Abstract
Rationale: Excessive neutrophilic airway inflammation is the central feature of bronchiectasis, but little is known about neutrophils in bronchiectasis.
Objectives: To assess blood neutrophil phenotype in patients with bronchiectasis while stable and during exacerbations.
Methods: In the clinically stable arm of this study, there were eight healthy volunteers, eight patients with mild bronchiectasis, and eight patients with severe bronchiectasis. In addition, six patients with severe bronchiectasis were compared with six patients with community-acquired pneumonia at the start and end of an exacerbation. We assessed neutrophils for spontaneous apoptosis, cell surface marker expression, degranulation, reactive oxygen species generation, phagocytosis, and killing of Pseudomonas aeruginosa (PAO1). In addition, blood neutrophil function was compared with airway neutrophil function in bronchiectasis.
Measurements and Main Results: In stable bronchiectasis, compared with healthy volunteers, blood neutrophils had significantly prolonged viability, delayed apoptosis, increased CD62L shedding, upregulated CD11b expression, increased myeloperoxidase release, and impaired neutrophil phagocytosis and killing of PAO1. Bronchiectatic airway neutrophils had significantly lower bacterial phagocytosis and killing than their matched autologous blood neutrophils. Both blood and airway neutrophil phagocytosis and killing were impaired at the start of an exacerbation and improved following antibiotic treatment. In pneumonia, there was a significant improvement in phagocytosis and killing after treatment with antibiotics. During infections, there was no difference in phagocytosis, but there was significantly increased bacterial killing at the start and end of infection in pneumonia compared with bronchiectasis exacerbations.
Conclusions: In bronchiectasis stable state, peripheral blood neutrophils are reprogrammed and have prolonged survival. This impairs their functional ability of bacterial phagocytosis and killing, thereby perpetuating the vicious circle in bronchiectasis.
Keywords: bronchiectasis, neutrophils, inflammation, infection
At a Glance Commentary
Scientific Knowledge on the Subject
Bronchiectasis is a predominantly neutrophilic airway disease, and despite this, there is persistent airway infection, leading to chronic inflammation. There are no studies that have assessed neutrophil phenotype and how this alters neutrophil function in bronchiectasis.
What This Study Adds to the Field
This is the first study that demonstrates that there is a distinct subset of neutrophils that are reprogrammed in bronchiectasis. Bronchiectasis neutrophils have prolonged survival and delayed apoptosis. In patients with stable bronchiectasis, blood neutrophils are preactivated and reprogrammed, leading to an inability to kill PAO1 as effectively as healthy control subjects or patients that have recovered from community-acquired pneumonia, leading to persistent infection and inflammation. Blood neutrophil phagocytosis and killing of PAO1 are further impaired at the start of exacerbations needing intravenous antibiotic therapy that improved following treatment.
Excessive neutrophilic airway inflammation is the central feature of bronchiectasis. This paradoxically both promotes bacterial colonization and perpetuates damage to the airways, creating a vicious circle of bacterial colonization and inflammation (1). The acute inflammatory response is a protective mechanism that is evolved to eliminate invading organisms and should ideally be self-limiting and lead to complete resolution (2–4). However, there is failure of resolution of inflammation in bronchiectasis, leading to irreversible damage and dilatation of the bronchial airways with loss of mucociliary function. The driver for persistent neutrophilic airway inflammation in bronchiectasis is unknown.
The traditional definition of the neutrophil, is that it is an effector of acute inflammation, and is short lived, with lifespan being measured in hours, and having a predefined set of functions (5). However, this concept has been developed primarily by studying blood neutrophils (before extravasation to the tissues). It is subject to challenge as evidence emerges that neutrophil lifespan is considerably prolonged in response to stimuli, such as granulocyte-macrophage colony–stimulating factor, tumor necrosis factor-α, IL, interferons, and bacterial products (6–10) with consequent functional significance. During inflammation, leukocytes leave the circulation; enter new environments; and are exposed to multiple factors, such as cytokines, endogenous growth factors, and bacterial products. Indeed, the activity of infiltrating neutrophils has been closely linked to disease evolution in a variety of clinical conditions (11, 12). Thereby these cells contribute not only to acute inflammatory reactions, but also to the evolution of a variety of chronic inflammatory diseases.
Despite being a predominantly neutrophilic disease, bronchiectasis results in patients having recurrent chest infections. In more severe bronchiectasis there are high levels of sputum myeloperoxidase, free elastase activity, and chemoattractants, such as IL-8, leukotriene B4, and C5a (7, 8), contributing to the persistent neutrophilic airway inflammation. Watt and colleagues (10) have shown there is prolonged airway neutrophil survival in bronchiectasis with less airway neutrophil apoptosis. Is this consequent to the altered inflammatory milieu in bronchiectasis? How does this impact on neutrophil function? There have been no studies to date assessing and phenotyping blood neutrophil function in bronchiectasis.
The aim of this study was to assess the phenotype of blood neutrophils in patients with bronchiectasis while stable and during exacerbations.
Methods
Three study groups were included. In study 1, all patients were in a stable state (no infective exacerbation of bronchiectasis for at least 4 wk before giving blood for this study) and included eight patients with mild bronchiectasis, eight with severe bronchiectasis, and eight healthy volunteers. Patients with bronchiectasis that took part in the study were not on any inhaled corticosteroids, long-term antibiotics, or immunosuppressive therapy. All participants had 60 ml of blood taken and underwent a bronchoscopy with a bronchoalveolar lavage targeted to the most affected segment with bronchiectasis.
In study 2, six patients with severe bronchiectasis were recruited during an exacerbation requiring intravenous antibiotics and patients were reviewed at Day 1 (start of antibiotic therapy) and Day 14 (end of antibiotic therapy). All patients had 60 ml of blood taken and sputum induced on Day 1 and Day 14. Patients in study 2 were not the same patients as in study 1.
In study 3, six patients with community-acquired pneumonia were recruited and reviewed at Day 1 (hospital admission) and Day 5 (when stable following antibiotic therapy). All patients had blood taken at Day 1 and Day 5. Patients required oral or intravenous antibiotics. We were not able to induce sputum in these patients.
Bronchiectasis Severity
The severity of bronchiectasis was based on the Bronchiectasis Severity Index (13). The Bronchiectasis Severity Index is a risk stratification tool for morbidity and mortality in bronchiectasis. The minimum score is 0 and the maximum score is 26. A score between 0 and 4 indicates mild disease; between 5 and 8 indicates moderate disease, and a score of greater than or equal to 9 indicates severe disease. The Bronchiectasis Severity Index was calculated in all patients with bronchiectasis taking part in the study.
