Abstract
The high prevalence of cholesterol gallstones, the availability of new information about pathogenesis, and the relevant health costs due to the management of cholelithiasis in both children and adults contribute to a growing interest in this disease. From an epidemiologic point of view, the risk of gallstones has been associated with higher risk of incident ischemic heart disease, total mortality, and disease-specific mortality (including cancer) independently from the presence of traditional risk factors such as body weight, lifestyle, diabetes, and dyslipidemia. This evidence points to the existence of complex pathogenic pathways linking the occurrence of gallstones to altered systemic homeostasis involving multiple organs and dynamics. In fact, the formation of gallstones is secondary to local factors strictly dependent on the gallbladder (that is, impaired smooth muscle function, wall inflammation, and intraluminal mucin accumulation) and bile (that is, supersaturation in cholesterol and precipitation of solid crystals) but also to “extra-gallbladder” features such as gene polymorphism, epigenetic factors, expression and activity of nuclear receptors, hormonal factors (in particular, insulin resistance), multi-level alterations in cholesterol metabolism, altered intestinal motility, and variations in gut microbiota. Of note, the majority of these factors are potentially manageable. Thus, cholelithiasis appears as the expression of systemic unbalances that, besides the classic therapeutic approaches to patients with clinical evidence of symptomatic disease or complications (surgery and, in a small subgroup of subjects, oral litholysis with bile acids), could be managed with tools oriented to primary prevention (changes in diet and lifestyle and pharmacologic prevention in subgroups at high risk), and there could be relevant implications in reducing both prevalence and health costs.
Keywords: cholesterol gallstones, gallbladder, bile, pathogenesis, primary prevention
Introduction
Gallstone disease is highly predominant in Western countries, where it has a prevalence of up to 15% in adults 1 and is one of the most common causes of hospital admission for gastrointestinal disease in European countries 2. Also, gallstone disease has high health costs, especially in the presence of gallstones that become symptomatic or cause complications 1, 3, 4. The presence of gallstones also generates concerns when occurring in children 5 and adolescents (<20 years of age) 6. Though less frequent than in adults, the prevalence of cholelithiasis in childhood is increasing. In England, an increased incidence of cholecystectomy (from 0.78 in 1997 to 2.7 per 100,000 in 2012) has been reported in children not more than 16 years old 7. In a Canadian population-based, retrospective cohort, the crude incidence of cholecystectomy in subjects under 18 years of age increased from 8.8 per 100,000 person-years in 1993 to 13.0 per 100,000 person-years in 2012 8, and a retrospective study in the US over a nine-year period ending in 2012 registered an increment of cholecystectomies due to pediatric non-hemolytic (cholesterol) gallstones of 216% 9. It has been suggested that this epidemiologic increment during childhood could be due to the increasing prevalence of obesity 6, 10– 16, physical inactivity, diabetes, and early pregnancy 6.
Cholesterol gallstones are found in more than 80% of patients with gallstone, and the pathogenesis involves both local (that is, gallbladder and bile) and systemic factors 17.
Elevated levels of pro-inflammatory proteins such as interleukin-6 (IL-6), IL-10, IL-12(p70), and IL-13 appear to be associated with the risk of gallstones 18. Data from epidemiologic studies suggest an association between gallstone disease and higher risk of incident ischemic heart disease 19, 20 and total mortality and disease-specific mortality (cardiovascular disease and cancer). These links were statistically confirmed after adjustment for potential confounders, including traditional risk factors (such as body weight, cigarette smoking, physical activity, diabetes, hypertension, and hypercholesterolemia) 19– 22. Thus, complex systemic pathways might link the development of gallstones to other metabolic abnormalities (that is, insulin resistance, obesity, type 2 diabetes, non-alcoholic fatty liver disease, and metabolic syndrome itself) 23– 32. The association might also involve extra-gallbladder tumors 33, 34 in the liver 35, stomach 36, and colon 37. In this scenario, cholesterol gallstone disease becomes the expression of systemic metabolic and non-metabolic abnormalities 38.
Pathogenic clues for adequate management of gallstone disease
Critical factors contributing to the formation of cholesterol gallstones are defective gallbladder motility, hypersecretion and accumulation of mucin gel in the gallbladder lumen with ongoing local immune-mediated inflammation, rapid phase transition of cholesterol from supersaturated hepatic bile, and precipitation of solid cholesterol crystals 17, 39. Additional features include gene polymorphism, increased hepatic cholesterol secretion, increased absorption of biliary and dietary cholesterol, sluggish intestinal motility, and qualitative, quantitative, or topographic changes of gut microbiota 17, 40. The complex and variable interactions of such pathogenic factors contributing to cholesterol cholelithiasis require a comprehensive discussion to correctly address the management of the disease.
The relevance of a genetic background in the pathogenesis of cholesterol gallstones is disclosed by studies addressing the family history of cholelithiasis 41, 42, evaluating selected ethnic groups 43, 44, and confirming the presence of specific gene polymorphisms 45– 61. The simple existence of predisposing genetic conditions, however, is not sufficient to promote cholesterol gallstone formation. This concept is supported by studies on twin pairs, where genetic factors play a role in no more than 25 to 30% of subjects with symptomatic gallstones 62, 63. Indeed, genes provide an increased risk of forming gallstones, but several environmental factors such as diet and physical activity 58, 64– 66 but also exposure to chemicals as heavy metals 67, 68 or pesticides 69, 70 (which also involve epigenetic mechanisms 17, 71) must play a crucial additional role in determining how many subjects will effectively develop gallstones. This aspect is particularly relevant for primary prevention by acting on environmental factors.
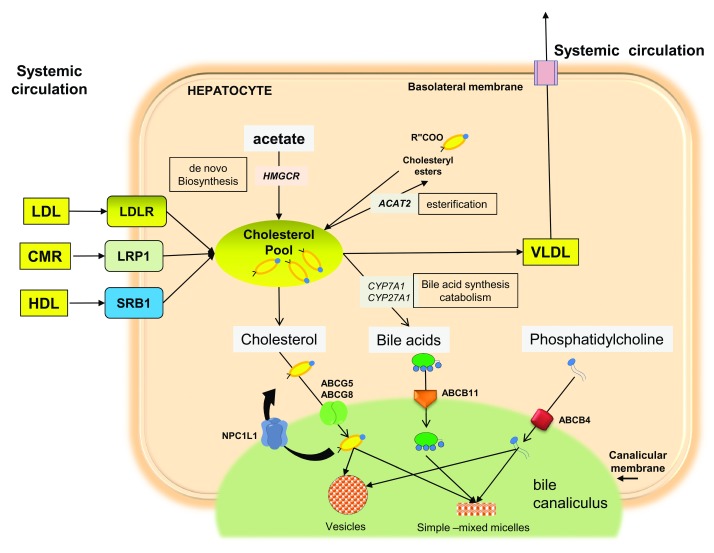
Figure 1 depicts pathways of cholesterol metabolism in the hepatocyte in humans. Cholesterol gallstones grow in the gallbladder and are made of aggregated solid cholesterol crystals originating from bile supersaturated with cholesterol, which is no longer solubilized by micelles and vesicles 40. During the process of cholesterol cholelithiasis, the homeostasis of cholesterol is highly disrupted, and events involve cholesterol intake, metabolism, and synthesis. Defects involve the cholesterol transporters ABCG5 and ABCG8 (which pump sterols out of hepatocytes into the biliary ducts and from enterocytes into the intestinal lumen 72), hormones (that is, estrogen 73, 74, insulin 30, and thyroid hormones 75), nuclear receptors 23, 76– 78, and factors governing the entero-hepatic recirculation of cholesterol 79– 81.
Figure 1. Pathways of cholesterol metabolism in the hepatocyte in humans.
Cholesterol can enter the hepatocytes as low-density lipoprotein (LDL) via the LDL receptor (LDLR), as chylomicron remnants (CMRs) via the prolow-density lipoprotein receptor-related protein 1 (LRP1), and as high-density lipoprotein (HDL) via the scavenger receptor class B member 1 (SRB1). The absorbed cholesterol and the cholesterol synthetized from acetate via the rate-limiting enzyme 3 hydroxy 3 methylglutaryl-coenzyme A reductase (HMGCR) contribute to the intrahepatic cholesterol pool. From here, cholesterol can follow different pathways: esterification (by the acyl-coenzyme A: cholesterol acyltransferase isoform 2, ACAT or SOAT1) and storage in the hepatocyte; incorporation into assembled very-low-density lipoprotein (VLDL) and secretion; catabolic synthesis of bile acids—via the rate-limiting enzymes cholesterol 7α hydroxylase (CYP7A1) and sterol 27 hydroxylase (CYP27A1)—and secretion in bile; and direct secretion into bile. Lipid secretion in bile requires distinct ATP-binding cassette (ABC) transporters at the canalicular membrane of the hepatocytes: the heterodimer ABCG5/G8 for cholesterol, ABCB11 for bile acids, and ABCB4 for phosphatidylcholine. In humans, NPC1L1 is at the canalicular membrane of hepatocytes and is responsible for the reuptake of cholesterol from bile back into the hepatocytes. The nuclear receptors in the hepatocyte (not shown) play a key role at different levels: the farnesoid X receptor (FXR or NR1H1) is controlled via the intestinal fibroblast growth factor 19 (FGF19) and its receptor (FGFR4) in the liver and regulates bile acid synthesis through the small heterodimer partner (SHP) 92, 93. The liver X receptor (LXR or NR1H3) regulates cholesterol synthesis (via the cytochrome P450 51A1, or CYP51A1), bile acid synthesis (via the UDP glucuronosyltransferase 1–3, or UGT1A3), and biliary cholesterol secretion (via transcriptional activation of ABCG5/G8) 94– 96. One to three lipids (that is, cholesterol, bile acids, and phospholipids) are secreted into the bile canaliculus, and they aggregate into the typical cholesterol carriers—that is, simple (1–2 nm), mixed micelles (4–8 nm), and small unilamellar (40–100 nm) or large multilamellar (300–500 nm) vesicles 97.
Women display an increased prevalence of gallstones compared with men because of the influence of estrogen on cholesterol metabolism 73. This effect involves the enhanced synthesis of cholesterol and decreased synthesis of bile acids (BAs), which are sterols synthesized from cholesterol, and represents the main catabolic pathway of cholesterol metabolism in humans. The step involves the upregulation of estrogen receptor 1 and G protein–coupled receptor 30 82, 83. A recent meta-analysis also confirmed a positive association between the intake of exogenous estrogen and the risk of cholelithiasis 74.
