Abstract
Freezing is recognized as the most effective method of maintaining a stable supply of various cell types for long-term storage. However, cells might be damaged by environmental changes during the freezing process. There are various factors that influence the function of cells cultured after cryopreservation and thawing. These factors include cryopreservation solutions, biomaterials, freezing methods, and the freezing and preservation temperatures. There is also a risk of infection with mycoplasma in liquid nitrogen phase. Therefore, it is necessary to consider more useful and safe methods for freezing and storing various cells. In this study, we investigated the effects of temperature during long-term storage (8 years at −80 °C and in liquid nitrogen phase) on the quality of various cells (human hepatocellular carcinoma cells, bovine carotid artery normal endothelial cells, mouse fibroblast cells 3T3, and mouse embryo fibroblast cells STO). We examined the cell viability of cryopreserved human hepatocellular carcinoma cells at −80 °C using culture medium containing 10% DMSO, Cell Banker 1, and Cell Banker 2 as cryopreservation solutions. Among these solutions, Cell Banker 1 showed the highest efficiency. The viability of human hepatocellular carcinoma and bovine carotid artery normal endothelial cells in the Cell Banker 1 stored at −80 °C was over 90%, which was the same as that in liquid nitrogen phase. The cells stored at −80 °C had a morphology similar to that of the cells stored at liquid nitrogen phase. The proliferation of cells stored at −80 °C and in liquid nitrogen phase was not significantly different. Furthermore, none of the cells were infected with mycoplasma. There was no marked difference in the albumin secretion between the human hepatocellular carcinoma cells stored at −80 °C and those in liquid nitrogen phase. The short tandem repeats of the human hepatocellular carcinoma cells stored at −80 °C were identical to those stored in liquid nitrogen phase. In this report, various cells stored long-term at −80 °C were able to be used effectively after long-term storage. These findings can be applied to drug discovery, cell medicine, and cell therapy.
Keywords: human and mammalian cells, cryopreservation, −80 °C, long-term storage, cell quality
Introduction
Freezing for long-term storage has proven to be one of the most effective methods of maintaining a stable supply of various cell types. However, cells may be damaged by environmental changes during the freezing process1,2. There are various factors that influence the function of cells cultured after cryopreservation and thawing, incuding the cryopreservation solution3–6, biomaterials7,8, freezing methods9,10, and freezing and preservation temperatures3–10. Among cryopreservation solutions, cryoprotective agents such as glycerol11,12, ethylene glycol13, and dimethyl sulfoxide (DMSO)14 are the most effective due to their high rate of penetration into cells. In addition, it has been reported that starch and oligosaccharides3 like trehalose15,16 and maltose4,6 are effective in suppressing damage to cells.
Cells cryopreserved on a collagen thin film7 or a carrier material8 can be directly applied to transplantation and drug discovery efforts. Freezing methods that reduce cell damage, like the vitrification method, have also been reported9,10. Vitrification is an effective cryopreservation technique of induced pluripotent stem cells (iPSCs)17 and embryonic stem cells (ESCs)9,10, but the cells become damaged if the osmotic pressure increases. Therefore, more effective and less toxic solutions, as well as more convenient techniques, are strongly desired. Taking into consideration the reduction in cell damage caused by ice crystal formation within the cell and solution troubles such as cell dehydration1,2, it would seem best to store cells in liquid nitrogen (LN2) phase and the vapor phase of LN2. However, long-term storage in LN2 phase carries a risk of mycoplasma infection, bacterial, and viral agents18,19. Therefore, it is necessary to consider more effective methods for freezing and storing various types of cells.
In this study, we investigated the effects of temperature during long-term storage (8 years at −80 °C or in LN2 phase) on the quality of various cells.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM) and antibiotics (penicillin, streptomycin) were purchased from GIBCO BRL, Life Technologies Inc. (Grand Island, NY, USA). Fetal bovine serum (FBS, BIO-WEST) was obtained from Funakoshi Co., Ltd. (Tokyo, Japan). Dulbecco’s phosphate buffered saline without calcium chloride and magnesium chloride (DPBS(−)) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other materials and chemicals not specified above were of the highest grade available.
Cells
HepG2 cells (human hepatocellular carcinoma cells, HB-8065) and STO cells (mouse embryo fibroblast cells, CRL-1503) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). HH cells (Bovine carotid artery normal endothelial cells, JCRB0099) and NIH 3T3 cells (Mouse fibroblast cells, clone 5611, JCRB0615) were obtained from the JCRB Cell Bank (Osaka, Japan).
