells in the non-FBS supplements. Across the types of supplements, we counted more floating SH-SY5Y cells in the non-FBS media than in FBS media (Fig 1 lower row). Figure 3 also displays the cellometry values from the SH-SY5Y wells when the cells were in 10% non-FBS supplemented media for 5 days. Whenever FBS was absent – regardless of the supplement type and regardless of the counting attached or total phenotype – the SH-SY5Y cells were fewer compared with controls grown in FBS (Fig. 3). The detriment was worse again in Cell-Ess® (Fig. 3). The percent of viable SH-SY5Ys by trypan blue exclusion was good (around 80%) and unchanged by any of these supplements (Fig. 3 insert), again indicating a lack of toxicity. Also, no differences were found between the supplements in regard to migration or cell diameter of SH-SY5Ys (not shown because they were not significantly different). The findings therefore showed all non-FBS supplements yielded less SH-SY5Y proliferation and more conversion to floating cells (Fig. 3), but again no cellular toxicity was indicated (Fig. 3).
Fig. 3.
Comparison of growth of SH-SY5Y cells in non-FBS supplements versus FBS. This was done after the last step in the progression, after the cells had been scraped in swaths and allowed to proliferate for 5 more days in their respective media (Table 1). Cells were stained and counted by cellometry at the end of the 5 days in their media. ‘Total’ refers to the sum of the portions of: attached cells (A) plus floater cells (not shown). Values represent the mean ± SEM. The asterisks indicate that all the alternatives yielded statistically lower values from FBS (Seradigm brand).
FBS: fetal bovine serum; SEM: standard error of the mean.
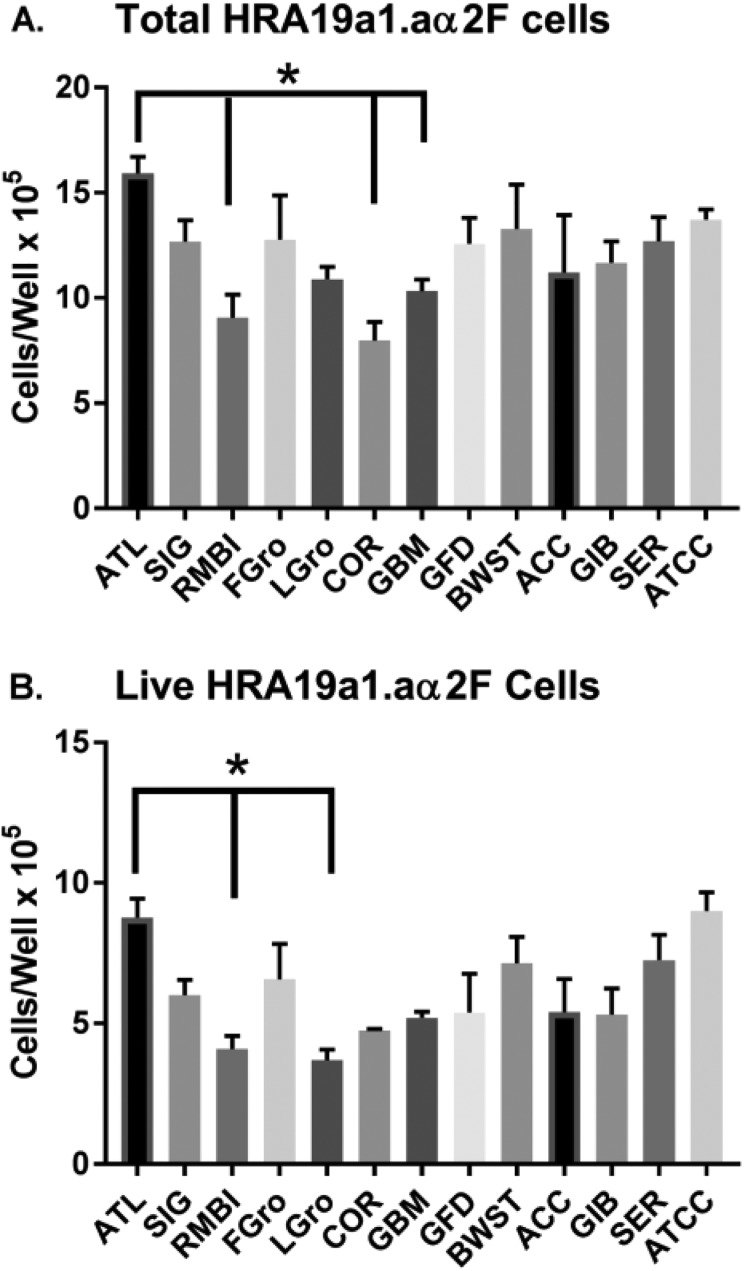
