Abstract
Purpose:
To report mortality of patients who were eligible for enrollment in the Collaborative Ocular Melanoma Study (COMS) clinical trials of medium-sized choroidal melanoma or large-sized choroidal melanoma but chose to defer treatment or receive no melanoma treatment.
Design:
Prospective nonrandomized multicenter cohort study as an adjunct to COMS randomized clinical trials.
Methods:
Patient follow-up procedures included examinations, correspondence, telephone contacts, and National Death Index searches. Primary outcome was patient death measured by all-cause mortality. Secondary outcomes were melanoma treatment and melanoma metastasis.
Results:
Of 77 patients eligible for COMS clinical trials who chose to defer or receive no melanoma treatment, 61 were appropriate candidates and 45 (74%) enrolled in the natural history study (NHS). In all, 42 patients (42 eyes) had medium melanoma, and the median follow-up was 5.3 years (range, 4–10.7 years). In all, 22 patients (52%) had subsequent melanoma treatment, and 20 (48%) had no melanoma treatment. For the 42 patients, Kaplan–Meier estimate of 5-year mortality was approximately 30% [95% confidence interval (CI), 18%–47%]. For COMS medium melanoma trial, 5-year mortality was 18% (95% CI, 16%–20%), not statistically significantly different from the NHS patients. After adjusting for differences in age and longest basal diameter, the 5-year risk of death for NHS patients versus COMS trial patients was 1.54 (95% CI, 0.93–2.56). Three patients had large melanoma. Melanoma metastasis was confirmed or suspected in 8 (42%) of 19 deaths.
Conclusion:
Greater mortality and higher risk of death for NHS patients are probative but not conclusive evidence of a beneficial, life-extending effect of medium melanoma treatment.
Keywords: Choroidal melanoma, COMS, melanoma, mortality, uveal melanoma
Reprinted from the American Journal of Ophthalmology, Vol 136, Straatsma BR, Diener-West M, Caldwell R, Engstrom Jr RE, For The Collaborative Ocular Melanoma Study Group, Mortality after deferral of treatment or no treatment for choroidal melanoma, Pages 47-54, Copyright (2003) with permission from Elsevier.
For more than a century, choroidal melanoma has been recognized as a primary intraocular cancer. Reports by Fuchs and others[1] in the 19th century established enucleation as the accepted treatment. In 1939, Weve[2] reported destruction of the melanoma with diathermy. In the second half of the 20th century, therapy of choroidal melanoma by globe-conserving treatments including radiation therapy, photocoagulation, local excision, and transpupillary thermotherapy were widely reported.[2,3,4,5,6,7,8,9,10,11,12,13]
Throughout this period of more than 100 years, diagnosis of choroidal melanoma was associated with treatment. Only case reports or small series were considered cases of choroidal melanoma in which treatment was deferred or declined.[14,15]
When the Collaborative Ocular Melanoma Study (COMS) commenced in 1985, the planning group identified a scarcity of information regarding the natural history of choroidal melanoma. However, a clinical trial of medium-sized or large choroidal melanoma in which some patients received no treatment was judged to be manifestly unethical. Nonetheless, the planners recognized that some patients, confronted with the diagnosis of cancer, elect to defer or decline definitive treatment. From the outset of COMS, information was assembled, as a natural history study (NHS), on patients who were eligible for COMS trials but chose to defer treatment or receive no treatment. This article reports the mortality rate among patients who agreed to follow-up in the NHS.
Materials and Methods
The NHS is a prospective, nonrandomized multicenter cohort study conducted as an adjunct to COMS randomized trials of iodine-125 brachytherapy versus enucleation for medium-sized choroidal melanoma and pre-enucleation radiation versus enucleation for large-sized choroidal melanoma.
COMS design and methods have been published.[16,17,18,19,20,21,22,23] Aspects relevant to the evaluation and follow-up of COMS-eligible patients who deferred treatment or had no treatment for choroidal melanoma are noted hereafter.
Patients were evaluated at 43 COMS clinical centers in the United States and Canada. All patients for whom the diagnosis of choroidal melanoma was confirmed clinically by a COMS-certified ophthalmologist during the enrollment period from November 1986 to July 31, 1998 were reported to COMS Coordinating Center (Baltimore, MD, USA). Diagnosis of choroidal melanoma was based on a brief medical and ocular history in addition to a comprehensive ophthalmic examination that addressed eligibility for COMS clinical trials and provided baseline descriptive information. Procedures included best-corrected visual acuity, description of melanoma size and location, A-scan and B-scan echograms, fundus photography, and fluorescein angiography. An oncologist or an internist elicited a detailed medical history, performed a cancer-oriented physical examination, and evaluated laboratory studies, chest radiograph, and, where appropriate, imaging or biopsy of the liver or other organs to rule out metastasis or other primary cancer.