Consent
Lothian Research Ethics Committee gave consent for the study (10/S1402/33).
Isolation of Blood and Airway Neutrophils
Freshly drawn blood was collected into 3.8% sodium citrate and granulocytes were subsequently isolated by dextran sedimentation and discontinuous Percoll gradient, as described (14). Sputum and bronchoalveolar lavage was washed, treated with sputolysin, and airway neutrophils isolated (described in detail in the online supplement). Anti-CD16 antibodies (Abcam) were used to identify neutrophils by flow cytometry.
Blood Neutrophil Apoptosis at 20 Hours
Freshly isolated blood neutrophils (at least 97% purity) was suspended at 10 × 106 cells/ml in Iscove's modified Dulbecco's media supplemented with 10% autologous serum and penicillin/streptomycin (×1). The assay was done on a 96-well flat-bottom plate. Following 20-hour incubation, apoptosis was examined by flow cytometry (Annexin-V [Roche] and propidium iodide [Sigma]; BD FACS Calibur) and confirmed by cytocentrifuge and Diff-Quick staining (Gamidor) (15).
Neutrophil Activation
Freshly isolated neutrophils were activated with N-formyl-methionyl-leucyl-phenylalanine, or vehicle control. Anti-CD62L (BD Pharmigen) and CD11b (Alexa Fluor) antibodies were added and the samples analyzed by flow cytometry (BD FACS Calibur) (15).
Neutrophil Degranulation and Myeloperoxidase Measurement
Neutrophils were activated with cytochalsin B (Sigma Aldrich) and N-formyl-methionyl-leucyl-phenylalanine (Sigma Aldrich). Supernatants were collected and stored at −80°C. We measured myeloperoxidase activity (Sigma Aldrich) (16) with a chromogenic substrate assay.
Reactive Oxygen Species Generation
Freshly isolated blood neutrophils were loaded with dihydrorhodhamine (Sigma Aldrich). N-formyl-methionyl-leucyl-phenylalanine was added and superoxide release was measured by flow cytometry (BD FACS Calibur) (15).
Neutrophil Phagocytosis and Killing of Green Fluorescent Protein–labeled Pseudomonas
GFP (green fluorescent protein) bacteria in the log phage were resuspended in phosphate-buffered saline at a final concentration of 108 bacteria/ml. Following this, bacteria were opsonized with autologous serum for 1 hour at 37°C. The neutrophils (10 × 106 per condition) were then cocultured with opsonized GFP PAO1 (1 × 108 bacteria per condition) and phagocytosis was measured by flow cytometry (BD FACS Calibur) (17). Cells were lysed with saponin (Sigma Aldrich) before serial dilutions were plated out on Pseudomonas isolation agar. Colony counts were performed after 24 hours incubation of plates at 37°C in 5% CO2 (see online supplement).
Bronchoscopy
All patients in study 1 underwent a bronchoscopy. Patients were sedated with midazolam ± fentanyl. Bronchoalveolar lavage was done and samples obtained for neutrophil studies.
Inducing Sputum
Sputum was induced as per standard protocol using 3% saline (18).
Statistical Analysis
Flow cytometry analysis was performed using FlowJo v10.0.4 (Tree Star). Results are presented as mean ± SEM. Paired and unpaired Student’s t tests were used to compare two groups, where applicable. Data were analyzed by one-way ANOVA with a Bonferroni multiple comparison post hoc test (GraphPad Prism version 6; GraphPad Software), when three groups were involved; significance was accepted with P < 0.05.
Results
Study Design
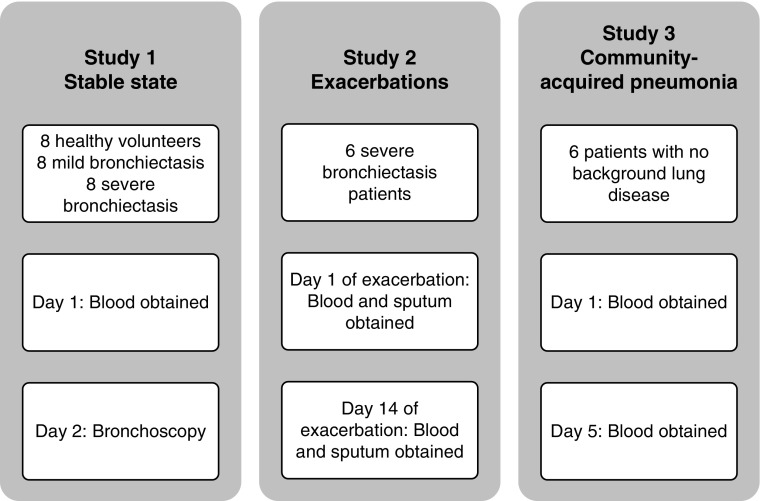
The groups examined, and the time points for collection of biologic samples, in the three elements of this study are shown in Figure 1.
Figure 1.
The three groups recruited for this study. Study 1: all study participants (healthy volunteers, volunteers with mild bronchiectasis, and volunteers with severe bronchiectasis [no exacerbations for at least 4 wk before taking part in the study]) had blood obtained and underwent bronchoscopy. Study 2: six patients with severe bronchiectasis had blood and sputum obtained at the start (Day 1) and end of antibiotic therapy (Day 14). Study 3: six patients with pneumonia had blood obtained at the start (Day 1) and end of antibiotic therapy (Day 5).
Baseline demographics of the participants for study 1 are shown in Table 1 (see Tables E1 and E2 in the online supplement for baseline demographics of study 2 and study 3 participants). In comparison with the patients with mild bronchiectasis, the severe group had significantly higher white cell counts, neutrophil counts, and C-reactive protein in blood; lower FEV1 % predicted, FVC % predicted, and transfer factor for the lung for carbon monoxide % predicted; higher rates of bacterial lung colonization; and a greater number of exacerbations and hospital admissions in the preceding year.
Table 1.