Cryopreservation and Thawing of HepG2 and Mammalian Cells
Cells were cultured on 60-mm culture dishes with 4 mL of culture medium at 37 °C in 5% CO2. The culture medium consists of DMEM supplemented with 10% FBS, 100 U/mL of penicillin, and 100 U/mL of streptomycin. The cells were passaged at least four times and then frozen in a cryopreservation solution. As cryopreservation solutions, the culture medium and 10% DMSO, Cell Banker 1, and Cell Banker 2 (Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan) were used. One milliliter of a cell suspension containing 1 × 106 cells was quickly transferred to a 2.0-mL cryotube and frozen at a cooling rate of 1 °C/min. After cooling to −80 °C, the cells were stored at −80 °C or in LN2 phase until use (typically over 8 years; HepG2 cells, August 16, 2008; STO cells, September 12, 2008; HH cells, August 8, 2008; and NIH 3T3 cells, August 5, 2006).
The frozen tubes were placed in a water bath at 37 °C to thaw until the ice crystals had nearly melted. The cell suspension was then diluted to 1:9 with ice-cold culture medium and centrifuged at 1,000 rpm for 5 min. The supernatant was removed, and the cells were resuspended in fresh medium. The cell viability was assessed using the trypan blue exclusion test. The final concentration of trypan blue (GIBCO BRL; Life Technologies Inc.) was 0.2%.
Culture and Proliferation Assay of HepG2 and Mammalian Cells
After long-term cryopreservation at −80 °C, thawed HepG2 cells and mammalian cells (2 × 105 live cells) were seeded into 60-mm culture dishes in 4 mL of the culture medium. The cells were incubated at 37 °C under a humidified 5% CO2 atmosphere, and the culture medium was changed every 24 h. For cell culture, cells were detached from a 60-mm culture dish with trypsin (GIBCO BRL; Life Technologies Inc.) and repeatedly seeded onto a new culture dish.
After 3 to 4 d, the cells attached and spread on 60-mm culture dishes and subcultured until 80% to 90% confluent. After trypsin treatment, cell viability and cell number were determined using a trypan blue exclusion assay. The passages were repeated one to five times. The attached cells were also stained with a kit to evaluate their viability, as described below.
Determination of Living and Dead Cells
The cells were stained with calcein acetoxymethyl ester (calcein AM, 4 mM in anhydrous DMSO) and ethidium homodimer 1 (EthD-1, 2 mM in DMSO/H2O 1:4 [v/v]; LIVE/DEAD® Viability/Cytotoxicity kit, for mammalian cells, L3224; Molecular Probes®; Life Technologies Inc.) to visualize living and dead cells. A total of 4 μL of the supplied 2 mM ethidium homodimer-1 (EthD-1) stock solution was transferred to 2 mL of DPBS(−), and then 1 μL of the supplied 4 mM calcein AM stock solution was added to 2 mL of the EthD-1 solution. The working solution containing 2 μM of calcein acetoxymethyl ester (AM) and 4 μM EthD-1 was then added directly to the cells. After the cells had been incubated for 20 min at 37 °C, calcein AM-positive green fluorescence in living cells and EthD-1-positive red fluorescence in dead cells were examined using a fluorescence microscope (Keyence Japan, Osaka, Japan).
Mycoplasma Tests of HepG2 Cells and Mammalian Cells
The cells cryopreserved at −80 °C and in LN2 were tested for mycoplasma contamination as below. Cells (2 × 105 cells) were cultured in a 60-mm dish with 4 mL of culture medium. After 48 h of culture, the culture medium was changed, and the cells were incubated for 48 h. Mycoplasma infection in the culture medium after 48 h was assessed using the MycoAlert kit in accordance with the manufacturer’s instructions (Lonza Rockland Inc., Rockland, ME, USA).
Quantitation of Albumin Secreted by the HepG2 Cells
HepG2 cells (2 × 105 cells) were cultured in a 60-mm dish, and the culture medium was changed every 24 h. After 48 h of culture, the cells were incubated in culture medium for 24 h. The level of albumin secretion was assessed based on the accumulation of albumin in the culture medium after 24 h using a human albumin enzyme-linked immunosorbent assay (ELISA) quantification kit in accordance with the manufacturer’s instructions (Bethyl Laboratories, Inc., Montgomery, TX, USA).
An Analysis of Short Tandem Repeats (STRs) for the Identification of HepG2 Cells
To determine whether the HepG2 cells that had been stored at −80 °C were genomically identical to those that had been stored in LN2 phase, the STRs of the genomes were analyzed20,21. The analysis of the STRs was outsourced to BEX Co., Ltd. (Tokyo, Japan) using the Cell ID System (Promega Corp., Madison, WI, USA).
