Abstract
Bronchopulmonary dysplasia (BPD), a disease affecting extremely premature infants, results from the disruption of normal pulmonary vascular and alveolar growth. Currently, there is no specific effective treatment. We report a case of a 10-mo-old female infant with BPD, who was admitted because of adenovirus pneumonia and acute respiratory distress syndrome (ARDS) with prolonged venovenous and arteriovenous extracorporeal membrane oxygenation (ECMO) support (total 125 d). The respiratory condition dramatically improved, and ECMO was removed 25 d after intratracheal delivery of maternal bone marrow-derived mesenchymal stem cells (BM-MSCs). Short tandem repeat examinations revealed that there was no maternal cells in the bronchial wash fluid. To our knowledge, this is the first human report of BM-MSC therapy reversal of the course of BPD superimposed with ARDS. We also suggest that BM-MSC therapy may not only be effective in the newborn stage but also works in infants and children with BPD.
Keywords: acute respiratory distress syndrome, bronchopulmonary dysplasia, extracorporeal membrane oxygenation, mesenchymal stem cell
Introduction
The incidence of bronchopulmonary dysplasia (BPD) is increasing with great advances in neonatal intensive care of extremely premature infants1. Disruption of normal pulmonary vascular and alveolar growth results in simplified pulmonary alveolar acini and vascular structures, reducing the surface area for gas exchange2,3. Secondary pulmonary hypertension is also common in severe cases4. The possible pathogenesis is altered vascular endothelial growth factor (VEGF) signaling and a decreased level of various pro-inflammatory cytokines (e.g., tumor necrosis factor-α, interleukin-1β, interleukin-6, and interleukin-16)5,6. Since there is no specific effective treatment, BPD remains a serious cause of morbidity and mortality in premature infants7.
Cell therapy with mesenchymal stem cells (MSCs) provides a new hope for treatment of BPD. MSCs, one type of adult stem cells, are identified according to the following consensus of minimal criteria: (1) adherence to plastic under standard culture conditions; (2) expression of CD105, CD73, and CD90 and lack of surface expression of CD45, CD34, CD14, CD11b, CD79, CD19, and human leukocyte antigen-antigen D related (HLA-DR); and (3) ability to differentiate into adipocytes, chondrocytes, and osteocytes in vitro8. MSCs have been used for treatment of soft tissue and bone injuries, autoimmune diseases, hematological diseases, diabetes mellitus, heart failure, central nervous system diseases, and lung diseases in early phase clinical trials9. They can be isolated from bone marrow (BM), adipose tissue, umbilical cord, and the placenta10,11. Four important mechanisms contribute to their therapeutic effect: (1) the ability to home to sites of inflammation following tissue injury, (2) the ability to differentiate into various cell types, (3) the ability to secrete multiple bioactive molecules capable of stimulating recovery of injured cells and inhibiting inflammation, and (4) the lack of immunogenicity and the ability to perform immunomodulatory functions12.
We report a case of an infant with severe BPD superimposed with acute respiratory distress syndrome (ARDS). The respiratory condition dramatically improved after intratracheal delivery of BM–derived MSCs (BM-MSCs).