Baseline Demographics of Study Participants
| Parameters | Healthy Volunteers |
Mild |
Severe |
P Value |
|---|---|---|---|---|
| (n = 8) | (n = 8) | (n = 8) | ||
| Age | 52 (6.8) | 55 (3.8) | 64 (2.2) | 0.09 |
| Sex, % female | 80 | 40 | 22 | <0.0001 |
| Cause of bronchiectasis, n (%) | ||||
| Idiopathic | — | 8 (100) | 8 (100) | — |
| Postinfective | — | 0 | 0 | — |
| Total WCC, ×109/L | 5.9 (0.5) | 6 (0.5) | 9.3 (1.1) | 0.005 |
| Neutrophils, ×109/L | 3.5 (0.3) | 3.3 (0.3) | 6.6 (1.1) | 0.001 |
| Eosinophils, ×109/L | 0.2 (0.06) | 0.2 (0.04) | 0.2 (0.06) | 0.7 |
| Monocytes, ×109/L | 0.5 (0.05) | 0.5 (0.03) | 0.7 (1) | 0.7 |
| ESR, mm/h | 4.8 (1) | 6.7 (1.8) | 19.6 (6.8) | 0.1 |
| CRP, mg/L | 3.2 (1.1) | 2.8 (0.5) | 16 (7.4) | 0.04 |
| FEV1 % predicted | — | 95 (5.5) | 55 (6.5) | 0.0001 |
| FVC % predicted | — | 111 (6) | 84 (6) | 0.008 |
| DlCO % predicted | — | 94 (4.9) | 74 (7.8) | 0.002 |
| Kco % predicted | — | 106 (4.5) | 100 (7.2) | 0.4 |
| Chronic bacterial lung colonization, n (%), bacteria | — | 3 (37.5), Haemophilus influenzae (3) | 5 (62.5), Pseudomonas aeruginosa (2), Streptococcus pneumoniae (2), H. influenzae (1) | <0.0001 |
| Exacerbations in the last year | — | 0.4 (0.3) | 4.2 (0.9) | 0.002 |
| Hospital admissions in the last year | — | 0 | 0.7 (0.2) | 0.008 |
Blood Neutrophil: Spontaneous Apoptosis
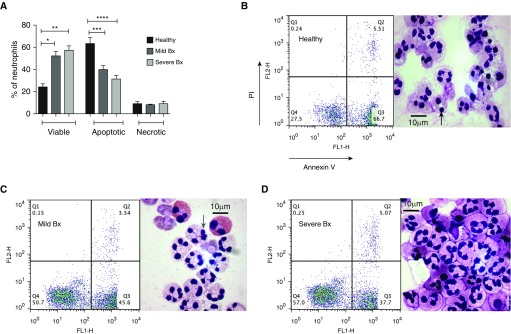
Healthy blood neutrophils undergo well-described spontaneous apoptosis in culture, a process that can be altered by exposure to survival factors and by activation (19). Blood neutrophils from patients with both mild and severe bronchiectasis, in stable state, had greater viability after 20 hours in culture than healthy control subjects (P = 0.002, P = 0.005, respectively) (Figure 2). A correspondingly lower number of apoptotic neutrophils was observed (P = 0.0003 for mild and P < 0.0001 for severe), with no difference in the percentage of necrotic cells. The proportion of apoptotic neutrophils from patients with severe bronchiectasis was reduced to approximately 50% of that of control subjects. There was no difference in the proportions of viable (P = 0.4) or apoptotic cells (P = 0.2) when comparing cells from patients with mild and severe bronchiectasis. The effect was confirmed by light microscopic counting and assessment of total cell numbers demonstrated that these percentages were not skewed by an artifact of cell loss (data not shown).
Figure 2.
Blood neutrophils from patients with mild and severe bronchiectasis survived longer and underwent less apoptosis when compared with healthy volunteers. Blood neutrophils from patients with mild and severe bronchiectasis in a stable state and healthy volunteers were cultured for 20 hours and cell viability (AnnV−/PI−), apoptosis (AnnV+/PI−), and necrosis (AnnV+/PI+) were assessed by flow cytometry. (A) n = 8 in each group; percentage of viable, apoptotic, and necrotic neutrophils in each group; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. One-way ANOVA with Bonferroni correction for multiple comparisons used for all three groups compared; comparing mild and severe bronchiectasis with healthy control subjects in viable, apoptotic, and necrotic neutrophils. (B–D) Representative flow cytometry plots and cytocentrifuge preparations at 20 hours. AnnV = annexin V; Bx = bronchiectasis; FL1-H and FL2-H = fluorescence indices–height; PI = propidium iodide. Black arrow = dark pyknotic apoptotic nucleus; gray arrow = multilobulated viable nucleus.
To determine whether disease-related factors in the autologous serum used in these cultures differentially impacted the rate of neutrophil apoptosis, serum swap studies were conducted (see Figures E1 and E2). First, spontaneous apoptosis was assessed in neutrophils from patients with bronchiectasis in the presence of serum from healthy volunteers. After 20 hours, there was a significant reduction in the percentage of viable neutrophils when cultured in healthy control serum, as opposed to autologous serum (43.4% ± 1.5 vs. 50.7% ± 3, respectively; P = 0.02) and a significant increase in the percentage of apoptotic neutrophils (43% ± 1.8 vs. 38% ± 2, respectively; P = 0.02). There was no change in the percentage of necrotic neutrophils. In contrast, the nature of the serum used had no effect on the apoptosis of neutrophils from healthy volunteers.
These data raise that possibility that a factor in healthy serum, deficient in serum from patients with bronchiectasis, can promote neutrophil apoptosis. However, the effect of serum was minimal compared with the overall disease-specific differences between the rates of spontaneous apoptosis of neutrophils, suggesting some level of reprogramming or activation of circulating blood neutrophils in bronchiectasis.
Blood Neutrophil: Cell Surface Expression of CD62L and CD11b
To evaluate the baseline activation status of unstimulated blood neutrophils from stable patients with bronchiectasis, surface expression of CD62L and CD11b were contrasted to heathy control subjects (Figure 3). Surface expression of CD62L on neutrophils from patients with mild and severe bronchiectasis was significantly lower than on healthy control neutrophils (P = 0.04 and P = 0.02, respectively). There was no difference between mild and severe bronchiectasis. Compatible with this, CD11b levels were significantly higher on neutrophils from patients with severe bronchiectasis compared with healthy control subjects (P = 0.01) and mild bronchiectasis (P = 0.01), but in this case neutrophils from patients with mild bronchiectasis were unchanged compared with control subjects.
Figure 3.
Blood neutrophils from stable patients with bronchiectasis are more activated in the unstimulated state than neutrophils from healthy volunteers. (A and B) Blood neutrophils were isolated, and cell surface markers CD62L and CD11b were measured by flow cytometry, when unstimulated. n = 8 in each group. (C) Blood neutrophils were isolated and myeloperoxidase measured by chromogenic assay, when unstimulated. n = 8 in each group. (D) Blood neutrophils were isolated and loaded with dihydrorhodamine, and spontaneous reactive oxygen species generation was measured by flow cytometry. n = 8 in each group. One-way ANOVA with Bonferroni correction for multiple comparisons was used for all four experiments. (A, C, and D) Comparison of severe and mild bronchiectasis with healthy volunteers (used as control). (B) Comparison of healthy volunteers and mild bronchiectasis with severe bronchiectasis (used as control). Pooled data are expressed as mean ± SEM. Bx = bronchiectasis; MPO = myeloperoxidase; ROS = reactive oxygen species.
