Abstract
Hypertensive retinopathy is seen frequently in patients with systemic hypertension and is usually asymptomatic. An acute rise in blood pressure may lead to exudative changes in the form of macular edema, hemorrhages, and serous macular detachment that can lead to visual decline. The authors report prompt resolution of exudative changes in a case of hypertensive retinopathy following intravitreal bevacizumab.
Keywords: Bevacizumab, disc edema, hypertensive retinopathy, macular edema, serous macular detachment
Hypertension affects a large part of world's population and is a single most important modifiable risk factor for stroke.[1] Retinal vasculature is well known to have similarities to cerebral and coronary vasculature and is frequently affected in patients with hypertension leading to hypertensive retinopathy (HTR). The pathogenesis of HTR includes vasoconstrictive phase in which the retinal arterioles constrict (leading to generalized or localized arteriolar attenuation), sclerotic phase in which vessel wall becomes thick (leading to arteriovenous crossing changes and silver/copper wiring), and exudative phase whereby lipids and blood exudate from retinal vessels due to breakage of inner blood retinal barrier.[2] In addition to HTR, hypertension may also lead to hypertensive optic neuropathy and choroidopathy.
Although most cases with HTR retain good vision, exudation phase may lead to macular edema, serous macular detachment, and hemorrhages leading to decreased visual acuity. Bevacizumab, a widely used anti-vascular endothelial-derived growth factor (VEGF) is known to reduce retinal vascular permeability and stabilize blood retina barrier.[3] Use of Bevacizumab has been reported sparsely in the setting of HTR.[4,5,6] We describe the use of intravitreal Bevacizumab in the setting of severe visual loss due to HTR.
Case Report
A 23-year-old female, known case of chronic kidney disease due to IgA nephropathy (diagnosed 6 months back) and resultant malignant hypertension, presented with complaints of sudden painless decrease in vision in her right eye since last 1 day. The blood pressure at presentation to us was 210/140 mm Hg while mean arterial blood pressure (MABP) was 136 mm Hg. The patient had been started on systemic corticosteroids 1 month back. The best-corrected visual acuity (BCVA) was 3/60 in the right eye and 6/18 in the left eye. Detailed fundus evaluation revealed severe HTR[7] leading to disc edema, macular edema, hard exudates, and retinal hemorrhages in both the eyes [Fig. 1a and b]. Swept source optical coherence tomography (SS-OCT) showed presence of disc edema, intraretinal and subretinal fluid, and intraretinal hyperreflective lesions corresponding to hard exudates in both the eyes [Fig. 1c and d]. In view of poor visual acuity, the patient was advised intravitreal injection of Bevacizumab (1.25 mg in 0.05 mL) in the right eye after informed consent. At 1 month after intravitreal injection, BCVA in the right and left eyes improved to 6/18 and 6/12, respectively. The blood pressure was 160/96 mm Hg while MABP was 101.3 mm Hg. Fundus showed marked improvement in disc edema, macular edema, and retinal hemorrhages in both the eyes [Fig. 2a and b]. SS-OCT showed complete resolution of intra- and subretinal fluid at the macula [Fig. 2c and d] along with disruption of outer retinal layers in the papillomacular bundle. The patient was advised regular follow-up and good control of blood pressures.
Figure 1.
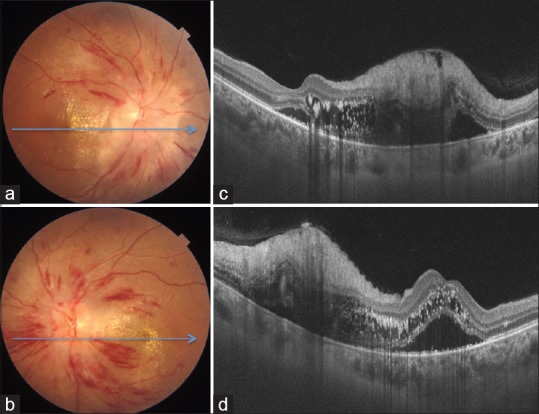
Color fundus photographs of right (a) and left eye (b) show features of severe hypertensive retinopathy (disc edema, retinal hemorrhages, macular edema, and hard exudates). Optical coherence tomography confirms optic disc and macular edema along with neurosensory detachment at the macula in both eyes (c and d)
Figure 2.
