Abstract
Primary cytomegalovirus (CMV) infection leads to strong innate and adaptive immune responses against the virus, which prevents serious disease. However, CMV infection can cause serious morbidity and mortality in individuals who are immunocompromised. The adaptive immune response to CMV is characterized by large populations of effector-memory (EM) T cells that are maintained lifelong, a process termed memory inflation. Recent findings indicate that infection with CMV leads to continuous differentiation of CMV-specific EM-like T cells and that high-dose infection accelerates this progression. Whether measures that counteract CMV infection, such as anti-viral drugs, targeting of latently infected cells, adoptive transfer of CMV-specific T cells, and vaccination strategies, are able to impact the progressive differentiation of CMV-specific EM-like cells is discussed.
Keywords: Cytomegalovirus, memory CD8 T cell, memory inflation, T cell differentiation
Introduction
Human cytomegalovirus (HCMV) is a highly prevalent virus that establishes a state of persistent infection 1. Primary infection rarely causes severe disease in immunocompetent individuals. However, infection of immunocompromised individuals (for example, untreated HIV and transplant patients) or congenitally infected children ultimately can result in serious disease and mortality 2. CMV is also considered to play a role in immune senescence, although its role herein is controversial 3, 4.
During primary HCMV infection, there is a strong natural killer cell response, which is succeeded by the formation of humoral and cellular immunity 5. CMV immunity comprises neutralizing antibodies and the generation of CMV-specific CD4 + and CD8 + T cells recognizing an extensive range of viral proteins. On average, the T-cell response to CMV is exceptionally high. About 10% of the memory T-cell compartment in blood is CMV specific 6 and therefore HCMV is considered one of the most immunogenic pathogens for humans. However, the range of T-cell frequencies in the blood of infected individuals is quite variable, ranging from barely detectable to very high (even above 40%), and this variance is likely caused by differences in the infectious dose and host-intrinsic factors. Importantly, HCMV infection has been demonstrated to be a major driver of the variation in the immune system by systems-level analysis 7.
Despite robust primary immune responses leading to control of primary infection, the virus is never cleared. The establishment of latent infection and subsequent recurrent viral reactivation from latency are related to numerous sophisticated immune evasion strategies of the virus. For example, CMV-encoded genes impair major histocompatibility complex (MHC) class I and II-restricted antigen processing and presentation, which suppresses CD8 + and CD4 + T-cell recognition 8, 9. CMV also prevents the activation of T cells by down-modulating co-stimulatory ligands on infected antigen-presenting cells 10, 11.
Although latent infection suggests a silent state, it has become evident that changes in the phenotype of virus-reactive cells occur during the course of persistent infection and that these changes are related to factors such as the initial dose of viral inoculum and aging. Here, we discuss recent findings regarding the differentiation of CMV-specific T cells and interventions that counteract CMV-associated perturbations that may impact T-cell differentiation.
Progressive differentiation of cytomegalovirus-specific effector-memory T cells
The T-cell response to CMV is exceptional because of the large numbers of functional effector-memory-like (EM-like) cells that are induced and maintained lifelong in blood and tissue. This phenomenon, termed memory inflation 12– 14, relates to the low-level persistence of the virus, as demonstrated by viral latency and intermittent viral reactivation. In healthy hosts, the infectious dose is a strong determinant of the degree of memory inflation that occurs 15. The circulating EM-like T cells that are induced upon CMV infection express markers such as KLRG1 and CD44, whereas expression of CD62L, CD127 (IL-7Rα), and the co-stimulatory molecules CD27 and CD28 is downregulated or lost 16– 18. In tissues, not only circulating EM-like CMV-specific T cells but also CMV-specific non-recirculating tissue-resident memory (TRM) T cells are present. These TRM T cells, considered a distinct memory population 19, are characterized by CD69 expression and, depending on the tissue, also express CD103 20. CMV-specific memory T cells with a central-memory (CM)-like phenotype (CD62L +, CD127 +, CD27 +, CD28 +, KLRG1 −, and IL-2 +) also exist and are thought to dominantly contribute to population expansion upon re-challenge 21. Systemic control of CMV infection likely depends on the collective contribution of the circulating and non-circulating CMV-specific T cells.