Blood Neutrophil: Degranulation and Reactive Oxygen Species Generation
To further evaluate the baseline activation status of blood neutrophils from stable patients with bronchiectasis, myeloperoxidase release from granules and superoxide generation were evaluated in unstimulated cells. A significantly higher level of myeloperoxidase was detected in the supernatant of neutrophils from patients with severe bronchiectasis, after 90 minutes in culture, compared with neutrophils from patients with mild bronchiectasis (P = 0.03) and healthy control subjects (P = 0.04), with the latter two groups comparable. However, on comparison of superoxide release at baseline, there was no significant difference between neutrophils from healthy volunteers, and patients with mild and severe bronchiectasis (P = 0.3) (Figure 3).
Blood Neutrophil: Phagocytosis and Killing of Pseudomonas aeruginosa
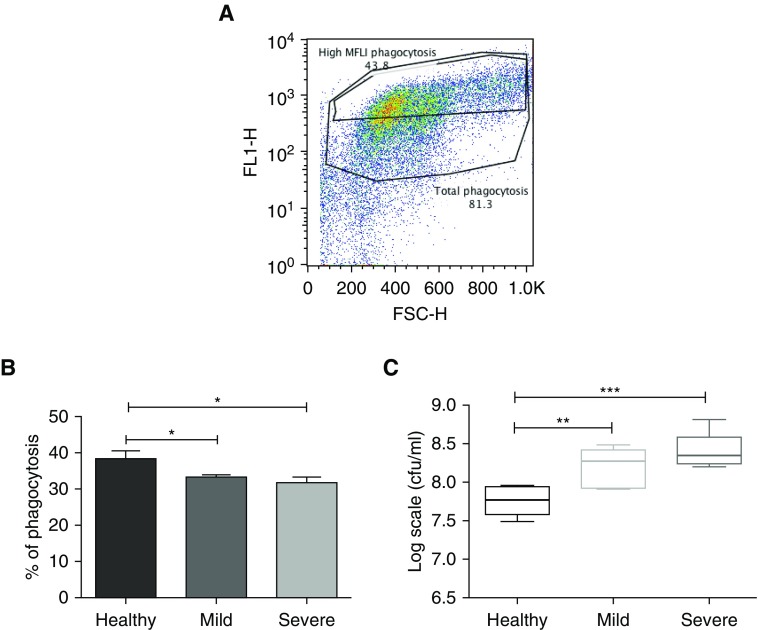
Having determined baseline differences in parameters that suggest differential activation of blood neutrophils from stable patients with bronchiectasis, their functional efficacy in uptake and killing of pathogens was evaluated using a GFP Pseudomonas aeruginosa strain PAO1 (Figure 4). Cells were spun at 300 × g for 5 minutes, supernatants discarded to remove free bacteria, and then cells lysed before plating. Phagocytosis was confirmed with z-stacking using confocal microscopy. In separate experiments phagocytosis was blocked by doing the experiments at 4°C, which demonstrated that at 4°C bacteria were still bound to cells but not internalized (data not shown).
Figure 4.
Impaired bacterial phagocytosis and killing by blood neutrophils from patients with bronchiectasis compared with in healthy control subjects. Blood neutrophils were isolated and cocultured with autologous serum-opsonized GFP (green fluorescent protein)-labeled Pseudomonas aeruginosa PAO1 (at a concentration of 108/ml) for 15 minutes. Bacterial phagocytosis was measured by flow cytometry and serial dilutions of lysed cells were plated on Pseudomonas isolation agar, with colony-forming units counted 24 hours after plating to assess killing. (A) Representative flow cytometry plot of phagocytosis, with high mean fluorescence intensity (MFLI). Gates distinguish total cells having phagocytosed GFP-labeled bacteria, with “High MFLI Phagocytosis” indicating cells that had phagocytosed the most bacteria. (B) Pooled % neutrophil phagocytosis data, showing means ± SEM of n = 8 per group for high MFLI gating. (C) Pooled bacterial killing in log scale units cfu/ml, data, showing median with interquartile range of n = 8 per group. (B and C) One-way ANOVA with Bonferroni correction for multiple comparisons used for both experiments, with P values representing the comparison of severe and mild bronchiectasis with healthy volunteers (used as control). *P < 0.05; **P < 0.01, ***P < 0.001. FL1-H = fluorescence index–height; FSC-H = forward scatter–height.
Data were analyzed by gating the overall phagocytosis first (Figure 4A). There was no difference in the overall phagocytosis between the groups. Next, we gated the neutrophils that had taken up much higher number of bacteria, as indicated by the mean fluorescence intensity (MFLI). This was done at 50% of the total phagocytosis and we called this high-MFLI phagocytosis. There was significantly higher phagocytosis (comparing only the high MFLI and not total phagocytosis) by blood neutrophils from healthy volunteers compared with those from patients with bronchiectasis, both the mild and severe groups (P = 0.04, P = 0.02, respectively). There was no difference between mild and severe groups (P = 0.4). Comparison of the subsequent neutrophil-mediated bacterial killing showed significantly lower numbers of bacteria following exposure to healthy control neutrophils when compared with neutrophils from patients with mild or severe bronchiectasis (P = 0.006, P = 0.0003, respectively). There was no difference between mild and severe groups (P = 0.1). These data demonstrate that despite increased activation, neutrophils from the blood of patients with bronchiectasis have impaired phagocytosis and killing capacity (Figure 4).
