Abstract
Rationale: Idiopathic pulmonary fibrosis (IPF) is a progressive, fibrotic interstitial lung disease characterized by (myo)fibroblast accumulation and collagen deposition. Resistance to Fas-induced apoptosis is thought to facilitate (myo)fibroblast persistence in fibrotic lung tissues by poorly understood mechanisms.
Objectives: To test the hypothesis that PTPN13 (protein tyrosine phosphatase-N13) is expressed by IPF lung (myo)fibroblasts, promotes their resistance to Fas-induced apoptosis, and contributes to the development of pulmonary fibrosis.
Methods: PTPN13 was localized in lung tissues from patients with IPF and control subjects by immunohistochemical staining. Inhibition of PTPN13 function in primary IPF and normal lung (myo)fibroblasts was accomplished by: 1) downregulation with TNF-α (tumor necrosis factor-α)/IFN-γ, 2) siRNA knockdown, or 3) a cell-permeable Fas/PTPN13 interaction inhibitory peptide. The role of PTPN13 in the development of pulmonary fibrosis was assessed in mice with genetic deficiency of PTP-BL, the murine ortholog of PTPN13.
Measurements and Main Results: PTPN13 was constitutively expressed by (myo)fibroblasts in the fibroblastic foci of patients with IPF. Human lung (myo)fibroblasts, which are resistant to Fas-induced apoptosis, basally expressed PTPN13 in vitro. TNF-α/IFN-γ or siRNA-mediated PTPN13 downregulation and peptide-mediated inhibition of the Fas/PTPN13 interaction in human lung (myo)fibroblasts promoted Fas-induced apoptosis. Bleomycin-challenged PTP-BL−/− mice, while developing inflammatory lung injury, exhibited reduced pulmonary fibrosis compared with wild-type mice.
Conclusions: These findings suggest that PTPN13 mediates the resistance of human lung (myo)fibroblasts to Fas-induced apoptosis and promotes pulmonary fibrosis in mice. Our results suggest that strategies aimed at interfering with PTPN13 expression or function may represent a novel strategy to reduce fibrosis in IPF.
Keywords: pulmonary fibrosis, (myo)fibroblasts, apoptosis resistance, PTPN13
At a Glance Commentary
Scientific Knowledge of the Subject
Idiopathic pulmonary fibrosis is an intractable lung disease in which apoptosis-resistant (myo)fibroblasts accumulate and persist in fibroblastic foci leading to ongoing collagen matrix deposition. The mechanisms that promote (myo)fibroblast resistance to apoptosis remain incompletely understood. Improved knowledge of these mechanisms could lead to new therapeutic approaches to control (myo)fibroblast accumulation.
What This Study Adds to the Field
Through the combined use of human lung tissues, isolated lung (myo)fibroblasts, and a mouse model of pulmonary fibrosis, our study has identified previously unknown roles for the protein tyrosine phosphatase, PTPN13, in the persistence of fibrotic lung (myo)fibroblasts and the development of pulmonary fibrosis. These findings suggest that interfering with PTPN13 expression or function may represent a novel strategy to prevent the development and progression of fibrotic lung diseases.
Idiopathic pulmonary fibrosis (IPF) is a parenchymal lung disease that results in progressive scarring of the alveolar-capillary units and eventual respiratory failure (1). With a median survival of approximately 3 years after diagnosis and limited therapies, the prognosis for patients with IPF is dismal (2, 3). Precise details regarding the initiating events remain poorly understood, although repetitive injury to the alveolar epithelium is thought to drive an aberrant repair process in which an unrestrained accumulation of fibroblasts and α-SMA (α-smooth muscle actin)-expressing myofibroblasts (which we collectively refer to as [myo]fibroblasts) within fibroblastic foci results in excessive paraseptal collagen deposition (1, 4). Recent studies have suggested that multiple cell types contribute to (myo)fibroblast accumulation in the granulation tissue that forms during alveolar injury (5–7). However, unlike normal granulation tissue resolution, where (myo)fibroblasts undergo apoptosis and are removed during repair (8), (myo)fibroblasts persist in the fibroblastic foci of patients with IPF because of a failure to undergo apoptosis (9–11). Thus, improved understanding of the mechanisms contributing to (myo)fibroblast apoptotic failure might reveal new therapeutic targets to reduce lung (myo)fibroblast accumulation and parenchymal fibrosis in patients with IPF.
The death receptor Fas is abundantly expressed on normal lung fibroblasts, and once a predefined threshold has been exceeded, its ligation induces apoptosis (12, 13). Lung (myo)fibroblasts from patients with IPF, however, are largely resistant to Fas-induced apoptosis (12, 14–16). TGF-β (transforming growth factor-β), in addition to its profibrotic activities, creates an antiinflammatory microenvironment through its ability to inhibit the production of proinflammatory cytokines, including TNF-α (tumor necrosis factor TNF-α) and IFN-γ. Our previous studies have shown that exposure of (myo)fibroblasts to the combination of TNF-α and IFN-γ (TNF-α/IFN-γ) overcomes their intrinsic resistance to Fas-induced apoptosis (12, 13). Furthermore, based on our earlier studies showing a paucity of TNF-α in the lungs of patients with IPF (17), we recently observed that intratracheal delivery of TNF-α to mice with established pulmonary fibrosis accelerates fibrotic resolution (18), whereas genetic TNF-α deficiency impedes fibrosis resolution (18). Together, these findings support the concept that a limited TNF-α-driven proinflammatory signal to the lungs beneficially promotes the resolution of pulmonary fibrosis, in part by sensitizing lung (myo)fibroblasts to Fas-induced apoptosis.
Seeking to understand the mechanisms by which TNF-α/IFN-γ promotes sensitivity of lung fibroblasts to Fas-induced apoptosis, we analyzed the transcriptomes of TNF-α/IFN-γ-exposed human primary lung (myo)fibroblasts. We found that among 603 differentially expressed transcripts, the mRNA encoding PTPN13 (protein tyrosine phosphatase-N13), an apoptosis inhibitory Fas-interacting tyrosine phosphatase (also known as FAP-1 in humans, and PTP-BL in mice) was reduced (13). Based on these findings, we hypothesized that PTPN13 mediates the resistance of lung (myo)fibroblasts to Fas-induced apoptosis and promotes pulmonary fibrosis. Herein, we address this hypothesis in fibrotic lung tissues and primary lung (myo)fibroblasts from patients with IPF, and in PTP-BL−/− mice.
Methods
Human Lung Tissues and Primary Lung (Myo)Fibroblasts
Lungs from nondiseased individuals were obtained from Tissue Transformation Technologies, as described (12). Lung samples from patients with interstitial lung diseases were obtained during diagnostic surgical lung biopsy or lung transplantation. Immunohistochemical staining for PTPN13 and α-SMA was conducted, as described (9). PTPN13 staining intensity was semiquantified by a board-certified pathologist using a three-tiered scaling system (absent = 0, intermediate = 1, strong = 2).
Primary cultures of normal lung (myo)fibroblasts from nondiseased, male and female human donors (Table 1) were isolated and cultured on plastic, as described (12). IPF (myo)fibroblasts were isolated from peripheral, lower lobe lung biopsies from patients with IPF. When cultured on rigid plastic substrata, fibroblasts increase α-SMA expression suggesting they exhibit features of (myo)fibroblasts, hence our definition. (Myo)fibroblast apoptosis and Fas DISC (death-inducing signaling complex) assembly were assessed, as described (12, 13) and in the online supplement. All human studies used deidentified samples and were approved by the National Jewish Health and University of Colorado Denver institutional review boards.
Table 1.