Case
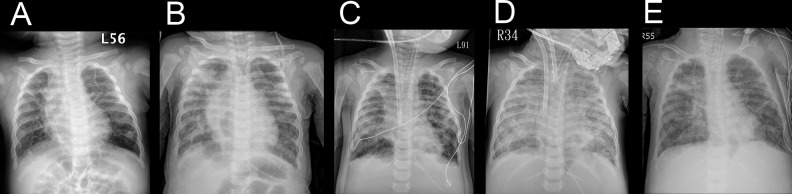
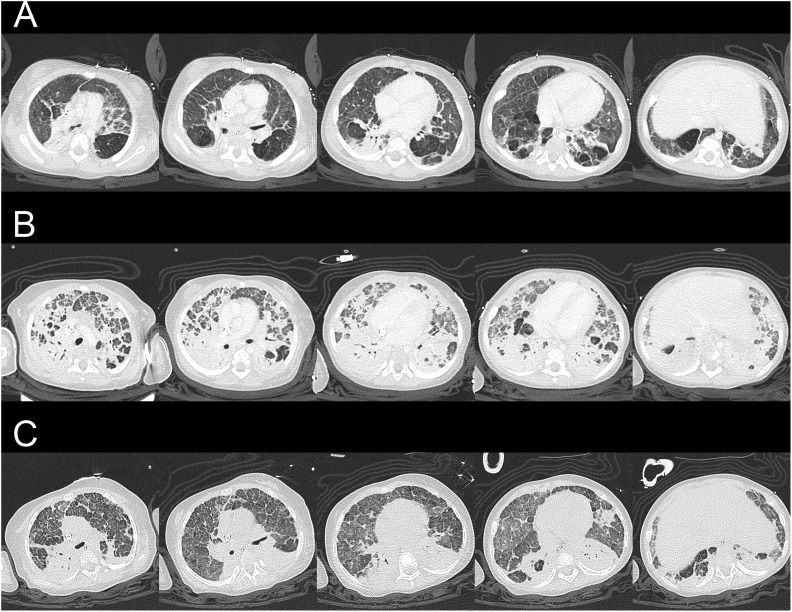
A 10-mo-old girl (gestational age: 25 wk and 4 d, birth body weight: 778 g, corrected age 6 and a half months) was admitted to our intensive care unit. She had a history of prolonged hospitalization due to BPD. Her baseline oxygen saturation was 90% in ambient air (Fig. 1A). She was intubated due to severe adenovirus pneumonia and ARDS. Chest radiography showed widespread reticular pattern and parenchymal bands (Fig. 1B). Pulmonary hypertension was suggested by echocardiography. A trial of inhaled nitric oxide and intravenous infusion of milrinone failed to improve oxygenation. Computed tomographic (CT) scan imaging of the chest was obtained (Fig. 2A). There were diffused coarse fibrotic bands, extensively thickened interstitium, subsegmental atelectasis, air trapping, and early cavitation in both lung fields. Sudden onset profound desaturation and bradycardia happened 22 d after admission. Spontaneous circulation recovered after 1-min bag-valve-mask ventilation as well as chest compression. Her saturation could hardly be maintained above 80% even with delivery of 100% oxygen.
Fig. 1.
Serial chest radiographs. Serial chest radiographs, anteroposterior view: (A) At 1 month before admission. (B) At the time of admission due to adenovirus pneumonia. (C) After cardiopulmonary resuscitation and venovenous ECMO implantation. (D) After conversion to venoarterial ECMO. (E) After intratracheal bone marrow–derived mesenchymal stem cell injection and extracorporeal membrane oxygenation removal. ECMO, extracorporeal membrane oxygenation.
Fig. 2.
Serial chest computed tomographic scan images: (A) At 17 d after admission, before venovenous ECMO implantation. (B) At 49 d after admission, before conversion to venoarterial ECMO implantation. (C) At 147 d after admission, 125 d after ECMO implantation, 25 d after intratracheal bone marrow–derived mesenchymal stem cell injection, before ECMO removal. Lung fibrosis dramatically improved after bone marrow-derived mesenchymal stem cell treatment. ECMO, extracorporeal membrane oxygenation.
Therefore, venovenous extracorporeal membrane oxygenation (ECMO) was implanted (a 15F double lumen cannula, blood flow 0.4 L/min; Fig. 1C). However, the blood flow gradually became unstable due to the presence of thrombosis and hemolysis. For better support, ECMO was changed to venoarterial (a 14F A-cannula and a 14F V-cannula, blood flow 0.9 L/min) after 35 d (Fig. 1D). Chest CT on 49 d after admission revealed deterioration and diffused fibrosis of both lung fields (Fig. 2B). Since she was ECMO dependent, we sought for MSC therapy.
Materials and Methods
Informed Consent
This study was approved by the Research Ethics Committee as a compassionate use (No. C1050901). Full understanding was confirmed, and written informed consent was obtained from the parent.
Culture Protocol for MSCs
Fifty milliliters of BM was aspirated from the donor’s posterior superior iliac crest. The BM sample was preserved in preservative-free heparinized Iscove’s modified dulbecco’s solution (Sigma-Aldrich) and filtrated through a 70-µm filter, then a 20-µm filter (Sigma-Aldrich) subsequently.
The diluted BM sample was carefully layered on Ficoll solution (Sigma-Aldrich) in a specific ratio. It was centrifuged at 4 °C, 2,400 rpm for 20 min. Mononuclear cells (MNCs) were aspirated through the interface. The MNCs were washed by low-glucose Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Waltham, MA, USA) twice.