To be eligible for COMS randomized medium choroidal melanoma (iodine-125 brachytherapy) clinical trial, the patient had to have a choroidal melanoma measuring from 2.5 to 10.0 mm in apical height and no more than 16.0 mm in longest basal diameter. (From November 1986 until November 1990, the apical height limits were from 3.1 to 8.0 mm.) Peripapillary tumors (with a border 2.0 mm or less from the optic disk) were eligible only when the tumor was within a 90° angle with the apex at the optic disk center and when the enrolling ophthalmologist was confident that a radioactive episcleral plaque could be placed to provide therapy to the entire tumor base and an appropriate margin.
As general eligibility requirements, patients had a primary choroidal melanoma in one eye only; were 21 years of age or older; had no other primary tumor and no history of cancer other than noninvasive nonmelanotic skin cancer or carcinoma in situ of the uterine cervix; had no coexisting disease that threatened survival for 5 years or longer; were judged by the examining oncologist or internist to be free of metastatic melanoma; had best-corrected visual acuity in the fellow eye of 20/200 or better; had the ability to give informed consent; and had no condition that would prevent return for posttreatment follow-up examinations. Previous treatment for choroidal or ciliary body melanoma in either eye, treatment for any condition secondary to the tumor, or fine needle aspiration biopsy of the melanoma rendered a patient ineligible for the clinical trial. Patients with extrascleral tumor extension of 2.0 mm or greater thickness, diffuse, ring, or multifocal tumors were ineligible.
To be eligible for COMS randomized large choroidal melanoma (pre-enucleation radiation) clinical trial, the patient had to have a choroidal melanoma 2.0 mm or greater in apical height and greater than 16.0 mm in longest basal diameter or greater than 10.0 mm in apical height regardless of the basal dimensions. (From November 1986 to November 1990, tumors 8.0–10.0 mm in apical height also were eligible for this clinical trial.) Throughout enrollment, patients with peripapillary choroidal melanoma (with a border 2.0 mm or less from the optic disk) greater than 8.0 mm in apical height were eligible.
General eligibility requirements were the same as the requirements for COMS medium choroidal melanoma clinical trial. To be eligible for the NHS, patients had to fulfill all requirements for enrollment in the medium choroidal melanoma or large choroidal melanoma clinical trial but (1) did not enroll (reported to COMS Coordinating Center) and (2) chose to receive no treatment for choroidal melanoma. Patients who declined treatment but subsequently received melanoma treatment within 45 days of COMS eligibility were excluded from the NHS.
The NHS protocol and patient consent forms were approved by the Human Subject Protection Committee at UCLA School of Medicine and by the Institutional Review Board of participating institutions. COMS Coordinating Center notified the NHS Coordinating Center (Los Angeles, CA, USA) of the study, identifying numbers and name codes of eligible patients who declined enrollment in a COMS trial and refused initial treatment. The NHS Coordinating Center communicated with COMS clinical centers, which solicited patient participation in the NHS. Patients who enrolled in the NHS signed an approved consent form that permitted periodic follow-up. Patients were identified only by study numbers and name codes assigned at the local clinical center. Follow-up procedures included examinations, correspondence, telephone contacts, and, when appropriate, searches of the National Death Index database. Information was collected on dates of subsequent treatment for choroidal melanoma as well as diagnosis and treatment for metastatic disease. For a patient who died, hospital and laboratory reports and histopathologic materials, when available, were reviewed by COMS Mortality Coding Committee to determine the metastatic status of the patient at the time of death.
The characteristics of patients enrolled and not enrolled in the NHS were compared. Similarly, the characteristics of NHS patients with medium tumors and patients enrolled in COMS medium tumor trial were also compared. Chi-square statistic was used to compare distributions of categorical variables. Survival estimates and associated 95% confidence intervals (CIs) were calculated using Kaplan–Meier method.[24] Cox proportional hazards modeling[25] was performed to investigate the association between survival and treatment and baseline demographic and tumor characteristics. Treatment for choroidal melanoma was handled as a time-dependent covariate.