Patient Demographics
| Characteristics | Healthy Volunteers (n = 6) | Patients |
|
|---|---|---|---|
| IPF (n = 8) | Organizing Pneumonia (n = 6) | ||
| Male sex, n (%) | 2 (33) | 5 (63) | 5 (83) |
| Age, yr, mean ± SD | 55 ± 28 | 65 ± 12 | 63 ± 16 |
| Age distribution, n (%) | |||
| 15 to <25 | 2 (33) | 0 (0) | 0 (0) |
| 25 to <50 | 0 (0) | 0 (0) | 1 (17) |
| 50 to <80 | 4 (67) | 8 (100) | 5 (83) |
Definition of abbreviation: IPF = idiopathic pulmonary fibrosis.
Two of the patients with organizing pneumonia presented with a mixed usual interstitial pneumonia/organizing pneumonia pathology.
Animal Care and Procedures
PTP-BL+/− embryos, generously provided by Dr. Michael Grusby, Harvard University TH Chan School of Public Health, were implanted into pseudopregnant females. PTP-BL−/− male mice and control C57BL/6J male mice (Jackson Laboratory) aged 8–10 weeks were studied. Mice were intratracheally instilled with 2.5 U/kg bleomycin (APP Pharmaceuticals), as described (18). Fibrotic parameters were assessed, as described (18, 19) and in the online supplement. All mouse procedures were approved by the National Jewish Health Institutional Animal Care and Use Committee.
Statistics
Data are presented as mean ± SEM. Statistical analyses were performed using GraphPad Software or R (R: a Language and Environment for Statistical Computing. http://www.R-project.org). Statistical comparisons of means were performed using Student’s t test or by ANOVA. We used the false discovery rate procedure to control for multiple testing (18). A P value less than 0.05 was regarded as statistically significant.
Results
PTPN13 Is Localized to Fibroblastic Foci in the Lungs of Patients with IPF
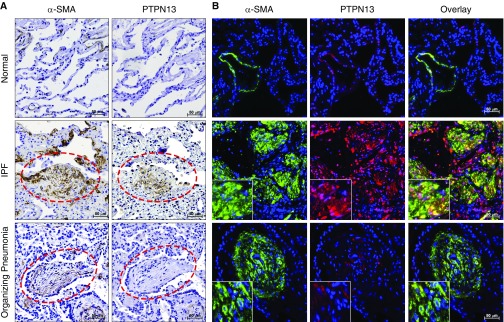
PTPN13 was first identified through its ability to inhibit Fas-induced apoptosis (20). Because primary lung fibroblasts from patients with IPF are impaired in their ability to undergo Fas-induced apoptosis (12, 16), we first determined if PTPN13 might be relevant to (myo)fibroblast resistance to Fas-induced apoptosis by investigating its expression in the fibroblastic foci of patients with IPF. Using both immunohistochemical staining of PTPN13 and α-SMA on serial sections and dual-label immunofluorescent staining on single sections, PTPN13 was found to be localized to both α-SMA-positive myofibroblasts and α-SMA-negative cells of fibroblastic morphology in the fibroblastic foci of patients with IPF (Figures 1A and 1B, middle row). Of the 13 IPF lung biopsies studied, 12 (92%) demonstrated PTPN13 staining in fibroblastic foci and PTPN13 expression was significantly increased (P < 0.001) in IPF lung tissues compared with normal subjects (see Figure E2 in the online supplement). As a disease control, we evaluated PTPN13 localization in lung biopsies from patients with organizing pneumonia, a subacute, nonprogressive, and largely temporary reparative disorder in which (myo)fibroblasts organize in the alveoli of affected patients. Unlike IPF, organizing pneumonia frequently resolves because of (myo)fibroblast apoptosis (9, 10). We observed minimal expression of PTPN13 in the luminal α-SMA-positive myofibroblasts in organizing pneumonia compared with IPF (Figures 1A and 1B, lower row). We also observed minimal expression of PTPN13 in normal lung parenchyma, although positive staining was detected in alveolar macrophages and some airway epithelial cells (see Figure E3). These findings support the notion that PTPN13 expression by lung (myo)fibroblasts in IPF contributes to their accumulation via resistance to Fas-induced apoptosis.
Figure 1.
PTPN13 (protein tyrosine phosphatase-N13) is associated with fibrotic foci in lung tissues from patients with idiopathic pulmonary fibrosis (IPF). (A) PTPN13 and α-SMA (α-smooth muscle actin) colocalization of immunohistochemical staining in serial sections of lung tissues from nondiseased human subjects (n = 6), patients with IPF (n = 8), and patients with organizing pneumonia (n = 6). (B) Coimmunofluorescent staining of α-SMA (green) and PTPN13 (red). Insets are twice the magnification of the other panels. Control nonimmune IgG staining is shown in Figure E1. ***P < 0.001, mean ± SEM. Red dashed ellipses indicate fibroblastic foci or fibroblastic buds.
TNF-α/IFN-γ Downregulates PTPN13 Expression in Human Lung (Myo)Fibroblasts
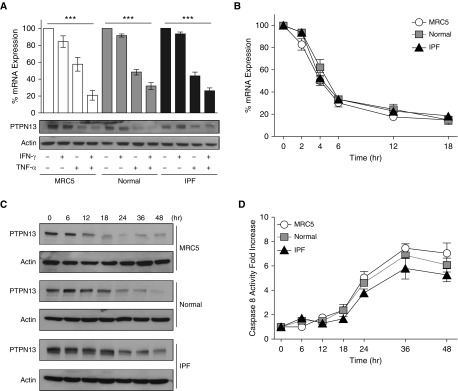
Given that PTPN13 is expressed in the fibroblastic foci in patients with IPF and exposure of primary human lung (myo)fibroblasts to TNF-α/IFN-γ overcomes their resistance to Fas-induced apoptosis (12, 14, 16, 21), we hypothesized that PTPN13 is basally expressed by cultured primary human lung (myo)fibroblasts, and exposure to TNF-α/IFN-γ downregulates PTPN13. Primary normal human and IPF lung (myo)fibroblasts along with human lung (myo)fibroblastic MRC5 cells were grown on rigid plastic tissue culture plates and stimulated with TNF-α, IFN-γ, or both cytokines. PTPN13 mRNA and protein levels were quantified by qPCR and Western blotting, respectively. PTPN13 mRNA and protein were found to be basally expressed at similar levels in primary normal human and IPF lung (myo)fibroblasts and MRC5 cells (Figures 2A and 2C). Likewise, basal PTPN13 mRNA expression as quantified by RNA-seq (see Figure E4) and protein levels were similar in multiple normal and IPF (myo)fibroblast lines (see Figure E5). Incubation with IFN-γ had little effect on PTPN13 expression (Figure 2A). However, exposure to TNF-α reduced PTPN13 expression by approximately 50% (Figure 2A), whereas incubation with both cytokines reduced PTPN13 expression by approximately 75% (Figure 2A).
Figure 2.
TNF-α (tumor necrosis factor-α)/IFN-γ decrease PTPN13 (protein tyrosine phosphatase-N13) expression in human lung myofibroblasts. (A, top panel) qPCR analysis of PTPN13 in normal human lung fibroblasts (normal), fibrotic human lung myofibroblasts (IPF), and MRC5 cells. (A, bottom panel) Western blot analysis of PTPN13 protein. (B) qPCR-based assessment of the time course of PTPN13 mRNA expression after TNF-α/IFN-γ exposure. (C) Western blot analysis of PTPN13 expression over 48 hours in MRC5, normal, and IPF fibroblasts exposed to TNF-α/IFN-γ. (D) Fas-induced caspase-8 activation after sensitization of myofibroblasts to TNF-α/IFN-γ over 48 hours before stimulation with agonistic anti–Fas-activating antibody for 4 hours. TNF-α was used at 10 ng/ml and IFN-γ at 50 U/ml. ***P < 0.001, mean ± SEM. n = 3 per cell line. IPF = idiopathic pulmonary fibrosis.