Results
Cryopreservation of HepG2 Cells in Various Solutions at −80 °C
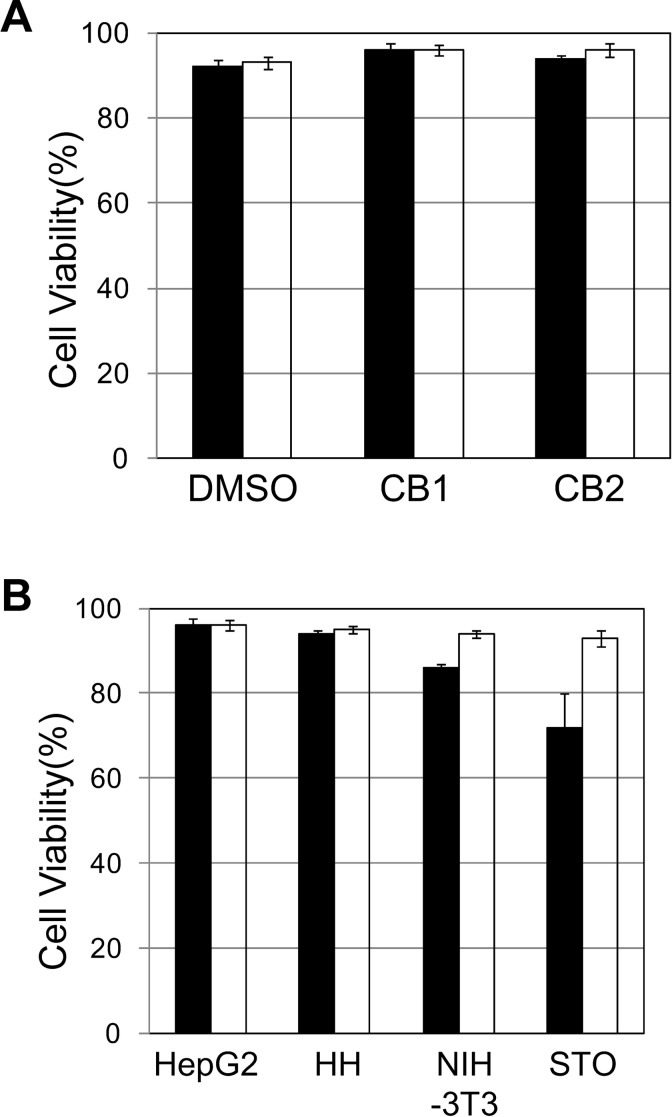
HepG2 cells were frozen and stored at −80 °C for 8 years. These cells were cryopreserved in culture medium containing 10% DMSO, Cell Banker 1, and Cell Banker 2. In order to investigate the effects of cryopreservation, we determined the cell viability immediately after thawing (Fig. 1A). The viability of HepG2 cells in the culture medium containing 10% DMSO, Cell Banker 1, and Cell Banker 2 was shown to be over 93% (Fig. 1A). The viability was highest with Cell Banker 1 (96%), so Cell Banker 1 was used in subsequent experiments.
Fig. 1.
Cell viability of human and mammalian cells cryopreserved at −80 °C and in liquid nitrogen (LN2) phase for a long period. HepG2, HH, NIH-3T3, and STO cells were frozen and stored at −80 °C or in LN2 phase for 8 years. (A) Cell viability of the cryopreserved HepG2 cells in different cryopreservation solutions. Dimethyl sulfoxide (DMSO): culture medium containing 10% DMSO; CB1: Cell Banker 1; CB2: Cell Banker 2. The cell storage settings were as follows: −80 °C (black column), and LN2 phase (open column). (B) Cell viability of human and mammalian cells cryopreserved with Cell Banker 1. HepG2: HepG2 cells; HH: HH cells; NIH-3T3: NIH-3T3 cells; STO: STO cells. The cell storage settings were as follows: −80 °C (black column) and LN2 phase (open column). The data represent the means and standard deviation (SD) of three independent experiments.
Long-term Cryopreservation of HepG2 and Mammalian Cells at −80 °C or in LN2 phase
HepG2 and mammalian cells were frozen and stored at −80 °C or in LN2 phase for 8 years. These cells were cryopreserved in Cell Banker 1. We investigated the cell functions at cryopreservation temperatures over a long period of time.
First, we determined the cell viability immediately after thawing (Fig. 1B). The viability of HepG2 cells and HH cells stored at −80 °C was over 94% (cell viability of HepG2 and HH cells was 96% and 94%, respectively), which was nearly the same as that of the cells stored in LN2 phase (cell viability of HepG2 and HH cells was 96% and 95%, respectively). However, the viability of the NIH 3T3 cells and STO cells stored at −80 °C (cell viability of NIH 3T3 and STO cells was 86% and 72%, respectively) was lower than that of the cells stored in LN2 (cell viability of NIH 3T3 and STO cells was 94% and 93%, respectively).