Results
A total of 77 patients were reported to COMS Coordinating Center as eligible for a COMS clinical trial but did not enroll and chose to receive no melanoma treatment. Of these, three patients were lost to follow-up shortly after COMS eligibility. Three patients received melanoma treatment within 45 days of eligibility and 10 were unsuitable (because of patient mental status, patient ineligibility, or inappropriate reporting) for enrollment in NHS; these patients were considered ineligible for NHS. In all, 45 (74%) of 61 remaining patients enrolled in the NHS and agreed to periodic follow-up. There was no statistically significant difference between NHS patients and nonenrolled patients with respect to demographic or tumor characteristics.
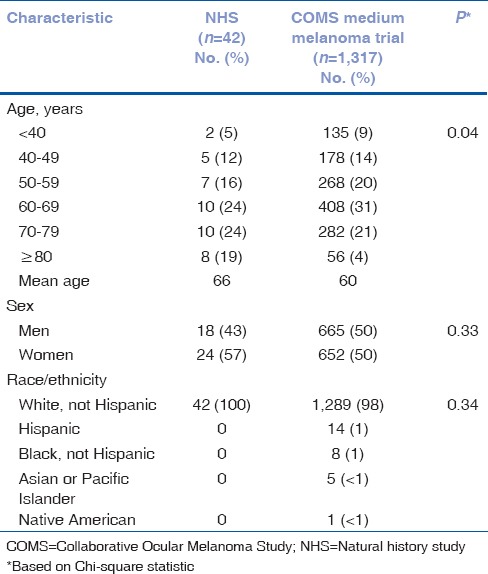
A total of 42 of the NHS patients had medium-sized melanoma. Demographic characteristics of these 42 NHS patients and the 1,317 patients enrolled in COMS medium melanoma trial are displayed in Table 1. The NHS patients were significantly older than COMS medium tumor trial patients but did not differ by sex or race.
Table 1.
NHS medium melanoma patients and COMS medium melanoma trial patients: demographic characteristics
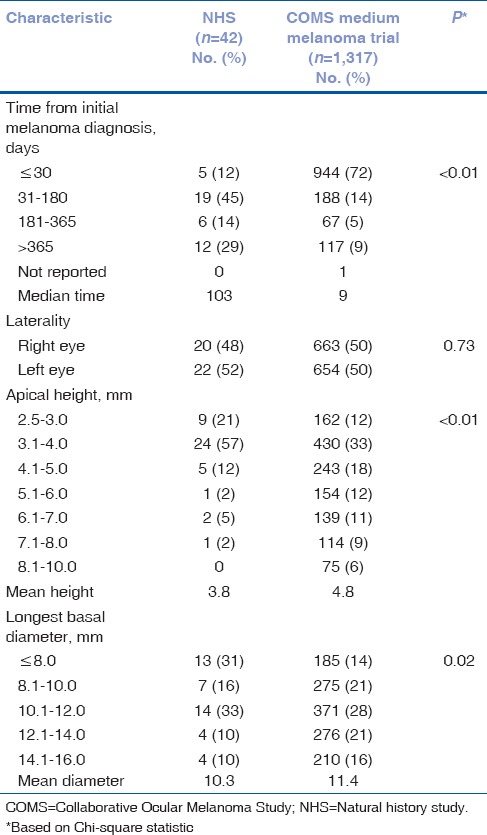
Distributions of time from initial diagnosis of choroidal melanoma to COMS evaluation and of tumor characteristics are provided in Table 2. The NHS patients had a significantly longer time from diagnosis to COMS evaluation; 12% of patients had received the diagnosis within the past month when compared with 72% of COMS medium melanoma trial patients. There was no difference by laterality of the eye with the tumor, but NHS patients had significantly smaller tumors, in both apical height and longest basal diameter. Both tumor height and diameter were, on average, 1 mm less for NHS patients.
Table 2.
NHS medium melanoma patients and COMS medium melanoma trial patients: tumor characteristics
For the 42 NHS patients with medium choroidal melanoma, the median follow-up was 5.3 years (range 4–10.7 years). In all, 22 (52%) of the 42 patients who declined treatment initially had subsequent melanoma treatment (eye-conserving radiation therapy or enucleation) at a mean ± standard deviation (SD) of 1.4 ± 1.4 years (median, 0.9 years; range, 0.2–5.1 years) after declining enrollment and were followed up for a mean ± SD of 5.2 ± 2.2 years (median, 5.0 years; range, 1.7–10.0 years). Fourteen of the 22 patients deferred treatment for more than 6 months. Of all 42 patients, Kaplan–Meier estimates of the proportion receiving treatment were 43%, 52%, and 56% by 2, 4, and 5 years, respectively. Twenty (48%) of the 42 patients had no melanoma treatment and were followed up for a mean ± SD of 5.4 ± 3.4 years (median, 5.1 years; range, 0.1–10.7 years).