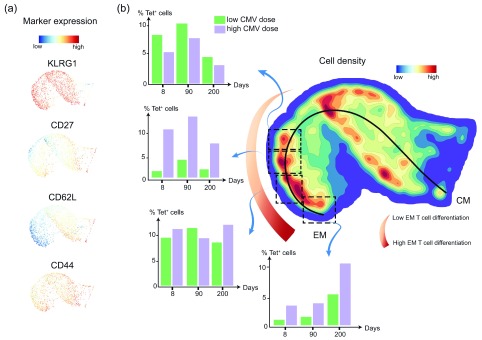
With the use of novel computational tools that allow the analysis of cytometry data with much finer detail 22, 23, we recently discovered that CMV infection continuously affects the differentiation of the virus-specific EM-like cells 24. Inflationary T cells seem to undergo progressive differentiation unremittingly, and this was most clearly observed upon high-dose infection. Quantification of inflationary CMV-specific CD8 + T cells in different stages of EM differentiation performed with the previously described Cytosplore data set 24 revealed that the differentiation of these EM-like inflationary CMV-specific T cells strikingly increases over time ( Figure 1). High-dose infection accelerated this progressive profile of EM differentiation compared with a lower dose. This quantification unequivocally shows that CMV infection causes progressive EM T-cell differentiation that continues throughout the life span of the host and that the grade of infection (for example, low versus high) impacts the degree of circulating EM T-cell differentiation. It is currently unknown whether TRM T-cell differentiation is impacted by aging and infectious dose.
Figure 1. Progressive differentiation of cytomegalovirus (CMV)-specific effector-memory (EM) CD8 + T cells and its relation to the initial viral inoculum.
C57BL/6 mice were infected intraperitoneally with a low (10 3 plaque-forming units, or PFU) or a high (10 5 PFU) dose of mouse CMV (MCMV)-Smith. Inflationary IE3-specific CD8 + T cells in blood were detected with major histocompatibility complex (MHC) class I tetramer (Tet) and stained for the cell surface markers CD62L, KLRG1, CD27, and CD44, which allow discrimination between central-memory (CM) and EM-like T cells at days 8, 90, and 200 after infection. ( a) IE3-specific cells were gated (Tet +) and an equally down-sampled number of cells per sample was analyzed by Cytosplore. Single-marker expression of IE3-specific CD8 + T cells is shown as Approximated t-distributed Stochastic Neighbor Embedding (A-tSNE) scatterplots to visualize the intensity of the markers. ( b) A-tSNE plot depicts the pooled phenotypical data of the cell surface markers visualized as cell density clusters of the IE3-specific CD8 + T cells of low- and high-dose MCMV-infected mice for day 8, 90, and 200 time points after infection. In the A-tSNE plot, the differentiation path from the CM and EM phenotype is specified. The black curved line indicates the ongoing shift toward a higher advanced EM phenotype. Clusters showing different points in the differentiation path were selected and indicated by dashed line boxes. The percentage of IE3-specific CD8 + T cells in the selected clusters was determined and displayed in the corresponding bar graphs for each time point and viral inoculum.
Progressive EM T-cell differentiation might be dependent on the differentiation of naïve and CM-like T cells into EM T cells 17 but as well on the stimulation and expansion of less EM-differentiated cells into more differentiated populations. CD27 expression, which is higher on less differentiated cells, gradually declines on the cell surface of inflationary EM T-cell populations over time. Thus, CD27 has a likely role in progressive EM T-cell differentiation and coincides with the requirement of CD27–CD70 interactions to support and maintain memory inflation 25. Moreover, CD27-expressing memory T cells can restore inflationary populations during latency 26. The anti-apoptotic molecule Bcl-2 accumulates over time and presumably is essential for the survival of the inflationary EM T cells 27. Correspondingly, the half-life of inflationary EM T cells is considerably longer than that of effector T cells, which have lower Bcl-2 levels 28. Together, these findings fuel the concept that less-differentiated EM-like T cells can accumulate into more differentiated EM cells and that these cells have a prolonged survival. Whether the cytokines IL-2 and IL-15, implicated in the maintenance of inflationary T cells 28, 29, also directly contribute to the differentiation of the T cells is unclear, but surely higher levels of CMV drive the T-cell differentiation to a more advanced EM phenotype.