We next sought to determine how these findings compared with the extent of variability among the gold standard positive control brands of FBS. Figure 4 shows how the numbers of HRA-19 cells grown in different FBS brands varied considerably. The overall ANOVA failed to confirm that these were statistically significant differences between FBS brands (p = 0.115), but the post-hoc analysis revealed the statistically highest proliferation occurred with ATL BIOL FBS brand and the lowest proliferation occurred with Corning FBS; findings which met Tukey’s correction versus ATL BIOL FBS (p = 0.0003; Fig. 4A). Also, two brands of FBS showed statistically lower levels of cell growth compared with ATL BIOL FBS: RMBI FBS (p = 0.001) and Gemini’s Benchmark FBS (p = 0.0026; Fig. 4A). Surprisingly, when we re-ran two of the non-FBS supplements in the same paradigm (Fetalgro® and Lipogro®), Fetalgro® alone supported the expansion of cells – and it did so just as well as FBS (Fig 4A). In Fig. 4B it can be seen that the viable HRA-19 cells confirm the same trend as with total cells – except two branded lots of serum met Tukey’s criteria by significantly lowering live cells versus ATL BIOL FBS: RMBI FBS (p = 0.0035) and Lipogro® (p = 0.002). Comparing Fig. 4A and 4B shows again that the percent viability was unchanged by any serum (hovering around 80%). Cell migration and mean cell diameters in the HRA-19 cell line also did not differ with source of serum (not shown because not significantly different).
Fig. 4.
Comparison of HRA19a1.aα2F cells grown for 5 days in different brands of sera. Sera was made 10% in basal media and used to culture the HRA19a1.aα2F cell line. Post-scraping cells were stained and counted by cellometry. Values represent the mean ± SEM. The asterisk and lines indicates that ATL BIOL FBS was statistically higher by Tukey’s correction (p < 0.004) than these other sources of serum.
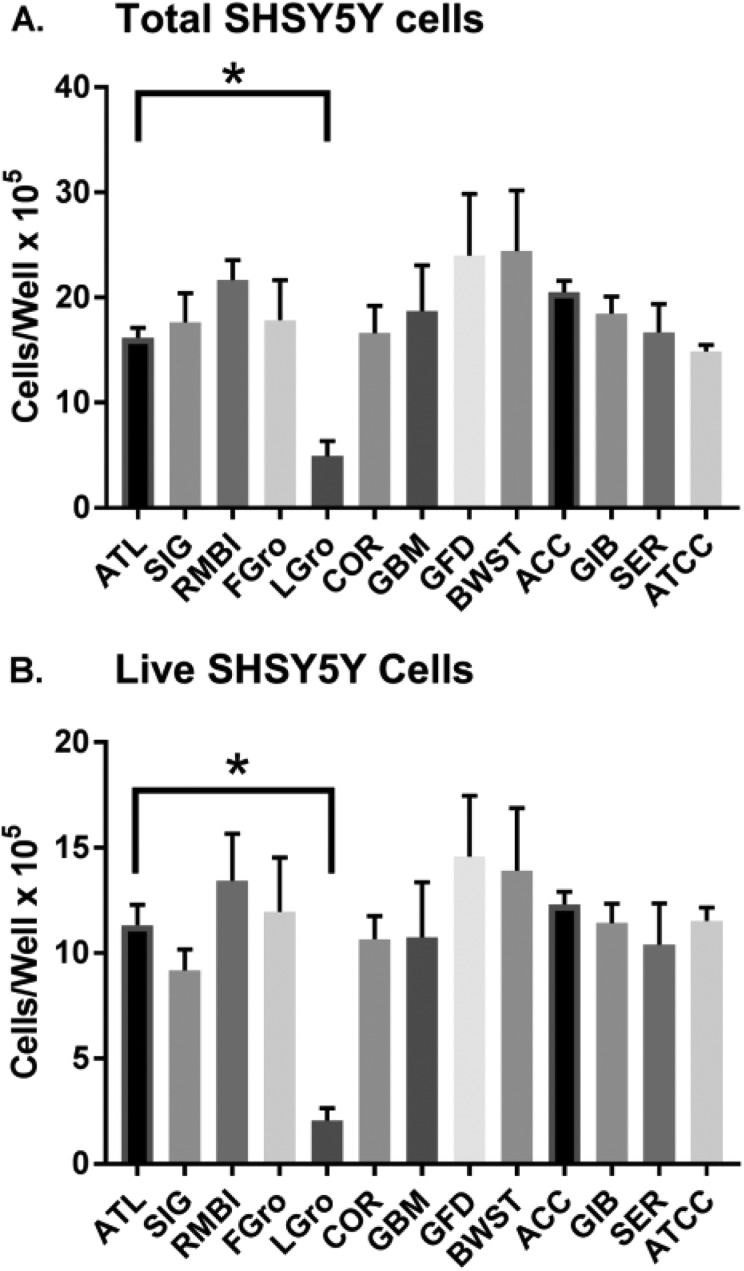