The pathogenesis of cholesterol gallstones is closely linked with frequent metabolic abnormalities involving insulin resistance 30, obesity, dyslipidemia (hypertrigliceridemia), type 2 diabetes 44, 84, and metabolic syndrome per se 85– 90. Insulin resistance produces several lithogenic effects; that is, it increases the activity of the rate-limiting step in cholesterol synthesis, hydroxyl-methyl-glutaryl coenzyme A reductase 91; modulates the expression of the ABCG5 and ABCG8 genes involved in the expression of cholesterol transporters (governing biliary cholesterol secretion); and dysregulates the liver transcription factor forkhead box protein O1 (FOXO1) 94, which modulates cholesterol homeostasis and high-density lipoprotein (HDL)-mediated reverse cholesterol transport to the liver 98. In the liver, insulin resistance influences the level and activity of the BA sensor nuclear receptor farnesoid X receptor (FXR) 23, 76, 77, which is involved in crucial pathways of cholesterol and BA metabolism. FXR also upregulates the hepatic expression of the ABCG5 and ABCG8 genes by activating the other BA sensor, liver X receptor (LXR) 78. Thus, FXR plays a protective role against the development of cholesterol gallstones and pharmacologic agents as 6-α-ethyl-ursodeoxycholic acid (6EUDCA) modulating the activity of this nuclear receptor could be a useful therapeutic tool 99. Also, results from animal models suggest that piperine (a potential cholesterol-lowering agent) could be useful in preventing cholesterol gallstone formation by inhibiting the expression of the cholesterol transporters ABCG5/8 and LXR 100.
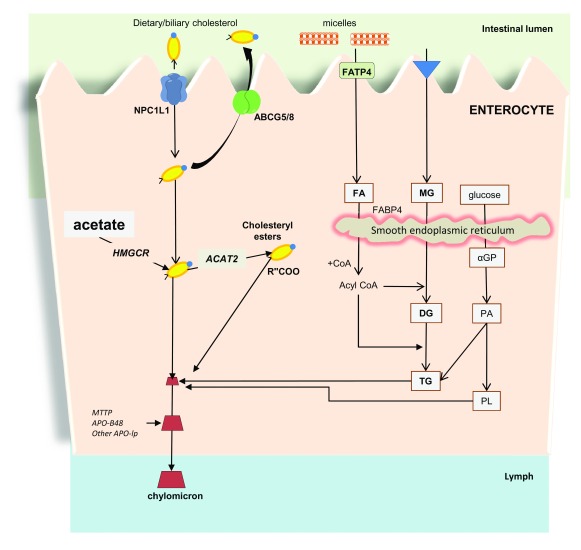
The systemic homeostasis of cholesterol is influenced by the small intestine; here, dietary cholesterol is absorbed ( Figure 2), and biliary cholesterol is reabsorbed during the entero-hepatic circulation of BA 101, with variable efficiency levels 102, 103. The step is co-regulated by genes determining the balance between influx of intraluminal cholesterol molecules crossing the enterocyte brush border membrane—via the Niemann-Pick C1-like 1 (NPC1L1) pathway—and efflux of enterocyte cholesterol into the intestinal lumen (via the ABCG5/ABCG8 pathway) 101. A large observational study showed that genetic variations in ABCG5/8 associated with decreased levels of plasma low-density lipoprotein (LDL) cholesterol are protective against myocardial infarction but increase the risk of symptomatic gallstone disease, suggesting that there is an intrinsic link between these two diseases and that this link is based on the activity of ABCG5/8 106. The coding variant rs11887534 (D19H) in ABCG8 is associated with a more efficient transport of cholesterol into bile 45. Of note, despite the presence of D19H polymorphism 107, 108, patients with gallstones have significantly lower cholesterol absorption 107, 108 and higher or unchanged 108 de novo synthesis of cholesterol 107. This peculiar metabolic feature could precede gallstone formation in groups at risk 107. Independently from the presence of obesity, cholesterol absorption by the small intestine can be reduced by the presence of insulin resistance, which is also able to increase cholesterol synthesis 109, 110.
Figure 2. Regulation of intestinal cholesterol absorption and role of the Niemann-Pick C1-like 1 protein (NPC1L1) at the enterocyte level in humans.
Bile acids enrich both hepatic and gallbladder bile. Sterols undergo micellar solubilization in the intestinal lumen, and uptake occurs at the enterocyte brush border. The NPC1L1, located at the apical membrane of the enterocytes, governs the active uptake of cholesterol and plant sterols across the brush border membrane of the enterocytes. Ezetimibe specifically inhibits the NPC1L1 pathway. The ATP-binding cassette transporters ABCG5/G8, also located at the enterocyte brush border, promote the active efflux of cholesterol and plant sterols from the enterocytes into the intestinal lumen for fecal excretion. In the enterocyte, new synthesis of cholesterol occurs from acetate (by 3-hydroxy-3-methylglutaryl-CoA reductase, or HMGCR). The esterification of intracellular cholesterol requires the acyl-CoA:cholesterol acyltransferase isoform 2 (ACAT2). Chylomicrons are assembled following triacylglycerol (TG) and phospholipid (PL) synthesis, steps requiring facilitated transport of fatty acids (FAs) and monoacylglycerol (MG) into the enterocytes, which include their FA binding protein 4 (FABP4)-mediated transport into the smooth endoplasmic reticulum. This step is followed by synthesis of diacylglycerol (DG) and TG, whereas PL synthesis is dependent on glucose transport into the smooth endoplasmic reticulum and synthesis of α-glycerophosphate (α-GP) and phosphatidic acid (PA). Apolipoprotein (APO)-B48 and activity of microsomal triglyceride transfer protein (MTTP) are required for assembly of chylomicrons before their secretion into the lymph. Thus, final chylomicron particles contain a core of triglycerides and cholesteryl esters surrounded by a surface enriched with PLs (mainly phosphatidylcholine), unesterified cholesterol, and apolipoproteins, including A-I, A-II, A-IV, B-48, C-I, and C-III. APO C-II and APO-E are acquired when the chylomicrons enter the circulation 96, 97, 104. Adapted from 105.
In animal studies, cholesterol gallstone formation can be prevented by ezetimibe, the selective inhibitor of the intestinal NPC1L1. The mechanism involves reduced amounts of absorbed cholesterol reaching the liver through the entero-hepatic circulation and, in turn, decreased biliary cholesterol saturation 79– 81. Additionally, deficiency of osteopontin (OPN) (a soluble cytokine and a matrix-associated protein detectable in the majority of tissues and body fluids) in OPN −/− mice on lithogenic diet reduces the intestinal absorption of cholesterol through a suppressed expression of NPC1L1, preventing cholesterol gallstone formation 111.
The potential effects of the intestinal microbiota on the pathogenesis of cholesterol gallstones should not be neglected. Gut bacterial communities collected from patients with gallstones exhibit increased phylum Proteobacteria and a decrement of genera Faecalibacterium, Lachnospira, and Roseburia 112. The cecum of patients with gallstones contains increased amounts of Gram-positive anaerobic bacteria with elevated 7α-dehydroxylation activity, a finding linked with increased concentrations of the more hydrophobic and lithogenic secondary BA deoxycholate 113. Increased concentrations of secondary BA could also depend on enrichment with the genus Oscillospira, negatively correlated with primary BA formation. An opposite trend exists for the phylum Bacteroidetes 114. A reduced richness and alpha diversity of microbiota, with lower levels of Firmicutes and decreased ratio of the phyla Firmicutes to Bacteroidetes, have been reported in mice fed a lithogenic diet and forming gallstones 115.
A number of studies have examined the role of defective gallbladder motility (that is, increased fasting and postprandial gallbladder volumes and slower postprandial emptying in response to meal) as a major risk factor for cholesterol gallstone formation 44, 116– 119. Indeed, intraluminal gallbladder stasis provides a sufficiently prolonged time for nucleation of excess biliary cholesterol and gallstone growth 120– 122. A sluggish motility function of the gallbladder also predisposes to gallstone recurrence after successful extracorporeal shock-wave lithotripsy or oral BA litholysis or both 123, 124. Abnormal gallbladder motor function is found in about one third of patients, independently of the physical presence of (small) stones 125– 130, and is a feature before gallstone occurrence 120, 125, 131, 132. Alterations in the gallbladder contractility are secondary, at least in part, to a direct toxic effect of unesterified biliary cholesterol on the gallbladder smooth muscle plasma membranes 133– 135. The steps involve the migration of intraluminal cholesterol into the muscularis propria, inhibition of action potentials, currents of Ca 2+ 136, decreased density of cholecystokinin-1R (CCK-1R) on the smooth muscle 137 and signal-transduction decoupling of the CCK-1R 122, 138– 140 (mainly due to CCK binding to cell receptors not followed by G-protein activation 138, 141– 143), and smooth muscle cell proliferation and inflammation in the gallbladder mucosa and lamina propria 119, 144.
A defective intrinsic innervation of the gallbladder has also been described with marked reduction of neurons, enteric glial cells, mast cells, and interstitial cells of Cajal in patients with gallstones 145. These alterations lead not only to altered postprandial contraction but also to defective interprandial relaxation 146, 147.
Of note, extra-gallbladder (that is, systemic) factors play a relevant role also in the modulation of fasting and postprandial gallbladder motility. Besides the existence of polymorphisms in the CCK-1R gene that might be associated with gallstone formation in humans 148, insulin resistance has been linked with defective gallbladder motility in lean, non-diabetic, and gallstone-free subjects 149. The gallbladder dysmotility is also described in women with polycystic ovary syndrome, a condition often linked with insulin resistance 150. Notably, the anti-diabetic agent metformin ameliorates the gallbladder motility defect 151, and prolonged therapy with this drug has been linked with reduced risk of gallstones in patients with diabetes 152.
The interprandial gallbladder relaxation leading to organ refilling is stimulated by the acid-induced duodenal release of vasointestinal peptide (VIP) and is regulated by the human protein fibroblast grow factor 19 (FGF19, FGF15 in mice) 153, located in the gallbladder epithelium but also in cholangiocytes 154 and in the ileum 155. Secreted BAs reach the terminal ileum and act as signaling factors, which activate FXR and, in turn, increase the intestinal FGF19 levels in the portal circulation. FGF19 binds to its receptor fibroblast growth factor receptor 4 (FGFR4)/co-receptor β-klotho found in the liver and also in the gallbladder 156. FGF19-FGFR4/β-klotho interaction in the gallbladder accounts for relaxation of the gallbladder smooth muscle and this feedback modulates gallbladder refilling between meals 119, 153. Intraluminal hydrophobic BAs also act as signaling agents for the G protein – coupled bile acid receptor 1 (GPBAR-1) 157, located in the gallbladder epithelium and smooth muscle 156, 158 and stimulating gallbladder relaxation independently of FGF19 159, through the activation of KATP channels 160.