All three types of lung (myo)fibroblasts behaved similarly (Figure 2A; see Figure E5). Because we have previously shown that TNF-α/IFN-γ sensitization to Fas-induced apoptosis takes approximately 24–36 hours (12), we compared the kinetics of cytokine-induced downregulation of PTPN13 with acquisition of sensitivity to Fas-induced apoptosis. Exposure to TNF-α/IFN-γ led to maximal down-regulation of PTPN13 mRNA and protein by 18–24 hours (Figures 2B and 2C) concurrent with acquisition of sensitivity to Fas-induced apoptosis as reflected by caspase-8 activation (Figure 2D) and mitochondrial depolarization (see Figure E6), which was used as an alternative apoptosis marker.
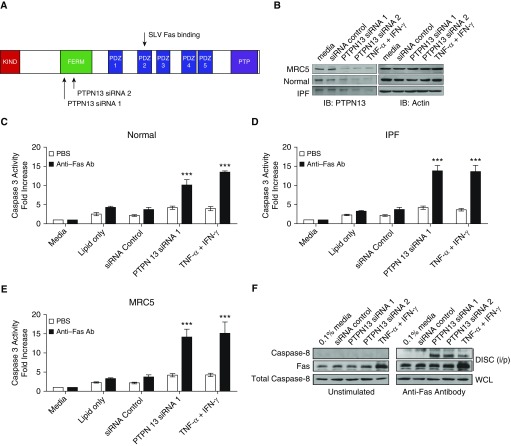
Reduced PTPN13 Expression or Impaired Interaction with Fas Overcomes Resistance of Lung (Myo)Fibroblasts to Fas-induced Apoptosis
To determine if reduced expression of PTPN13 is sufficient to overcome the resistance of human (myo)fibroblasts to Fas-induced apoptosis, we knocked-down PTPN13 with two different siRNAs (Figure 3A). Normal, IPF and MRC5 lung (myo)fibroblasts were transfected with PTPN13 or nontargeting siRNAs before assessing their ability to undergo Fas-induced apoptosis. Both PTPN13 siRNAs reduced PTPN13 protein expression by greater than 80% and to a level similar to TNF-α/IFN-γ treatment (Figure 3B). Figures 3C–3E show that PTPN13 knockdown sensitized normal, IPF, and MRC5 (myo)fibroblasts to Fas-induced proapoptotic caspase-3 activation, whereas the nontargeting siRNA had no effect on either PTPN13 protein expression or susceptibility to Fas-induced apoptosis (Figures 3B–3E). Similar data were obtained for caspase-8 activation (see Figure E7).
Figure 3.
Decreased PTPN13 (protein tyrosine phosphatase-N13) expression sensitizes human lung myofibroblasts to Fas-induced apoptosis. (A) Illustration of protein domains within PTPN13. Two PTPN13-specific siRNAs are directed toward the FERM domain. (B) Western blot analysis of siRNA-mediated knockdown of PTPN13. (C–E) Fas-induced caspase-3 activation in PTPN13 siRNA-treated normal, idiopathic pulmonary fibrosis, and MRC5 lung myofibroblasts, respectively. (F) Decreased expression of PTPN13 by siRNA or TNF-α (tumor necrosis factor-α)/IFN-γ promotes the DISC (death-inducing signaling complex) assembly after Fas stimulation. Coprecipitated caspase-8 was recruited to the DISC after Fas ligation in lung fibroblasts pretreated with PTPN13 siRNA or with TNF-α/IFN-γ. The quantitative increase in immunoprecipitated Fas after stimulation with TNF-α/IFN-γ is caused by increased Fas expression as previously reported (13). ***P < 0.001, mean ± SEM. n = 3 per cell line. Ab = antibody; IPF = idiopathic pulmonary fibrosis; PBS = phosphate-buffered saline; WCL = whole-cell lysate.
Fas-induced apoptosis is initiated when the adapter protein FADD and procaspase-8 are recruited to the cytoplasmic domain of Fas to form the DISC (22). We tested the hypothesis that PTPN13 knockdown overcomes the resistance of human lung (myo)fibroblasts to Fas-induced apoptosis by enabling DISC assembly. PTPN13-specific siRNAs were used to knockdown PTPN13 in MRC5 lung (myo)fibroblasts before stimulation with agonistic anti-Fas antibody and assessment of DISC assembly by coimmunoprecipitation of caspase-8 with Fas. Figure 3F shows that PTPN13 knockdown was sufficient to increase Fas-induced recruitment of caspase-8, whereas the nontargeting siRNA was without effect. Together, these data indicate that PTPN13 expression is necessary for the resistance of normal, IPF, and MRC5 human lung (myo)fibroblasts to Fas-induced signaling and apoptosis.
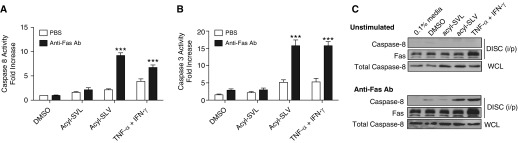
Previous studies have shown that Fas contains a C-terminal tripeptide sequence (SLV) that interacts with PTPN13 via PDZ domain 2 (Figure 3A) (23). To confirm that PTPN13 is necessary for the resistance to Fas-induced fibroblast apoptosis, we interfered with the interaction between Fas and PTPN13 with a previously validated cell-permeable acyl-SLV peptide (23). Pretreatment of MRC5 (myo)fibroblasts with acyl-SLV resulted in a similar level of Fas-induced caspase-8 and caspase-3 activation to that accomplished with TNF-α/IFN-γ stimulation (Figures 4A and 4B), whereas pretreatment with the control acyl-SVL peptide was without effect (Figures 4A and 4B). Similarly, the acyl-SLV peptide, but not the acyl-SVL peptide, enabled DISC assembly (Figure 4C). We confirmed the specificity and effectiveness of the SLV peptide in blocking the Fas-PTPN13 interaction using an AlphaScreen assay and found that the SLV peptide, but not the SVL peptide, inhibited the interaction between the PDZ1/2 domain of PTPN13 and the C-terminal SLV sequence of Fas (see Figure E8). Together, these findings indicate that downregulation of PTPN13 expression by TNF-α/IFN-γ exposure, PTPN13 siRNA, or interference of the Fas-PTPN13 interaction restores Fas-induced DISC assembly and apoptotic signaling in human lung (myo)fibroblasts.
Figure 4.
Reduced PTPN13 (protein tyrosine phosphatase-N13) expression or decreased association with Fas enables assembly of the Fas-induced DISC (death-inducing signaling complex) in MRC5 myofibroblasts. (A and B) Inhibition of Fas/PTPN13 interactions with acylated-SLV peptide augments Fas-induced caspase-8 (A) and caspase-3 (B) activation. (C) Inhibition of Fas/PTPN13 interactions with acylated-SLV peptide promotes the formation of the DISC after Fas ligation. Both peptides were used at a concentration of 10 μM. ***P < 0.001, mean ± SEM. n = 3 per cell line. Ab = antibody; PBS = phosphate-buffered saline; TNF = tumor necrosis factor; WCL = whole-cell lysate.