The BM-MNCs were added to DMEM containing 25 U/mL heparin (Sigma-Aldrich) in 1:1 ratio and plated in T25 flasks at 5 × 107 nucleated cells per flask in 5 mL regular growth medium (Gibco). During the first 2 wk incubation, 5 mL of fresh growth medium was added once weekly for cell adherence and initial expansion. After adherent cells reached approximately 60% to 70% confluence, they were detached with 0.25% trypsin-ethylenediamineteraacetic acid (Gibco) and replated at 1:3 in regular growth medium to allow for continuing passing. Cultured MSCs were harvested at the end of the second passage.
Quality Control
Sterility and contamination: Aerobe, anaerobe, and fungus cultures were performed for sterility.
Immunophenotyping: The harvested cells were stained with phycoerythrin-conjugated antibodies (Becton Dickinson, Franklin Lakes, NJ, USA) against human antigens CD10, CD13, CD14, CD29, CD34, CD44, CD90/Thy-1, CD117/c-kit, CD166, AC133, human leukocyte antigens (HLA) A, B, C loci, HLA-DR, and glycophorin A and analyzed by a Becton Dickinson FACS Caliber flow cytometry system.
Differentiation: (1) Adipogenic differentiation: Adipogenic medium consisted of high-glucose DMEM, (Gibco) supplemented with 0.5 mM isobutyl-1-methylxanthine (IBMX; Sigma-Aldrich, St. Louis, MO, USA), 1 µM dexamethasone (Sigma-Aldrich), 10 ng/mL insulin (Sigma-Aldrich), 50 µM indomethacin (Sigma-Aldrich), and 10% fetal bovine serum (FBS, Gibco). To induce adipocyte differentiation, hMSCs were transferred into a 15-mL polypropylene tube and centrifuged at 1,000 rpm for 5 min, to form a pelleted micromass at the bottom of the tube. The cells were then treated with adipogenic medium (Gibco) for 4 wk with medium changes twice weekly. Adipogenic differentiation was assessed by the cellular accumulation of neutral lipid vacuoles after cells were fixed with 4% formaldehyde (Sigma-Aldrich), stained with oil-red O (Sigma-Aldrich). (2) Chondrogenic differentiation: Chondrogenic medium consisted of low-glucose DMEM, (Gibco) supplemented with 1 mM sodium pyruvate (Sigma-Aldrich), 0.1 µM dexamethasone (Sigma-Aldrich), 0.1 mM acetylsalicylic acid (AsA, Sigma-Aldrich), 10 ng/mL transforming growth factor-β (TGF-β1; R&D Systems, Minneapolis, MN, USA), and 1× ITS premix (Sigma-Aldrich; 5 µg/mL insulin, 5 µg/mL transferrin [Sigma-Aldrich], and 25 ng/mL selenius acid). Similar MSC processing was used as in adipogenic differentiation. Chondrogenic differentiation was evaluated after pellets had been transferred into 96-well plates, fixed with 4% formaldehyde (Sigma-Aldrich), and stained with 1% Alcian blue (Sigma-Aldrich).
Administration
After harvest, concentrated BM-MSCs were diluted to 20 mL with 0.9% saline. The patient was put in supine position and was deeply sedated. A 3-mm flexible bronchoscope was inserted through the endotracheal tube. The diluted BM-MSCs were equally administered into both bronchi from the working channel. After administration, sputum suctioning was avoided for 24 h.
Follow-Up
ECMO and ventilation parameters were adjusted according to clinical conditions. Blood gas was checked and recorded daily. Short tandem repeat (STR) examination of bronchial wash fluid was used to evaluate engraftment after obvious improvement in ventilation function. Chest CT was performed to evaluate lung condition.
Results
Intratracheal administration of BM-MSCs (6.25 × 106 cells/kg; total dose: 5 × 107 cells, body weight: 8.0 kg) was performed 100 d after ECMO implantation.