Whether progressive EM T-cell differentiation is a non-functional response against the persistent presence of CMV antigens or a functional adaptation of the CMV-reactive T cells to keep control over CMV infection is unknown, but it may affect cellular function and senescence. In humans, latent CMV infection in older individuals resulted in lower protection rates after vaccination with an influenza vaccine 30– 32; however, neutral effects of CMV infection were also observed 33– 36. Possibly related to some of these studies is the finding that high-dose but not low-dose CMV infection impairs the development of a heterologous anti-viral T-cell response 24, 37, 38. In contrast to these possible negative outcomes of persistent CMV infection, recent studies are indicating positive effects. For example, it was shown that old mice infected with mouse CMV (MCMV) had a broader T-cell response compared with non-infected old mice after challenge with Listeria monocytogenes 39. Notably, positive effects of CMV infection early after infection have been well documented and may relate to a heightened innate immune activation status 24, 35, 40. Together, these studies imply that the progressive differentiation of CMV-specific EM-like T cells may be either negatively or positively affecting host immunity. CMV latency and its impact on T-cell responses thus may reflect a virus–host balance that can be impacted by the infectious dose and aging. Nevertheless, it is likely that lowering of the viral load is key to diminishing putative T-cell senescence, as lower viral loads lead to a reduction in EM T-cell differentiation 15, 41.
Measures to counteract cytomegalovirus-associated perturbations and their impact on T-cell differentiation
In cases where CMV-associated perturbations are known to be a negative factor (for example, in congenital infection and viral reactivation after transplantation), measures to reduce the burden of CMV infection are being investigated. Several approaches, such as anti-viral drugs, treatments targeting latently infected cells, adoptive transfer of CMV-specific T cells, and (prophylactic) vaccines, have been developed.
Anti-viral drugs targeting CMV are commonly used for transplantation patients with clinical reactivation of CMV upon transplantation 42. In these patients, the use of anti-viral drugs can reduce viral load 43, but not much is known about the effect of anti-viral drugs on the differentiation of CMV-specific EM T cells. Whether anti-viral therapy can be used to reduce EM T-cell differentiation and improve heterologous immunity was recently experimentally assessed by Beswick et al. 44. Administration of valaciclovir to mice with an established MCMV infection resulted in a reduction of the magnitude of the MCMV-specific CD8 + T-cell response. This was accompanied by a less-differentiated phenotype of the residual CD8 + T cells compared with mice that received no anti-viral treatment. Treatment with valaciclovir also reduced influenza A viral loads upon challenge and reduced the differentiation of influenza-specific CD8 + T cells. However, CMV can adapt to become resistant to anti-viral treatment 45, suggesting that treatment with anti-viral drugs might not generally be effective in the long term.
A sophisticated way to target latent infection can be to manipulate the mechanisms used by CMV to avoid detection by the immune system 46. The viral protein UL138, expressed during latency, results in loss of multidrug resistance-associated protein-1 (MRP1) 47. The treatment of latently infected monocytes with vincristine, a cytotoxic agent normally exported by MRP1, resulted in specific ablation of these cells. Also, other genes involved in CMV latency (for example, US28 encoding a cell surface G-protein-coupled receptor 48, 49 and LUNA encoding a motif with deSUMOylase activity 50) could be targeted to clear CMV. Such treatments may be used to eliminate latently infected cells before transplantation. However, because a wide range of cells is latently infected during CMV infection, this therapy could result in unwanted side effects. But it is likely that a substantial reduction in latently infected cells, if effective, will diminish memory inflation and the ongoing EM T-cell differentiation.
Adoptive transfer of HCMV-specific T cells is a method used to restore CMV-specific immunity in transplant recipients and has been shown to reduce the risk for HCMV infection or reactivation or both 51, 52. For this type of treatment, it is expected that different subsets of CMV-specific T cells being transferred have different effects on protective immunity and viral load 53. Some studies have examined the relationship of the T-cell phenotype with the clinical outcome 54– 56. Positive correlations were found between population expansion and the number of CM-like cells within the transferred population. Weeks after transfer, the majority of the expanded CD8 + T cells nevertheless become highly differentiated. In-depth studies are required to assess the impact and level of progressive EM T-cell differentiation that occurs in adoptive T-cell transfer settings.
