To the Editor:
Bronchopulmonary dysplasia (BPD) is the most common long-term pulmonary complication associated with extreme preterm birth (1). BPD is a complex lung injury syndrome caused by a combination of prenatal and postnatal insults associated with prematurity that result in altered lung development (2). A major challenge to development of adequate prevention and treatment strategies is limited understanding of BPD endotypes, which are subtypes with distinct pathobiological mechanisms (1). Moreover, characterization of the molecular landscape of late-stage human lung development and how it is affected by premature birth remains incomplete, in part owing to limited availability of human tissue samples.
To address these limitations, we explored the potential of tracheal aspirate–derived mesenchymal stromal cells (MSCs) to reflect the molecular landscape of the preterm lung. Earlier reports have demonstrated that these cells are lung-resident stromal cells and can be expanded in culture (3). We applied weighted gene coexpression network analysis (WGCNA) to explore the highly dimensional gene expression space of premature tracheal aspirate–derived MSC lines (4). The gene regulatory network analysis revealed gene modules that correlated with the corrected gestational age (CGA) at MSC collection and the severity of BPD, suggesting that tracheal aspirate–derived MSC transcriptomes retain transcriptional dynamics of the preterm lung. Some of the results of this study were previously reported in the form of an abstract (5).
Methods
Twenty MSC lines were derived from tracheal aspirates collected during routine endotracheal suctioning of 19 intubated newborns born between 23 and 42 weeks as previously described (3). Tracheal aspirates were collected in 1 ml of normal saline and stored at 4°C for no longer than 12 hours. Cells were isolated from the tracheal aspirate and plated on untreated tissue culture plastic in regular Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum, 1% glutamine, and 1% antibiotics. After 2–4 weeks, when the cellular outgrowth reached 80% confluency, the cells were trypsinized and purified using fluorescence-activated cell sorting with an MSC phenotyping kit, which selects for cells expressing CD73, CD90, and CD105 and excludes cells expressing CD14, CD20, CD34, and CD45 (130-095-198; Miltenyi Biotec). All MSC lines have a doubling time of approximately 24 hours and, when tested, differentiated into osteoblasts, adipocytes, and chondroblasts, regardless of the CGA at collection (A1007201, A1041001, and A1007101, respectively; Thermo Fisher Scientific). BPD severity was defined as previously reported (6). Respiratory support and oxygen dependence at 36 weeks postmaturational age were determined by chart review; an oxygen reduction test was not performed. MSC line transcriptome sequencing on three (in a few cases, two) technical replicates (global r = 0.97) was performed at passages 6–13 using the HiSeq 2500 system (Illumina) for single-end 50-bp read (mean read depth, 44 × 106; mean mapping rate, 96%). Gene expression data were preprocessed to include genes with over 0.5 count per million in at least two replicates. WGCNA analysis was then performed on log-transformed transcripts per million values after batch correction with the Bioconductor sva package (version 3.22.0) because these data originated from different batches (7).
Results and Discussion
We selected 20 tracheal aspirate–derived MSC lines to reflect a spectrum of gestational maturity and BPD. We defined intrauterine development MSCs (n = 14) as those lines generated from samples collected in the same canonical stage of lung development in which the patient was born: canalicular (22–26 wk GA) (n = 4), saccular (26 wk and 1 d to 36 wk GA) (n = 6), or alveolar (36 wk and 1 d to 42 wk GA) (n = 4). MSC lines generated from samples collected in a subsequent lung development stage from the stage at birth were defined as extrauterine development MSCs (n = 6). Ten MSC lines collected at or before 32 weeks CGA were obtained from patients with BPD (any degree). Although MSCs are ubiquitous in human tissues, the transcriptomic signature of MSCs varies as a function of their anatomic source (8). Principal component analysis of whole-genome transcriptomes revealed cosegregation of the tracheal aspirate–derived MSC lines with two human lung fibroblast cell lines (IMR90, CCD34) and no clustering with a dermal fibroblast cell line (BJ1), thus suggesting their lung tissue specificity.
To study the complex relationships between the MSC transcriptome and various clinical variables, we applied WGCNA analysis to the 20 RNA-sequencing profiles. WGCNA relies on the assumption that strongly correlated expression levels of a group of genes (modules) indicate that those genes may work cooperatively in related pathways and could contribute to a specific phenotype. To identify relevant module–trait relationships, correlations between the modules and selected clinical traits were calculated. We identified two modules of particular interest. The GA module (69 genes) had a significant direct correlation with birth GA (r = 0.65; P = 5 ×10−8) and CGA (r = 0.68; P = 8 ×10−9) at the time of MSC collection. The BPD module (286 genes) significantly correlated with BPD severity (r = 0.55; P = 9 ×10−6), days of mechanical ventilation (MV) at the time of collection (r = 0.54; P = 2 ×10−5), and total days of MV (r = 0.54; P = 4 ×10−5) during the patient’s course.