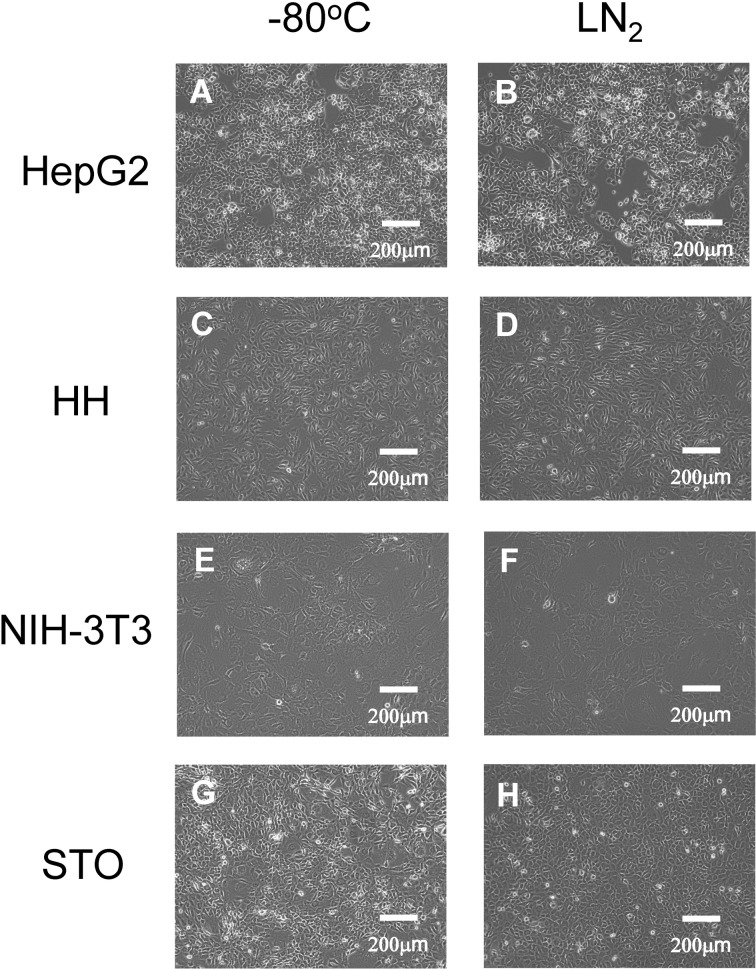
Second, HepG2 cells and mammalian cells stored at −80 °C and in LN2 phase were observed under a phase-contrast microscope (Fig. 2). The morphology of the cells stored at −80 °C (Fig. 2A, C, E, and G) was similar to that of the cells stored in LN2 phase (Fig. 2B, D, F, and H).
Fig. 2.
Phase-contrast photomicrographs of the human and other mammalian cells. HepG2, HH, NIH-3T3, and STO cells at a density of 5 × 105 (A, and B), 2 × 105 (C, and D), 2 × 105 (E, and F), and 2 × 105 cells (G, and H), respectively, were seeded onto 60-mm culture dishes and then cultured for 4 d. The cell storage settings were as follows: −80 °C (A, C, E, and G) and liquid nitrogen (LN2) phase (B, D, F, and H). Scale bar: 200 μm.
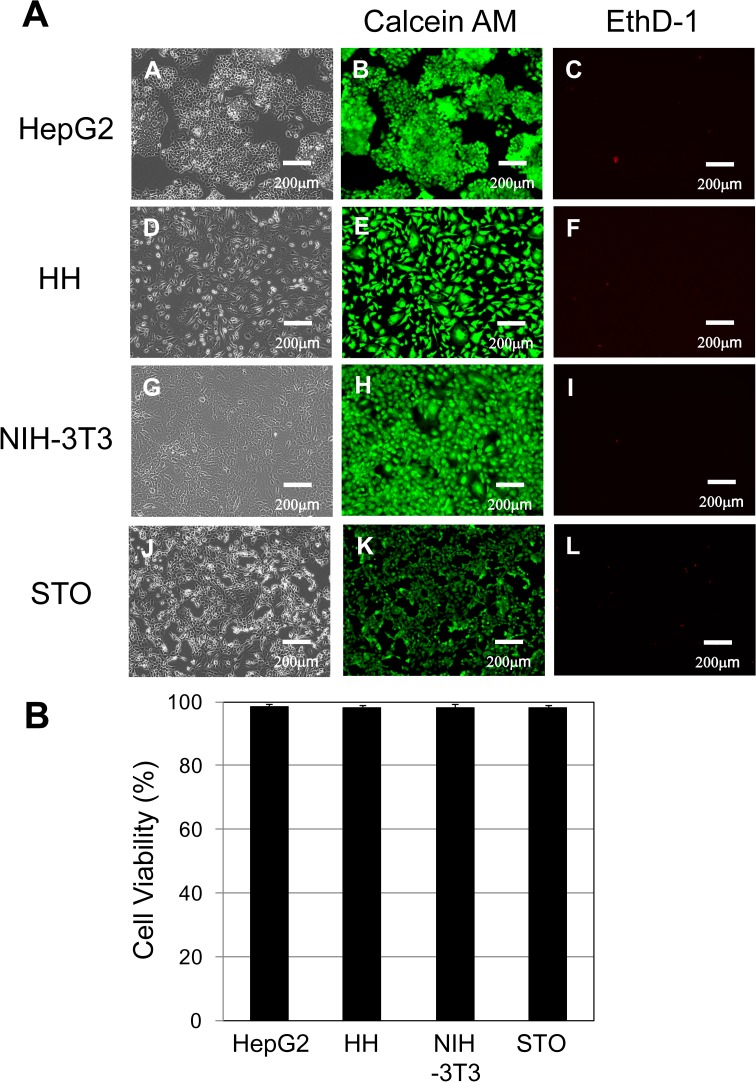
Third, after thawing, HepG2 and mammalian cells were stained with calcein AM and EthD-1 to discriminate between living and dead cells (Fig. 3A). Calcein AM-positive green fluorescence in the living cells and EthD-1-positive red fluorescence in the dead cells were observed using a fluorescence microscope. The viability of the HepG2 cells and the mammalian cells stored at −80 °C was over 98% (Fig. 3B).