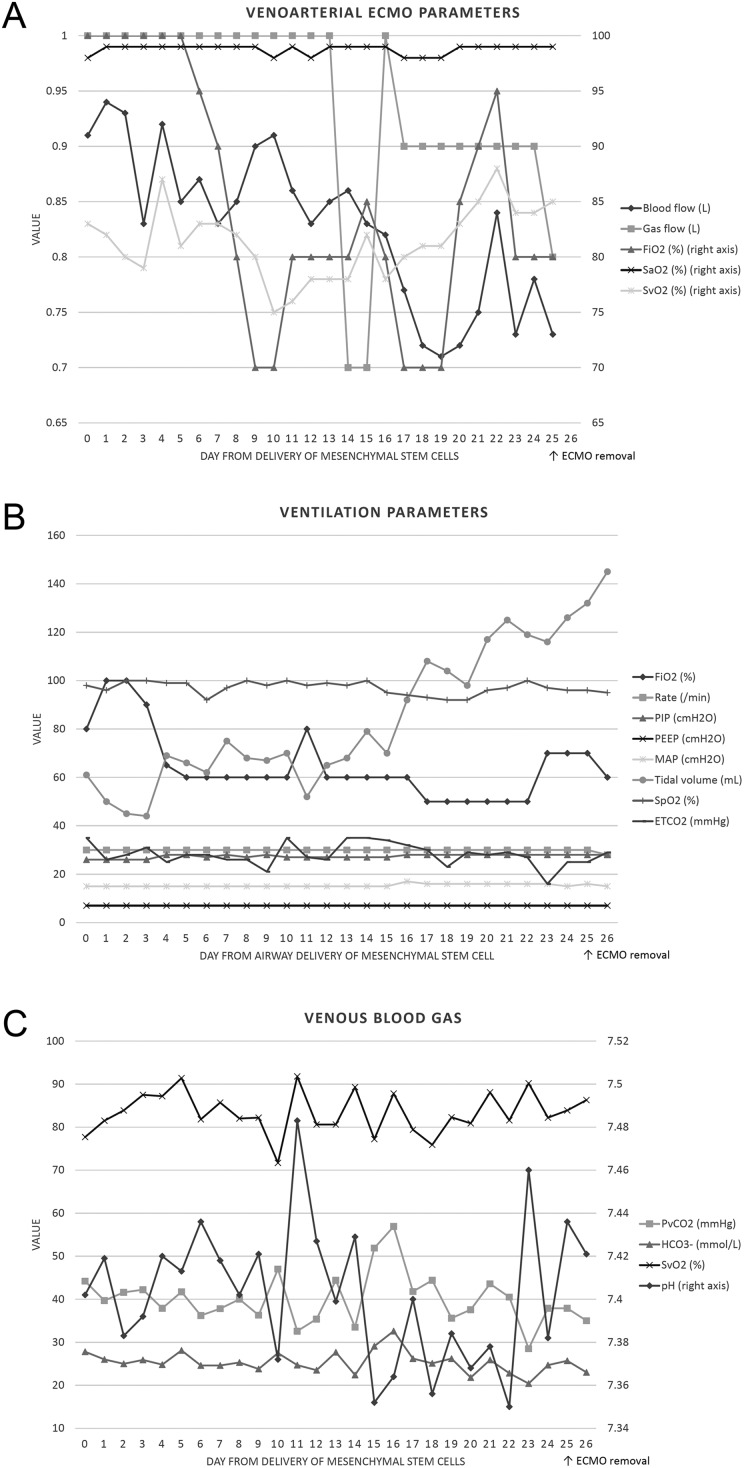
Dramatic improvement in oxygen demand and tidal volume was obvious under similar settings of peak inspiratory pressure, positive end-expiratory pressure, and mean airway pressure (Fig. 3). A much improved lung condition could be seen in the chest CT (Fig. 2C). STR examination 23 d after delivery revealed no maternal cell in bronchial wash fluid. ECMO was removed 25 d after delivery of BM-MSCs (Fig. 1E). She received tracheostomy and was discharged for long-term care. The delivered oxygen fraction before discharge was only 0.3.
Fig. 3.
Venoarterial extracorporeal membrane oxygenation, ventilation, and venous blood gas parameters after delivery of bone marrow–derived mesenchymal stem cells. (A) Venoarterial ECMO parameters: FiO2 and blood flow gradually decreased after delivery of MSCs. (B) Ventilation parameters: FiO2 was 100% after delivery of MSCs but could be adjusted to 60% to 70% after 5 d. Tidal volume improved from 40 to 60 mL to over 140 mL in similar settings of PIP, PEEP, and MAP. (C) Venous blood gas parameters: Blood gas values remained stable during venoarterial ECMO support. ECMO, extracorporeal membrane oxygenation; ETCO2, end-tidal carbon dioxide; FiO2, fraction of inspired oxygen; , bicarbonate; MAP, mean airway pressure; MSC, mesenchymal stem cell; PEEP, positive end-expiratory pressure; PIP, peak inspiratory pressure; PvCO2, partial pressure of venous carbon dioxide; SaO2, arterial oxygen saturation; SpO2, peripheral oxygen saturation; SvO2, venous oxygen saturation.