Based on time since COMS eligibility, vital status was known at 5 and 10 years for 32 and 20 of the 42 NHS medium melanoma patients, respectively; Kaplan–Meier estimate of 5-year mortality was approximately 30% (95% CI, 18%–47%; Fig. 1). A total of 16 medium melanoma patients had died: 7 (44%) of the 16 patients had deferred melanoma treatment and 9 (56%) had no melanoma treatment. After accounting for treatment as a time-dependent covariate, there was no clinically or statistically significant difference in mortality between groups defined by deferred treatment versus no treatment (risk ratio = 1.28; 95% CI, 0.50–3.31). Similarly, none of the baseline characteristics, other than age of the patient, was significantly correlated with decreased survival.
Figure 1.
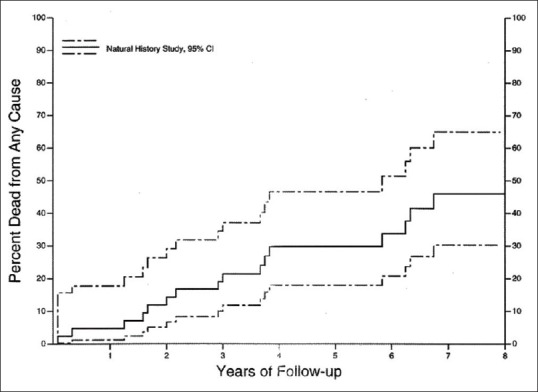
Natural history study. Cumulative proportion of patients with medium choroidal melanoma who died by time after determination of eligibility for Collaborative Ocular Melanoma Study medium melanoma trial; enrolled natural history study patients (n = 42) with 95% confidence interval (CI)
A comparison of overall time to death for NHS medium melanoma patients versus COMS patients in the medium tumor trial is shown in Fig. 2. The unadjusted risk ratio for death was 1.79 (95% CI, 1.08–2.97). After adjusting for baseline age and longest basal diameter, the adjusted risk ratio was 1.54 (95% CI, 0.93–2.56).
Figure 2.
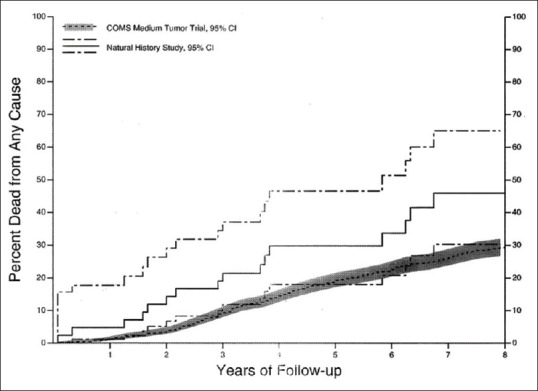
Natural history study and Collaborative Ocular Melanoma Study (COMS) medium melanoma trial. Cumulative proportion of patients with medium choroidal melanoma who died by time after determination of eligibility for COMS medium melanoma trial; enrolled COMS medium tumor trial patients (n = 1,317) and natural history study patients (n = 42) with 95% CI
Three NHS patients (three white men, age 71, 78, and 86 years) had large melanoma. Two patients had melanoma treatment (enucleation) 48 and 73 days after COMS eligibility and were observed until death at 1.5 and 4 years, respectively. Patient 3 had no melanoma treatment and was followed up until death at 12.1 years. Because only three NHS patients had large melanoma, findings were not compared with the demographic and tumor characteristics for the 1,003 patients enrolled in COMS large melanoma trial.[19,20,21]
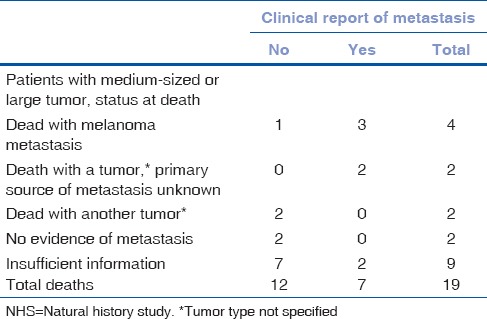
All 19 deaths (16 patients with medium melanoma and 3 patients with large melanoma) were reviewed and classified by COMS Mortality Coding Committee[23] as shown in Table 3; 4 deaths with histopathologic confirmation of melanoma metastasis, 2 had a malignant tumor (source of metastasis uncertain), 2 had another tumor, 2 had no evidence of metastasis, and 9 had insufficient information to support presence or absence of metastasis. Melanoma metastasis was confirmed histopathologically or suspected in 6 (32%) of the 19 deaths (5 medium and 1 large); clinical diagnosis of melanoma metastasis was established by imaging or other tests in an additional 2 medium tumor cases, totaling 8 (42%) cases of metastasis in 19 deaths.