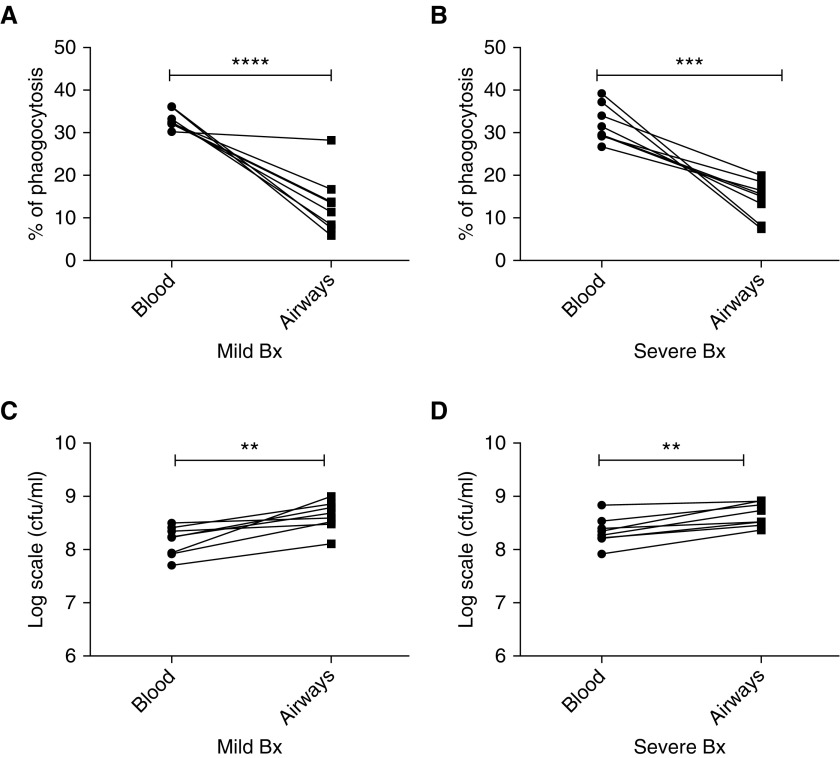
Comparison of Antibacterial Function of Blood and Airway Neutrophils from Patients with Bronchiectasis
Having established differential function of blood neutrophils isolated from patients with bronchiectasis as compared with healthy control subjects, the antibacterial function of bronchoalveolar lavage–derived airway neutrophils from these patients was then evaluated (Figure 5). Significantly lower levels of bacterial phagocytosis were observed for bronchoalveolar lavage–isolated neutrophils, when compared with donor-matched blood neutrophils, both from patients with mild and severe bronchiectasis (P < 0.0001, P = 0.0004, respectively). Comparison of the bacterial killing capacity showed a compatible significantly lower antibacterial function of bronchoalveolar lavage–derived neutrophils compared with autologous blood neutrophils from the same patient, both in patients with mild and severe bronchiectasis (P = 0.003, P = 0.001, respectively). No differences between the antimicrobial functions of neutrophils from patients with mild and severe bronchiectasis were observed. This demonstrates that bronchoalveolar lavage neutrophils have impaired function compared with blood neutrophils in bronchiectasis.
Figure 5.
Impaired antibacterial function of BAL-derived neutrophils compared with matched blood neutrophils from patients with mild and severe bronchiectasis. Neutrophils were isolated from BAL and blood from patients with mild and severe bronchiectasis, and cocultured with serum opsonized green fluorescent protein PAO1 for 15 minutes (blood neutrophils) or 60 minutes (BAL neutrophils). Bacterial phagocytosis was measured by flow cytometry, and serial dilutions of lysed cells were plated on Pseudomonas isolation agar, with colony-forming units counted to assess killing. (A) Mild and (B) severe bronchiectasis: matched neutrophil phagocytosis data in blood and BAL-derived neutrophils in the same patient; n = 8 per group. (C) Mild and (D) severe bronchiectasis: matched bacterial killing in log scale units cfu/ml, in blood and BAL-derived neutrophils in the same patient; n = 8 per group. Two-way ANOVA showed no significant difference in the antibacterial activity between mild and severe bronchiectasis; P = 0.2. **P < 0.01; ***P < 0.001; ****P < 0.0001. Bx = bronchiectasis.
Comparison of Antibacterial Function of Blood Neutrophils from Patients with Bronchiectasis in Stable and Exacerbation States
The observation that blood neutrophils from patients with bronchiectasis had a higher baseline level of activation, with impaired antibacterial function, raised the question of the extent to which this might be further modulated by disease exacerbations. Matched samples collected from the same individuals at the beginning and end of an exacerbation were analyzed and compared with unmatched stable state control subjects. There was significantly higher bacterial phagocytosis by neutrophils collected at the end of an exacerbation compared those from the beginning of exacerbation (P = 0.02). There was significantly more phagocytosis in the stable state compared with start of exacerbation (P = 0.01). There was no difference in phagocytosis between the stable state and the end of exacerbation (P = 0.9) (Figure 6).
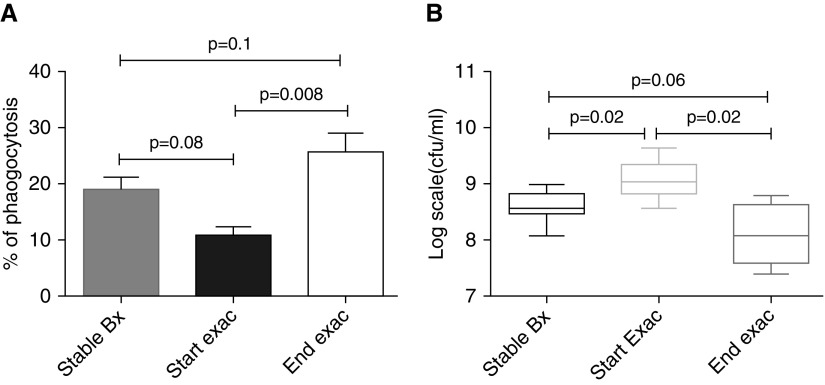
Figure 6.
Phagocytosis and killing of bacteria by blood neutrophils significantly improves after antibiotic therapy in bronchiectasis. (A and B) n = 8 in stable state and n = 6 at the start and end of exacerbation. Blood neutrophils were isolated and phagocytosis and killing of green fluorescent protein PAO1 assessed as previously described. Unpaired Student’s t tests were used for comparison of stable state with start and end of exacerbation, and paired Student’s t tests were used for comparison of start with end of exacerbation. Pooled data are presented as mean ± SEM for phagocytosis data and median (interquartile range) for bacterial killing data. Bx = bronchiectasis; exac = exacerbation.
There was significantly higher killing at the end of exacerbation compared with beginning of exacerbation (P = 0.03). There was significantly more killing in the stable state compared with start of exacerbation (P = 0.02). There was no difference in bacterial killing between the stable state and the end of exacerbation (P = 0.3). This demonstrates that blood neutrophil function is restored to levels comparable with the stable state in bronchiectasis, at the end of treatment of exacerbations.
Phagocytosis and Killing by Sputum-derived Neutrophils during Exacerbations and Comparison with Stable State
Having established that blood neutrophil function improved after treatment with antibiotics, the extent to which sputum-derived neutrophils had a similar response was assessed. There was significantly higher phagocytosis at the end of exacerbation compared with cells from the same donor taken at the beginning (P = 0.008). There was more phagocytosis in the stable state compared with start of exacerbation although this observation, between unmatched donors, failed to reach statistical significance (P = 0.08). There was no difference in phagocytosis between the stable state and the end of exacerbation (P = 0.1).