Discussion
MSCs are regulators of lung growth13. Exogenous MSCs are home to injured tissues and exert their immunomodulatory ability with the secretion of cytokines (e.g., VEGF, interferon-γ, interleukin-10, hepatocyte growth factor), stimulating angiogenesis and anti-inflammation, and tissue repair14. Several hyperoxia animal model studies proved the beneficial effect of BM-MSCs with different routes of administration, intratracheally, intravenously, or even intraperitoneally, in treatment of BPD15–23. BM-MSC therapy for BPD has never been reported in clinical trial. There was only 1 small-scale phase 1 dose escalation clinical trial using human umbilical cord–derived MSC therapy for BPD in Korea16. Intratracheal injection was performed in 9 extremely preterm infants (3 with 1 × 107 cells/kg, 6 with 2 × 107 cells/kg) at a mean of 10.4 d after birth. There was no adverse effect. BPD severity was lower in the recipients.
In addition, preclinical data support the use of MSCs for treatment of ARDS24. Early phase clinical trials are ongoing25. MSC therapy for refractory ARDS on ECMO was previously reported in 2 adult patients (2 × 106 cells/kg), whose conditions subsequently improved with resolution of respiratory, hemodynamic, and multiorgan failure26. The potential of MSC therapy in lung diseases is promising.
After intratracheal delivery of BM-MSC treatment, a delay in recovery was noticed (Fig. 3). Tidal volume improved slightly at day 4 (from 40 to 60 mL before treatment to 60 to 80 mL during day 4 to 14) and dramatically increased after day 14 (from 80 mL at day 14 to 145 mL at day 26). This phenomenon is similar to the finding of the only phase 1 clinical trial for BPD: respiratory severity score only mildly improved during the first 7 d after transplantation of MSCs and differed much more obviously at day 1416.
MSCs do not engraft and have no risk of rejection or malignancy15,18. Previous studies demonstrated that delivery of conditioned medium of MSCs is also therapeutic15,19. It is compatible with our finding that no maternal cell was found in STR analysis. The effect of MSCs is likely to be paracrine27.
Delivery of MSCs is safe. There is no difference in acute infusional toxicity and fever, organ system–related adverse events (cardiovascular, gastrointestinal, renal, pulmonary, neurological, hematological, infection, and malignancy), or death, in comparison to the control group in a systematic review and meta-analysis of clinical trials18. However, it is still a serious concern that a relatively large volume (20 mL) of 0.9% saline is needed to dilute the MSCs for passing and flushing the narrow working channel of a 3 mm bronchoscope in an infant with poor lung condition (tidal volume 40 to 60 mL at the day of delivery). Full ECMO support of respiratory function facilitates this procedure in our case. No desaturation happened during or soon after delivery. MSCs that are more concentrated might be used for patients without ECMO support.
This is only a case study of MSC treatment. Although the results are promising, other contributing factors should still be considered, including successful control of infection, restricting fluid intake, and lung protective ventilation strategy. Further clinical trials are needed to validate the safety and efficacy.
Conclusion
To our knowledge, this is the first human report of BM-MSC therapy in treatment of BPD superimposed with ARDS. We also suggest that MSC therapy may not only be effective in the newborn stage but also works in infants and children with BPD.
Footnotes
Ethical Approval: This study was approved by our institutional review board as a compassionate use (No. C1050901).
Statement of Human and Animal Rights: The study was performed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2005.
Statement of Informed Consent: Written informed consent was obtained from the parent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Partially funded by Sharing Society in ECMO Foundation.