ACC: Access Cell Culture; ATCC: American Type Culture Collection; ATL: Atlanta Biologicals; BWST: Biowest; COR: Corning; FBS: fetal bovine serum; FGro: Fetalgro®; GBM: FBS Benchmark® from Gemini Bio Products; GFD: FBS Foundation® from Gemini Bio Products; GIB: FBS from Gibco; LGro: Lipogro®; RMBI: Rocky Mountain Biologicals; SEM: standard error of the mean; SER: FBS from Seradigm; SIG: Sigma.
Figure 5 shows the same 11 FBS brands tested on SH-SY5Y cells. The Fig. 5A panel focuses on just the total cell numbers, while panel 5B zooms-in to show the living cells based on trypan blue exclusion. With SH-SY5Ys, an overall one-way ANOVA reached marginal significance between all brands of serum: Fig. 5A (p = 0.052) and Fig. 5B (p = 0.046). The post-hoc analysis revealed the SH-SY5Y total cell numbers in ATL BIOL FBS were statistically higher than in Lipogro® (p < 0.0001; which met Tukey’s correction criteria; Fig. 5A). Post-hoc analysis also revealed statistically higher total cell numbers by RMBI FBS and Access FBS compared with Lipogro®. Total SH-SY5Y cell numbers in RMBI and Access FBS were not different than any of the other FBS samples (Fig. 5A and 5B) suggesting that these brands simply showed the lowest inter-well variance for some reason, making the artefactual appearance of superiority to Lipogro®. Comparing Fig. 5A and 5B, the viabilities of attached cells were realized to be constant at around 80%, regardless of the source of serum. Also, mean cell diameters and migration of the SH-SY5Y cells did not vary based on serum brand (data not shown).
Fig. 5.
Comparison of SH-SY5Y cells grown for 5 days in different sources of sera. Sera was made 10% in basal media and used to culture the SH-SY5Y cell line post-scraping. Cells were stained and counted by cellometry. Values represent the mean ± SEM. The asterisk and lines indicate that ATL BIOL FBS was statistically higher by Tukey’s correction (p < 0.004) than the other sources of serum.