There was significantly higher bacterial killing at the end of exacerbation compared with the beginning (P = 0.02). There was more killing in the stable state compared with start of exacerbation (P = 0.02). There was no significant difference in killing between stable state and end of exacerbation (P = 0.06), demonstrating that sputum neutrophil function is restored to stable state after treatment with antibiotics in exacerbations (Figure 7).
Figure 7.
Phagocytosis and killing of bacteria by airway neutrophils significantly improves after antibiotic therapy in bronchiectasis. (A and B) n = 8 in stable state and n = 6 at the start and end of exacerbation. Airway neutrophils were isolated and phagocytosis and killing of green fluorescent protein PAO1 assessed as previously described. Unpaired Student’s t tests were used for comparison of stable state with start and end of exacerbation, and paired Student’s t tests were used for comparison of start with end of exacerbation. Pooled data are presented as mean ± SEM for phagocytosis data and median (interquartile range) for bacterial killing data. Bx = bronchiectasis; exac = exacerbation.
Community-acquired Pneumonia
Comparison of bronchiectasis versus pneumonia
To determine the disease specificity of the neutrophil phenotypes determined for individuals with bronchiectasis, six patients with pneumonia (with no comorbidities) who were admitted to hospital were also recruited and studied. Baseline demographics of this group are available in Table E1.
Phagocytosis and killing of GFP PAO1 by blood neutrophils
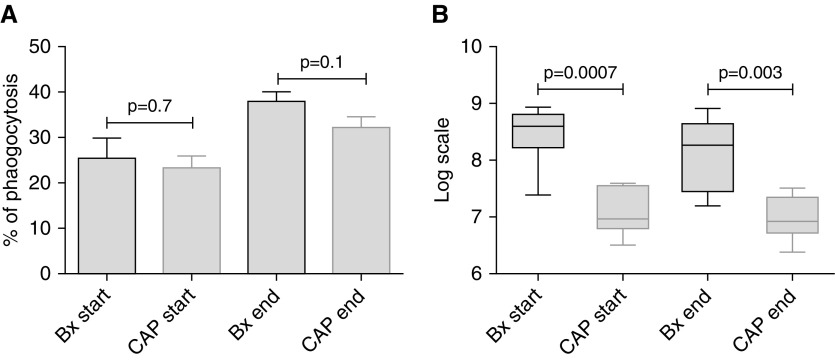
The phagocytic and antibacterial properties of matched blood neutrophils collected at the beginning and Day 5 of treatment of pneumonia, from the same patients, were compared. There was significant improvement in phagocytosis (P = 0.03) and killing (P = 0.03) at the end of treatment in patients with pneumonia.
Comparison of phagocytosis by blood neutrophils collected in community-acquired pneumonia and bronchiectasis exacerbation showed no statistically significant difference at the beginning (P = 0.7) or end of infection (P = 0.1), between the two conditions. This suggested that the phagocytosis phenotype may not be disease specific, but a more general consequence of severe infection. In contrast, there was significantly lower bacterial killing in patients with bronchiectasis both at the start (P = 0.0007) and end of exacerbation (P = 0.003) (Figure 8). These data demonstrate a bronchiectasis-specific neutrophil bacterial killing defect that may be exacerbated by impaired phagocytosis. Furthermore, at the end of bronchiectasis exacerbations treated with antibiotics, this remains highly impaired in comparison with neutrophils from healthy volunteers with a recent pneumonia.
Figure 8.
There is significantly higher bacterial killing at the start and end of pneumonia compared with bronchiectasis. (A and B) n = 6 in each group. Blood neutrophils were isolated and phagocytosis and killing of green fluorescent protein PAO1 assessed as previously described. Unpaired Student’s t tests were used for all comparisons. Pooled data are presented as mean ± SEM for phagocytosis data and median (interquartile range) for bacterial killing data. Bx = bronchiectasis; CAP = community-acquired pneumonia.
Discussion
We studied the functional phenotype of blood neutrophils in mild and severe bronchiectasis and compared it with the activity of blood neutrophils isolated from healthy volunteers. In patients with bronchiectasis, we also assessed the function of airway neutrophils and compared it with blood neutrophil function from the same patient. In addition, we studied neutrophil function during bronchiectasis exacerbations and compared it with neutrophil function in healthy patients admitted with community-acquired pneumonia. The authors chose experiments with P. aeruginosa, because this organism is associated with a worse outcome in bronchiectasis (13). Future work investigating other pathogens in bronchiectasis is warranted.
Blood neutrophils from patients with bronchiectasis (both in mild and severe groups) had prolonged survival and delayed apoptosis when compared with healthy volunteers. Further studies demonstrated that when blood neutrophils from patients with bronchiectasis were treated with serum from healthy volunteers, there was a reduction in viability and increased apoptosis in bronchiectasis neutrophils. However, the reverse was not true when treating healthy volunteers’ neutrophils with bronchiectasis serum. The overall effect of delayed apoptosis and prolonged survival of bronchiectasis neutrophils was more pronounced than the effect of serum swap. The reason for this remains to be determined, but the effect was minimal compared with the overall disease-specific differences between the rates of spontaneous apoptosis of neutrophils, suggesting some level of reprogramming or activation of circulating blood neutrophils in bronchiectasis. There was more CD62L shedding in mild and severe bronchiectasis compared with healthy volunteers. There was higher CD11b expression and myeloperoxidase release in severe bronchiectasis compared with healthy volunteers and mild bronchiectasis. There was no difference in reactive oxygen species generation in the three groups. There was significantly higher phagocytosis and bacterial killing of PAO1 in healthy volunteers compared with mild and severe bronchiectasis. Although the significantly higher proportion of females in the healthy volunteers group compared with patients with bronchiectasis could be considered a minor limitation of this study, we have no reason to believe that this had any impact on the results of the study (20). Studies have shown that older adults have impaired neutrophil migration that contributes to poorer outcomes during infection (21, 22). In this study, both in the stable state (healthy volunteers and patients with bronchiectasis) and during exacerbations (patients with pneumonia and patients with severe bronchiectasis), there was no significant difference in the age of the study groups.