Fig. 3.
Cell viability of living/dead cells in the human and mammalian cells. (A) HepG2, HH, NIH-3T3, and STO cells at a density of 2 × 105 cells were inoculated onto 60-mm dishes for 4 d (phase contrast; A, D, G, and J). HepG2, HH, NIH-3T3, and STO cells were stained with calcein acetoxymethyl ester (calcein AM) and ethidium homodimer-1 (EthD-1) to visualize living and dead cells. Calcein AM-positive green fluorescence (B, E, H, and K) in living cells and EthD-1-positive red fluorescence (C, F, I, and L) in dead cells were observed with a fluorescence microscope. Scale bar: 200 μm. (B) Cell viability of human and mammalian cells attached onto 60-mm dishes. HepG2: HepG2 cells; HH: HH cells; NIH-3T3: NIH-3T3 cells; STO: STO cells. The cell storage settings were as follows: −80 °C (black column). The data represent the means and SD of three independent experiments.
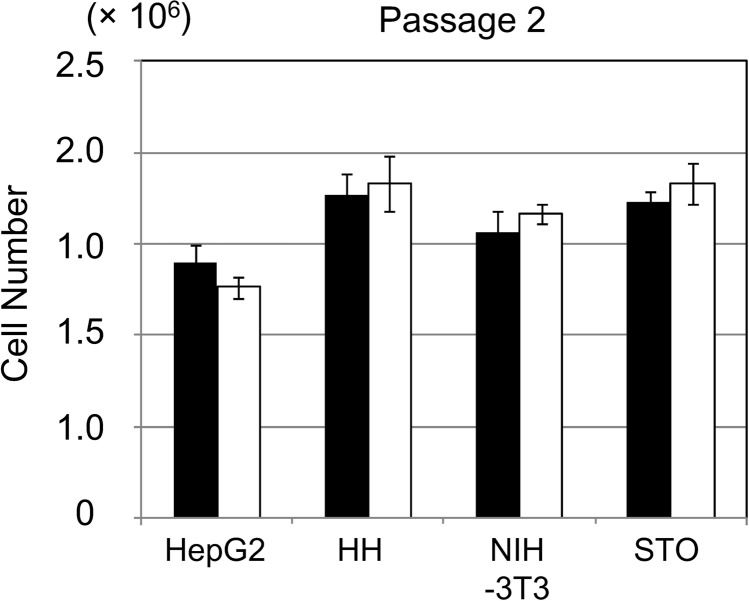
Fourth, to evaluate the proliferation of HepG2 cells and mammalian cells stored at −80 °C and in LN2 phase, we cultured these cells on new culture dishes for 4 d. After cell cryopreservation and thawing, the passages were repeated twice (Fig. 4). The proliferation of HepG2 cells and mammalian cells was not significantly different between those stored at −80 °C and those stored in LN2 phase.
Fig. 4.
Proliferation of HepG2 cells and mammalian cells at −80 °C and in liquid nitrogen (LN2) phase. HepG2, HH, NIH-3T3, and STO cells at a density of 2 × 105 cells were seeded onto 60-mm culture dishes and then cultured for 4 d. After cell cryopreservation and thawing, the passages were repeated twice. HepG2: HepG2 cells; HH: HH cells; NIH-3T3: NIH-3T3 cells; STO: STO cells. The cell storage settings were as follows: −80 °C (black column) and LN2 phase (open column). The data represent the means and standard deviation (SD) of three independent experiments.
Fifth, the cells cryopreserved at −80 °C or in LN2 phase were tested for mycoplasma contamination (Table 1). After 48 h of culture, the culture medium was changed, and the cells were incubated for 48 h. The results showed that the cultured cells were negative (MycoAlertTM ratio of the HepG2 cells and mammalian cells < 0.67) for mycoplasma compared to the positive control (MycoAlert ratio of positive control = 95). The cells cryopreserved at −80 °C and in LN2 phase were not infected with mycoplasma.
Table 1.