ACC: Access Cell Culture; ATCC: American Type Culture Collection; ATL: Atlanta Biologicals; BWST: Biowest; COR: Corning; FBS: fetal bovine serum; FGro: Fetalgro®; GBM: FBS Benchmark® from Gemini Bio Products; GFD: FBS Foundation® from Gemini Bio Products; GIB: FBS from Gibco; LGro: Lipogro®; RMBI: Rocky Mountain Biologicals; SEM: standard error of the mean; SER: FBS from Seradigm; SIG: Sigma.
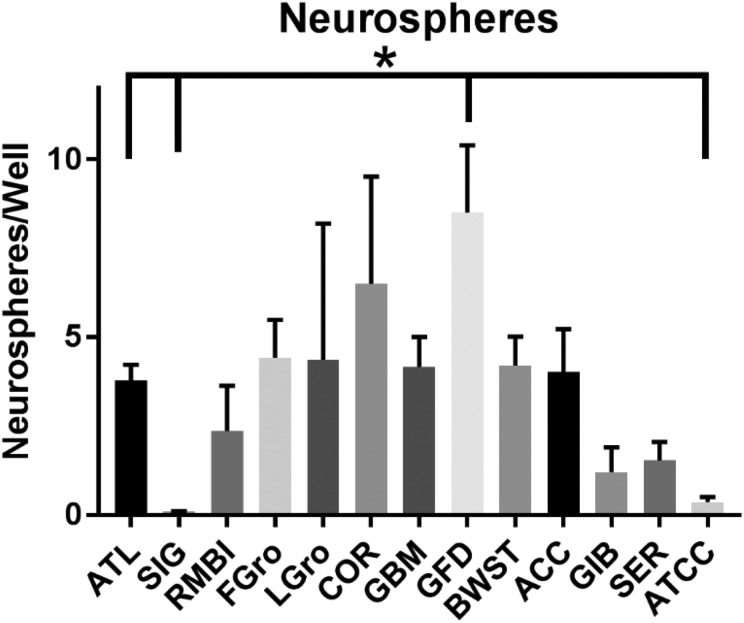
Spheroid counts were also important because these structures exist in relationship to amount and duration of our cells in 10% of even reference FBS. Differences thus emerge in the numbers of neurospheres to distinguish some of the branded lots of serum (Fig. 6; overall one-way ANOVA p = 0.0078). The brand producing the most neurospheres was the Gemini Foundation FBS brand, which yielded twice the number of neurospheres found with ATL BIOL FBS (p = 0.004). There were also more neurospheres in SH-SY56Y cells grown in Fetalgro®. In contrast, two other FBS brands seemed to significantly lower the numbers of neurospheres compared with ATL BIOL FBS: Sigma (p = 0.001) and ATCC (p = 0.002; Fig. 6). The Gemini Foundation brand seemed to promote the highest number of tumorspheres with HRA-19, but the numbers of tumorspheres from well to well were variable and low (data not shown).
Fig. 6.
Neurospheres in SH-SY5Y cells grown in different FBS sources. Sera was made 10% in basal media and used to culture the SH-SY5Y cell line 5 days post-scraping the swaths. The wells are the same as in Fig. 5 except counted for neurospheres before they were dispersed for cellometry. Microscopic observations were made of each well at low (100×) magnification in a systematic manner so that all the neurospheres in each well could be counted. Values represent the mean ± SEM. The asterisk indicates ATL BIOL FBS was statistically different in neurosphere numbers by Tukey’s correction (p < 0.004) from the other sources of serum
ACC: Access Cell Culture; ATCC: American Type Culture Collection; ATL: Atlanta Biologicals; BWST: Biowest; COR: Corning; FBS: fetal bovine serum; FGro: Fetalgro®; GBM: FBS Benchmark® from Gemini Bio Products; GFD: FBS Foundation® from Gemini Bio Products; GIB: FBS from Gibco; LGro: Lipogro®; RMBI: Rocky Mountain Biologicals; SEM: standard error of the mean; SER: FBS from Seradigm; SIG: Sigma.
