Figure 4.
LRRTM2 Binding to HS Is Required for its Role in Presynaptic Differentiation
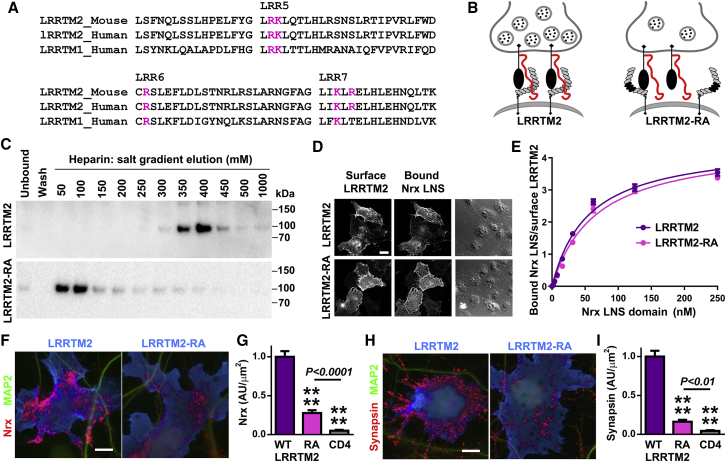
(A) The proposed HS binding region in LRRTM is shown, with residues in pink mutated to Ala in LRRTM2-RA.
(B) Schematic model. LRRTM2 binds to protein and HS domains of Nrx; reduction of HS binding by the LRRTM2-RA mutation may result in maintenance of some LRRTM2-Nrx complexes by the protein domain interactions and disruption of other complexes.
(C) LRRTM2 ectodomain binds heparin, and binding is reduced by the RA mutation. Elution at higher salt indicates stronger binding.
(D and E) LRRTM2 RA mutation does not affect binding to the Nrx LNS domain. Binding of Nrx LNS domain was measured on COS7 cells expressing LRRTM2 or LRRTM2-RA. Scatchard analysis of this cell-based binding revealed no significant difference (p > 0.1).
(F–I) LRRTM2-RA is deficient at inducing presynaptic differentiation. Clustering of native Nrx (F and G) and synapsin (H and I) in contacting axons induced by LRRTM2 on COS7 cells was impaired by the RA mutation. Measures are integrated intensity of Nrx or synapsin puncta per transfected COS7 cell-axon contact area lacking MAP2 dendrite contact. ∗∗∗∗p < 0.0001 by Kruskal-Wallis and Dunn’s tests compared to wild-type LRRTM2 (G, n = 43–48 and I, n = 31–33) from 3-4 independent experiments. Surface levels of LRRTM2 and LRRTM2-RA did not vary in these co-culture assays (Figure S4).
Error bars represent SEM. Scale bars: 20 μm. See also Figure S4.