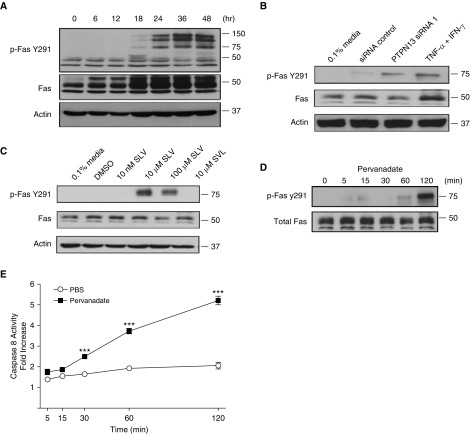
PTPN13 Tyrosine Phosphatase Activity Protects (Myo)Fibroblasts from Fas-induced Apoptosis by Dephosphorylating Fas
Fas contains two tyrosine residues (Y232 and Y291) that upon phosphorylation (24, 25), promote Fas oligomerization and apoptosis (26, 27). Because PTPN13 is a protein tyrosine phosphatase, we tested the hypothesis that TNF-α/IFN-γ-induced PTPN13 downregulation or siRNA silencing would increase Fas tyrosine phosphorylation and sensitivity to Fas-induced apoptosis. First, we incubated MRC5 (myo)fibroblasts with TNF-α/IFN-γ and assessed phosphorylation of Fas residues Y232 and Y291 by Western blotting. Exposure to TNF-α/IFN-γ increased phosphorylation of Fas Y291 (Figure 5A) coincident with both the downregulation of PTPN13 expression and the acquisition of sensitivity to Fas-induced apoptosis (Figures 2C and 2D). The appearance of slower migrating phospho-Fas bands and SDS-stable higher order receptor complexes (Figure 5A) is consistent with previous studies showing that Fas undergoes post-translational palmitoylation and phosphorylation in cells destined to undergo apoptosis (28). We confirmed that Fas undergoes tyrosine phosphorylation of both monomeric and higher order complexes by immunoprecipitation with antiphosphotyrosine antibody and immunoblotting with anti-Fas (see Figure E9). Phosphorylation of Fas Y232 was not detected (data not shown). Next, to determine if Fas phosphorylation was mediated by PTPN13, we silenced PTPN13 expression or interfered with its interaction with Fas with the acyl-SLV peptide. Figures 5B and 5C show that both approaches led to similar increases in Fas Y291 phosphorylation in lung (myo)fibroblasts.
Figure 5.
PTPN13 (protein tyrosine phosphatase-N13) decreases Fas tyrosine phosphorylation and Fas-induced apoptosis. (A) Western blot depicting the time-dependent increase in phosphorylation of Fas at Y291 after tumor necrosis factor-α/IFN-γ sensitization. (B) Western blot analysis showing increased tyrosine phosphorylation of Fas at Y291 after siRNA1-mediated PTPN13 knockdown. (C) Western blot showing increased phosphorylation of Fas at Y291 after treatment with acylated-SLV peptide. (D) Western blot analysis illustrating increased phosphorylation of Fas at Y291 after pervanadate treatment. (E) Caspase-8 activation in MRC5 myofibroblasts is significantly increased after pretreatment with pervanadate for 2 hours before stimulation with agonistic anti-Fas antibody. ***P < 0.001, mean ± SEM . n = 3. PBS = phosphate-buffered saline; TNF = tumor necrosis factor.
To determine if reduced protein tyrosine phosphatase activity accounted for the increase in Fas Y291 phosphorylation, we incubated MRC5 myofibroblasts with pervanadate, a broad-spectrum PTP inhibitor, and evaluated its effect on Fas Y291 phosphorylation. Figures 5D and 5E show that pervanadate increased Fas Y291 phosphorylation and overcame the resistance of the cells to Fas-induced apoptosis. Thus, the resistance of human lung (myo)fibroblasts to Fas-induced apoptosis is caused by PTPN13-mediated dephosphorylation of Fas Y291.
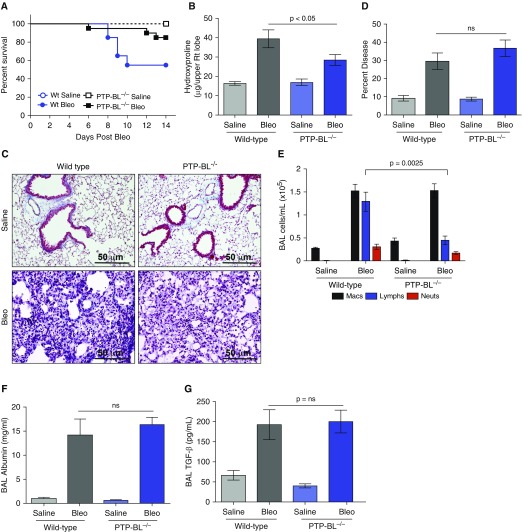
PTP-BL Deficiency Reduces Bleomycin-induced Pulmonary Fibrosis in Mice
To assess the in vivo role of PTPN13 in pulmonary fibrosis, we studied the consequences of intratracheal instillation of bleomycin (2.5 U/kg) in mice lacking PTP-BL, the highly conserved ortholog of PTPN13 (29). Whereas wild-type (Wt) mice developed significant mortality 8–10 days after bleomycin, mortality in PTP-BL−/− mice was reduced (Figure 6A). The amount of lung collagen, as visualized by trichrome staining (Figure 6D) and lung hydroxyproline measurements, was significantly (P = 0.031) reduced in bleomycin-challenged PTP-BL−/− mice at 14 days (Figure 6B). However, histologic examination (Figure 6C) and stereologic quantification of lung inflammation (Figure 6F) in the lungs of bleomycin-instilled mice revealed extensive lung injury with infiltration of macrophages, thickened alveolar septae, protein exudation, and alveolar collapse in both mouse strains (P = 0.642). Analysis of BAL leukocyte numbers also revealed similar increases in macrophages and neutrophils in bleomycin-instilled Wt and PTP-BL−/− mice, although lymphocyte numbers were significantly reduced in PTP-BL−/− mice (Figure 6E). Furthermore, BAL fluid levels of albumin (Figure 6F; P = 0.288) and TGF-β (Figure 6G; P = 0.641) were not significantly different between PTP-BL−/− mice and Wt mice. Thus, PTP-BL deficiency had little effect on the development of inflammation and acute lung injury after bleomycin exposure, but significantly inhibited the ensuing collagen accumulation (i.e., fibrosis).
Figure 6.
PTP-BL deficiency reduces bleomycin-induced pulmonary fibrosis in mice. (A) PTP-BL−/− mice have decreased mortality after bleomycin instillation compared with wild-type control animals. (B and C) Bleomycin-instilled PTP-BL−/− mice had a significantly reduced percentage of lung collagen compared with bleomycin-instilled wild-type mice as determined by assessment of collagen deposition identified by hydroxyproline and Masson trichrome staining (blue). Hydroxyproline levels were significantly reduced in bleomycin-instilled PTP-BL−/− mice compared with wild-type mice. (D) The percentage of diseased lung assessed by quantitative stereology was similar in bleomycin-instilled wild-type and PTP-BL−/− mice. (E) PTP-BL−/− mice have decreased lymphocyte infiltration into the airspaces after bleomycin instillation. (F) BAL albumin and (G) transforming growth factor-β levels were similar in bleomycin-instilled PTP-BL−/− and wild-type control animals. Values are mean ± SEM difference. n = 10 mice per group. All endpoints were measured at Day 14. Bleo = bleomycin; ns = not significant; TGF = transforming growth factor; Wt = wild type.
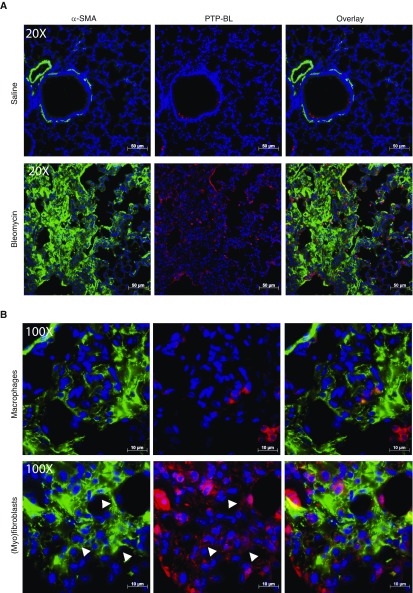
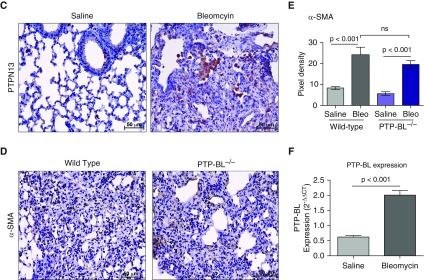
Although PTPN13/PTP-BL was originally described as a mediator of resistance to Fas-induced apoptosis, it has also been shown to participate in additional signaling pathways that may be of relevance to the reduced fibrosis seen in PTP-BL−/− mice. To further investigate it’s in vivo function, we assessed PTP-BL expression and localization in saline- and bleomycin-instilled Wt mice (Figure 7C). Figure 7F shows that total lung PTP-BL mRNA levels were significantly increased (P < 0.001) in response to bleomycin instillation in comparison with saline-instilled control mice. Similar to that seen in human lung tissues, PTP-BL was expressed by lung macrophages and a few epithelial cells. However, in contrast to IPF lung tissues wherein PTPN13 was robustly expressed in (myo)fibroblasts in fibroblastic foci, PTP-BL was less robustly expressed in the (myo)fibroblasts of the fibrotic lesions in bleomycin-instilled mice (Figures 7A and 7B). In addition, quantification of lung myofibroblast density by immunohistochemistry for α-SMA unexpectedly indicated that lung (myo)fibroblast density was not significantly (P > 0.05) affected by whole-body PTP-BL deficiency (Figures 7D and 7E).