The existence of impaired gallbladder refilling due to altered interprandial motility might have a role in the pathogenesis of gallstones. In the intedigestive period, fasting rhythmic fluctuations physiologically decrease the gallbladder volume by 20 to 30% 161, 162. An altered interprandial gallbladder motility 120, 163, mainly secondary to less frequent migrating myoelectric complex cycles and abnormal motilin release, has been described in patients with cholesterol gallstone as compared with healthy subjects 119, 125, 128, 163. The interprandial motility defect would be able to increase the hepatic secretion of lithogenic bile to the small intestine, with faster recycling of BAs and increased hydrophobicity of the BA pool 164, a mechanism predisposing to cholesterol crystallization and stone growth 165. As suggested by animal studies, CCK incretion might also have a role in the modulation of fasting gallbladder motility linked with an impaired small intestinal motility since an increment of fasting gallbladder volume, a prolonged intestinal transit time, and an increased intestinal cholesterol absorption have been detected in CCK knockout mice 166.
Future perspectives: from therapy to prevention
The majority of patients with gallstone disease remain asymptomatic throughout their life 167, 168 and should be managed with watchful waiting 40. If symptoms or complications occur or if subjects are at high clinical risk of complications, the current therapeutic approach is surgery, which remains the mainstay treatment 169, by laparoscopy, small incision, or (in selected cases) laparotomy 40, 170. In a small (10%) subgroup of patients with small (<5 mm), pure-cholesterol radiotransparent stones in a functioning gallbladder with a patent cystic duct, litholysis with oral ursodeoxycholic acid (UDCA) (12 to 15 mg/kg per day up to 12 to 24 months) could be considered 40. Nevertheless, the risk of post-dissolution gallstone recurrence is high (about 10% per year and up to 50% by five years 171).
Cholecystectomy has a low risk of mortality (30-day mortality 0.15%, close to that of the general population in a Swedish study 172) but is not a neutral event. About 10% of patients can develop non-specific gastrointestinal symptoms following cholecystectomy (that is, the “post-cholecystectomy syndrome”) mainly due, in the first three years, to gastric diseases, including peptic ulcer, hiatus hernia, and gastro-esophageal reflux. In these cases, however, a misdiagnosis of pre-existing clinical conditions is possible. In a longer time window, the most common cause is the presence of stones within the biliary tree 173.
Although laparoscopic cholecystectomy has a low surgical risk and is the most common elective abdominal surgery performed in the US 174, a series of complications is possible, ranging from conversion to open cholecystectomy (the most frequent) to bile leak, bile duct injury, and (in few cases) death 175. It was recently suggested that surgical complications are more likely in patients with low socioeconomic status, who appear to be more vulnerable. In fact, patients from low-income populations show higher rates of 30-day mortality, in-hospital complications, readmission for complications, hospital costs, and length of stay in comparison with the general population 176.
Increased health costs have also been described in the case of delayed cholecystectomy, as compared with early cholecystectomy 177, and of emergency surgical procedure 178 and laparoscopic cholecystectomy, as compared with small-incision open cholecystectomy (with similar quality of life after these last two procedures) 179.
The robotic-assisted laparoscopic cholecystectomy appears to be an interesting and emerging technology. However, in the case of benign gallbladder diseases, this procedure does not seem to be more effective or safer than conventional laparoscopic cholecystectomy, which should be preferred because of lower costs 180.
Finally, the potential metabolic consequences of cholecystectomy have recently been discussed. Cholecystectomy might disrupt trans-intestinal flow of BAs acting as signaling (hormonal) agents via FXR, GPBAR-1 intestinal receptors in the absence of their natural reservoir (the gallbladder) 181. This aspect has practical implications, and cholecystectomy is suggested after adequate selection of patients on the basis of clinical and epidemiological evidence. It is mandatory that the surgical procedure be restricted to patients with specific symptoms (that is, colicky pain) or complications of cholelithiasis or to subjects at high risk requiring prophylactic cholecystectomy 181.
In conclusion, given the limits, risks, and costs of the currently available therapeutic approaches, the critical role played by systemic factors in the risk of gallstone formation and in the pathogenesis of cholesterol gallstone disease might offer interesting possibilities for primary prevention 64, 71, 105, 182, 183 aimed at reducing gallstone prevalence in subjects at risk and the related health costs.
A major intervention in the general population should include lifestyle changes 64, 184– 189, including dietary models able to possibly reduce the risk of gallstones mainly acting on lipid metabolism and metabolic pathways leading to gallstone formation 188 (that is, a low-carbohydrate diet with enriched vegetable proteins 188, nuts 190, and vegetable oils 191, 192 with moderate alcohol intake 193), and adequate physical activity 187 aimed at maintaining a normal body weight.
In fact, the risk of developing gallstones appears to increase with some dietary factors (that is, increased energy intake, low dietary fiber content, highly refined sugars, high fructose and fat intake, and low vitamin C intake) and to decrease with others (that is, olive oil consumption, ω-3 fatty acids, high intake of monounsaturated fats and fiber, vegetables, vitamin C supplementation, fruit, and moderate alcohol consumption) 64. In a large French cohort, the high adherence to the Mediterranean diet was linked with a significantly lower risk of cholecystectomy 185. Physical activity also positively influences several metabolic features related with the hepatobiliary–gut axis, and beneficial effects are anticipated by regular physical activity on several metabolic disorders, including cholesterol gallstone disease 184.
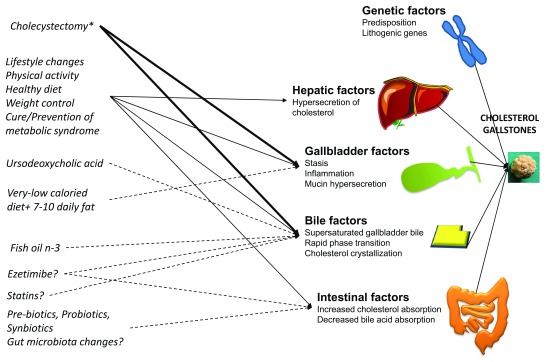
Owing to the scarcity of strong evidence of effectiveness, pharmacological prevention of gallstones is not advisable in the general population 169. In theory, however, each pathogenic factor involved in gallstone formation could be considered a potential therapeutic target for prevention or treatment of cholesterol cholelithiasis ( Figure 3). In particular, a protective role is possible for the hydrophilic bile acid UDCA 194– 199. Other therapeutic options (alone or in combination) are promising but not yet supported by strong evidence: statins or inhibitors (or both) of the intestinal absorption of cholesterol such as ezetimibe 79, 95, 201– 207; nuclear receptor modulators 99, 208– 212 such as 6EUDCA, a synthetic derivative of UDCA acting as FXR modulator 99, or piperine, an alkaloid able to inhibit ABCG5/8 and LXR expression in the liver reducing biliary cholesterol secretion 100; possible modulators of gut microbiota 112; dietary/lifestyle models 64, 65, 184, 213; pharmacologic agents acting on gallbladder hypomotility 121, 214, 215; and inflammatory cytokine modulators 216– 218.
Figure 3. Pathogenetic factors involved in the formation of cholesterol gallstones and therapeutic opportunities.
Increased hepatic hypersecretion of cholesterol represents the primary cause of cholesterol gallstone formation developing in the background of genetic predisposition. Gallbladder factors include defective motility due to a form of leiomyopathy developing in response to excess biliary cholesterol, local immune-mediated inflammation, and hypersecretion of mucin in the gallbladder lumen. Bile factors include the accumulation of supersaturated, concentrated gallbladder bile, a predisposing factor to rapid phase transitions of cholesterol to solid crystals, aggregation, and conglomeration within the mucin gel. Intestinal factors increase absorption of cholesterol and reduce absorption of bile acids. Therapeutic interventions include cholecystectomy, which radically cures gallbladder and bile abnormalities (and is effective in the case of pigment gallstones); general lifestyle recommendations; dietary changes; regular physical activity; and cure and prevention of metabolic abnormalities. Ursodeoxycholic acid is reserved to a subgroup of symptomatic uncomplicated gallstone patients with small stones, radiolucent (x-ray) in a functioning gallbladder and patent cystic duct. Also, in the high-risk group of patients undergoing rapid weight loss (that is, bariatric surgery or very-low calorie diet), ursodeoxycholic acid and a daily fat content of 7 to 10 g are recommended to improve gallbladder emptying and to prevent the formation of cholesterol gallstones following rapid weight reduction. All other therapeutic options are currently not supported by strong scientific evidence or require further studies 40, 94, 105, 169, 200.