Table 3.
Status at death coded by the Mortality Coding Committee and by premortum clinical report of metastasis: 19 NHS patients
Discussion
The NHS presents long-term follow-up of patients who were eligible for COMS clinical trials for medium-sized or large choroidal melanoma but who deferred treatment or had no melanoma treatment. Three strengths of the study are evident. First, the NHS study was conducted in conjunction with COMS; hence, collection and recording of patient demographic data, ophthalmic examination information, and general medical examination results were performed in a uniform and consistent manner.
Second, COMS ophthalmologists, photographers, echographers, and other personnel completed certification and recertification procedures. Periodic monitoring by extramural site visitors provided assurance of quality control throughout the years of the study. Diagnosis of melanoma was made in every case by a COMS-certified ophthalmologist on the basis of all available information. Consequently, patient data were reliable, and accuracy of melanoma diagnosis was enhanced.
Third, the high degree of accuracy of melanoma diagnosis by COMS ophthalmologists was verified by histopathology of eyes enucleated in the COMS clinical trials. COMS Pathology Review Committee received histopathologic sections for evaluation from all eyes assigned to enucleation. Clinical diagnosis of choroid melanoma was confirmed by histopathology in 1,527 (99.7%) of 1,532 eyes assigned to enucleation.[26] Because of randomization, 99.7% accuracy of diagnosis may be attributed to patients who underwent brachytherapy and reasonably assumed for patients who were eligible for COMS clinical trials but chose to defer or decline melanoma treatment.
With these strengths, use of COMS eligibility as a criterion for participation in NHS provided an optimal foundation for assessment of choroidal melanoma natural history. Furthermore, use of COMS eligibility as a criterion for NHS eligibility enabled reasonable comparison between outcomes of untreated and treated choroidal melanoma patients.
Limitations of NHS relate to the relatively small number of patients and the fact that this group was “self-selected” and not randomized. In addition, patients who elected to defer or decline treatment for melanoma often elected to have minimal or no contact with physicians. Consequently, information from the subset of 45 patients who enrolled in NHS and agreed to follow-up is incomplete. Even with these limitations, natural history information on 45 patients is greater than any previous report on survival following delayed treatment or no treatment of choroidal melanoma.[14,15]
Clinically relevant inferences of this study relate to mortality of NHS patients and the comparison of this outcome with mortality of COMS clinical trial participants. In NHS, the 42 patients with medium melanoma who deferred treatment or received no treatment had a 5-year mortality of 30% (95% CI, 18%–47%). Although there was substantial variation in survival among these 42 patients, this estimate of mortality may be a useful guide in counseling patients who have medium melanoma and additional life-threatening medical conditions.
In a comparison of outcome, the 1,317 patients in COMS medium melanoma trial who received protocol treatment had a 5-year mortality of 18% (95% CI, 16%–20%),[23] at the lower boundary of 95% CI for NHS patients and not a statistically significant difference.
Overlapping 95% CIs may have stemmed from the discrepant sample sizes. The relatively small sample of 42 NHS patients resulted in reduced power to detect a statistically significant difference. Post hoc estimate of power to detect a statistically significant difference between 18% and 30% (a difference of 12%) was only 44%, but there was an 80% power to detect a difference of 20% or more.
With regard to other factors that may have affected the comparison of outcome, NHS patients were older than patients in COMS medium melanoma trial, had a longer interval between melanoma diagnosis and eligibility, and had somewhat smaller sized melanomas at the time of COMS eligibility. After adjusting for baseline age and the longest basal diameter of the melanoma, the adjusted risk ratio for death in NHS versus COMS patients was 1.54 (95% CI, 0.93–2.56). With this analysis, age and tumor size may have influenced 5-year mortality, but the net effect and its direction cannot be estimated.