Mycoplasma Tests of Human and other Mammalian Cells at −80 °C and in LN2 Phase for a Long-Term Period.
| Cells | Cryopreservation Temperature | MycoAlerttm Plus Ratio | Conclusion |
|---|---|---|---|
| HepG2 | −80 °C | 0.45 | Negative |
| HepG2 | LN2 | 0.65 | Negative |
| HH | −80 °C | 0.58 | Negative |
| HH | LN2 | 0.52 | Negative |
| NIH/3T3 | −80 °C | 0.67 | Negative |
| NIH/3T3 | LN2 | 0.48 | Negative |
| STO | −80 °C | 0.61 | Negative |
| STO | LN2 | 0.56 | Negative |
| Positive Control | 95 | ||
| Negative Control | 0.17 |
Note. The cells cryopreserved at −80 °C and in liquid nitrogen (LN2) phase were tested for mycoplasma contamination as below. Cells (2 × 105 cells) were cultured in a 60-mm dish with 4 mL of culture medium. After 48 h of culture, the culture medium was changed, and the cells were incubated for 48 h. Mycoplasma infection in the culture medium after 48 h was assessed using the MycoAlert kit in accordance with the manufacturer’s instructions (Lonza Rockland Inc., Rockland, ME, USA).
The Liver Function and STRs of HepG2 Cells at −80 °C and in LN2 phase
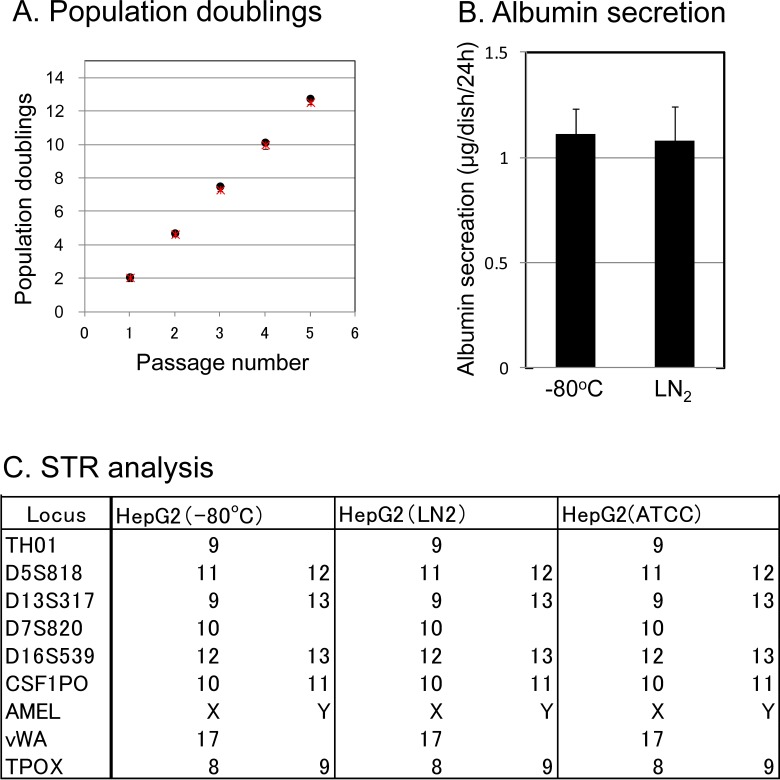
We performed a population doublings (PD) test over 1 to 5 passages (Fig. 5A) and measured the quantification of albumin secreted by the cells (Fig. 5B) and conducted an STR analysis (Fig. 5C) on the HepG2 cells stored at −80 °C or in LN2 phase. HepG2 cells at 2 × 105 cells were seeded onto 60-mm dishes and then cultured for 4 d (1 passage per day; Fig. 5A). The population doublings of HepG2 cells and mammalian cells were not significantly different between the cells stored at −80 °C and those in LN2 phase (Fig. 5A). To examine the liver function of the HepG2 cells, we also evaluated the amount of albumin secretion in the medium over 24 h after 3 d of culture (Fig. 5B). There was no marked difference in the albumin secretion of the HepG2 cells stored at −80 °C (1.1 μg/dish/24 h) and those stored in LN2 phase (1.1 μg/dish/24 h; Fig. 5B). The STRs of the HepG2 cells stored at −80 °C and in LN2 phase were identical, indicating that the cells were derived from the same origin HepG2 cells (ATCC; Fig. 5C).
Fig. 5.