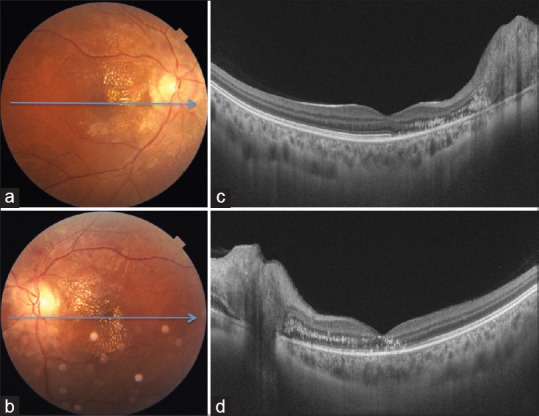
Color fundus photographs of right (a) and left eye (b) 1 month after intravitreal bevacizumab in the right eye show resolution of hemorrhages and marked decrease in optic disc and macular edema. Optical coherence tomography shows complete resolution of intra- and subretinal fluid in both eyes (c and d)
Discussion
The retina and optic disc are frequently affected in patients with malignant hypertension. Typically, the visual loss is mild. In some cases, the visual decline may be marked because of disc edema, macular edema, and/or neurosensory detachment at the macula. This is because of breakdown of inner blood retinal barrier leading to exudation of blood and lipids.[7] Bevacizumab being an anti-VEGF agent has anti-permeability action and has been shown to be effective in HTR.[4,5,6] In view of markedly decreased visual acuity in the right eye, the patient was treated with intravitreal injection of Bevacizumab after informed consent. This led to prompt and dramatic resolution of retinal changes in the right eye along with improvement in visual acuity. Surprisingly, a dramatic reduction in edema and hemorrhages was noted in the left eye as well. Intravitreal Bevacizumab is known to have some effect on the fellow eye as it is absorbed systemically as well.[8]
Although changes in HTR especially those resulting from exudation tend to regress with appropriate control of blood pressure, no randomized controlled studies have evaluated whether hypertensive control will reverse HTR. While macular edema and intraretinal hemorrhage resolve in 6–12 weeks once underlying cause is removed, hard exudates may take even longer to disappear. This may leave behind permanent damage to retinal layers limiting final visual outcome. This along with severe visual decline prompted us to treat the patient with intravitreal Bevacizumab that resulted in quick anatomical and visual recovery.
Conclusion
To conclude, Bevacizumab maybe an effective treatment modality in patients with visual loss due to HTR.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289:2363–9. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- 2.Tso MO, Jampol LM. Pathophysiology of hypertensive retinopathy. Ophthalmology. 1982;89:1132–45. doi: 10.1016/s0161-6420(82)34663-1. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson PN, Schachat A. P.A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Grafe's Arch Clin Exp Ophthalmol. 2010;248:915–30. doi: 10.1007/s00417-010-1315-z. [DOI] [PubMed] [Google Scholar]
- 4.Kim EY, Lew HM, Song JH. Effect of intravitreal bevacizumab (Avastin(®) therapy in malignant hypertensive retinopathy: A report of two cases. J Ocul Pharmacol Ther. 2012;28:318–22. doi: 10.1089/jop.2011.0113. [DOI] [PubMed] [Google Scholar]
- 5.Salman AG. Intravitreal bevacizumab in persistent retinopathy secondary to malignant hypertension. Saudi J Ophthalmol. 2013;27:25–9. doi: 10.1016/j.sjopt.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Halafi AM. Tremendous result of bevacizumab in malignant hypertensive retinopathy. Oman J Ophthalmol. 2015;8:61. doi: 10.4103/0974-620X.149872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong TY, Mitchell P. Hypertensive retinopathy. N Engl J Med. 2004;351:2310–7. doi: 10.1056/NEJMra032865. [DOI] [PubMed] [Google Scholar]
- 8.Hanhart J, Tiosano L, Averbukh E, Banin E, Hemo I, Chowers I. Fellow eye effect of unilateral intravitreal bevacizumab injection in eyes with diabetic macular edema. Eye. 2014;28:646. doi: 10.1038/eye.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]