Figure 7.
PTP-BL is modestly expressed in α-SMA (α-smooth muscle actin)-positive fibroblasts in lung tissues from fibrotic wild-type mice. (A) Coimmunofluorescent staining of α-SMA (green) and PTP-BL (red) shows some dual staining α-SMA+/PTPN13+ myofibroblasts. (B) Higher magnification shows positive PTP-BL staining in macrophages (top) and fibroblasts (bottom). White arrowheads highlight cells in which α-SMA and PTP-BL are colocalized. (C) Immunohistochemical localization of PTP-BL in saline-instilled (left) and bleomycin-instilled (right) wild-type mice. (D) Immunohistochemical staining of α-SMA in bleomycin-instilled wild-type (left) and PTP-BL−/− mice (right). (E) Semiquantitative assessment of α-SMA immunostaining to identify (myo)fibroblasts was not significantly different in bleomycin-treated wild-type and PTP-BL–deficient mice. (F) PTP-BL mRNA expression in whole-lung tissue from saline- and bleomycin-instilled wild-type mice. (A–C and F) n = 5 mice per group. (D and E) n = 10 mice per group. Bleo = bleomycin; ns = not significant; PTPN13 = protein tyrosine phosphatase-N13.
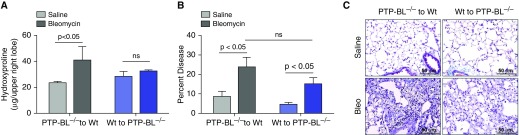
PTPN13 (humans) and PTP-BL (mice) are expressed in both hematopoietic and nonhematopoietic (i.e., stromal) cells. Thus, it was possible that the reduced fibrosis seen in PTP-BL−/− mice may be caused by loss of PTP-BL in hematopoietic cells, a possibility strengthened by the findings that PTPN13/PTP-BL is expressed in macrophages (Figures 7A and 7B; see Figure E3); and that PTP-BL−/− mice recruited fewer lymphocytes into their lungs in response to bleomycin instillation compared with Wt mice (Figure 6E). To address this possibility, we created bone marrow chimeric mice in which PTP-BL−/− bone marrow cells were transferred into lethally irradiated Wt recipients (PTP-BL−/− to Wt) and vice versa (Wt to PTP-BL−/−). All groups of mice exhibited >95% chimerism at 8 weeks as reflected by CD45.1 and CD45.2 expression levels on peripheral blood leukocytes (data not shown). The mice were then instilled with bleomycin and assessed for inflammatory, injury-associated, and fibrotic parameters at 2 weeks. Figures 8A–8C show that the lungs of chimeric Wt to PTP-BL−/− mice phenocopied whole-body PTP-BL−/− mice (Figure 6) and exhibited a significant reduction in fibrosis. In contrast, chimeric PTP-BL−/− to Wt mice phenocopied the typical fibrotic response seen in Wt mice (Figures 6 and 8A–8C). Both Wt to Wt and PTP-BL−/− to PTP-BL−/− control mice developed the expected fibrotic and nonfibrotic responses, respectively (data not shown). Collectively, our findings support the conclusion that loss of PTP-BL in nonhematopoietic stromal cells is responsible for the reduced fibrosis seen in the lungs of PTP-BL−/− mice.
Figure 8.
PTP-BL deficiency in stromal, but not hematopoietic, cells phenocopies the reduced bleomycin-induced pulmonary fibrosis seen in whole-body PTP-BL–deficient mice. (A) Lung hydroxyproline levels and (B) stereology-quantified lung injury in saline- and bleomycin-instilled PTP-BL−/− to wild-type (Wt) and Wt to PTP-BL−/− mice. (C) Trichrome-stained sections from saline- and bleomycin-instilled PTP-BL−/− to Wt and Wt to PTP-BL−/− mice. Values are mean ± SEM. n = 5 mice per group. Bleo = bleomycin; ns = no significant difference.
Discussion
Resistance to apoptosis is thought to contribute to (myo)fibroblast persistence in the lung parenchyma of patients with IPF (9–11). Here we show that the protein tyrosine phosphatase PTPN13 is expressed by (myo)fibroblasts in the fibroblastic foci of patients with IPF and contributes to their resistance to Fas-induced apoptosis. We also show that genetic deficiency of PTP-BL, the mouse ortholog of human PTPN13, reduced bleomycin-induced pulmonary fibrosis in mice, although the mechanism may differ. Additionally, the proinflammatory cytokines TNF-α/IFN-γ, which sensitize lung fibroblasts to Fas-induced apoptosis, downregulate PTPN13 expression and augment proapoptotic Fas tyrosine phosphorylation and signaling. Taken together, these findings suggest that a therapeutic approach aimed at reducing PTPN13 expression, activity, or interaction with Fas might serve as novel strategies to facilitate lung (myo)fibroblast apoptosis in the lungs of patients with IPF and reduce pulmonary fibrosis.
PTPN13 was abundantly detected in α-SMA-negative fibroblasts and α-SMA-positive (myo)fibroblasts in the fibroblastic foci of IPF patients but was absent from these cells in the fibrotic buds in organizing pneumonia, a reversible inflammatory/fibrotic lung disease (30). These findings may be significant because unlike the apoptosis-resistant (myo)fibroblasts in the fibroblastic foci of patients with IPF (9, 10, 31), some of the (myo)fibroblasts in the fibrotic buds in organizing pneumonia are apoptotic, presumably as the fibrotic buds undergo resolution (10). These findings therefore provided a compelling impetus to test the hypothesis that PTPN13 contributes to the resistance of human lung fibroblasts to apoptosis and pulmonary fibrosis.
PTPN13 mRNA and protein were found to be basally expressed at similar levels by primary normal and IFP lung (myo)fibroblasts and MRC5 cells cultured in vitro on ultrarigid plastic, a finding that we confirmed by querying publicly available transcriptomic datasets (GSE44723, GSE47460, GSE2052) containing normal and IPF (myo)fibroblasts, and normal and fibrotic whole-lung tissue. Although conceptually one might have expected cultured IPF lung (myo)fibroblasts to express higher levels of PTPN13 than cultured normal lung fibroblasts, these findings are consistent with current knowledge about the consequences of culture of fibroblasts on rigid plastic surfaces, which rapidly result in the development of myofibroblast features, including increased expression of α-SMA and stretch fiber formation. Furthermore, in unpublished studies (E.F. Redente and D.W.H. Riches) using hydrogels of increasing Young’s modulus (i.e., stiffness), we observed that PTPN13 can be regulated by substratum stiffness. Thus, by virtue of their ability to basally express PTPN13, thereby mimicking (myo)fibroblasts in the fibroblastic foci of patients with IPF, these collective findings reinforce the suitability of cultured human lung (myo)fibroblasts as a model to study the mechanisms underlying the resistance of fibrotic lung fibroblasts to Fas-induced apoptosis.