Taken together, the greater mortality and higher relative risk of death for NHS patients are evidence that tends to prove the beneficial life-extending effect of medium melanoma treatment. Although the evidence is probative and not conclusive, it forms a reasonable basis for recommending ocular treatment of medium melanoma. Moreover, the similarity of 5-year outcome emphasizes the need for long-term follow-up (5 years or more) to determine the efficacy of melanoma treatment by any means.
In NHS, the component of all-cause mortality related to metastatic melanoma is difficult to establish, because information from records at time of death or autopsy is not complete for all patients. However, COMS Mortality Coding Committee reviewed all available information for the 16 and 3 deaths in NHS medium and large tumor patients, respectively [Table 3]. Melanoma metastasis was confirmed or suspected histologically in 6 deaths (5 medium and 1 large) and diagnosed premortum in 2 additional medium tumor cases, totaling 8 (42.1%) cases of metastasis in 19 deaths. Fewer than half of the decedents in each group were judged to have melanoma metastasis at time of death, but over half of the patients with premortum melanoma metastasis were judged to have died from confirmed or suspected melanoma metastasis.
Mortality coding, similar 5-year mortality for treated and not treated medium melanoma, and calculations of tumor doubling time suggest that metastatic dissemination may occur at an early stage of choroidal melanoma.[27,28,29,30,31] If metastasis has occurred, ocular treatment will control the ocular tumor but have no effect on the growth of metastatic tumors or long-term survival.
To increase long-term survival, ocular treatment of choroidal melanoma must be combined with measures to detect and treat micrometastasis. Illustrating an approach to detection of micrometastasis, Eide et al. recently reported a prospective study of uveal melanoma patients in whom bone marrow aspirates, studied with the immunomagnetic rosette method, detected tumor cells in 47 (28.7%) of 161 patients. (Eide N, Overgaard R, Jebsen P, et al. Evaluation of 9.2.27 melanoma antibody in micrometastases in malignant uveal melanoma and a comparison of the test results in the eye tumor, the bone marrow and peripheral blood. Presented at the Jules Gonin Club meeting, September 1, 2002.) Treatment of occult micrometastasis is burdened by the limited understanding of fundamental factors that initiate and control the metastatic process in uveal melanoma.[32] However, promising research in this area is directed to (1) genomic study of aberrations in chromosomes 3, 6, and 8[33,34,35] and investigation of tumor suppresser genes; (2) proteomic research to discover products of tumor suppresser genes and evaluate the metalloproteinases;[36] and (3) immunotherapeutic strategies to enhance melanoma-specific cytotoxic response.[37,38]
In summary, 42 of 45 NHS patients had medium melanoma with an all-cause 5-year mortality of 30% (95% CI, 18%–47%). In comparison, 1,317 patients in the COMS medium melanoma trial had an all-cause 5-year mortality of 18% (95% CI, 16%–20%), a difference that was not statistically significant. After adjustment for patient age and tumor size, the relative risk of death for NHS medium melanoma patients was approximately 50% higher than COMS medium melanoma trial patients (risk ratio = 1.54; 95% CI, 0.93–2.56). Greater mortality and higher 5-year death rate of NHS patients are probative although not conclusive evidence of a life-extending benefit of treatment for medium choroidal melanoma. Among 19 deaths in NHS (16 medium, 3 large), melanoma metastasis was confirmed or suspected in 8 (42%) of 19 deaths. To increase long-term survival, there is a need to combine ocular treatment of choroidal melanoma with measures to detect and treat micrometastatic disease.