Having established that blood neutrophil phagocytosis and killing is impaired irrespective of disease severity, we wanted to explore whether airway neutrophil function was impaired. We demonstrated that phagocytosis and killing of PAO1 is impaired in airway neutrophils compared with blood neutrophils in both mild and severe bronchiectasis. Although the etiology of bronchiectasis is heterogeneous, to address this as a confounding factor, for the purpose of this study we included patients with idiopathic bronchiectasis only. Despite the neutrophils being preactivated, the phagocytic and bactericidal ability of blood and airway neutrophils is impaired (more marked in the airways), which suggests that the inflammatory milieu with myeloperoxidase and proteinase excess in patients with bronchiectasis is perhaps a contributory factor for this (8), leading to impaired function. It is known that inflammatory reprogramming of neutrophils leads to increased viability (23). To the authors best knowledge airways neutrophil function in other disease, such as chronic obstructive pulmonary disease and cystic fibrosis conditions, remains limited. Studies on bronchoalveolar lavage fluid neutrophils from other chronic lung diseases is warranted in the future. We hypothesize that airway neutrophils are also reprogrammed in bronchiectasis.
In acute infective exacerbations of bronchiectasis, phagocytic and bactericidal activity of blood and airways neutrophil function improved with antibiotic therapy and was restored to levels comparable with the stable state. On comparison of bronchiectasis exacerbations and patients admitted with community-acquired pneumonia, there was no difference in blood neutrophil phagocytosis between the two groups; there was, however, significantly higher bacterial killing of PAO1 at the start and end of infection in patients with community-acquired pneumonia compared with bronchiectasis exacerbations. Indeed, even at the end of treatment with antibiotics during bronchiectasis exacerbations, although phagocytosis and killing ability of neutrophils are restored to stable state, it was still lesser than in patients recovered from community-acquired pneumonia suggesting that even at the end of exacerbation, there is failure of resolution of inflammation in bronchiectasis. This is important, because reduced functional activity of neutrophils would mean slower and lesser clearance of bacteria in bronchiectasis. Reduced phagocytosis and killing was not secondary to antipseudomonal antibodies because only 25% were positive for P. aeruginosa in the severe group and in subgroup analysis excluding P. aeruginosa, killing was still impaired (data not shown). We hypothesize that this failure of resolution could be secondary to persistence of reprogrammed neutrophils at the sites of inflammation or impaired clearance of these neutrophils.
Bronchiectasis is a predominantly neutrophilic condition, where despite excess neutrophils, patients suffer daily cough, chronic sputum production, and recurrent chest infections. The lung is continuously exposed to inhaled pathogen and it is the primary and secondary defense of the lung that maintains the microbiome of the lung. The excessive neutrophilic airway inflammation leads to damage of the bronchial wall and paradoxically promotes more airway inflammation and bacterial infection, creating a vicious circle (1, 24). During natural resolution, polymorphonuclear neutrophils are required for antimicrobial defense (3), but these cells must then apoptose and are removed from the inflammatory site by macrophages (25). Is this natural resolution impaired in bronchiectasis? There are no studies exploring the resolution mechanism in bronchiectasis or perhaps the lack of it in the literature to date.
Apoptotic neutrophil death in situ has multiple proresolution actions (26–28). Bronchiectasis neutrophils live longer and undergo delayed apoptosis compared with healthy volunteers. Given the number of neutrophils in bronchiectasis airways, late neutrophil apoptosis could have devastating consequences. Slowing neutrophil apoptosis would mean delayed removal by macrophages and delaying the process of resolution, thereby perpetuating the ongoing inflammation. Vandivier and colleagues (29) had previously demonstrated that neutrophil elastase cleaves phosphatidylserine receptor on neutrophils in cystic fibrosis and bronchiectasis, thereby impairing its clearance by macrophages. This along with the data from our study suggest that bronchiectasis neutrophils undergo an alternative method of cell death. Could this be NETosis or secondary necrosis? It is known that inefficient clearance of apoptotic cells results in secondary necrosis of cells and exacerbation of the inflammatory response (30). Recent studies have demonstrated that in cystic fibrosis, there is increased NETosis as these neutrophils engage less in apoptosis (31). This needs to be studied further in bronchiectasis.
Our studies demonstrate that bronchiectasis neutrophils are reprogrammed. There is emerging evidence of subpopulations of mature and immature neutrophils coexisting in the circulation performing immunosuppressive or proinflammatory functions (32). These have been identified as low-density neutrophils and are known to have phenotypic and functional heterogeneity (32). Low-density neutrophils have not been investigated in this study. Is there a release of chronic immature neutrophils in bronchiectasis or is there an imbalance between mature and immature neutrophils released? Antibiotics improve neutrophil function during exacerbations. However, neutrophils are preactivated even in the stable state. Do antiinflammatory or proresolution mediators have a role as a long-term treatment in bronchiectasis? This needs to be investigated further.
Overall, there was higher C-reactive protein, CD11b, and myeloperoxidase generation in severe disease compared with mild disease. For the rest of the other parameters measured there was no difference in viability, apoptosis, CD62L, reactive oxygen species generation, phagocytosis, and killing between mild and severe groups. These suggest that the effect of the disease on peripheral blood neutrophil activity and function is more pronounced than disease severity. However, we accept that a limitation of the study is the size of the study and therefore further differences may have been found with a larger sample size. We accept that exacerbation data from the same patients in study 1 would be ideal but this is a technical difficulty. It was difficult to predict exacerbations in the stable patients and hence we recruited consecutive patients who had an exacerbation and presented to hospital. The data remain a valuable addition despite this. Another limitation is we assessed patients following 14 days of antibiotic therapy in patients with bronchiectasis. A different time point was chosen for patients with pneumonia at Day 5. All patients with pneumonia were, however, clinically improved at Day 5. These time points were chosen to reflect end of antibiotic therapy.
To the authors’ best knowledge, this is the first study demonstrating that peripheral blood neutrophils are reprogrammed in bronchiectasis. This is important preliminary work and would lead to further research in the area. Key future work would be to assess the airway neutrophil function. In this study, we have shown that in the vicious circle of infection and inflammation, the dynamics of cell death and clearance are not altered, leading to overwhelming neutrophilic inflammation and thereby cytokine release. Neutrophil apoptosis and clearance and how this can be modified needs to be investigated further.
Conclusions
In the stable state, peripheral blood neutrophils are reprogrammed and persist longer in bronchiectasis. This impairs their functional ability of bacterial phagocytosis and killing, thereby perpetuating the vicious circle in bronchiectasis.
Acknowledgments
Acknowledgment
The authors thank T. Tolker Nielsen, University of Copenhagen, for providing the green fluorescent protein Pseudomonas aeruginosa; the QMRI Flow Cytometry and Cell Sorting Facility for assistance with flow cytometric analysis; and Dr. John McCafferty, Royal Infirmary of Edinburgh, for his help and guidance during the bronchoscopies.
Footnotes
Supported by Chief Scientist Office (P.B.) and by Medical Research Council Senior Non-clinical Fellowship G1002046 (D.J.D.) and grant MR/K013386/1 (A.G.R).