Future experimental and clinical studies are certainly needed to clarify the real usefulness of these potentially innovative preventive tools in selected groups of subjects.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Jill Koshiol, Infections and Immunoepidemiology Branch, Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute (NCIM), Maryland, USA
Cynthia Ko, Division of Gastroenterology, University of Washington, Washington, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Everhart JE, Ruhl CE: Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136(4):1134–44. 10.1053/j.gastro.2009.02.038 [DOI] [PubMed] [Google Scholar]
- 2. Farthing M, Roberts SE, Samuel DG, et al. : Survey of digestive health across Europe: Final report. Part 1: The burden of gastrointestinal diseases and the organisation and delivery of gastroenterology services across Europe. United European Gastroenterol J. 2014;2(6):539–43. 10.1177/2050640614554154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sandler RS, Everhart JE, Donowitz M, et al. : The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122(5):1500–11. 10.1053/gast.2002.32978 [DOI] [PubMed] [Google Scholar]
- 4. Shaffer EA: Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep. 2005;7(2):132–40. 10.1007/s11894-005-0051-8 [DOI] [PubMed] [Google Scholar]
- 5. Akhtar-Danesh GG, Doumouras AG, Bos C, et al. : Factors Associated With Outcomes and Costs After Pediatric Laparoscopic Cholecystectomy. JAMA Surg. 2018;153(6):551–7. 10.1001/jamasurg.2017.5461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chilimuri S, Gaduputi V, Tariq H, et al. : Symptomatic Gallstones in the Young: Changing Trends of the Gallstone Disease-Related Hospitalization in the State of New York: 1996 - 2010. J Clin Med Res. 2017;9(2):117–23. 10.14740/jocmr2847w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khoo AK, Cartwright R, Berry S, et al. : Cholecystectomy in English children: evidence of an epidemic (1997-2012). J Pediatr Surg. 2014;49(2):284–8; discussion 288. 10.1016/j.jpedsurg.2013.11.053 [DOI] [PubMed] [Google Scholar]
- 8. Murphy PB, Vogt KN, Winick-Ng J, et al. : The increasing incidence of gallbladder disease in children: A 20year perspective. J Pediatr Surg. 2016;51(5):748–52. 10.1016/j.jpedsurg.2016.02.017 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Walker SK, Maki AC, Cannon RM, et al. : Etiology and incidence of pediatric gallbladder disease. Surgery. 2013;154(4):927–31; discussion 931-3. 10.1016/j.surg.2013.04.040 [DOI] [PubMed] [Google Scholar]
- 10. Koebnick C, Smith N, Black MH, et al. : Pediatric obesity and gallstone disease. J Pediatr Gastroenterol Nutr. 2012;55(3):328–33. 10.1097/MPG.0b013e31824d256f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fradin K, Racine AD, Belamarich PF: Obesity and symptomatic cholelithiasis in childhood: epidemiologic and case-control evidence for a strong relation. J Pediatr Gastroenterol Nutr. 2014;58(1):102–6. 10.1097/MPG.0b013e3182a939cf [DOI] [PubMed] [Google Scholar]
- 12. Mehta S, Lopez ME, Chumpitazi BP, et al. : Clinical characteristics and risk factors for symptomatic pediatric gallbladder disease. Pediatrics. 2012;129(1):e82–8. 10.1542/peds.2011-0579 [DOI] [PubMed] [Google Scholar]
- 13. Kaechele V, Wabitsch M, Thiere D, et al. : Prevalence of gallbladder stone disease in obese children and adolescents: influence of the degree of obesity, sex, and pubertal development. J Pediatr Gastroenterol Nutr. 2006;42(1):66–70. 10.1097/01.mpg.0000187816.31213.06 [DOI] [PubMed] [Google Scholar]
- 14. Svensson J, Makin E: Gallstone disease in children. Semin Pediatr Surg. 2012;21(3):255–65. 10.1053/j.sempedsurg.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 15. Kim HY, Kim SH, Cho YH: Pediatric Cholecystectomy: Clinical Significance of Cases Unrelated to Hematologic Disorders. Pediatr Gastroenterol Hepatol Nutr. 2015;18(2):115–20. 10.5223/pghn.2015.18.2.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kratzer W, Walcher T, Arnold F, et al. : Gallstone prevalence and risk factors for gallstone disease in an urban population of children and adolescents. Z Gastroenterol. 2010;48(6):683–7. 10.1055/s-0028-1109957 [DOI] [PubMed] [Google Scholar]
- 17. Di Ciaula A, Wang DQH, Portincasa P: An update on the pathogenesis of cholesterol gallstone disease. Curr Opin Gastroenterol. 2018;34(2):71–80. 10.1097/MOG.0000000000000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Z, Kemp TJ, Gao YT, et al. : Association of circulating inflammation proteins and gallstone disease. J Gastroenterol Hepatol. 2018. 10.1111/jgh.14265 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Lv J, Qi L, Yu C, et al. : Gallstone Disease and the Risk of Ischemic Heart Disease. Arterioscler Thromb Vasc Biol. 2015;35(10):2232–7. 10.1161/ATVBAHA.115.306043 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Zheng Y, Xu M, Li Y, et al. : Gallstones and Risk of Coronary Heart Disease: Prospective Analysis of 270 000 Men and Women From 3 US Cohorts and Meta-Analysis. Arterioscler Thromb Vasc Biol. 2016;36(9):1997–2003. 10.1161/ATVBAHA.116.307507 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Zheng Y, Xu M, Heianza Y, et al. : Gallstone disease and increased risk of mortality: Two large prospective studies in US men and women. J Gastroenterol Hepatol. 2018. 10.1111/jgh.14264 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Shabanzadeh DM, Sørensen LT, Jørgensen T: Gallstone disease and mortality: a cohort study. Int J Public Health. 2017;62(3):353–60. 10.1007/s00038-016-0916-7 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Biddinger SB, Haas JT, Yu BB, et al. : Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med. 2008;14(7):778–82. 10.1038/nm1785 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Ruhl CE, Everhart JE: Association of diabetes, serum insulin, and C-peptide with gallbladder disease. Hepatology. 2000;31(2):299–303. 10.1002/hep.510310206 [DOI] [PubMed] [Google Scholar]
- 25. Scragg RK, Calvert GD, Oliver JR: Plasma lipids and insulin in gall stone disease: a case-control study. Br Med J (Clin Res Ed). 1984;289(6444):521–5. 10.1136/bmj.289.6444.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Misciagna G, Guerra V, Di Leo A, et al. : Insulin and gall stones: a population case control study in southern Italy. Gut. 2000;47(1):144–7. 10.1136/gut.47.1.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang Y, Sung E, Ryu S, et al. : Insulin resistance is associated with gallstones even in non-obese, non-diabetic Korean men. J Korean Med Sci. 2008;23(4):644–50. 10.3346/jkms.2008.23.4.644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin IC, Yang YW, Wu MF, et al. : The association of metabolic syndrome and its factors with gallstone disease. BMC Fam Pract. 2014;15:138. 10.1186/1471-2296-15-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shebl FM, Andreotti G, Meyer TE, et al. : Metabolic syndrome and insulin resistance in relation to biliary tract cancer and stone risks: a population-based study in Shanghai, China. Br J Cancer. 2011;105(9):1424–9. 10.1038/bjc.2011.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Di Ciaula A, Garruti G, Wang DQ: Role of insulin resistance in the formation of cholesterol gallstones.In: Gallstones - Recent advances in epidemiology, pathogenesis, diagnosis and management Edited by: Wang DQ-H, Portincasa P. New York: Nova Science Publishers;2016;357–372. [Google Scholar]
- 31. Lv J, Yu C, Guo Y, et al. : Gallstone Disease and the Risk of Type 2 Diabetes. Sci Rep. 2017;7(1):15853. 10.1038/s41598-017-14801-2 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Qiao QH, Zhu WH, Yu YX, et al. : Nonalcoholic fatty liver was associated with asymptomatic gallstones in a Chinese population. Medicine (Baltimore). 2017;96(38):e7853. 10.1097/MD.0000000000007853 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Nogueira L, Freedman ND, Engels EA, et al. : Gallstones, cholecystectomy, and risk of digestive system cancers. Am J Epidemiol. 2014;179(6):731–9. 10.1093/aje/kwt322 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Schmidt M, Småstuen MC, Søndenaa K: Increased cancer incidence in some gallstone diseases, and equivocal effect of cholecystectomy: A long-term analysis of cancer and mortality. Scand J Gastroenterol. 2012;47(12):1467–74. 10.3109/00365521.2012.719928 [DOI] [PubMed] [Google Scholar]
- 35. Wang Y, Xie LF, Lin J: Gallstones and cholecystectomy in relation to risk of liver cancer. Eur J Cancer Prev. 2018. 10.1097/CEJ.0000000000000421 [DOI] [PubMed] [Google Scholar]
- 36. Kang SH, Kim YH, Roh YH, et al. : Gallstone, cholecystectomy and risk of gastric cancer. Ann Hepatobiliary Pancreat Surg. 2017;21(3):131–7. 10.14701/ahbps.2017.21.3.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shabanzadeh DM, Sørensen LT, Jørgensen T: Association Between Screen-Detected Gallstone Disease and Cancer in a Cohort Study. Gastroenterology. 2017;152(8):1965–1974.e1. 10.1053/j.gastro.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 38. Grundy SM: Cholesterol gallstones: a fellow traveler with metabolic syndrome? Am J Clin Nutr. 2004;80(1):1–2. 10.1093/ajcn/80.1.1 [DOI] [PubMed] [Google Scholar]
- 39. Portincasa P, Wang DQH: Gallstones.In: Yamada's Atlas of Gastroenterology 5th. Edited by: Podolsky KD, Camilleri M, Fitz JG et al.UK: Wiley-Blackwell;2016;335–353. [Google Scholar]
- 40. Portincasa P, Di Ciaula A, de Bari O, et al. : Management of gallstones and its related complications. Expert Rev Gastroenterol Hepatol. 2016;10(1):93–112. 10.1586/17474124.2016.1109445 [DOI] [PubMed] [Google Scholar]
- 41. Sarin SK, Negi VS, Dewan R, et al. : High familial prevalence of gallstones in the first-degree relatives of gallstone patients. Hepatology. 1995;22(1):138–41. 10.1002/hep.1840220122 [DOI] [PubMed] [Google Scholar]
- 42. Hsing AW, Bai Y, Andreotti G, et al. : Family history of gallstones and the risk of biliary tract cancer and gallstones: a population-based study in Shanghai, China. Int J Cancer. 2007;121(4):832–8. 10.1002/ijc.22756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Redinger RN, Small DM: Bile composition, bile salt metabolism and gallstones. Arch Intern Med. 1972;130(4):618–30. 10.1001/archinte.1972.03650040142013 [DOI] [PubMed] [Google Scholar]
- 44. Portincasa P, Moschetta A, Palasciano G: Cholesterol gallstone disease. Lancet. 2006;368(9531):230–9. 10.1016/S0140-6736(06)69044-2 [DOI] [PubMed] [Google Scholar]