Population doublings, liver function, and short tandem repeats (STRs) of HepG2 Cells at −80 °C and in liquid nitrogen (LN2) phase. HepG2 cells were frozen and stored at −80 °C or in LN2 for 8 years. The cell storage settings were as follows: −80 °C and LN2 phase. (A) A population doublings (PD) test in the HepG2 cells during 1 to 5 passages. HepG2 cells at a density of 2 × 105 cells were inoculated onto 60-mm dishes for 4 d, and the passages were repeated 1 to 5 times. The cell storage settings were as follows: −80 °C (black circle) and LN2 phase (red asterisk). (B) Albumin secretion in the HepG2 cells at −80 °C and in LN2 phase. We quantified the albumin secreted by HepG2 cells at −80 °C and in LN2 phase. HepG2 cells at 2 × 105 cells were seeded onto 60-mm dishes and then cultured for 3 d. The amount of albumin secreted into the medium was measured by enzyme-linked immunosorbent assay (ELISA) after 24 h of accumulation on day 3 of culture. The data represent the mean ± standard deviation (SD) of triplicate experiments. (C) The STR analysis to confirm the identity of HepG2 cells. We performed an STR analysis to confirm that the HepG2 cells stored at −80 °C and in LN2 phase were of ATCC origin. The allele data are presented as a table on the right side of each image. The locus indicates the name of the gene analyzed. Genes presented by one datum point were homo and those presented by two data points were hetero.
Discussion
There have been many reports of cells cryopreserved in LN2 or in other cryopreservation solutions3–6,11–16 or with different freezing methods9,10. We previously reported that oligosaccharides3 and sericin4 were effective cryoprotectants against primary human hepatocytes with greater freezing damage.
However, few reports5,6,22 have described the effects of cryopreservation of human and mammalian cells at −80 °C. Although we reported on the cryopreservation of adipose-derived stem cells6,22 and iPS cells5 for short periods, such as 1 month, the quality of cells cryopreserved for longer periods must also be evaluated. Therefore, in this study, we examined the outcomes of human and mammalian cells frozen and stored at −80 °C or in LN2 phase over 8 years ago.
The viability of HepG2 cells stored at −80 °C was evaluated using three types of cryopreservation solutions (Fig. 1A). The Cell Banker 1 solution resulted in the highest viability, so other mammalian cells were also evaluated using this solution. The cell viabilities of HepG2 cells and mammalian cells stored in LN2 phase were over 93% (Fig. 1B), whereas those of cells stored at −80 °C were lower, depending on the type of cell (Fig. 1B). Since the cell viability varies by cell type, it is necessary to optimize the composition of the cell cryopreservation solution. The cells frozen and stored at −80 °C and in LN2 phase were thawed and then cultured, and the survival of the adhered cells was evaluated using calcein AM and EthD-1 (Fig. 3A). All of the adhered cells maintained their original morphology. No marked differences were observed in the cell morphology due to storage temperature. In addition, the cell viability of the adherent cells stored at −80 °C was over 98%, indicating that long-term storage at −80 °C is effective. Furthermore, the quality of cells passaged frequently was maintained, as there were no differences in the cell proliferation due to long-term storage temperature (Fig. 4).
Infection with mycoplasma was also evaluated in these cryopreserved cells. No mycoplasma infection was observed in any cells stored at −80 °C or in LN2 phase (Table 1). In contrast to LN2 phase, cells stored at −80 °C are kept in a freezing box under a gas phase; as such, the risk of contamination18,19 with agents like the mycoplasma, bacteria, and virus agents can be avoided. Therefore, large volumes of cells can be cryopreserved for a long time with easy handling and high safety at a low cost by simply transferring cells into the −80 °C freezer.
Finally, we examined the function and conducted an STR analysis of HepG2 cells stored at −80 °C and in LN2 phase (Fig. 5A, B, and C). There were no marked differences in the function of HepG2 cells by cryopreservation setting (−80 °C and in LN2 phase). The STR results confirmed that the cells were equivalent, supporting the effectiveness of frozen storage at −80 °C.
In this study, evaluating the efficacy of the preservation of frozen human and mammalian cells not only for the short-term but also for the long-term is extremely important in the fields of medicine and drug discovery. We hope that our findings will be useful for fundamental and clinical studies using cells. In conclusion, various cells stored for a long time at −80 °C were able to be effectively used. These findings are expected to be applicable in drug discovery tests, cell medicine, and cell therapy.
Acknowledgments
The authors would like to thank Ms. Rina Yokota at Nagoya University for assistance. This work was supported in part by JSPS KAKENHI Grant No. 15H03039 (Grant-in-Aid for Scientific Research (B)), and Sasakawa Scientific Research Grant from The Japan Science Society. The authors declare no conflicts of interest.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported in part by JSPS KAKENHI Grant No. 15H03039 (Grant-in-Aid for Scientific Research (B)), and Sasakawa Scientific Research Grant from The Japan Science Society.