Longer-term studies were undertaken with the best growth supplements to assess their long-term capacities. For these studies we selected only the best supplements: ATCC FBS, Gibco FBS, Biowest FBS, ATL BIOL FBS, Seradigm FBS, and the Fetalgro® alternative. These were chosen because all appeared equivalent in terms of promoting healthy looking cells that were largely neurosphere-free, and good proliferation during the earlier studies (Figs. 1 –6). Over the first 2 weeks since thawing the cells, the five FBS brands and Fetalgro® continued to look identical and support good cell growth. However, by the third passage in Fetalgro®, the C2BBe1 cells began propagating much slower than the others. Another problem was that FetalGro® led to many neurospheres. A mixture of 5% Fetalgro® plus 5% Biowest FBS was tried in the hope of ‘perking-up’ the cells that had been maintained in 10% Fetalgro®, but this did not work. After this was done, the Biowest FBS and ATL BIOL FBS were selected for full growth curve comparisons (both 10%, just following the cells more closely). These top two sources of FBS surprisingly differed visually in what appeared to be debris (Biowest FBS was debris-free while ATL BIOL FBS was debris-rich). It turned out that once the growth curves were analyzed, the cell doubling times in these two sources of FBS were indistinguishable. HRA-19 doubling times were 40.0–48.5 hours (Biowest versus ATL BIOL, respectively); as were the SH-SY5Y doubling times, 45.9–30.9 hours, respectively; as were the C2BBe1 doubling times, 50.0–79.6 hours, respectively.
Taking it all into account, we found good high functional equivalency with 5 brands of FBS out of the 11 brands of FBS initially screened. The best of the non-FBS supplements in terms of cell proliferation was FetalGro®.
Discussion
FBS has long been the gold standard for promoting the growth and differentiation of mammalian cell types. Yet, animal advocacy voices like the German newspaper, Süddeutsche Zeitung, have for years run articles on the ethical problems of FBS harvesting (Süddeutsche Zeitung,Tuesday, 11 August 2015, contributors: Christian Baars, Leo Klimm). Many of us ignored this activism, but were surprised by revelations on ISIA’s website to learn that dedicated herds of cattle in a controllable environment have never existed. Instead, pregnant cattle are gleaned from abattoirs and stockyards in circumstances that are difficult to control. Besides this, it seems that the reluctance of American herders to lose their pregnant animals has recently driven the FBS industry to overseas herds. No one disputes that sterile techniques and product certification have improved the quality of FBS since the ISIA was founded, but does this mean there is now acceptable batch-to-batch reproducibility in the FBS supply line29? With pricing of FBS approaching the value of gold, there are cheaper non-FBS alternatives on the market. Although the ISIA is mandated to improve the quality of FBS, we scientists need to test how changes in the FBS industry play on our cell cultures. That is why our research study was undertaken. We know of no other study that has done similarly.
The totally non-animal product tested was Cell-Ess® (Essential Pharmaceuticals). Cell-Ess® is a newer product and only appears in one previous publication30, as a feeder booster to increase the protein yield of monoclonal antibody-producing human cells. Another newly marketed product comes from human platelet donors, a lysate product called GroPro® (Zenbio Inc.). GroPro® has been reported17 very effective maintaining the growth and stem cell phenotype of limbal explant cultures. Other studies have shown that human platelet lysates have many growth factors and the capability to support a large-scale expansion of bone marrow mesenchymal stem cells for therapeutic application12,31. Concern exists though about the batch-to-batch consistency of human platelet lysates32. Our third product, FetalGro®, was developed along the rationale that if neonatal calves could be used instead of fetuses, their serum would lead to more scientific bleedings which might be viewed more ethically than FBS13. RMBI is a pioneer in the field by using an adult bovine serum mixture containing proprietary lipoproteins, named LipoGro®, and in recent years has introduced their beta-version, FetalGro®. FetalGro® is reported to be one of the better replacements for FBS in terms of maintaining six human cell lines13.