Prophylactic vaccination strategies have the potential to reduce the viral load of CMV. Several different CMV vaccination platforms have been developed 57, 58. Most of these concentrated on eliciting antibodies but some (also) aimed to induce robust CMV-specific T-cell immunity 59– 61. Vaccination with live-attenuated and replication-deficient CMV vectors seems to induce CD8 + T-cell responses undergoing less memory inflation, including the induction of the associated EM cell phenotype 62, 63. Vaccination with synthetic long peptides containing MCMV epitopes induces strong and polyfunctional CD8 + T-cell responses, but whereas responses to these epitopes are inflationary in the MCMV setting, they are non-inflationary in the peptide vaccine setting, which corresponds with their reduced EM-like phenotype 64, 65. Nevertheless, such vaccines are able to reduce viral load upon challenge with virulent CMV. It will be of interest to decipher the significance of the T-cell differentiation phenotype in relation to the effectiveness of CMV vaccines.
In conclusion, CMV infection results in a progressive differentiation of viral-specific EM T cells. The consequences of such a progressive differentiation may have both detrimental and beneficial effects on the virus–host balance and require further investigation. Such investigations may reveal opportunities to optimize immune function in CMV-seropositive people. Whether progressive differentiation is a distinctive property of the EM-like CMV-specific T cells undergoing inflation or whether progressive differentiation also occurs in other T-cell subsets (for example, TRM T cells) and in other infection settings remains to be explored.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Paul Moss, Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK
Ian Humphreys, Division of Infection Immunity, School of Medicine/Systems Immunity University Research Institute, Cardiff University, Cardiff, UK
Christopher Snyder, Department of Immunology and Microbiology, Thomas Jefferson University, Philadelphia, PA, USA
Funding Statement
This work was funded by a grant from NWO-TTW (project 15380, awarded to RA) and by a Horizon 2020 MSCA grant (project 675743, acronym ISPIC) from the European Commission.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. Cannon MJ, Schmid DS, Hyde TB: Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20(4):202–13. 10.1002/rmv.655 [DOI] [PubMed] [Google Scholar]
- 2. Griffiths P, Baraniak I, Reeves M: The pathogenesis of human cytomegalovirus. J Pathol. 2015;235(2):288–97. 10.1002/path.4437 [DOI] [PubMed] [Google Scholar]
- 3. Arens R, Remmerswaal EB, Bosch JA, et al. : 5 th International Workshop on CMV and Immunosenescence - A shadow of cytomegalovirus infection on immunological memory. Eur J Immunol. 2015;45(4):954–7. 10.1002/eji.201570044 [DOI] [PubMed] [Google Scholar]
- 4. Sansoni P, Vescovini R, Fagnoni FF, et al. : New advances in CMV and immunosenescence. Exp Gerontol. 2014;55:54–62. 10.1016/j.exger.2014.03.020 [DOI] [PubMed] [Google Scholar]
- 5. Picarda G, Benedict CA: Cytomegalovirus: Shape-Shifting the Immune System. J Immunol. 2018;200(12):3881–9. 10.4049/jimmunol.1800171 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Sylwester AW, Mitchell BL, Edgar JB, et al. : Broadly targeted human cytomegalovirus-specific CD4 + and CD8 + T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202(5):673–85. 10.1084/jem.20050882 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Brodin P, Jojic V, Gao T, et al. : Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160(1–2):37–47. 10.1016/j.cell.2014.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Arens R: Rational design of vaccines: learning from immune evasion mechanisms of persistent viruses and tumors. Adv Immunol. 2012;114:217–43. 10.1016/B978-0-12-396548-6.00009-3 [DOI] [PubMed] [Google Scholar]
- 9. Powers C, DeFilippis V, Malouli D, et al. : Cytomegalovirus immune evasion. Curr Top Microbiol Immunol. 2008;325:333–59. 10.1007/978-3-540-77349-8_19 [DOI] [PubMed] [Google Scholar]
- 10. Arens R, Loewendorf A, Her MJ, et al. : B7-mediated costimulation of CD4 T cells constrains cytomegalovirus persistence. J Virol. 2011;85(1):390–6. 10.1128/JVI.01839-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moutaftsi M, Mehl AM, Borysiewicz LK, et al. : Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood. 2002;99(8):2913–21. 10.1182/blood.V99.8.2913 [DOI] [PubMed] [Google Scholar]