- 45. Buch S, Schafmayer C, Völzke H, et al. : A genome-wide association scan identifies the hepatic cholesterol transporter ABCG8 as a susceptibility factor for human gallstone disease. Nat Genet. 2007;39(8):995–9. 10.1038/ng2101 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Goodloe R, Brown-Gentry K, Gillani NB, et al. : Lipid trait-associated genetic variation is associated with gallstone disease in the diverse Third National Health and Nutrition Examination Survey (NHANES III). BMC Med Genet. 2013;14:120. 10.1186/1471-2350-14-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Joshi AD, Andersson C, Buch S, et al. : Four Susceptibility Loci for Gallstone Disease Identified in a Meta-analysis of Genome-Wide Association Studies. Gastroenterology. 2016;151(2):351–363.e28. 10.1053/j.gastro.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Grünhage F, Acalovschi M, Tirziu S, et al. : Increased gallstone risk in humans conferred by common variant of hepatic ATP-binding cassette transporter for cholesterol. Hepatology. 2007;46(3):793–801. 10.1002/hep.21847 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Wang Y, Jiang ZY, Fei J, et al. : ATP binding cassette G8 T400K polymorphism may affect the risk of gallstone disease among Chinese males. Clin Chim Acta. 2007;384(1–2):80–5. 10.1016/j.cca.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 50. Jiang ZY, Parini P, Eggertsen G, et al. : Increased expression of LXR alpha, ABCG5, ABCG8, and SR-BI in the liver from normolipidemic, nonobese Chinese gallstone patients. J Lipid Res. 2008;49(2):464–72. 10.1194/jlr.M700295-JLR200 [DOI] [PubMed] [Google Scholar]
- 51. Kuo KK, Shin SJ, Chen ZC: Significant association of ABCG5 604Q and ABCG8 D19H polymorphisms with gallstone disease. Br J Surg. 2008;95(8):1005–11. 10.1002/bjs.6178 [DOI] [PubMed] [Google Scholar]
- 52. Rudkowska I, Jones PJ: Polymorphisms in ABCG5/G8 transporters linked to hypercholesterolemia and gallstone disease. Nutr Rev. 2008;66(6):343–8. 10.1111/j.1753-4887.2008.00042.x [DOI] [PubMed] [Google Scholar]
- 53. Katsika D, Magnusson P, Krawczyk M, et al. : Gallstone disease in Swedish twins: Risk is associated with ABCG8 D19H genotype. J Intern Med. 2010;268(3):279–85. 10.1111/j.1365-2796.2010.02249.x [DOI] [PubMed] [Google Scholar]
- 54. von Kampen O, Buch S, Nothnagel M, et al. : Genetic and functional identification of the likely causative variant for cholesterol gallstone disease at the ABCG5/8 lithogenic locus. Hepatology. 2013;57(3):2407–17. 10.1002/hep.26009 [DOI] [PubMed] [Google Scholar]
- 55. von Schönfels W, Buch S, Wölk M, et al. : Recurrence of gallstones after cholecystectomy is associated with ABCG5/8 genotype. J Gastroenterol. 2013;48(3):391–6. 10.1007/s00535-012-0639-3 [DOI] [PubMed] [Google Scholar]
- 56. Xu HL, Cheng JR, Andreotti G, et al. : Cholesterol metabolism gene polymorphisms and the risk of biliary tract cancers and stones: A population-based case-control study in Shanghai, China. Carcinogenesis. 2011;32(1):58–62. 10.1093/carcin/bgq194 [DOI] [PubMed] [Google Scholar]
- 57. Hirobe-Jahn S, Harsch S, Renner O, et al. : Association of FXR gene variants with cholelithiasis. Clin Res Hepatol Gastroenterol. 2015;39(1):68–79. 10.1016/j.clinre.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 58. Martinez-Lopez E, Curiel-Lopez F, Hernandez-Nazara A, et al. : Influence of ApoE and FABP2 polymorphisms and environmental factors in the susceptibility to gallstone disease. Ann Hepatol. 2015;14(4):515–23. [PubMed] [Google Scholar]
- 59. Chuang SC, Hsi E, Lee KT: Mucin genes in gallstone disease. Clin Chim Acta. 2012;413(19–20):1466–71. 10.1016/j.cca.2012.06.015 [DOI] [PubMed] [Google Scholar]
- 60. Chen Q, Li WJ, Wan YY, et al. : Fibroblast growth factor receptor 4 Gly388Arg polymorphism associated with severity of gallstone disease in a Chinese population. Genet Mol Res. 2012;11(1):548–55. 10.4238/2012.March.8.3 [DOI] [PubMed] [Google Scholar]
- 61. Chuang SC, Hsi E, Wang SN, et al. : Polymorphism at the mucin-like protocadherin gene influences susceptibility to gallstone disease. Clin Chim Acta. 2011;412(23–24):2089–93. 10.1016/j.cca.2011.07.015 [DOI] [PubMed] [Google Scholar]
- 62. Nakeeb A, Comuzzie AG, Martin L, et al. : Gallstones: Genetics versus environment. Ann Surg. 2002;235(6):842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Katsika D, Grjibovski A, Einarsson C, et al. : Genetic and environmental influences on symptomatic gallstone disease: A Swedish study of 43,141 twin pairs. Hepatology. 2005;41(5):1138–43. 10.1002/hep.20654 [DOI] [PubMed] [Google Scholar]
- 64. Di Ciaula A, Garruti G, Frühbeck G, et al. : The Role Of Diet In The Pathogenesis Of Cholesterol Gallstones. Curr Med Chem. 2017. 10.2174/0929867324666170530080636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Di Ciaula A, Portincasa P: Diet and contaminants: Driving the rise to obesity epidemics? Curr Med Chem. 2017. 10.2174/0929867324666170518095736 [DOI] [PubMed] [Google Scholar]
- 66. Stokes CS, Krawczyk M, Lammert F: Gallstones: Environment, lifestyle and genes. Dig Dis. 2011;29(2):191–201. 10.1159/000323885 [DOI] [PubMed] [Google Scholar]
- 67. Unisa S, Jagannath P, Dhir V, et al. : Population-based study to estimate prevalence and determine risk factors of gallbladder diseases in the rural Gangetic basin of North India. HPB (Oxford). 2011;13(2):117–25. 10.1111/j.1477-2574.2010.00255.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Parviainen A, Suárez-Grau JM, Pérez-López R, et al. : Combined microstructural and mineralogical phase characterization of gallstones in a patient-based study in SW Spain - Implications for environmental contamination in their formation. Sci Total Environ. 2016;573:433–43. 10.1016/j.scitotenv.2016.08.110 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Su Y, Dai Y, Lin Y, et al. : Serum organochlorine pesticide residues and risk of gallstone disease: A case-control study in Xiamen. Ann Epidemiol. 2012;22(8):592–7. 10.1016/j.annepidem.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 70. Ji G, Xu C, Sun H, et al. : Organochloride pesticides induced hepatic ABCG5/G8 expression and lipogenesis in Chinese patients with gallstone disease. Oncotarget. 2016;7(23):33689–702. 10.18632/oncotarget.9399 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Di Ciaula A, Wang DQ, Bonfrate L, et al. : Current views on genetics and epigenetics of cholesterol gallstone disease. Cholesterol. 2013;2013: 298421. 10.1155/2013/298421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Patel SB, Graf GA, Temel RE: ABCG5 and ABCG8: More than a defense against xenosterols. J Lipid Res. 2018;59(7):1103–13. 10.1194/jlr.R084244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lavoie JM: Dynamics of hepatic and intestinal cholesterol and bile acid pathways: The impact of the animal model of estrogen deficiency and exercise training. World J Hepatol. 2016;8(23):961–75. 10.4254/wjh.v8.i23.961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang S, Wang Y, Xu J, et al. : Is the oral contraceptive or hormone replacement therapy a risk factor for cholelithiasis: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96(14):e6556. 10.1097/MD.0000000000006556 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Bonde Y, Plösch T, Kuipers F, et al. : Stimulation of murine biliary cholesterol secretion by thyroid hormone is dependent on a functional ABCG5/G8 complex. Hepatology. 2012;56(5):1828–37. 10.1002/hep.25861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Aguilar-Olivos NE, Carrillo-Córdova D, Oria-Hernández J, et al. : The nuclear receptor FXR, but not LXR, up-regulates bile acid transporter expression in non-alcoholic fatty liver disease. Ann Hepatol. 2015;14(4):487–93. [PubMed] [Google Scholar]
- 77. Modica S, Gadaleta RM, Moschetta A: Deciphering the nuclear bile acid receptor FXR paradigm. Nucl Recept Signal. 2010;8:e005. 10.1621/nrs.08005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Uppal H, Zhai Y, Gangopadhyay A, et al. : Activation of liver X receptor sensitizes mice to gallbladder cholesterol crystallization. Hepatology. 2008;47(4):1331–42. 10.1002/hep.22175 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Wang HH, Portincasa P, de Bari O, et al. : Prevention of cholesterol gallstones by inhibiting hepatic biosynthesis and intestinal absorption of cholesterol. Eur J Clin Invest. 2013;43(4):413–26. 10.1111/eci.12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zúñiga S, Molina H, Azocar L, et al. : Ezetimibe prevents cholesterol gallstone formation in mice. Liver Int. 2008;28(7):935–47. 10.1111/j.1478-3231.2008.01808.x [DOI] [PubMed] [Google Scholar]
- 81. Wang HH, Portincasa P, Mendez-Sanchez N, et al. : Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology. 2008;134(7):2101–10. 10.1053/j.gastro.2008.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. de Bari O, Wang TY, Liu M, et al. : Estrogen induces two distinct cholesterol crystallization pathways by activating ERα and GPR30 in female mice. J Lipid Res. 2015;56(9):1691–700. 10.1194/jlr.M059121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang HH, Liu M, Clegg DJ, et al. : New insights into the molecular mechanisms underlying effects of estrogen on cholesterol gallstone formation. Biochim Biophys Acta. 2009;1791(11):1037–47. 10.1016/j.bbalip.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Stinton LM, Myers RP, Shaffer EA: Epidemiology of gallstones. Gastroenterol Clin North Am. 2010;39(2):157–69, vii. 10.1016/j.gtc.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 85. Grundy SM, Barnett JP: Metabolic and health complications of obesity. Dis Mon. 1990;36(12):641–731. 10.1016/0011-5029(90)90015-J [DOI] [PubMed] [Google Scholar]
- 86. Grundy SM: Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler Thromb Vasc Biol. 2005;25(11):2243–4. 10.1161/01.ATV.0000189155.75833.c7 [DOI] [PubMed] [Google Scholar]
- 87. Grundy SM, Cleeman JI, Daniels SR, et al. : Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52. 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 88. Eckel RH, Grundy SM, Zimmet PZ: The metabolic syndrome. Lancet. 2005;365(9468):1415–28. 10.1016/S0140-6736(05)66378-7 [DOI] [PubMed] [Google Scholar]
- 89. Tsai CJ, Leitzmann MF, Willett WC, et al. : Prospective study of abdominal adiposity and gallstone disease in US men. Am J Clin Nutr. 2004;80(1):38–44. 10.1093/ajcn/80.1.38 [DOI] [PubMed] [Google Scholar]