References
- 1. Mazur P. Kinetics of water loss from cells at subzero temperatures and the likelihood of intracellular freezing. J Gen Physiol. 1963;47:347–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mazur P, Leibo SP, Chu EH. A two-factor hypothesis of freezing injury. Evidence from Chinese hamster tissue-culture cells. Exp Cell Res. 1972;71(2):345–355. [DOI] [PubMed] [Google Scholar]
- 3. Miyamoto Y, Suzuki S, Nomura K, Enosawa S. Improvement of hepatocyte viability after cryopreservation by supplementation of long-chain oligosaccharide in the freezing medium in rats and humans. Cell Transplant. 2006;15(10):911–919. [DOI] [PubMed] [Google Scholar]
- 4. Miyamoto Y, Teramoto N, Hayashi S, Enosawa S. An improvement in the attaching capability of cryopreserved human hepatocytes by a proteinaceous high molecule, sericin, in the serum-free solution. Cell Transplant. 2010;19(6):701–706. [DOI] [PubMed] [Google Scholar]
- 5. Miyamoto Y, Noguchi H, Yukawa H, Oishi K, Matsushita K, Iwata H, Hayashi S. Cryopreservation of induced pluripotent stem cells. Cell Med. 2012;3(1–3):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miyamoto Y, Oishi K, Yukawa H, Noguchi H, Sasaki M, Iwata H, Hayashi S. Cryopreservation of human adipose tissue-derived stem/progenitor cells using the silk protein sericin. Cell Transplant. 2012;21(2–3):617–622. [DOI] [PubMed] [Google Scholar]
- 7. Miyamoto Y, Enosawa S, Takeuchi T, Takezawa T. Cryopreservation in situ of cell monolayers on collagen vitrigel membrane culture substrata: ready-to-use preparation of primary hepatocytes and ES cells. Cell Transplant. 2009;18(5):619–626. [DOI] [PubMed] [Google Scholar]
- 8. Miyamoto Y, Ikeya T, Enosawa S. Preconditioned cell array optimized for a three-dimensional culture of hepatocytes. Cell Transplant. 2009;18(5):677–681. [DOI] [PubMed] [Google Scholar]
- 9. Fujioka T, Yasuchika K, Nakamura Y, Nakatsuji N, Suemori H. A simple and efficient cryopreservation method for primate embryonic stem cells. Int J Dev Biol. 2004;48(10):1149–1154. [DOI] [PubMed] [Google Scholar]
- 10. Reubinoff BE, Pera MF, Vajta G, Trounson AO. Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Hum Reprod. 2001;16(10):2187–2194. [DOI] [PubMed] [Google Scholar]
- 11. Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164(4172):666. [DOI] [PubMed] [Google Scholar]
- 12. Biagini G, Poppi V, Cocchia D, Ruboli G, Damiani R, Laschi R. Skin storage in liquid nitrogen. An ultrastructural investigation. J Cutan Pathol. 1979;6(1):5–17. [DOI] [PubMed] [Google Scholar]
- 13. Macpherson JW, Chatterjee S, Friars GW. Frozen turkey semen. Can J Comp Med. 1969;33(1):37–38. [PMC free article] [PubMed] [Google Scholar]
- 14. Lovelock JE, Bishop MW. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature. 1959;183(4672):1394–1395. [DOI] [PubMed] [Google Scholar]
- 15. Chen Y, Foote RH, Brockett CC. Effect of sucrose, trehalose, hypotaurine, taurine, and blood serum on survival of frozen bull sperm. Cryobiology. 1993;30(4):423–431. [DOI] [PubMed] [Google Scholar]
- 16. Crowe JH, Crowe LM. Preservation of mammalian cells-learning nature’s tricks. Nat Biotechnol. 2000;18(2):145–146. [DOI] [PubMed] [Google Scholar]
- 17. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. [DOI] [PubMed] [Google Scholar]
- 18. Bielanski A, Algire J, Randall GC, Surujballi O. Risk of transmission of Mycobacterium avium ssp. paratuberculosis by embryo transfer of in vivo and in vitro fertilized bovine embryos. Theriogenology. 2006;66(2):260–266. [DOI] [PubMed] [Google Scholar]
- 19. Bielanski A, Vajta G. Risk of contamination of germplasm during cryopreservation and cryobanking in IVF units. Hum Reprod. 2009:24(10):2457–2467. [DOI] [PubMed] [Google Scholar]
- 20. Masters JR, Thomson JA, Daly-Burns B, Reid YA, Dirks WG, Packer P, Toji LH, Ohno T, Tanabe H, Arlett CF, et al. Short tandem repeat profiling provides an international reference standard for human cell lines. Proc Natl Acad Sci U S A. 2001;98(14):8012–8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Himeda T, Hosomi T, Okuwa T, Muraki Y, Ohara Y. Saffold virus type 3 (SAFV-3) persists in HeLa cells. PLoS One. 2013;8(1):e53194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oishi K, Noguchi H, Yukawa H, Miyazaki T, Kato R, Kitagawa Y, Ueda M, Hayashi S. Cryopreservation of mouse adipose tissue-derived stem/progenitor cells. Cell Transplant. 2008;17(1–2):35–41. [DOI] [PubMed] [Google Scholar]