- 12. Karrer U, Sierro S, Wagner M, et al. : Memory inflation: continuous accumulation of antiviral CD8 + T cells over time. J Immunol. 2003;170(4):2022–9. 10.4049/jimmunol.170.4.2022 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. O'Hara GA, Welten SP, Klenerman P, et al. : Memory T cell inflation: understanding cause and effect. Trends Immunol. 2012;33(2):84–90. 10.1016/j.it.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 14. Klenerman P: The (gradual) rise of memory inflation. Immunol Rev. 2018;283(1):99–112. 10.1111/imr.12653 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Redeker A, Welten SP, Arens R: Viral inoculum dose impacts memory T-cell inflation. Eur J Immunol. 2014;44(4):1046–57. 10.1002/eji.201343946 [DOI] [PubMed] [Google Scholar]
- 16. Munks MW, Cho KS, Pinto AK, et al. : Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J Immunol. 2006;177(1):450–8. 10.4049/jimmunol.177.1.450 [DOI] [PubMed] [Google Scholar]
- 17. Snyder CM, Cho KS, Bonnett EL, et al. : Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29(4):650–9. 10.1016/j.immuni.2008.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Hertoghs KM, Moerland PD, van Stijn A, et al. : Molecular profiling of cytomegalovirus-induced human CD8 + T cell differentiation. J Clin Invest. 2010;120(11):4077–90. 10.1172/JCI42758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Welten SPM, Sandu I, Baumann NS, et al. : Memory CD8 T cell inflation vs tissue-resident memory T cells: Same patrollers, same controllers? Immunol Rev. 2018;283(1):161–75. 10.1111/imr.12649 [DOI] [PubMed] [Google Scholar]
- 20. Mueller SN, Mackay LK: Tissue-resident memory T cells: Local specialists in immune defence. Nat Rev Immunol. 2016;16(2):79–89. 10.1038/nri.2015.3 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Redeker A, Welten SP, Baert MR, et al. : The Quantity of Autocrine IL-2 Governs the Expansion Potential of CD8 + T Cells. J Immunol. 2015;195(10):4792–801. 10.4049/jimmunol.1501083 [DOI] [PubMed] [Google Scholar]
- 22. Hollt T, Pezzotti N, van Unen V, et al. : CyteGuide: Visual Guidance for Hierarchical Single-Cell Analysis. IEEE Trans Vis Comput Graph. 2018;24(1):739–48. 10.1109/TVCG.2017.2744318 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. van Unen V, Höllt T, Pezzotti N, et al. : Visual analysis of mass cytometry data by hierarchical stochastic neighbour embedding reveals rare cell types. Nat Commun. 2017;8(1): 1740. 10.1038/s41467-017-01689-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Redeker A, Remmerswaal EBM, van der Gracht ETI, et al. : The Contribution of Cytomegalovirus Infection to Immune Senescence Is Set by the Infectious Dose. Front Immunol. 2017;8:1953. 10.3389/fimmu.2017.01953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Welten SP, Redeker A, Franken KL, et al. : CD27-CD70 costimulation controls T cell immunity during acute and persistent cytomegalovirus infection. J Virol. 2013;87(12):6851–65. 10.1128/JVI.03305-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quinn M, Turula H, Tandon M, et al. : Memory T cells specific for murine cytomegalovirus re-emerge after multiple challenges and recapitulate immunity in various adoptive transfer scenarios. J Immunol. 2015;194(4):1726–36. 10.4049/jimmunol.1402757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Torti N, Walton SM, Brocker T, et al. : Non-hematopoietic cells in lymph nodes drive memory CD8 T cell inflation during murine cytomegalovirus infection. PLoS Pathog. 2011;7(10):e1002313. 10.1371/journal.ppat.1002313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baumann NS, Torti N, Welten SPM, et al. : Tissue maintenance of CMV-specific inflationary memory T cells by IL-15. PLoS Pathog. 2018;14(4):e1006993. 10.1371/journal.ppat.1006993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bachmann MF, Wolint P, Walton S, et al. : Differential role of IL-2R signaling for CD8 + T cell responses in acute and chronic viral infections. Eur J Immunol. 2007;37(6):1502–12. 10.1002/eji.200637023 [DOI] [PubMed] [Google Scholar]