- 90. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. 10.1161/circ.106.25.3143 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 91. Nepokroeff CM, Lakshmanan MR, Ness GC, et al. : Regulation of the diurnal rhythm of rat liver beta-hydroxy-beta-methylglutaryl coenzmye A reductase activity by insulin, glucagon, cyclic AMP and hydrocortisone. Arch Biochem Biophys. 1974;160(2):387–96. 10.1016/0003-9861(74)90412-3 [DOI] [PubMed] [Google Scholar]
- 92. Garruti G, Wang HH, Bonfrate L, et al. : A pleiotropic role for the orphan nuclear receptor small heterodimer partner in lipid homeostasis and metabolic pathways. J Lipids. 2012;2012:304292. 10.1155/2012/304292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Di Ciaula A, Garruti G, Lunardi Baccetto R, et al. : Bile Acid Physiology. Ann Hepatol. 2017;16(Suppl. 1: s3–105.):s4–s14. 10.5604/01.3001.0010.5493 [DOI] [PubMed] [Google Scholar]
- 94. Lammert F, Gurusamy K, Ko CW, et al. : Gallstones. Nat Rev Dis Primers. 2016;2:16024. 10.1038/nrdp.2016.24 [DOI] [PubMed] [Google Scholar]
- 95. de Bari O, Wang HH, Portincasa P, et al. : Ezetimibe prevents the formation of oestrogen-induced cholesterol gallstones in mice. Eur J Clin Invest. 2014;44(12):1159–68. 10.1111/eci.12350 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 96. de Bari O, Neuschwander-Tetri BA, Liu M, et al. : Ezetimibe: its novel effects on the prevention and the treatment of cholesterol gallstones and nonalcoholic Fatty liver disease. J Lipids. 2012;2012:302847. 10.1155/2012/302847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wang DQH, Neuschwander-Tetri BA, Portincasa P: The Biliary System. Second Edition. Morgan & Claypool Life Sciences;2017. 10.4199/C00147ED2V01Y201611ISP071 [DOI] [Google Scholar]
- 98. Lee SX, Heine M, Schlein C, et al. : FoxO transcription factors are required for hepatic HDL cholesterol clearance. J Clin Invest. 2018;128(4):1615–26. 10.1172/JCI94230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yu DD, Andrali SS, Li H, et al. : Novel FXR (farnesoid X receptor) modulators: Potential therapies for cholesterol gallstone disease. Bioorg Med Chem. 2016;24(18):3986–93. 10.1016/j.bmc.2016.06.039 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 100. Song XY, Xu S, Hu JF, et al. : Piperine prevents cholesterol gallstones formation in mice. Eur J Pharmacol. 2015;751:112–7. 10.1016/j.ejphar.2015.01.038 [DOI] [PubMed] [Google Scholar]
- 101. Wang DQ: Regulation of intestinal cholesterol absorption. Annu Rev Physiol. 2007;69:221–48. 10.1146/annurev.physiol.69.031905.160725 [DOI] [PubMed] [Google Scholar]
- 102. Kesäniemi YA, Ehnholm C, Miettinen TA: Intestinal cholesterol absorption efficiency in man is related to apoprotein E phenotype. J Clin Invest. 1987;80(2):578–81. 10.1172/JCI113107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bosner MS, Lange LG, Stenson WF, et al. : Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J Lipid Res. 1999;40(2):302–8. [PubMed] [Google Scholar]
- 104. Wang DQ, Cohen DE: Absorption and Excretion of Cholesterol and Other Sterols.In: Lipidology in the Treatment and Prevention of Cardiovascular Disease (Clinical Lipidology: A Companion to Braunwald’s Heart Disease) 1. Edited by: Ballantyne CM. Philadelphia: Elsevier Saunders;2008;26–44. Reference Source [Google Scholar]
- 105. Di Ciaula A, Wang DQ, Garruti G, et al. : Therapeutic reflections in cholesterol homeostasis and gallstone disease: A review. Curr Med Chem. 2014;21(12):1435–47. 10.2174/09298673113206660271 [DOI] [PubMed] [Google Scholar]
- 106. Stender S, Frikke-Schmidt R, Nordestgaard BG, et al. : The ABCG5/8 cholesterol transporter and myocardial infarction versus gallstone disease. J Am Coll Cardiol. 2014;63(20):2121–8. 10.1016/j.jacc.2013.12.055 [DOI] [PubMed] [Google Scholar]
- 107. Krawczyk M, Lütjohann D, Schirin-Sokhan R, et al. : Phytosterol and cholesterol precursor levels indicate increased cholesterol excretion and biosynthesis in gallstone disease. Hepatology. 2012;55(5):1507–17. 10.1002/hep.25563 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 108. Renner O, Lütjohann D, Richter D, et al. : Role of the ABCG8 19H risk allele in cholesterol absorption and gallstone disease. BMC Gastroenterol. 2013;13:30. 10.1186/1471-230X-13-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Paramsothy P, Knopp RH, Kahn SE, et al. : Plasma sterol evidence for decreased absorption and increased synthesis of cholesterol in insulin resistance and obesity. Am J Clin Nutr. 2011;94(5):1182–8. 10.3945/ajcn.110.006668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gylling H, Hallikainen M, Pihlajamäki J, et al. : Insulin sensitivity regulates cholesterol metabolism to a greater extent than obesity: Lessons from the METSIM Study. J Lipid Res. 2010;51(8):2422–7. 10.1194/jlr.P006619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lin J, Shao WQ, Chen QZ, et al. : Osteopontin deficiency protects mice from cholesterol gallstone formation by reducing expression of intestinal NPC1L1. Mol Med Rep. 2017;16(2):1785–92. 10.3892/mmr.2017.6774 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 112. Wu T, Zhang Z, Liu B, et al. : Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC Genomics. 2013;14:669. 10.1186/1471-2164-14-669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Thomas LA, Veysey MJ, Murphy GM, et al. : Octreotide induced prolongation of colonic transit increases faecal anaerobic bacteria, bile acid metabolising enzymes, and serum deoxycholic acid in patients with acromegaly. Gut. 2005;54(5):630–5. 10.1136/gut.2003.028431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Keren N, Konikoff FM, Paitan Y, et al. : Interactions between the intestinal microbiota and bile acids in gallstones patients. Environ Microbiol Rep. 2015;7(6):874–80. 10.1111/1758-2229.12319 [DOI] [PubMed] [Google Scholar]
- 115. Wang Q, Jiao L, He C, et al. : Alteration of gut microbiota in association with cholesterol gallstone formation in mice. BMC Gastroenterol. 2017;17(1):74. 10.1186/s12876-017-0629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 116. Palasciano G, Portincasa P, Vinciguerra V, et al. : Gallstone prevalence and gallbladder volume in children and adolescents: an epidemiological ultrasonographic survey and relationship to body mass index. Am J Gastroenterol. 1989;84(11):1378–82. [PubMed] [Google Scholar]
- 117. Palasciano G, Serio G, Portincasa P, et al. : Gallbladder volume in adults, and relationship to age, sex, body mass index, and gallstones: a sonographic population study. Am J Gastroenterol. 1992;87(4):493–7. [PubMed] [Google Scholar]
- 118. Portincasa P, Di Ciaula A, Palmieri VO, et al. : Ultrasonographic study of gallbladder and gastric dynamics in obese people after oral cholestyramine.In: Cholestatic liver diseases: new strategies for prevention and treatment of hepatobiliary and cholestatic liver diseases Dordrecht: Kluwer Academic Publisher;1994;323–327. Reference Source [Google Scholar]
- 119. Portincasa P, Di Ciaula A, Wang HH, et al. : Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology. 2008;47(6):2112–26. 10.1002/hep.22204 [DOI] [PubMed] [Google Scholar]
- 120. Portincasa P, Di Ciaula A, vanBerge-Henegouwen GP: Smooth muscle function and dysfunction in gallbladder disease. Curr Gastroenterol Rep. 2004;6(2):151–62. 10.1007/s11894-004-0043-0 [DOI] [PubMed] [Google Scholar]
- 121. van Erpecum KJ, Venneman NG, Portincasa P, et al. : Review article: agents affecting gall-bladder motility--role in treatment and prevention of gallstones. Aliment Pharmacol Ther. 2000;14 Suppl 2:66–70. 10.1046/j.1365-2036.2000.014s2066.x [DOI] [PubMed] [Google Scholar]
- 122. Lavoie B, Nausch B, Zane EA, et al. : Disruption of gallbladder smooth muscle function is an early feature in the development of cholesterol gallstone disease. Neurogastroenterol Motil. 2012;24(7):e313–24. 10.1111/j.1365-2982.2012.01935.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Portincasa P, van Erpecum KJ, van De Meeberg PC, et al. : Apolipoprotein E4 genotype and gallbladder motility influence speed of gallstone clearance and risk of recurrence after extracorporeal shock-wave lithotripsy. Hepatology. 1996;24(3):580–7. 10.1002/hep.510240320 [DOI] [PubMed] [Google Scholar]
- 124. Pauletzki J, Althaus R, Holl J, et al. : Gallbladder emptying and gallstone formation: a prospective study on gallstone recurrence. Gastroenterology. 1996;111(3):765–71. 10.1053/gast.1996.v111.pm8780583 [DOI] [PubMed] [Google Scholar]
- 125. Portincasa P, Di Ciaula A, Baldassarre G, et al. : Gallbladder motor function in gallstone patients: sonographic and in vitro studies on the role of gallstones, smooth muscle function and gallbladder wall inflammation. J Hepatol. 1994;21(3):430–40. 10.1016/S0168-8278(05)80324-1 [DOI] [PubMed] [Google Scholar]
- 126. Masclee AA, Jansen JB, Driessen WM, et al. : Plasma cholecystokinin and gallbladder responses to intraduodenal fat in gallstone patients. Dig Dis Sci. 1989;34(3):353–9. 10.1007/BF01536255 [DOI] [PubMed] [Google Scholar]
- 127. Pauletzki J, Cicala M, Holl J, et al. : Correlation between gall bladder fasting volume and postprandial emptying in patients with gall stones and healthy controls. Gut. 1993;34(10):1443–7. 10.1136/gut.34.10.1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Stolk MF, van Erpecum KJ, Renooij W, et al. : Gallbladder emptying in vivo, bile composition, and nucleation of cholesterol crystals in patients with cholesterol gallstones. Gastroenterology. 1995;108(6):1882–8. 10.1016/0016-5085(95)90153-1 [DOI] [PubMed] [Google Scholar]
- 129. van Erpecum KJ, van Berge Henegouwen GP, Stolk MF, et al. : Fasting gallbladder volume, postprandial emptying and cholecystokinin release in gallstone patients and normal subjects. J Hepatol. 1992;14(2–3):194–202. 10.1016/0168-8278(92)90158-L [DOI] [PubMed] [Google Scholar]
- 130. Pomeranz IS, Shaffer EA: Abnormal gallbladder emptying in a subgroup of patients with gallstones. Gastroenterology. 1985;88(3):787–91. 10.1016/0016-5085(85)90152-0 [DOI] [PubMed] [Google Scholar]
- 131. Pomeranz IS, Davison JS, Shaffer EA: The effects of prosthetic gallstones on gallbladder function and bile composition. J Surg Res. 1986;41(1):47–52. 10.1016/0022-4804(86)90007-7 [DOI] [PubMed] [Google Scholar]
- 132. Colecchia A, Sandri L, Bacchi-Reggiani ML, et al. : Is it possible to predict the clinical course of gallstone disease? Usefulness of gallbladder motility evaluation in a clinical setting. Am J Gastroenterol. 2006;101(11):2576–81; quiz 2672. 10.1111/j.1572-0241.2006.00793.x [DOI] [PubMed] [Google Scholar]
- 133. Conter RL, Roslyn JJ, Porter-Fink V, et al. : Gallbladder absorption increases during early cholesterol gallstone formation. Am J Surg. 1986;151(1):184–91. 10.1016/0002-9610(86)90030-9 [DOI] [PubMed] [Google Scholar]