Exposure of normal and IFP lung (myo)fibroblasts and MRC5 cells to TNF-α/IFN-γ downregulated PTPN13 expression coincident with the acquisition of sensitivity to Fas-induced apoptosis. PTPN13 expression has been shown to be regulated at multiple transcriptional and post-transcriptional levels (32–36). Additionally, we have shown that PTPN13 downregulation in lymphoid cell lines involves p62-dependent targeting to autophagosomes in cells that exhibit high autophagic flux (37). Interestingly, and consistent with the notion that impaired autophagy may result in the maintenance of PTPN13 expression, recent studies have suggested that the fibroblastic foci of patients with IPF are impaired in their ability to undergo autophagy via a mechanism involving the profibrotic cytokine, TGF-β (38, 39). Clearly many questions remain about how PTPN13 expression is controlled in vivo and future studies using freshly isolated fibroblasts and (myo)fibroblasts from normal and IPF lung tissue, which would not be impacted by in vitro culture, may help address this fundamental knowledge gap.
Seeking to understand how downregulation of PTPN13 function promoted Fas-induced (myo)fibroblast apoptosis, we investigated the role of its tyrosine phosphatase activity in Fas-signaling. PTPN13 downregulation or interference with its interaction with Fas concordantly increased phosphorylation of Fas tyrosine residue 291 (Y291) and sensitized (myo)fibroblasts to Fas-induced apoptosis. Pervanadate, a pan-tyrosine phosphatase inhibitor, mimicked both events, suggesting that PTPN13 suppresses apoptosis by dephosphorylating Fas phospho-Y291. Y291 (which, using a different numbering system, is also referred to as Y275 [40]) has been shown to be involved in Fas trafficking to the cell surface and in promoting susceptibility to Fas-induced apoptosis (26, 27, 40). In addition, Y291 is a key residue in a consensus internalization motif. Thus, it is plausible that phosphorylation of Y291 may mask this internalization motif, blocking internalization and increasing Fas cell surface expression and susceptibility to apoptosis (41, 42).
To explore the role of PTPN13 in vivo, we investigated fibrosis in mice lacking PTP-BL, the murine ortholog of PTPN13. Loss of PTP-BL had no effect on bleomycin-induced acute lung injury, inflammation, or TGF-β production. However, the ensuing fibrosis, as reflected by lung hydroxyproline levels and collagen staining, was reduced in PTP-BL−/− mice, through an effect on stromal cells. PTP-BL mRNA was detected in the lungs of bleomycin-instilled mice, although PTP-BL protein was only modestly detected in (myo)fibroblasts in fibrotic areas of Wt mice compared with the robust expression seen in the fibroblastic foci of patients with IPF. Although future studies in PTP-BL−/− mice should help resolve these seemingly discordant findings between humans and mice, we speculate that they may be related to differences in the mechanical properties of lung tissues in the transient, resolving bleomycin model of pulmonary fibrosis and the persistent, progressive fibrosis seen in IPF. In contrast to the persistently fibrotic lung tissues of patients with IPF, which have been reported to have a mean stiffness (Young’s modulus) of 16.5 kPa (43), the reversibly fibrotic lung tissues of bleomycin-instilled mice are considerably more compliant, with a median stiffness of approximately 3 kPa (44). Thus, it is conceivable that the reduced intensity of PTP-BL protein expression in lung tissues from bleomycin-instilled mice compared with the robust PTPN13 expression lung tissues from patients with IPF may be caused by difference in matrix stiffness. Consistent with this notion, we found that although murine (myo)fibroblasts expressed only modest PTP-BL staining in fibrotic lung tissues, primary murine lung fibroblasts were capable of expressing high levels of PTP-BL when cultured on rigid plastic dishes (see Figure E10).
PTPN13 binds to human Fas through a C-terminal SLV sequence that interacts with the PDZ2 domain of PTPN13, and via a second interaction with Fas Y291 (23, 40). Although human Fas contains both motifs, mouse Fas lacks the SLV sequence. Thus, mouse Fas and PTP-BL do not interact in the same way as their human orthologs, and PTP-BL does not inhibit Fas-induced apoptosis in mice (45). Consistent with these findings, (myo)fibroblast density in bleomycin-induced fibrotic lung tissues from Wt mice was not significantly different from that of PTP-BL−/− mice. Thus, the reduction in the fibrosis seen in PTP-BL−/− mice may represent an additional and previously unknown role for PTP-BL and potentially its human ortholog in collagen synthesis or turnover. PTPN13 and PTP-BL have both been shown to control additional signaling pathways including nuclear factor-κB, mitogen-activated protein kinase, signal transducer and activator of transcription, epidermal growth factor receptor signaling (29, 46–48), and Src-family kinases (49, 50). Because many of these pathways have also been implicated in the development of pulmonary fibrosis, PTP-BL deficiency might attenuate the fibrotic response by also affecting these pathways. Furthermore, Schickel and colleagues (36) reported that the micro-RNA, miR-200c, basally represses PTPN13 in normal epithelial cells. However, during their transition to mesenchymal-like cells, miR-200c expression is repressed leading to increased PTPN13 expression, acquired resistance to Fas-induced apoptosis, and development of an invasive fibroblast phenotype. Thus, it is plausible that PTP-BL deficiency may also reduce fibroblast invasiveness and contribute to the reduced fibrotic lung phenotype. Consistent with this view, Yang and colleagues (51) showed that miR-200 expression is reduced in the lungs of patients with IPF and that pulmonary delivery of miR-200c in mice reduced the development of bleomycin-induced pulmonary fibrosis (51). Thus, miR-200c-induced downregulation of PTP-BL/PTPN13 could plausibly contribute to the reduction of fibrosis in these mice.
Why (myo)fibroblasts undergo apoptosis and clearance during normal repair processes yet persist in the lungs of patients with IPF remains poorly understood. Inflammation, together with elevated levels of TNF-α and IFN-γ, accompany acute lung injury (52) before normal repair. Analogous to the epigenetically controlled development of fibroblast anatomic memory (53), it seems plausible that recruited fibroblasts could develop memory of TNF-α/IFN-γ exposure during acute lung injury, perhaps via downregulation of PTPN13 expression, thereby becoming poised to undergo apoptosis once the repair process is complete. In contrast to acute lung injury, persistent and progressive fibrosis in patients with IPF develops in the absence of a robust acute inflammatory response (1), related in part to the TGF-β-rich, antiinflammatory environment of the fibrotic lung, which suppresses TNF-α and IFN-γ expression (17, 54). Indeed, we proposed (12, 13, 18) that fibroblast persistence in vivo may be caused by limited availability of TNF-α, IFN-γ, and other factors (e.g., prostaglandin E2) (55, 56), that are required to sensitize fibrotic lung (myo)fibroblasts to apoptosis. Consistent with this idea, we recently showed that lung delivery of recombinant TNF-α accelerates the resolution of established fibrosis in the bleomycin model (18). Intratracheal administration of IFN-γ has similarly been shown to reduce fibrosis in this model (57). The notion that proinflammatory cytokines may play a role in fibrosis resolution is further supported by the finding of persistent fibrosis that we and other have seen in the absence of TNF-α and IFN-γ, as demonstrated in bleomycin-challenged TNF-α−/− mice and CXCR3−/− mice, the latter which fail to recruit IFN-γ producing natural killer cells (18, 58).
In summary, our findings reveal novel mechanisms by which PTPN13 promotes the resistance of lung (myo)fibroblasts to Fas-induced apoptosis and contributes to the development of pulmonary fibrosis. Although further studies are required to dissect the complexity of PTPN13 involvement, the current data obtained from the fibrotic lung tissues of patients with IPF, primary human lung (myo)fibroblasts, and PTP-BL−/− mice suggest that PTPN13 may be a novel and relevant therapeutic target to reduce the progression of fibrosis in patients with IPF. Furthermore, because PTPN13 interacts with Fas and not other death receptors, therapeutically targeting the Fas-PTPN13 interaction interface may be a more specific approach to interfere with the function of PTPN13 in the resistance of (myo)fibroblasts to Fas-induced apoptosis, without interfering with its other functions (e.g., its role as a tumor-suppressor in gut epithelia) (35).