In two endothelial cell lines, the results with GroPro® and FetalGro® (Figs. 1 and 2) were nearly as good as with FBS for up to 3 weeks. Then, after three passages in FetalGro® (we did not try longer with GroPro®), the endothelial cells seemed to stop dividing. The same was found in SH-SY5Y cells, which also showed more neurospheres in FetalGro® at third passage. The study was repeated multiple times. Of course, other variations could be tried in the algorithm in hope of making non-FBS supplements work better. For instance, increasing to 20% GroPro® or FetalGro® might be a way. But, the hurdle remains doubtful to be overcome with the neuronal cell line, SH-SY5Y, because the non-FBS supplements were quite inferior to the FBS. The positive news with SH-SY5Y cells was that these non-FBS supplements did not induce toxicity. However, they were inferior to FBS by almost all other measures (Figs. 1 and 3 short-term, longer-term with FetalGro®, etc.). Hence, if the goal is expansion of a neuronal type of cell with a non-FBS growth supplement, it would appear none of the ones we tested were close to FBS.
The branded lots of FBS were also not equivalent (Figs 4 –6). We searched the literature and found one prior comparable peer-reviewed report about FBS variability that was published before the ISIA was formed20 (and none since then). That 1999 study tested 12 lots of FBS in a different paradigm of growth, and reported that only 5 of the lots were capable of supporting the output goal of their study (that output goal was secretion of apolipoprotein CIII from their hepatoma cell line)20. If we compare that study with ours, then the success rate of our study is not really better than in 199920. Given that the FBS industry has been undergoing so many changes over past 10 years, it cannot be proven that the formation of the ISIA in 2006 has led to an obvious improvement in getting rid of the inter-lot variability. So, is it time for the FBS industry to consider acquiring their own dedicated herds to finally secure a scientifically designed and virus-free source of FBS for sale? Did the list prices on the FBS brands serve as an indicator of more robust growth rates or better differentiation of the cells? The answer was no. Nothing indicated that the brands of FBS with higher list prices resulted in better performing cells.
The higher numbers of neurospheres in some FBS brands and in FetalGro® (Fig. 6) maybe especially noteworthy. Neurospheres are a phenotype formed in SH-SY5Y cells in response to low concentrations of FBS in the media, especially lower than 10%24. Therefore, the brands of FBS that induced the least number of neurospheres (Fig. 4) may be assumed to be the ones with the highest concentration of growth factors.
In summary, we tested three categories of substitutes for FBS: (1) a serum-free and xeno-free material marketed under the name Cell-Ess®; (2) a human platelet lysate marketed under the name GroPro®; and (3) two neonatal bovine serum products mixed with purified growth factors marketed under the names Lipogro® and FetalGro®. Cell-Ess® was found to be the least effective on our three human cell lines. Based on an earlier report30 of its inability to maintain immunoglobulin producing cells, Cell-Ess® may therefore be no better than a feeder or initial booster of monoclonal antibody-producing cells. GroPro® and FetalGro® were better, which at least closely compares to the performance of FBS with our endothelial cell lines (at least for the 2-weekly cell passages). Although these two non-FBS growth supplements faltered by the third cell passage at week 3, they were within range of the batch-to-batch short-comings found among some FBS branded lots. To the extent that stem cells need be expanded for safety reasons prior to human use by using some type of non-FBS growth supplements, then GroPro® or FetalGro® seem to be the better alternatives. Keeping in mind, however, that where safely is not an issue – also if the ethical concerns of fetal harvesting are not paramount – then, the gold standard for most cell biologists still is FBS.
Acknowledgements
We thank Drs Hannah Broome, Jerry Reagan, and Angela Reiken (Mississippi College) for kindly gifting us their purchased brands of FBS.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: We have no conflicting interests to declare with vendors, agencies, or other interest groups. The sera came to us free of charge on a trial basis, unencumbered by material transfer agreements, restrictions on publication, or mention of intellectual property. Each vendor of FBS was provided the courtesy of a detailed scientific synopsis delivered approximately 2 years prior to the paper being written or submitted.
Funding: The authors disclosed receipt of the following financial support for the research and/or authorship of this article: We thank Mississippi College for the funding for these studies.