Financial support and sponsorship
This study was supported by the National Eye Institute and the National Cancer Institute, National Institutes of Health, Bethesda, Maryland, and Research to Prevent Blindness, New York, New York.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Fuchs E. Das Sarkom des Uvealtractus. Wien: Wilhelm Braumüller; 1882. After Reese AB. Tumors of the Eye. New York: Harper and Row; 1963. [Google Scholar]
- 2.Weve H. On diathermy in ophthalmic practice. Trans Ophthalmol Soc U K. 1939;59:43–80. [Google Scholar]
- 3.Stallard HB. Radiotherapy for malignant melanoma of the choroid. Br J Ophthalmol. 1966;50:147–55. doi: 10.1136/bjo.50.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lommatzsch PK. Treatment of choroidal melanomas with 106Ru/106Rh beta-ray applicators. Surv Ophthalmol. 1974;19:85–100. [PubMed] [Google Scholar]
- 5.Davidorf FH, Makley TA, Lang JR. Radiotherapy of malignant melanoma of the choroid. Trans Am Acad Ophthalmol Otolaryngol. 1976;81:849–61. [PubMed] [Google Scholar]
- 6.Packer S, Rotman M. Radiotherapy of choroidal melanoma with iodine-125. Ophthalmology. 1980;87:582–90. doi: 10.1016/s0161-6420(80)35194-4. [DOI] [PubMed] [Google Scholar]
- 7.Gragoudas ES, Goitein M, Verhey L, Munzenreider J, Urie M, Suit H, et al. Proton beam irradiation of uveal melanoma (results of a 5 1/2 year study) Arch Ophthalmol. 1982;100:928–34. doi: 10.1001/archopht.1982.01030030936007. [DOI] [PubMed] [Google Scholar]
- 8.Char DH, Saunders W, Castro JR, Quivey JM, Irvine AR, Stone RD, et al. Helium ion therapy for choroidal melanoma. Ophthalmology. 1983;90:1219–25. doi: 10.1016/s0161-6420(83)34405-5. [DOI] [PubMed] [Google Scholar]
- 9.Shields JA, Augsburger JJ, Brady LW, Day JL. Cobalt plaque therapy of posterior uveal melanoma. Ophthalmology. 1982;89:1201–7. doi: 10.1016/s0161-6420(82)34658-8. [DOI] [PubMed] [Google Scholar]
- 10.Meyer-Schwickerath G. The preservation of vision by treatment of intraocular tumors with light coagulation. Arch Ophthalmol. 1961;66:36–44. doi: 10.1001/archopht.1961.00960010460004. [DOI] [PubMed] [Google Scholar]
- 11.Boniuk M, Girard LJ. Malignant melanoma of the choroid treated with photocoagulation, transscleral diathermy and implanted radon seeds. Am J Ophthalmol. 1965;59:212–6. [PubMed] [Google Scholar]
- 12.Foulds WS, Damato BE. Alternatives to enucleation in the management of choroidal melanoma. Aust NZ J Ophthalmol. 1986;14:19–27. doi: 10.1111/j.1442-9071.1986.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 13.Shields CL, Shields JA, Perez N, Singh AD, Cater J. Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases. Ophthalmology. 2002;109:225–34. doi: 10.1016/s0161-6420(01)00902-2. [DOI] [PubMed] [Google Scholar]
- 14.Saraux H, Belosse B. Histoire naturelle des mélanomesmalins de la choroïde. J Fr Ophtalmol. 1988;11:169–75. [PubMed] [Google Scholar]
- 15.Augsburger JJ, Vrabec TR. Impact of delayed treatment in growing posterior uveal melanomas. Arch Ophthalmol. 1993;111:1382–16. doi: 10.1001/archopht.1993.01090100090033. [DOI] [PubMed] [Google Scholar]
- 16.Collaborative Ocular Melanoma Study Group. Design and methods of a clinical trial for a rare condition (the Collaborative Ocular Melanoma Study. COMS report no. 3) Control Clin Trials. 1993;14:362–91. doi: 10.1016/0197-2456(93)90052-f. [DOI] [PubMed] [Google Scholar]
- 17.Springfield, VA: National Technical Information Service; 1995. Collaborative Ocular Melanoma Study Group. COMS Manual of Procedures, PB95–179693. [Google Scholar]
- 18.Straatsma BR, Fine SL, Earle JD, Hawkins BS, Diener-West M, McLaughlin JA for the collaborative ocular melanoma study research group. Enucleation versus plaque irradiation for choroidal melanoma. Ophthalmology. 1988;95:1000–4. doi: 10.1016/s0161-6420(88)33086-1. [DOI] [PubMed] [Google Scholar]
- 19.Collaborative Ocular Melanoma Study Group. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma, I (characteristics of patients enrolled and not enrolled. COMS report no. 9) Am J Ophthalmol. 1998;125:767–78. doi: 10.1016/s0002-9394(98)00038-5. [DOI] [PubMed] [Google Scholar]