- 30. Wald A, Selke S, Magaret A, et al. : Impact of human cytomegalovirus (CMV) infection on immune response to pandemic 2009 H1N1 influenza vaccine in healthy adults. J Med Virol. 2013;85(9):1557–60. 10.1002/jmv.23642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Turner JE, Campbell JP, Edwards KM, et al. : Rudimentary signs of immunosenescence in Cytomegalovirus-seropositive healthy young adults. Age (Dordr). 2014;36(1):287–97. 10.1007/s11357-013-9557-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trzonkowski P, Myśliwska J, Szmit E, et al. : Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination--an impact of immunosenescence. Vaccine. 2003;21(25–26):3826–36. 10.1016/S0264-410X(03)00309-8 [DOI] [PubMed] [Google Scholar]
- 33. van den Berg SPH, Wong A, Hendriks M, et al. : Negative Effect of Age, but Not of Latent Cytomegalovirus Infection on the Antibody Response to a Novel Influenza Vaccine Strain in Healthy Adults. Front Immunol. 2018;9:82. 10.3389/fimmu.2018.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. den Elzen WP, Vossen AC, Cools HJ, et al. : Cytomegalovirus infection and responsiveness to influenza vaccination in elderly residents of long-term care facilities. Vaccine. 2011;29(29–30):4869–74. 10.1016/j.vaccine.2011.03.086 [DOI] [PubMed] [Google Scholar]
- 35. Furman D, Jojic V, Sharma S, et al. : Cytomegalovirus infection enhances the immune response to influenza. Sci Transl Med. 2015;7(281):281ra43. 10.1126/scitranslmed.aaa2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strindhall J, Ernerudh J, Mörner A, et al. : Humoral response to influenza vaccination in relation to pre-vaccination antibody titres, vaccination history, cytomegalovirus serostatus and CD4/CD8 ratio. Infect Dis (Lond). 2016;48(6):436–42. 10.3109/23744235.2015.1135252 [DOI] [PubMed] [Google Scholar]
- 37. Mekker A, Tchang VS, Haeberli L, et al. : Immune senescence: relative contributions of age and cytomegalovirus infection. PLoS Pathog. 2012;8(8):e1002850. 10.1371/journal.ppat.1002850 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Cicin-Sain L, Brien JD, Uhrlaub JL, et al. : Cytomegalovirus infection impairs immune responses and accentuates T-cell pool changes observed in mice with aging. PLoS Pathog. 2012;8(8):e1002849. 10.1371/journal.ppat.1002849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smithey MJ, Venturi V, Davenport MP, et al. : Lifelong CMV infection improves immune defense in old mice by broadening the mobilized TCR repertoire against third-party infection. Proc Natl Acad Sci U S A. 2018;115(29):E6817–E6825. 10.1073/pnas.1719451115 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Barton ES, White DW, Cathelyn JS, et al. : Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447(7142):326–9. 10.1038/nature05762 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Trgovcich J, Kincaid M, Thomas A, et al. : Cytomegalovirus Reinfections Stimulate CD8 T-Memory Inflation. PLoS One. 2016;11(11):e0167097. 10.1371/journal.pone.0167097 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Ahmed A: Antiviral treatment of cytomegalovirus infection. Infect Disord Drug Targets. 2011;11(5):475–503. 10.2174/187152611797636640 [DOI] [PubMed] [Google Scholar]
- 43. Maffini E, Giaccone L, Festuccia M, et al. : Treatment of CMV infection after allogeneic hematopoietic stem cell transplantation. Expert Rev Hematol. 2016;9(6):585–96. 10.1080/17474086.2016.1174571 [DOI] [PubMed] [Google Scholar]
- 44. Beswick M, Pachnio A, Lauder SN, et al. : Antiviral therapy can reverse the development of immune senescence in elderly mice with latent cytomegalovirus infection. J Virol. 2013;87(2):779–89. 10.1128/JVI.02427-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chou S: Approach to drug-resistant cytomegalovirus in transplant recipients. Curr Opin Infect Dis. 2015;28(4):293–9. 10.1097/QCO.0000000000000170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wills MR, Poole E, Lau B, et al. : The immunology of human cytomegalovirus latency: could latent infection be cleared by novel immunotherapeutic strategies? Cell Mol Immunol. 2015;12(2):128–38. 10.1038/cmi.2014.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weekes MP, Tan SY, Poole E, et al. : Latency-associated degradation of the MRP1 drug transporter during latent human cytomegalovirus infection. Science. 2013;340(6129):199–202. 10.1126/science.1235047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krishna BA, Poole EL, Jackson SE, et al. : Latency-Associated Expression of Human Cytomegalovirus US28 Attenuates Cell Signaling Pathways To Maintain Latent Infection. mBio. 2017;8(6): pii: e01754-17. 