- 134. Roslyn JJ, Doty J, Pitt HA, et al. : Enhanced gallbladder absorption during gallstone formation: the roles of cholesterol saturated bile and gallbladder stasis. Am J Med Sci. 1986;292(2):75–80. 10.1097/00000441-198608000-00002 [DOI] [PubMed] [Google Scholar]
- 135. Corradini SG, Elisei W, Giovannelli L, et al. : Impaired human gallbladder lipid absorption in cholesterol gallstone disease and its effect on cholesterol solubility in bile. Gastroenterology. 2000;118(5):912–20. 10.1016/S0016-5085(00)70177-6 [DOI] [PubMed] [Google Scholar]
- 136. Jennings LJ, Xu QW, Firth TA, et al. : Cholesterol inhibits spontaneous action potentials and calcium currents in guinea pig gallbladder smooth muscle. Am J Physiol. 1999;277(5 Pt 1):G1017–26. 10.1152/ajpgi.1999.277.5.G1017 [DOI] [PubMed] [Google Scholar]
- 137. Zhu J, Han TQ, Chen S, et al. : Gallbladder motor function, plasma cholecystokinin and cholecystokinin receptor of gallbladder in cholesterol stone patients. World J Gastroenterol. 2005;11(11):1685–9. 10.3748/wjg.v11.i11.1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yu P, Chen Q, Xiao Z, et al. : Signal transduction pathways mediating CCK-induced gallbladder muscle contraction. Am J Physiol. 1998;275(2 Pt 1):G203–11. 10.1152/ajpgi.1998.275.2.G203 [DOI] [PubMed] [Google Scholar]
- 139. Xiao ZL, Chen Q, Amaral J, et al. : CCK receptor dysfunction in muscle membranes from human gallbladders with cholesterol stones. Am J Physiol. 1999;276(6 Pt 1):G1401–7. 10.1152/ajpgi.1999.276.6.G1401 [DOI] [PubMed] [Google Scholar]
- 140. Cong P, Pricolo V, Biancani P, et al. : Effects of cholesterol on CCK-1 receptors and caveolin-3 proteins recycling in human gallbladder muscle. Am J Physiol Gastrointest Liver Physiol. 2010;299(3):G742–50. 10.1152/ajpgi.00064.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Yu P, de Petris G, Biancani P, et al. : Cholecystokinin-coupled intracellular signaling in human gallbladder muscle. Gastroenterology. 1994;106(3):763–70. 10.1016/0016-5085(94)90713-7 [DOI] [PubMed] [Google Scholar]
- 142. Yu P, Harnett KM, Biancani P, et al. : Interaction between signal transduction pathways contributing to gallbladder tonic contraction. Am J Physiol. 1993;265(6 Pt 1):G1082–9. 10.1152/ajpgi.1993.265.6.G1082 [DOI] [PubMed] [Google Scholar]
- 143. Yu P, Chen Q, Harnett KM, et al. : Direct G protein activation reverses impaired CCK signaling in human gallbladders with cholesterol stones. Am J Physiol. 1995;269(5 Pt 1):G659–65. 10.1152/ajpgi.1995.269.5.G659 [DOI] [PubMed] [Google Scholar]
- 144. Wang HH, Portincasa P, Wang DQ: Molecular pathophysiology and physical chemistry of cholesterol gallstones. Front Biosci. 2008;13:401–23. 10.2741/2688 [DOI] [PubMed] [Google Scholar]
- 145. Villanacci V, Del Sordo R, Salemme M, et al. : The enteric nervous system in patients with calculous and acalculous gallbladder. Dig Liver Dis. 2016;48(7):792–5. 10.1016/j.dld.2016.03.014 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 146. Amaral J, Xiao ZL, Chen Q, et al. : Gallbladder muscle dysfunction in patients with chronic acalculous disease. Gastroenterology. 2001;120(2):506–11. 10.1053/gast.2001.21190 [DOI] [PubMed] [Google Scholar]
- 147. Chen Q, Amaral J, Oh S, et al. : Gallbladder relaxation in patients with pigment and cholesterol stones. Gastroenterology. 1997;113(3):930–7. 10.1016/S0016-5085(97)70189-6 [DOI] [PubMed] [Google Scholar]
- 148. Miyasaka K, Takata Y, Funakoshi A: Association of cholecystokinin A receptor gene polymorphism with cholelithiasis and the molecular mechanisms of this polymorphism. J Gastroenterol. 2002;37 Suppl 14:102–6. 10.1007/BF03326426 [DOI] [PubMed] [Google Scholar]
- 149. Nakeeb A, Comuzzie AG, Al-Azzawi H, et al. : Insulin resistance causes human gallbladder dysmotility. J Gastrointest Surg. 2006;10(7):940–8; discussion 948-9. 10.1016/j.gassur.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 150. Diamanti-Kandarakis E, Dunaif A: Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. 10.1210/er.2011-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Isik S, Ozcan HN, Ozuguz U, et al. : Impaired gallbladder motility and the effect of metformin therapy in patients with polycystic ovary syndrome. Clin Endocrinol (Oxf). 2012;76(3):373–8. 10.1111/j.1365-2265.2011.04210.x [DOI] [PubMed] [Google Scholar]
- 152. Liao K-F, Chuang HY, Lai SW: Metformin Use Correlates with Reduced Risk of Gallstones in Diabetic Patients: A 12-Year Follow-up Study. Front Pharmacol. 2017;8:765. 10.3389/fphar.2017.00765 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 153. Choi M, Moschetta A, Bookout AL, et al. : Identification of a hormonal basis for gallbladder filling. Nat Med. 2006;12(11):1253–5. 10.1038/nm1501 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 154. Barrera F, Azócar L, Molina H, et al. : Effect of cholecystectomy on bile acid synthesis and circulating levels of fibroblast growth factor 19. Ann Hepatol. 2015;14(5):710–21. [PubMed] [Google Scholar]
- 155. Zweers SJ, Booij KA, Komuta M, et al. : The human gallbladder secretes fibroblast growth factor 19 into bile: towards defining the role of fibroblast growth factor 19 in the enterobiliary tract. Hepatology. 2012;55(2):575–83. 10.1002/hep.24702 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 156. Housset C, Chrétien Y, Debray D, et al. : Functions of the Gallbladder. Compr Physiol. 2016;6(3):1549–77. 10.1002/cphy.c150050 [DOI] [PubMed] [Google Scholar]
- 157. Maruyama T, Miyamoto Y, Nakamura T, et al. : Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun. 2002;298(5):714–9. 10.1016/S0006-291X(02)02550-0 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 158. Keitel V, Cupisti K, Ullmer C, et al. : The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology. 2009;50(3):861–70. 10.1002/hep.23032 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 159. Li T, Holmstrom SR, Kir S, et al. : The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol Endocrinol. 2011;25(6):1066–71. 10.1210/me.2010-0460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Lavoie B, Balemba OB, Godfrey C, et al. : Hydrophobic bile salts inhibit gallbladder smooth muscle function via stimulation of GPBAR1 receptors and activation of K ATP channels. J Physiol. 2010;588(Pt 17):3295–305. 10.1113/jphysiol.2010.192146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Luiking YC, Peeters TL, Stolk MF, et al. : Motilin induces gall bladder emptying and antral contractions in the fasted state in humans. Gut. 1998;42(6):830–5. 10.1136/gut.42.6.830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Portincasa P, Peeters TL, van Berge-Henegouwen GP, et al. : Acute intraduodenal bile salt depletion leads to strong gallbladder contraction, altered antroduodenal motility and high plasma motilin levels in humans. Neurogastroenterol Motil. 2000;12(5):421–30. 10.1046/j.1365-2982.2000.00217.x [DOI] [PubMed] [Google Scholar]
- 163. Stolk MF, van Erpecum KJ, Peeters TL, et al. : Interdigestive gallbladder emptying, antroduodenal motility, and motilin release patterns are altered in cholesterol gallstone patients. Dig Dis Sci. 2001;46(6):1328–34. 10.1023/A:1010635901414 [DOI] [PubMed] [Google Scholar]
- 164. Vanberge-Henegouwen GP, Venneman NG, Portincasa P, et al. : Relevance of hereditary defects in lipid transport proteins for the pathogenesis of cholesterol gallstone disease. Scand J Gastroenterol Suppl. 2004;39(241):60–9. 10.1080/00855920410011022 [DOI] [PubMed] [Google Scholar]
- 165. Van Erpecum KJ, Portincasa P, Gadellaa M, et al. : Effects of bile salt hydrophobicity on crystallization of cholesterol in model bile. Eur J Clin Invest. 1996;26(7):602–8. 10.1046/j.1365-2362.1996.1910532.x [DOI] [PubMed] [Google Scholar]
- 166. Wang HH, Liu M, Portincasa P, et al. : Lack of endogenous cholecystokinin promotes cholelithogenesis in mice. Neurogastroenterol Motil. 2016;28(3):364–75. 10.1111/nmo.12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Attili AF, De Santis A, Capri R, et al. : The natural history of gallstones: the GREPCO experience. The GREPCO Group. Hepatology. 1995;21(3):655–60. 10.1016/0270-9139(95)90514-6 [DOI] [PubMed] [Google Scholar]
- 168. Schmidt M, Hausken T, Glambek I: A 24-year controlled follow-up of patients with silent gallstones showed no long-term risk of symptoms or adverse events leading to cholecystectomy. Scand J Gastroenterol. 2011;46(7–8):949–54. 10.3109/00365521.2011.571710 [DOI] [PubMed] [Google Scholar]
- 169. European Association for the Study of the Liver (EASL). Electronic address: easloffice@easloffice.eu: EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol. 2016;65(1):146–81. 10.1016/j.jhep.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 170. Overby DW, Apelgren KN, Richardson W, et al. : SAGES guidelines for the clinical application of laparoscopic biliary tract surgery. Surg Endosc. 2010;24(10):2368–86. 10.1007/s00464-010-1268-7 [DOI] [PubMed] [Google Scholar]
- 171. Rabenstein T, Radespiel-Tröger M, Höpfner L, et al. : Ten years experience with piezoelectric extracorporeal shockwave lithotripsy of gallbladder stones. Eur J Gastroenterol Hepatol. 2005;17(6):629–39. 10.1097/00042737-200506000-00007 [DOI] [PubMed] [Google Scholar]