- 20.Collaborative Ocular Melanoma Study Group. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma, II (initial mortality findings. COMS report no. 10) Am J Ophthalmol. 1998;125:779–96. doi: 10.1016/s0002-9394(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 21.Collaborative Ocular Melanoma Study Group. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma, III (local complications and observations following enucleation: COMS report no. 11) Am J Ophthalmol. 1998;126:362–72. doi: 10.1016/s0002-9394(98)00091-9. [DOI] [PubMed] [Google Scholar]
- 22.Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, II (characteristics of patients enrolled and not enrolled. COMS report no. 17) Arch Ophthalmol. 2001;119:951–65. doi: 10.1001/archopht.119.7.951. [DOI] [PubMed] [Google Scholar]
- 23.Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III (initial mortality findings. COMS report no. 18) Arch Ophthalmol. 2001;119:969–82. doi: 10.1001/archopht.119.7.969. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 25.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–202. [Google Scholar]
- 26.Collaborative Ocular Melanoma Study Group. Histopathologic characteristics of uveal melanoma in eyes enucleated from the collaborative ocular melanoma study. COMS report no. 6. Am J Ophthalmol. 1998;125:745–66. doi: 10.1016/s0002-9394(98)00040-3. [DOI] [PubMed] [Google Scholar]
- 27.Laquis SJ, Freeman JM, Fleming JC, Wilson MW, Haik BG. A rapid growing choroidal melanoma. Am J Ophthalmol. 2002;133:580. doi: 10.1016/s0002-9394(01)01399-x. [DOI] [PubMed] [Google Scholar]
- 28.Friberg TR, Fineberg E, McQuaig S. Extremely rapid growth of a primary choroidal melanoma. Arch Ophthalmol. 1983;101:1375–7. doi: 10.1001/archopht.1983.01040020377006. [DOI] [PubMed] [Google Scholar]
- 29.Manschot WA. The natural history of uveal melanomas and its therapeutic consequences. Doc Ophthalmol. 1980;50:83–99. doi: 10.1007/BF00161154. [DOI] [PubMed] [Google Scholar]
- 30.Manschot WA, Lee WR, van Strik R. Uveal melanoma: Updated considerations on current management modalities. Int Ophthalmol. 1995–96;19:203–9. doi: 10.1007/BF00132688. [DOI] [PubMed] [Google Scholar]
- 31.Eskelin S, Pyrhönen S, Summanen P, Hahka-Kemppinen M, Kivelä T. Tumor doubling time in metastatic malignant melanoma of the uvea. Ophthalmology. 2000;107:1443–9. doi: 10.1016/s0161-6420(00)00182-2. [DOI] [PubMed] [Google Scholar]
- 32.Rennie I. Melanomas, metastases, and survival.([Editorial]) Br J Ophthalmol. 1993;77:685–7. doi: 10.1136/bjo.77.11.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sisley K, Rennie IG, Cottam DW, Potter AM, Potter CW, Rees RC. Cytogenetic findings in six posterior uveal melanomas. Genes Chrom Cancer. 1990;2:205–9. doi: 10.1002/gcc.2870020307. [DOI] [PubMed] [Google Scholar]
- 34.Prescher G, Bornfeld N, Becher R. Nonrandom chromosomal abnormalities in primary uveal melanoma. J Natl Cancer Inst. 1990;82:1765–9. doi: 10.1093/jnci/82.22.1765. [DOI] [PubMed] [Google Scholar]
- 35.Sisley K, Cottam DC, Rennie IG, Parsons MA, Potter AM, Potter CW, et al. Non-random abnormalities of chromosomes 3, 6 and 8 associated with posterior uveal melanoma. Genes Chrom Cancer. 1992;5:197–200. doi: 10.1002/gcc.2870050304. [DOI] [PubMed] [Google Scholar]
- 36.Cottam DW, Rennie IG, Woods K, Parsons MA, Bunning RAD, Rees RC. Gelatinolytic metalloproteinase secretion patterns in ocular melanoma. Ophthalmol Vis Sci. 1992;33:1923–27. [PubMed] [Google Scholar]
- 37.Frost PH, Butterfield LH, Dissette VB, Economou JS, Bonavida B. Immunosensitization of melanoma tumor cells to non-MHC Fas-mediated killing by MART-1-specific CTL cultures. J Immunol. 2001;166:3564–73. doi: 10.4049/jimmunol.166.5.3564. [DOI] [PubMed] [Google Scholar]
- 38.Ribas A, Butterfield LH, Hu B, Dissette VB, Meng WS, Koh A, et al. Immune deviation and Fas-mediated deletion limit antitumor activity after multiple dendritic cell vaccinations in mice. Cancer Res. 2000;60:2218–24. [PubMed] [Google Scholar]