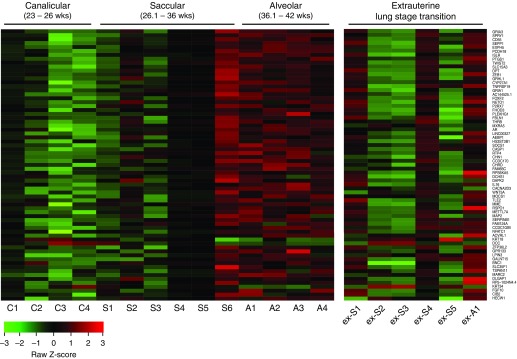
Because the extrauterine MSCs reflect an extrauterine lung developmental stage transition, the GA module gene expression profiles are displayed in two separate heat maps for intrauterine and extrauterine development MSC lines, respectively (Figure 1). Visual representation of the expression level of GA module member genes in intrauterine development MSCs suggested transitions in gene expression that correlated with known histologic transitions of lung development (Figure 1, left heat map). The first transition occurred between the canalicular and saccular stages, and another marked the transition of the saccular stage to the alveolar. Interestingly, a transition was also seen within the saccular stage, at around 32 weeks. The presence of novel molecular substages within the human pseudoglandular stage has previously been reported (9). In this letter, we report the possible presence of early (26 wk and 1 d to 32 wk) and late (32 wk and 1 d to 36 wk) molecular substages during the saccular stage.
Figure 1.
Heat maps depicting relative gestational age (GA) module member gene expression for individual mesenchymal stromal cell (MSC) lines. All 69 genes are listed and ranked by intramodular connectivity (highest at the top). In the left heat map, intrauterine development MSC lines are ranked by increasing GA and are grouped according to the canonical stages of lung development at the time of birth/MSC collection. In the right heat map, extrauterine development MSC lines are ranked by increasing GA and are grouped according to the canonical stage of lung development at the time of MSC collection, which for these MSC lines differs from the stage at birth. A = alveolar; C = canalicular; ex-A = extrauterine alveolar; ex-S = extrauterine saccular; S = saccular.
In contrast to intrauterine development MSCs, extrauterine development MSCs presented a high degree of heterogeneity of gene expression within GA modules (Figure 1, right heat map), likely reflecting the heterogeneous clinical course over a significantly higher number of MV days (P < 0.01) between intrauterine (4.3 ± 4.2 d) and extrauterine development (13.5 ± 3.7 d) MSCs. This finding would be consistent with the notion that GA module gene expression reflects the development trajectory of MSCs, which is likely to be disrupted in the extrauterine environment. Within the GA module, gene ontology (GO) processes relevant for lung development were enriched: regulation of animal organ formation (GO:0003156; P = 4.9 ×10−6), cell surface receptor signaling pathway (GO:0007166; P = 1.99 ×10−5), and response to transforming growth factor-β (GO:0071559; P = 1.09 ×10−4). A protein–protein interaction analysis (STRING database) identified developmental stage–specific signaling pathways (e.g., transforming growth factor-β, fibroblast growth factor, and Wnt/β-catenin) and transcription factors (e.g., Foxf2 [forkhead box F2], Twist2 [Twist family BHLH transcription factor 2]) (10, 11).
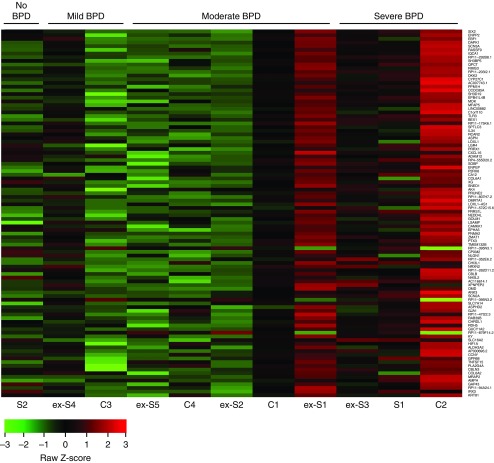
The BPD module demonstrated a clear correlation of the MSC transcriptome with BPD clinical severity, as displayed in the heat map of the top 100 BPD module genes for lines collected from newborns born at 32 weeks GA or less (Figure 2). Interestingly, there was noticeable heterogeneity, particularly among MSCs from patients with moderate BPD. For example, extrauterine saccular (ex-S) MSC lines ex-S1 and ex-S2 were derived from two siblings of a triplet gestation and have very different expression profiles. We suspect that this finding reflects patient heterogeneity not captured by the clinical definition of BPD and could be a future strategy for identifying BPD endotypes. Within the BPD module, the most significantly enriched GO term was extracellular matrix organization (GO:0030198; P = 9.26 ×10−6), and STRING analysis identified a strong enrichment of various extracellular matrix components (e.g., LOXL1 [lysyl oxidase-like 1], COL6A1 [collagen type VI alpha 1], ITGA7 [integrin subunit alpha 7]) known to be part of a functionally interconnected network perturbed in BPD (12).