References
- 1. Stoll BJ, Hansen N, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sánchez PJ, O’Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID, et al. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics. 2010;126(3):443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baker CD, Abman SH. Impaired pulmonary vascular development in bronchopulmonary dysplasia. Neonatology. 2015;107(4):344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker CD, Alvira CM. Disrupted lung development and bronchopulmonary dysplasia: opportunities for lung repair and regeneration. Curr Opin Pediatr. 2014;26(3):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kwon HW, Kim HS, An HS, Kwon BS, Kim GB, Shin SH, Kim EK, Bae EJ, Noh CI, Choi JH. Long-term outcomes of pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Neonatology. 2016;110(3):181–189. [DOI] [PubMed] [Google Scholar]
- 5. Thebaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med. 2007;175(10):978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mailaparambil B, Krueger M, Heizmann U, Schlegel K, Heinze J, Heinzmann A. Genetic and epidemiological risk factors in the development of bronchopulmonary dysplasia. Dis Markers. 2010;29(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gien J, Kinsella JP. Pathogenesis and treatment of bronchopulmonary dysplasia. Curr Opin Pediatr 2011;23(3):305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 9. Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion. 2014;54(5):1418–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22(7):1338–1345. [DOI] [PubMed] [Google Scholar]
- 12. Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol. 2012;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collins JJ, Thebaud B. Lung mesenchymal stromal cells in development and disease: to serve and protect? Antioxid Redox Signal. 2014;21(13):1849–1862. [DOI] [PubMed] [Google Scholar]
- 14. Antunes MA, Laffey JG, Pelosi P, Rocco PR. Mesenchymal stem cell trials for pulmonary diseases. J Cell Biochem. 2014;115(6):1023–1032. [DOI] [PubMed] [Google Scholar]
- 15. Mobius MA, Thebaud B. Cell therapy for bronchopulmonary dysplasia: promises and perils. Paediatr Respir Rev. 2016;20:33–41. [DOI] [PubMed] [Google Scholar]
- 16. Chang YS, Ahn SY, Yoo HS, Sung SI, Choi SJ, Oh WI, Park WS. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr. 2014;164(5):966–972 e6. [DOI] [PubMed] [Google Scholar]
- 17. Chang YS, Choi SJ, Ahn SY, Sung DK, Sung SI, Yoo HS, Oh WI, Park WS. Timing of umbilical cord blood derived mesenchymal stem cells transplantation determines therapeutic efficacy in the neonatal hyperoxic lung injury. PLoS One. 2013;8(1):e52419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ; Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7(10):e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hansmann G, Fernandez-Gonzalez A, Aslam M, Vitali SH, Martin T, Mitsialis SA, Kourembanas S. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ. 2012;2(2):170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luan Y, Ding W, Ju ZY, Zhang ZH, Zhang X, Kong F. Bone marrow-derived mesenchymal stem cells protect against lung injury in a mouse model of bronchopulmonary dysplasia. Mol Med Rep. 2015;11(3):19451950. [DOI] [PubMed] [Google Scholar]
- 21. van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, Rey-Parra GJ, Galipeau J, Haromy A, Eaton F, Chen M, Hashimoto K, Abley D, Korbutt G, Archer SL, Thébaud B. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180(11):1131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang H, Fang J, Su H, Yang M, Lai W, Mai Y, Wu Y. Bone marrow mesenchymal stem cells attenuate lung inflammation of hyperoxic newborn rats. Pediatr Transplant. 2012;16(6):589–598. [DOI] [PubMed] [Google Scholar]
- 23. Zhang X, Wang H, Shi Y, Peng W, Zhang S, Zhang W, Xu J, Mei Y, Feng Z. Role of bone marrow-derived mesenchymal stem cells in the prevention of hyperoxia-induced lung injury in newborn mice. Cell Biol Int. 2012;36(6):589–594. [DOI] [PubMed] [Google Scholar]
- 24. Horie S, Masterson C, Devaney J, Laffey JG. Stem cell therapy for acute respiratory distress syndrome: a promising future? Curr Opin Crit Care. 2016;22(1):14–20. [DOI] [PubMed] [Google Scholar]
- 25. Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, Rogers AJ, Levitt J, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3(1):24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simonson OE, Mougiakakos D, Heldring N, Bassi G, Johansson HJ, Dalén M, Jitschin R, Rodin S, Corbascio M, El Andaloussi S, Wiklander OP, Nordin JZ, Skog J, Romain C, Koestler T, Hellgren-Johansson L, Schiller P, Joachimsson PO, Hägglund H, Mattsson M, Lehtiö J, Faridani OR, et al. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Transl Med. 2015;4(10):1199–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fung ME, Thebaud B. Stem cell-based therapy for neonatal lung disease: it is in the juice. Pediatr Res. 2014;75(1–1):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]