Footnotes
Supported by VA Merit Award BX003471 (D.W.H.R.), VA Career Development Award BX002401 (E.F.R.), and Public Health Service grants HL114754 (D.W.H.R.) and HL126732 (S.D.L. and D.W.H.R., Multiple Principal Investigators) from the NHLBI of the NIH.
Author Contributions: A.B., E.F.R., R.C.K., S.D.G., C.D.C., B.L.E., B.P.B., and M.W.W. designed and planned experiments, analyzed the data, and contributed to writing the manuscript. R.M.T. and G.P.C. designed and planned experiments, provided patient samples, analyzed the data, and contributed to writing the manuscript. D.C.-E. performed the statistical analyses. S.D.L., L.A.O., A.T., and D.W.H.R. planned the project, designed experiments, analyzed the data, and contributed to writing the manuscript. All authors reviewed the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201707-1497OC on May 4, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 2.Bjoraker JA, Ryu JH, Edwin MK, Myers JL, Tazelaar HD, Schroeder DR, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157:199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 3.King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164:1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 4.White ES, Lazar MH, Thannickal VJ. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J Pathol. 2003;201:343–354. doi: 10.1002/path.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoyles RK, Derrett-Smith EC, Khan K, Shiwen X, Howat SL, Wells AU, et al. An essential role for resident fibroblasts in experimental lung fibrosis is defined by lineage-specific deletion of high-affinity type II transforming growth factor β receptor. Am J Respir Crit Care Med. 2011;183:249–261. doi: 10.1164/rccm.201002-0279OC. [DOI] [PubMed] [Google Scholar]
- 6.Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, et al. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188:820–830. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 9.Cha SI, Groshong SD, Frankel SK, Edelman BL, Cosgrove GP, Terry-Powers JL, et al. Compartmentalized expression of c-FLIP in lung tissues of patients with idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;42:140–148. doi: 10.1165/rcmb.2008-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lappi-Blanco E, Soini Y, Pääkkö P. Apoptotic activity is increased in the newly formed fibromyxoid connective tissue in bronchiolitis obliterans organizing pneumonia. Hai. 1999;177:367–376. doi: 10.1007/pl00007654. [DOI] [PubMed] [Google Scholar]
- 11.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:350–356. doi: 10.1513/pats.200601-001TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frankel SK, Cosgrove GP, Cha SI, Cool CD, Wynes MW, Edelman BL, et al. TNF-alpha sensitizes normal and fibrotic human lung fibroblasts to Fas-induced apoptosis. Am J Respir Cell Mol Biol. 2006;34:293–304. doi: 10.1165/rcmb.2005-0155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wynes MW, Edelman BL, Kostyk AG, Edwards MG, Coldren C, Groshong SD, et al. Increased cell surface Fas expression is necessary and sufficient to sensitize lung fibroblasts to Fas ligation-induced apoptosis: implications for fibroblast accumulation in idiopathic pulmonary fibrosis. J Immunol. 2011;187:527–537. doi: 10.4049/jimmunol.1100447. [DOI] [PubMed] [Google Scholar]
- 14.Moodley YP, Caterina P, Scaffidi AK, Misso NL, Papadimitriou JM, McAnulty RJ, et al. Comparison of the morphological and biochemical changes in normal human lung fibroblasts and fibroblasts derived from lungs of patients with idiopathic pulmonary fibrosis during FasL-induced apoptosis. J Pathol. 2004;202:486–495. doi: 10.1002/path.1531. [DOI] [PubMed] [Google Scholar]
- 15.Sisson TH, Ajayi IO, Subbotina N, Dodi AE, Rodansky ES, Chibucos LN, et al. Inhibition of myocardin-related transcription factor/serum response factor signaling decreases lung fibrosis and promotes mesenchymal cell apoptosis. Am J Pathol. 2015;185:969–986. doi: 10.1016/j.ajpath.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka T, Yoshimi M, Maeyama T, Hagimoto N, Kuwano K, Hara N. Resistance to Fas-mediated apoptosis in human lung fibroblast. Eur Respir J. 2002;20:359–368. doi: 10.1183/09031936.02.00252602. [DOI] [PubMed] [Google Scholar]
- 17.Martinez JA, King TE, Jr, Brown K, Jennings CA, Borish L, Mortenson RL, et al. Increased expression of the interleukin-10 gene by alveolar macrophages in interstitial lung disease. Am J Physiol. 1997;273:L676–L683. doi: 10.1152/ajplung.1997.273.3.L676. [DOI] [PubMed] [Google Scholar]
- 18.Redente EF, Keith RC, Janssen W, Henson PM, Ortiz LA, Downey GP, et al. Tumor necrosis factor-α accelerates the resolution of established pulmonary fibrosis in mice by targeting profibrotic lung macrophages. Am J Respir Cell Mol Biol. 2014;50:825–837. doi: 10.1165/rcmb.2013-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redente EF, Jacobsen KM, Solomon JJ, Lara AR, Faubel S, Keith RC, et al. Age and sex dimorphisms contribute to the severity of bleomycin-induced lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2011;301:L510–L518. doi: 10.1152/ajplung.00122.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato T, Irie S, Kitada S, Reed JC. FAP-1: a protein tyrosine phosphatase that associates with Fas. Science. 1995;268:411–415. doi: 10.1126/science.7536343. [DOI] [PubMed] [Google Scholar]
- 21.Moodley YP, Misso NL, Scaffidi AK, Fogel-Petrovic M, McAnulty RJ, Laurent GJ, et al. Inverse effects of interleukin-6 on apoptosis of fibroblasts from pulmonary fibrosis and normal lungs. Am J Respir Cell Mol Biol. 2003;29:490–498. doi: 10.1165/rcmb.2002-0262OC. [DOI] [PubMed] [Google Scholar]
- 22.Muzio M, Chinnaiyan AM, Kischkel FC, O’Rourke K, Shevchenko A, Ni J, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 23.Yanagisawa J, Takahashi M, Kanki H, Yano-Yanagisawa H, Tazunoki T, Sawa E, et al. The molecular interaction of Fas and FAP-1. A tripeptide blocker of human Fas interaction with FAP-1 promotes Fas-induced apoptosis. J Biol Chem. 1997;272:8539–8545. doi: 10.1074/jbc.272.13.8539. [DOI] [PubMed] [Google Scholar]
- 24.Gradl G, Grandison P, Lindridge E, Wang Y, Watson J, Rudert F. The CD95 (Fas/APO-1) receptor is phosphorylated in vitro and in vivo and constitutively associates with several cellular proteins. Apoptosis. 1996;1:131–140. [Google Scholar]
- 25.Reinehr R, Becker S, Eberle A, Grether-Beck S, Häussinger D. Involvement of NADPH oxidase isoforms and Src family kinases in CD95-dependent hepatocyte apoptosis. J Biol Chem. 2005;280:27179–27194. doi: 10.1074/jbc.M414361200. [DOI] [PubMed] [Google Scholar]
- 26.Eberle A, Reinehr R, Becker S, Keitel V, Häussinger D. CD95 tyrosine phosphorylation is required for CD95 oligomerization. Apoptosis. 2007;12:719–729. doi: 10.1007/s10495-006-0003-2. [DOI] [PubMed] [Google Scholar]
- 27.Foehr ED, Lorente G, Vincent V, Nikolich K, Urfer R. FAS associated phosphatase (FAP-1) blocks apoptosis of astrocytomas through dephosphorylation of FAS. J Neurooncol. 2005;74:241–248. doi: 10.1007/s11060-004-7202-x. [DOI] [PubMed] [Google Scholar]