Figure 2.
Heat map depicting relative bronchopulmonary dysplasia (BPD) module member gene expression for individual mesenchymal stromal cell lines. Genes are ranked by intramodular connectivity (top 100 of 286 are shown here, with highest at the top). The BPD module correlates with BPD, and thus lines chosen for this analysis were all mesenchymal stromal cells from patients born at gestational age less than 32 weeks. C = canalicular; ex-S = extrauterine saccular; S = saccular.
One limitation of this study is that our cohort, though representing a large spectrum of gestational maturity, is relatively small. Nonetheless, the transcriptomes of tracheal aspirate–derived MSCs show a clear association with late-stage human lung development and the severity of BPD. The translational potentials of our findings are important because tracheal aspirate–derived MSCs represent a novel ex vivo system to study the premature human lung. Further studies are necessary to investigate the predictive potential of the MSC molecular landscape and to validate it as a biomarker for BPD.
Footnotes
Supported by Massachusetts General Hospital and Brigham and Women’s Hospital intramural funds. Funding for bioinformatics analyses conducted by R.S.K. at the Harvard Chan Bioinformatics Core was provided by the Harvard Stem Cell Institute Center for Stem Cell Bioinformatics, as well as by Harvard Catalyst, The Harvard Clinical and Translational Science Center (NIH award UL1 RR025758 and financial contributions from participating institutions). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the NIH.
Author Contributions: R.S.: designed experiments and analyses, conducted experiments, acquired and analyzed data, and drafted the manuscript; J.L.: designed analyses, analyzed data, and edited the manuscript; R.S.K.: analyzed data and edited the manuscript; C.Z.: analyzed data; A.I.: designed and conducted experiments; H.L.: designed analyses and analyzed data; Y.S.: helped to design experiments; and P.H.L.: designed analyses, analyzed data, edited the manuscript, and provided final approval of the submitted version.
Originally Published in Press as DOI: 10.1164/rccm.201801-0024LE on May 14, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.McEvoy CT, Jain L, Schmidt B, Abman S, Bancalari E, Aschner JL. Bronchopulmonary dysplasia: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann Am Thorac Soc. 2014;11(Suppl 3):S146–S153. doi: 10.1513/AnnalsATS.201312-424LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jobe AH. Mechanisms of lung injury and bronchopulmonary dysplasia. Am J Perinatol. 2016;33:1076–1078. doi: 10.1055/s-0036-1586107. [DOI] [PubMed] [Google Scholar]
- 3.Hennrick KT, Keeton AG, Nanua S, Kijek TG, Goldsmith AM, Sajjan US, et al. Lung cells from neonates show a mesenchymal stem cell phenotype. Am J Respir Crit Care Med. 2007;175:1158–1164. doi: 10.1164/rccm.200607-941OC. [DOI] [PubMed] [Google Scholar]
- 4.Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. 2005;4:Article 17. doi: 10.2202/1544-6115.1128. [DOI] [PubMed] [Google Scholar]
- 5.Spadafora R, Khetani R, Shi Y, Lerou PH.A new human ex-vivo model to investigate the late stage of lung development. Presented at the Pediatric Academic Societies annual meeting. May 6–9, 2017. San Francisco, CA [Google Scholar]
- 6.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 7.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–883. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemos DR, Duffield JS. Tissue-resident mesenchymal stromal cells: Implications for tissue-specific antifibrotic therapies. Sci Transl Med. 2018;10:eaan5174. doi: 10.1126/scitranslmed.aan5174. [DOI] [PubMed] [Google Scholar]
- 9.Kho AT, Bhattacharya S, Tantisira KG, Carey VJ, Gaedigk R, Leeder JS, et al. Transcriptomic analysis of human lung development. Am J Respir Crit Care Med. 2010;181:54–63. doi: 10.1164/rccm.200907-1063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda Y, Davé V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87:219–244. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- 11.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mižíková I, Morty RE. The extracellular matrix in bronchopulmonary dysplasia: target and source. Front Med (Lausanne) 2015;2:91. doi: 10.3389/fmed.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]