References
- 1. Van der Valk J, Mellor D, Brands R, Fischer R, Gruber F, Gstraunthaler G, Hellebrekers L, Hyllner J, Jonker FH, Prieto P, et al. The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicology In Vitro. 2004;18(1):1–12. [DOI] [PubMed] [Google Scholar]
- 2. Van der Valk J, Bieback K, Buta C, Cochrane B, Dirks WG, Fu J, Hickman JJ, Hohensee C, Kolar R, Liebsch M, et al. Fetal bovine serum (FBS): Past - Present - Future. ALTEX. 2018;35(1):99–118. [DOI] [PubMed] [Google Scholar]
- 3. Davis D, Hirschi SD. Fetal bovine serum: what you should ask your supplier and why. BioProcessing J. 2014;13(1):1–3. [Google Scholar]
- 4. Gstraunthaler G, Lindl T, van der Valk J. A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology. 2013;65(5):791–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xia H, Vijayaraghavan B, Belák S, Liu L. Detection and Identification of the Atypical Bovine Pestiviruses in Commercial Foetal Bovine Serum Batches. PLoS One. 2011;6(12):e28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gagnieur L, Cheval J, Gratigny M, Hébert C, Muth E, Dumarest M, Eloit M. Unbiased analysis by high throughput sequencing of the viral diversity in fetal bovine serum and trypsin used in cell culture. Biologicals. 2014;42(3):145–152. [DOI] [PubMed] [Google Scholar]
- 7. Pinheiro de Oliveira TF, Fonseca AA, Camargos MF, de Oliveira AM, Pinto Cottorello AC, Souza A dos R, de Almeida IG, Heinemann MB. Detection of contaminants in cell cultures, sera and trypsin. Biologicals. 2013;41(6):407–14. [DOI] [PubMed] [Google Scholar]
- 8. Gray JS, Birmingham JM, Fenton JI. Got black swimming dots in your cell culture? Identification of Achromobacter as a novel cell culture contaminant. Biologicals. 2010;38(2):273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell. 2014;14(2):141–5. [DOI] [PubMed] [Google Scholar]
- 10. Karnieli O, Friedner OM, Allickson JG, Zhang N, Jung S, Fiorentini D, Abraham E, Eaker SS, Yong tan K, Chan A, Griffiths S, Wehn AK, Oh S, Karnieli O. A consensus introduction to serum replacements and serum-free media for cellular therapies. Cytotherapy. 2017;19(2):155–169. [DOI] [PubMed] [Google Scholar]
- 11. Brunner D, Frank J, Appl H, Schöffl H, Pfaller W, Gstraunthaler G. Serum-free cell culture: the serum-free media interactive online database. ALTEX. 2010;27(1):53–62. [DOI] [PubMed] [Google Scholar]
- 12. Astori G, Amati E, Bambi F, Bernardi M, Chieregato K, Schäfer R, Sella S, Rodeghiero F. Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: present and future. Stem Cell Res Ther. 2016;7(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fang CY, Wu CC, Fang CL, Chen WY, Chen CL. Long-term growth comparison studies of FBS and FBS alternatives in six head and neck cell lines. PLoS One. 2017;12(6): e0178960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gottipamula S, Ashwin KM, Muttigi MS, Kannan S, Kolkundkar U, Seetharam RN. Isolation, expansion and characterization of bone marrow-derived mesenchymal stromal cells in serum-free conditions. Cell Tissue Res. 2014;356(1):123–35. [DOI] [PubMed] [Google Scholar]
- 15. Lu HF, Chai C, Lim TC, Leong MF, Lim JK, Gao S, Lim KL, Wan ACA. A defined xeno-free and feeder-free culture system for the derivation, expansion and direct differentiation of transgene-free patient-specific induced pluripotent stem cells. Biomaterials. 2014;35(9):2816–26. [DOI] [PubMed] [Google Scholar]
- 16. Burnouf T, Strunk D, Koh MBC, Schallmoser K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016;76:371–87. [DOI] [PubMed] [Google Scholar]