- 172. Sandblom G, Videhult P, Crona Guterstam Y, et al. : Mortality after a cholecystectomy: A population-based study. HPB (Oxford). 2015;17(3):239–43. 10.1111/hpb.12356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Isherwood J, Oakland K, Khanna A: A systematic review of the aetiology and management of post cholecystectomy syndrome. Surgeon. 2018; pii: S1479-666X(18)30045-3. 10.1016/j.surge.2018.04.001 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 174. Stinton LM, Shaffer EA: Epidemiology of gallbladder disease: Cholelithiasis and cancer. Gut Liver. 2012;6(2):172–87. 10.5009/gnl.2012.6.2.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Alexander HC, Bartlett AS, Wells CI, et al. : Reporting of complications after laparoscopic cholecystectomy: A systematic review. HPB (Oxford). 2018.;20(9):786–794. 10.1016/j.hpb.2018.03.004 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 176. Lu P, Yang NP, Chang NT, et al. : Effect of socioeconomic inequalities on cholecystectomy outcomes: A 10-year population-based analysis. Int J Equity Health. 2018;17(1):22. 10.1186/s12939-018-0739-7 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 177. Sánchez-Carrasco M, Rodríguez-Sanjuán JC, Martín-Acebes F, et al. : Evaluation of Early Cholecystectomy versus Delayed Cholecystectomy in the Treatment of Acute Cholecystitis. HPB Surg. 2016;2016: 4614096. 10.1155/2016/4614096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Sutton AJ, Vohra RS, Hollyman M, et al. : Cost-effectiveness of emergency versus delayed laparoscopic cholecystectomy for acute gallbladder pathology. Br J Surg. 2017;104(1):98–107. 10.1002/bjs.10317 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 179. Rosenmüller MH, Nilsson E, Lindberg F, et al. : Costs and quality of life of small-incision open cholecystectomy and laparoscopic cholecystectomy - an expertise-based randomised controlled trial. BMC Gastroenterol. 2017;17(1):48. 10.1186/s12876-017-0601-1 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 180. Han C, Shan X, Yao L, et al. : Robotic-assisted versus laparoscopic cholecystectomy for benign gallbladder diseases: A systematic review and meta-analysis. Surg Endosc. 2018.. 10.1007/s00464-018-6295-9 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 181. Di Ciaula A, Garruti G, Wang DQ, et al. : Cholecystectomy and risk of metabolic syndrome. Eur J Intern Med. 2018;53:3–11. 10.1016/j.ejim.2018.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Portincasa P, Di Ciaula A, Grattagliano I: Preventing a Mass Disease: The Case of Gallstones Disease: Role and Competence for Family Physicians. Korean J Fam Med. 2016;37(4):205–13. 10.4082/kjfm.2016.37.4.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Di Ciaula A, Wang DQ, Wang HH, et al. : Targets for current pharmacologic therapy in cholesterol gallstone disease. Gastroenterol Clin North Am. 2010;39(2):245–64, viii-ix. 10.1016/j.gtc.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Molina-Molina E, Lunardi Baccetto R, Wang DQ, et al. : Exercising the hepatobiliary-gut axis. The impact of physical activity performance. Eur J Clin Invest. 2018;48(8):e12958. 10.1111/eci.12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Barré A, Gusto G, Cadeau C, et al. : Diet and Risk of Cholecystectomy: A Prospective Study Based on the French E3N Cohort. Am J Gastroenterol. 2017;112(9):1448–56. 10.1038/ajg.2017.216 [DOI] [PubMed] [Google Scholar]
- 186. Shabanzadeh DM, Sørensen LT, Jørgensen T: Determinants for clinical events in gallstone carriers unaware of their gallstones. J Gastroenterol Hepatol. 2017;32(3):721–6. 10.1111/jgh.13531 [DOI] [PubMed] [Google Scholar]
- 187. Zhang YP, Zhao YL, Sun YL, et al. : Physical Activity and the Risk of Gallstone Disease: A Systematic Review and Meta-analysis. J Clin Gastroenterol. 2017;51(9):857–68. [DOI] [PubMed] [Google Scholar]
- 188. Lander EM, Wertheim BC, Koch SM, et al. : Vegetable protein intake is associated with lower gallbladder disease risk: Findings from the Women's Health Initiative prospective cohort. Prev Med. 2016;88:20–6. 10.1016/j.ypmed.2016.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Jessri M, Rashidkhani B: Dietary patterns and risk of gallbladder disease: A hospital-based case-control study in adult women. J Health Popul Nutr. 2015;33(1):39–49. [PMC free article] [PubMed] [Google Scholar]
- 190. Brown RC, Gray AR, Tey SL, et al. : Associations between Nut Consumption and Health Vary between Omnivores, Vegetarians, and Vegans. Nutrients. 2017;9(11): pii: E1219. 10.3390/nu9111219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Linos AD, Daras V, Linos DA, et al. : Dietary and other risk factors in the aetiology of cholelithiasis: A case control study. HPB Surg. 1989;1(3):221–7. 10.1155/1989/56539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Yago MD, González V, Serrano P, et al. : Effect of the type of dietary fat on biliary lipid composition and bile lithogenicity in humans with cholesterol gallstone disease. Nutrition. 2005;21(3):339–47. 10.1016/j.nut.2004.06.028 [DOI] [PubMed] [Google Scholar]
- 193. Mostofsky E, Mukamal KJ, Giovannucci EL, et al. : Key Findings on Alcohol Consumption and a Variety of Health Outcomes From the Nurses' Health Study. Am J Public Health. 2016;106(9):1586–91. 10.2105/AJPH.2016.303336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Boerlage TC, Haal S, Maurits de Brauw L, et al. : Ursodeoxycholic acid for the prevention of symptomatic gallstone disease after bariatric surgery: Study protocol for a randomized controlled trial (UPGRADE trial). BMC Gastroenterol. 2017;17(1):164. 10.1186/s12876-017-0674-x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 195. Magouliotis DE, Tasiopoulou VS, Svokos AA, et al. : Ursodeoxycholic Acid in the Prevention of Gallstone Formation After Bariatric Surgery: An Updated Systematic Review and Meta-analysis. Obes Surg. 2017;27(11):3021–30. 10.1007/s11695-017-2924-y [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 196. Coupaye M, Calabrese D, Sami O, et al. : Evaluation of incidence of cholelithiasis after bariatric surgery in subjects treated or not treated with ursodeoxycholic acid. Surg Obes Relat Dis. 2017;13(4):681–5. 10.1016/j.soard.2016.11.022 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 197. Abdallah E, Emile SH, Elfeki H, et al. : Role of ursodeoxycholic acid in the prevention of gallstone formation after laparoscopic sleeve gastrectomy. Surg Today. 2017;47(7):844–50. 10.1007/s00595-016-1446-x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 198. Adams LB, Chang C, Pope J, et al. : Randomized, Prospective Comparison of Ursodeoxycholic Acid for the Prevention of Gallstones after Sleeve Gastrectomy. Obes Surg. 2016;26(5):990–4. 10.1007/s11695-015-1858-5 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 199. Stokes CS, Gluud LL, Casper M, et al. : Ursodeoxycholic acid and diets higher in fat prevent gallbladder stones during weight loss: a meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2014;12(7):1090–1100.e2; quiz e61. 10.1016/j.cgh.2013.11.031 [DOI] [PubMed] [Google Scholar]
- 200. Portincasa P, Wang DQH: Gallstones.In: Yamada's Textbook of Gastroenterology 6th. Edited by: Podolsky KD, Camilleri M, Fitz JG, et al UK: Wiley-Blackwell;2015;1808–1834. 10.1002/9781118512074.ch89 [DOI] [Google Scholar]
- 201. Portincasa P, Wang DQ: Effect of Inhibition of Intestinal Cholesterol Absorption on the Prevention of Cholesterol Gallstone Formation. Med Chem. 2017;13(5):421–9. 10.2174/1573406413666170209122851 [DOI] [PubMed] [Google Scholar]
- 202. Cariati A, Piromalli E: Limits and perspective of oral therapy with statins and aspirin for the prevention of symptomatic cholesterol gallstone disease. Expert Opin Pharmacother. 2012;13(9):1223–7. 10.1517/14656566.2012.685161 [DOI] [PubMed] [Google Scholar]
- 203. Ahmed MH, Byrne CD: Potential therapeutic uses for ezetimibe beyond lowering LDL-c to decrease cardiovascular events. Diabetes Obes Metab. 2010;12(11):958–66. 10.1111/j.1463-1326.2010.01261.x [DOI] [PubMed] [Google Scholar]
- 204. Ahmed MH, Hamad MA, Routh C, et al. : Statins as potential treatment for cholesterol gallstones: an attempt to understand the underlying mechanism of actions. Expert Opin Pharmacother. 2011;12(17):2673–81. 10.1517/14656566.2011.629995 [DOI] [PubMed] [Google Scholar]
- 205. Castro-Torres IG, de Jesús Cárdenas-Vázquez R, Velázquez-González C, et al. : Future therapeutic targets for the treatment and prevention of cholesterol gallstones. Eur J Pharmacol. 2015;765:366–74. 10.1016/j.ejphar.2015.08.045 [DOI] [PubMed] [Google Scholar]
- 206. Suuronen S, Niskanen L, Paajanen P, et al. : Declining cholecystectomy rate during the era of statin use in Finland: a population-based cohort study between 1995 and 2009. Scand J Surg. 2013;102(3):158–63. 10.1177/1457496913492463 [DOI] [PubMed] [Google Scholar]
- 207. Chiu HF, Chen CC, Kuo HW, et al. : Statin use and the risk of gallstone disease: a population-based case-control study. Expert Opin Drug Saf. 2012;11(3):369–74. 10.1517/14740338.2012.653560 [DOI] [PubMed] [Google Scholar]
- 208. Liu M, Liu C, Chen H, et al. : Prevention of cholesterol gallstone disease by schaftoside in lithogenic diet-induced C57BL/6 mouse model. Eur J Pharmacol. 2017;815:1–9. 10.1016/j.ejphar.2017.10.003 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 209. Cheng S, Zou M, Liu Q, et al. : Activation of Constitutive Androstane Receptor Prevents Cholesterol Gallstone Formation. Am J Pathol. 2017;187(4):808–18. 10.1016/j.ajpath.2016.12.013 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 210. Moschetta A, Bookout AL, Mangelsdorf DJ: Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med. 2004;10(12):1352–8. 10.1038/nm1138 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 211. Juran BD, Lazaridis KN: Is the FXR the fix for cholesterol gallstone disease? Hepatology. 2005;42(1):218–21. 10.1002/hep.20776 [DOI] [PubMed] [Google Scholar]
- 212. He J, Nishida S, Xu M, et al. : PXR prevents cholesterol gallstone disease by regulating biosynthesis and transport of bile salts. Gastroenterology. 2011;140(7):2095–106. 10.1053/j.gastro.2011.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 213. Diella G, Di Ciaula A, Lorusso MP, et al. : Distinct Effects of two Almond Cultivars on Agreeability and Gastrointestinal Motility in Healthy Subjects: more than mere Nutraceuticals. J Gastrointestin Liver Dis. 2018;27(1):31–9. 10.15403/jgld.2014.1121.271.dll [DOI] [PubMed] [Google Scholar]
- 214. Wang HH, Portincasa P, Wang DQ: Update on the Molecular Mechanisms Underlying the Effect of Cholecystokinin and Cholecystokinin-1 Receptor on the Formation of Cholesterol Gallstones. Curr Med Chem. 2017. 10.2174/0929867324666170619104801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Tharp KM, Khalifeh-Soltani A, Park HM, et al. : Prevention of gallbladder hypomotility via FATP2 inhibition protects from lithogenic diet-induced cholelithiasis. Am J Physiol Gastrointest Liver Physiol. 2016;310(10):G855–64. 10.1152/ajpgi.00316.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 216. Wu X, Liang X, DU Y, et al. : Prevention of gallstones by Lidan Granule: Insight into underlying mechanisms using a guinea pig model. Biomed Rep. 2016;5(1):50–6. 10.3892/br.2016.672 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 217. Shan D, Fang Y, Ye Y, et al. : EGCG reducing the susceptibility to cholesterol gallstone formation through the regulation of inflammation. Biomed Pharmacother. 2008;62(10):677–83. 10.1016/j.biopha.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 218. Kam DM, Webb PA, Sandman G, et al. : A novel 5-lipoxygenase inhibitor prevents gallstone formation in a lithogenic prairie dog model. Am Surg. 1996;62(7):551–5; discussion 555–6. [PubMed] [Google Scholar]