Author Contributions: P.B. performed the experiments, collected and interpreted the data, and wrote the manuscript. D.J.D. contributed to experimental design, interpretation of data, and writing of the manuscript. B.J.M. contributed to experimental design and writing of the manuscript. A.G.R. contributed to experimental design, interpretation of data, and writing of the manuscript. A.T.H. contributed to experimental design, interpretation of data, and writing of the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201712-2423OC on May 7, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Cole PJ. A new look at the pathogenesis, management of persistent bronchial sepsis: a 'vicious circle' hypothesis and its logical therapeutic connotations. Davies RJ. Strategies for the management of chronic bacterial sepsis. Oxford: Medicine Publishing Foundation; 1984. pp. 1–20. [Google Scholar]
- 2.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 5.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 6.Burdon PC, Martin C, Rankin SM. Migration across the sinusoidal endothelium regulates neutrophil mobilization in response to ELR + CXC chemokines. Br J Haematol. 2008;142:100–108. doi: 10.1111/j.1365-2141.2008.07018.x. [DOI] [PubMed] [Google Scholar]
- 7.Jones HR, Robb CT, Perretti M, Rossi AG. The role of neutrophils in inflammation resolution. Semin Immunol. 2016;28:137–145. doi: 10.1016/j.smim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Maskrey BH, Megson IL, Whitfield PD, Rossi AG. Mechanisms of resolution of inflammation: a focus on cardiovascular disease. Arterioscler Thromb Vasc Biol. 2011;31:1001–1006. doi: 10.1161/ATVBAHA.110.213850. [DOI] [PubMed] [Google Scholar]
- 9.Robb CT, Regan KH, Dorward DA, Rossi AG. Key mechanisms governing resolution of lung inflammation. Semin Immunopathol. 2016;38:425–448. doi: 10.1007/s00281-016-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watt AP, Brown V, Courtney J, Kelly M, Garske L, Elborn JS, et al. Neutrophil apoptosis, proinflammatory mediators and cell counts in bronchiectasis. Thorax. 2004;59:231–236. doi: 10.1136/thx.2003.008037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikami M, Llewellyn-Jones CG, Bayley D, Hill SL, Stockley RA. The chemotactic activity of sputum from patients with bronchiectasis. Am J Respir Crit Care Med. 1998;157:723–728. doi: 10.1164/ajrccm.157.3.9606120. [DOI] [PubMed] [Google Scholar]
- 12.Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2012;186:657–665. doi: 10.1164/rccm.201203-0487OC. [DOI] [PubMed] [Google Scholar]
- 13.Chalmers JD, Goeminne P, Aliberti S, McDonnell MJ, Lonni S, Davidson J, et al. The bronchiectasis severity index: an international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576–585. doi: 10.1164/rccm.201309-1575OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haslett C, Guthrie LA, Kopaniak MM, Johnston RB, Jr, Henson PM. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985;119:101–110. [PMC free article] [PubMed] [Google Scholar]
- 15.Lucas CD, Allen KC, Dorward DA, Hoodless LJ, Melrose LA, Marwick JA, et al. Flavones induce neutrophil apoptosis by down-regulation of Mcl-1 via a proteasomal-dependent pathway. FASEB J. 2013;27:1084–1094. doi: 10.1096/fj.12-218990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stockley RA, Bayley DL. Validation of assays for inflammatory mediators in sputum. Eur Respir J. 2000;15:778–781. doi: 10.1034/j.1399-3003.2000.15d24.x. [DOI] [PubMed] [Google Scholar]
- 17.Katzenmeyer KN, Bryers JD. Multivalent artificial opsonin for the recognition and phagocytosis of gram-positive bacteria by human phagocytes. Biomaterials. 2011;32:4042–4051. doi: 10.1016/j.biomaterials.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiszhar Z, Horvath I. Induced sputum analysis: step by step. Breathe. 2013;9:300–306. [Google Scholar]
- 19.Pasteur MC, Bilton D, Hill AT British Thoracic Society Non-CF Bronchiectasis Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65:577. doi: 10.1136/thx.2010.142778. [DOI] [PubMed] [Google Scholar]
- 20.Spitzer JA, Zhang P. Gender differences in neutrophil function and cytokine-induced neutrophil chemoattractant generation in endotoxic rats. Inflammation. 1996;20:485–498. doi: 10.1007/BF01487041. [DOI] [PubMed] [Google Scholar]
- 21.Sapey E, Patel JM, Greenwood HL, Walton GM, Hazeldine J, Sadhra C, et al. Pulmonary infections in the elderly lead to impaired neutrophil targeting, which is improved by simvastatin. Am J Respir Crit Care Med. 2017;196:1325–1336. doi: 10.1164/rccm.201704-0814OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drew W, Wilson DV, Sapey E. Inflammation and neutrophil immunosenescence in health and disease: targeted treatments to improve clinical outcomes in the elderly. Exp Gerontol. 2018;105:70–77. doi: 10.1016/j.exger.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Chakravarti A, Rusu D, Flamand N, Borgeat P, Poubelle PE. Reprogramming of a subpopulation of human blood neutrophils by prolonged exposure to cytokines. Lab Invest. 2009;89:1084–1099. doi: 10.1038/labinvest.2009.74. [DOI] [PubMed] [Google Scholar]
- 24.Barker AF. Bronchiectasis. N Engl J Med. 2002;346:1383–1393. doi: 10.1056/NEJMra012519. [DOI] [PubMed] [Google Scholar]
- 25.Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12:1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 26.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Köckritz-Blickwede M, Chow OA, Nizet V. Fetal calf serum contains heat-stable nucleases that degrade neutrophil extracellular traps. Blood. 2009;114:5245–5246. doi: 10.1182/blood-2009-08-240713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bratton DL, Henson PM. Neutrophil clearance: when the party is over, clean-up begins. Trends Immunol. 2011;32:350–357. doi: 10.1016/j.it.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vandivier RW, Fadok VA, Hoffmann PR, Bratton DL, Penvari C, Brown KK, et al. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest. 2002;109:661–670. doi: 10.1172/JCI13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med. 1999;160:S5–S11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- 31.Gray RD, Hardisty G, Regan KH, Smith M, Robb CT, Duffin R, et al. Delayed neutrophil apoptosis enhances NET formation in cystic fibrosis. Thorax. 2018;73:134–144. doi: 10.1136/thoraxjnl-2017-210134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scapini P, Marini O, Tecchio C, Cassatella MA. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol Rev. 2016;273:48–60. doi: 10.1111/imr.12448. [DOI] [PubMed] [Google Scholar]