10.1128/mBio.01754-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krishna BA, Spiess K, Poole EL, et al. : Targeting the latent cytomegalovirus reservoir with an antiviral fusion toxin protein. Nat Commun. 2017;8: 14321. 10.1038/ncomms14321 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Poole EL, Kew VG, Lau JCH, et al. : A Virally Encoded DeSUMOylase Activity Is Required for Cytomegalovirus Reactivation from Latency. Cell Rep. 2018;24(3):594–606. 10.1016/j.celrep.2018.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Holmes-Liew CL, Holmes M, Beagley L, et al. : Adoptive T-cell immunotherapy for ganciclovir-resistant CMV disease after lung transplantation. Clin Transl Immunology. 2015;4(3):e35. 10.1038/cti.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bao L, Cowan MJ, Dunham K, et al. : Adoptive immunotherapy with CMV-specific cytotoxic T lymphocytes for stem cell transplant patients with refractory CMV infections. J Immunother. 2012;35(3):293–8. 10.1097/CJI.0b013e31824300a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Smith CJ, Quinn M, Snyder CM: CMV-Specific CD8 T Cell Differentiation and Localization: Implications for Adoptive Therapies. Front Immunol. 2016;7:352. 10.3389/fimmu.2016.00352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Peggs KS, Thomson K, Samuel E, et al. : Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis. 2011;52(1):49–57. 10.1093/cid/ciq042 [DOI] [PubMed] [Google Scholar]
- 55. Scheinberg P, Melenhorst JJ, Brenchley JM, et al. : The transfer of adaptive immunity to CMV during hematopoietic stem cell transplantation is dependent on the specificity and phenotype of CMV-specific T cells in the donor. Blood. 2009;114(24):5071–80. 10.1182/blood-2009-04-214684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Berger C, Jensen MC, Lansdorp PM, et al. : Adoptive transfer of effector CD8 + T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118(1):294–305. 10.1172/JCI32103 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Schleiss MR, Permar SR, Plotkin SA: Progress toward Development of a Vaccine against Congenital Cytomegalovirus Infection. Clin Vaccine Immunol. 2017;24(12): pii: e00268-17. 10.1128/CVI.00268-17 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Wang D, Fu TM: Progress on human cytomegalovirus vaccines for prevention of congenital infection and disease. Curr Opin Virol. 2014;6:13–23. 10.1016/j.coviro.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 59. Tang A, Freed DC, Li F, et al. : Functionally inactivated dominant viral antigens of human cytomegalovirus delivered in replication incompetent adenovirus type 6 vectors as vaccine candidates. Hum Vaccin Immunother. 2017;13(12):2763–71. 10.1080/21645515.2017.1308988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. John S, Yuzhakov O, Woods A, et al. : Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine. 2018;36(12):1689–99. 10.1016/j.vaccine.2018.01.029 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Morello CS, Kelley LA, Munks MW, et al. : DNA immunization using highly conserved murine cytomegalovirus genes encoding homologs of human cytomegalovirus UL54 (DNA polymerase) and UL105 (helicase) elicits strong CD8 T-cell responses and is protective against systemic challenge. J Virol. 2007;81(14):7766–75. 10.1128/JVI.00633-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Beswick M, Pachnio A, Al-Ali A, et al. : An attenuated temperature-sensitive strain of cytomegalovirus ( tsm5) establishes immunity without development of CD8 + T cell memory inflation. J Med Virol. 2013;85(11):1968–74. 10.1002/jmv.23688 [DOI] [PubMed] [Google Scholar]
- 63. Snyder CM, Cho KS, Bonnett EL, et al. : Sustained CD8+ T cell memory inflation after infection with a single-cycle cytomegalovirus. PLoS Pathog. 2011;7(10):e1002295. 10.1371/journal.ppat.1002295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Panagioti E, Redeker A, van Duikeren S, et al. : The Breadth of Synthetic Long Peptide Vaccine-Induced CD8 + T Cell Responses Determines the Efficacy against Mouse Cytomegalovirus Infection. PLoS Pathog. 2016;12(9):e1005895. 10.1371/journal.ppat.1005895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Panagioti E, Boon L, Arens R, et al. : Enforced OX40 Stimulation Empowers Booster Vaccines to Induce Effective CD4 + and CD8 + T Cell Responses against Mouse Cytomegalovirus Infection. Front Immunol. 2017;8:144. 10.3389/fimmu.2017.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]