- 17. Suri K, Gong HK, Yuan C, Kaufman SC. human platelet lysate as a replacement for fetal bovine serum in limbal stem cell therapy. Curr Eye Res. 2016;41(10):1266–73. [DOI] [PubMed] [Google Scholar]
- 18. Hu W, He Y, Xiong Y, Lu H, Chen H, Hou L, Qiu Z, Fang Y, Zhang S. Derivation, expansion, and motor neuron differentiation of human-induced pluripotent stem cells with non-integrating episomal vectors and a defined xenogeneic-free culture system. Mol Neurobiol. 2016;53(3):1589–1600. [DOI] [PubMed] [Google Scholar]
- 19. Nguyen MT, Okina E, Chai X, Hian Tan K, Hovatta O, Ghosh S, Tryggvason K. Differentiation of human embryonic stem cells to endothelial progenitor cells on laminins in defined and xeno-free systems. Stem Cell Reports. 2016;7:802–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clavey V, Copin C, Mariotte MC, Baugé E, Chinetti G, Fruchart J, Fruchart JC, Dallongeville J, Staels B. Cell culture conditions determine apolipoprotein CIII secretion and regulation by fibrates in human hepatoma HepG2 cells. Cellular Physiol Biochem. 1999; 9(3):139–49. [DOI] [PubMed] [Google Scholar]
- 21. Odelstad L, Pahlman S, Nilsson K, Larsson E, Lackgren G, Johansson KE, Hjerten S, Grotte G. Neuron-specific enolase in relation to differentiation in human neuroblastoma. Brain Res. 1981;224(1):69–82. [DOI] [PubMed] [Google Scholar]
- 22. Kovalevich J, Langford D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol. 2013;1078:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xie HR, Hu LS, Li GY. SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chinese Med J (Engl). 2010;123(8):1086–92. [PubMed] [Google Scholar]
- 24. Chakrabarti L, Abou-Antoun T, Vukmanovic S, Sandler AD. Reversible adaptive plasticity: a mechanism for neuroblastoma cell heterogeneity and chemo-resistance. Frontiers in oncology. 2012; 2:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hämmerle B, Yañez Y, Palanca S, Cañete A, Burks DJ, Castel V, Font de Mora J. Targeting neuroblastoma stem cells with retinoic acid and proteasome inhibitor. PLoS One. 2013;8(10):e76761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. El-Zein O, Usta J, Kreydiyyeh SI. The appearance of a leptin effect on glucose absorption in Caco-2 cells depends on their differentiation level. Cellular Physiol Biochem. 2015; 37(2):491–500. [DOI] [PubMed] [Google Scholar]
- 27. Cai Y, Xu C, Chen P, Hu J, Hu R, Huang M, Bi H. Development, validation, and application of a novel 7-day Caco-2 cell culture system. J Pharmacol Toxicol Methods. 2014;70(2):175–81. [DOI] [PubMed] [Google Scholar]
- 28. Kirkland SC, Henderson K. Endocrine and mucous differentiation by a cloned human rectal adenocarcinoma cell line (HRA-19) in vitro: inhibition by TGF-beta 1. J Cell Sci. 1994;107 (Pt 4):1041–46. [DOI] [PubMed] [Google Scholar]
- 29. Versteegen R. Serum: what, when, and where? BioProcess J. 2016; 15(1):1–4. [Google Scholar]
- 30. Elhofy A. Novel Cell-Ess supplement used as a feed or as an initial boost to CHO serum-free media results in a significant increase in protein yield and production. Computational and Structural Biotechnol J. 2016;14:319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gottipamula S, Sharma A, Krishnamurthy S, Majumdar A Sen, Seetharam RN. Human platelet lysate is an alternative to fetal bovine serum for large-scale expansion of bone marrow-derived mesenchymal stromal cells. Biotechnol Letters. 2012;34(7):1367–74. [DOI] [PubMed] [Google Scholar]
- 32. Hemeda H, Giebel B, Wagner W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy. 2014;16(2):170–80. [DOI] [PubMed] [Google Scholar]