- 28.Feig C, Tchikov V, Schütze S, Peter ME. Palmitoylation of CD95 facilitates formation of SDS-stable receptor aggregates that initiate apoptosis signaling. EMBO J. 2007;26:221–231. doi: 10.1038/sj.emboj.7601460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakahira M, Tanaka T, Robson BE, Mizgerd JP, Grusby MJ. Regulation of signal transducer and activator of transcription signaling by the tyrosine phosphatase PTP-BL. Immunity. 2007;26:163–176. doi: 10.1016/j.immuni.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Cottin V, Cordier JF. Cryptogenic organizing pneumonia. Semin Respir Crit Care Med. 2012;33:462–475. doi: 10.1055/s-0032-1325157. [DOI] [PubMed] [Google Scholar]
- 31.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6:231ra47. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irie S, Li Y, Kanki H, Ohyama T, Deaven LL, Somlo S, et al. Identification of two Fas-associated phosphatase-1 (FAP-1) promoters in human cancer cells. DNA Seq. 2001;11:519–526. doi: 10.3109/10425170109041336. [DOI] [PubMed] [Google Scholar]
- 33.Huang W, Zhu C, Wang H, Horvath E, Eklund EA. The interferon consensus sequence-binding protein (ICSBP/IRF8) represses PTPN13 gene transcription in differentiating myeloid cells. J Biol Chem. 2008;283:7921–7935. doi: 10.1074/jbc.M706710200. [DOI] [PubMed] [Google Scholar]
- 34.Ying J, Li H, Cui Y, Wong AH, Langford C, Tao Q. Epigenetic disruption of two proapoptotic genes MAPK10/JNK3 and PTPN13/FAP-1 in multiple lymphomas and carcinomas through hypermethylation of a common bidirectional promoter. Leukemia. 2006;20:1173–1175. doi: 10.1038/sj.leu.2404193. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Shen D, Parsons DW, Bardelli A, Sager J, Szabo S, et al. Mutational analysis of the tyrosine phosphatome in colorectal cancers. Science. 2004;304:1164–1166. doi: 10.1126/science.1096096. [DOI] [PubMed] [Google Scholar]
- 36.Schickel R, Park SM, Murmann AE, Peter ME. miR-200c regulates induction of apoptosis through CD95 by targeting FAP-1. Mol Cell. 2010;38:908–915. doi: 10.1016/j.molcel.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gump JM, Straskiewicz L, Morgan MJ, Bamberg A, Riches DW, Thorburn A. Autophagy variation within a cell population determines cell fate via selective autophagy of Fap-1. Nat Cell Biol. 2013;16:47–54. doi: 10.1038/ncb2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Araya J, Kojima J, Takasaka N, Ito S, Fujii S, Hara H, et al. Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304:L56–L69. doi: 10.1152/ajplung.00213.2012. [DOI] [PubMed] [Google Scholar]
- 39.Patel AS, Lin L, Geyer A, Haspel JA, An CH, Cao J, et al. Autophagy in idiopathic pulmonary fibrosis. PLoS One. 2012;7:e41394. doi: 10.1371/journal.pone.0041394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ivanov VN, Lopez Bergami P, Maulit G, Sato TA, Sassoon D, Ronai Z. FAP-1 association with Fas (Apo-1) inhibits Fas expression on the cell surface. Mol Cell Biol. 2003;23:3623–3635. doi: 10.1128/MCB.23.10.3623-3635.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carbone CJ, Zheng H, Bhattacharya S, Lewis JR, Reiter AM, Henthorn P, et al. Protein tyrosine phosphatase 1B is a key regulator of IFNAR1 endocytosis and a target for antiviral therapies. Proc Natl Acad Sci USA. 2012;109:19226–19231. doi: 10.1073/pnas.1211491109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chesarino NM, McMichael TM, Hach JC, Yount JS. Phosphorylation of the antiviral protein interferon-inducible transmembrane protein 3 (IFITM3) dually regulates its endocytosis and ubiquitination. J Biol Chem. 2014;289:11986–11992. doi: 10.1074/jbc.M114.557694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186:866–876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuppen E, Nagata S, Wieringa B, Hendriks W. No evidence for involvement of mouse protein-tyrosine phosphatase-BAS-like Fas-associated phosphatase-1 in Fas-mediated apoptosis. J Biol Chem. 1997;272:30215–30220. doi: 10.1074/jbc.272.48.30215. [DOI] [PubMed] [Google Scholar]
- 46.Wieckowski E, Atarashi Y, Stanson J, Sato TA, Whiteside TL. FAP-1-mediated activation of NF-kappaB induces resistance of head and neck cancer to Fas-induced apoptosis. J Cell Biochem. 2007;100:16–28. doi: 10.1002/jcb.20922. [DOI] [PubMed] [Google Scholar]
- 47.Hoover AC, Strand GL, Nowicki PN, Anderson ME, Vermeer PD, Klingelhutz AJ, et al. Impaired PTPN13 phosphatase activity in spontaneous or HPV-induced squamous cell carcinomas potentiates oncogene signaling through the MAP kinase pathway. Oncogene. 2009;28:3960–3970. doi: 10.1038/onc.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scrima M, De Marco C, De Vita F, Fabiani F, Franco R, Pirozzi G, et al. The nonreceptor-type tyrosine phosphatase PTPN13 is a tumor suppressor gene in non-small cell lung cancer. Am J Pathol. 2012;180:1202–1214. doi: 10.1016/j.ajpath.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 49.Glondu-Lassis M, Dromard M, Lacroix-Triki M, Nirdé P, Puech C, Knani D, et al. PTPL1/PTPN13 regulates breast cancer cell aggressiveness through direct inactivation of Src kinase. Cancer Res. 2010;70:5116–5126. doi: 10.1158/0008-5472.CAN-09-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu JH, Chen R, Yi W, Cantin GT, Fearns C, Yang Y, et al. Protein tyrosine phosphatase PTPN13 negatively regulates Her2/ErbB2 malignant signaling. Oncogene. 2008;27:2525–2531. doi: 10.1038/sj.onc.1210922. [DOI] [PubMed] [Google Scholar]
- 51.Yang S, Banerjee S, de Freitas A, Sanders YY, Ding Q, Matalon S, et al. Participation of miR-200 in pulmonary fibrosis. Am J Pathol. 2012;180:484–493. doi: 10.1016/j.ajpath.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 53.Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006;2:e119. doi: 10.1371/journal.pgen.0020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strieter RM, Starko KM, Enelow RI, Noth I, Valentine VG Idiopathic Pulmonary Fibrosis Biomarkers Study Group. Effects of interferon-gamma 1b on biomarker expression in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2004;170:133–140. doi: 10.1164/rccm.200312-1670OC. [DOI] [PubMed] [Google Scholar]
- 55.Huang SK, White ES, Wettlaufer SH, Grifka H, Hogaboam CM, Thannickal VJ, et al. Prostaglandin E(2) induces fibroblast apoptosis by modulating multiple survival pathways. FASEB J. 2009;23:4317–4326. doi: 10.1096/fj.08-128801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maher TM, Evans IC, Bottoms SE, Mercer PF, Thorley AJ, Nicholson AG, et al. Diminished prostaglandin E2 contributes to the apoptosis paradox in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;182:73–82. doi: 10.1164/rccm.200905-0674OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giri SN, Hyde DM, Marafino BJ., Jr Ameliorating effect of murine interferon gamma on bleomycin-induced lung collagen fibrosis in mice. Biochem Med Metab Biol. 1986;36:194–197. doi: 10.1016/0885-4505(86)90124-6. [DOI] [PubMed] [Google Scholar]
- 58.Jiang D, Liang J, Hodge J, Lu B, Zhu Z, Yu S, et al. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest. 2004;114:291–299. doi: 10.1172/JCI16861. [DOI] [PMC free article] [PubMed] [Google Scholar]