Abstract
Rice seedlings (Oryza sativa) that have died from drought cannot be rescued by watering afterward, but pre-treatment with exogenous acetic acid enabled the plants to produce shoots again after being watered (hereinafter referred to as “drought resilience”). To elucidate the metabolism of acetic acid, we treated rice plants with 13C-labeled acetic acid and traced 13C-labeled metabolites using LC-MS and 13C-NMR techniques. The LC-MS and 13C-NMR spectral data of the root extracts indicated that the acetic acid treatment was absorbed into the plants and then was metabolized to gamma-aminobutyric acid (GABA) by glutamic acid decarboxylase (GAD). GABA accumulation in the roots took place in advance of that in the shoots, and the survival rate against drought stress increased in proportion to the amount of GABA accumulated in the shoots. Therefore, GABA accumulation in shoots may be a key step in drought resilience induced by the acetic acid treatment.
Keywords: drought stress, drought resilience, acetic acid, Oryza sativa, gamma-aminobutyric acid (GABA), glutamic acid decarboxylase (GAD)
Introduction
Water deficit caused by global climate change affects various species and greatly reduces the yield of crop plants.1,2) To maintain stable food production, it is important to clarify how plants resist drought stress. It is well known that plants change their metabolism by expressing various stress-responsive genes and acquire stress tolerance when they sense the stress via signal cascades.3) For instance, wheat plants exposed to osmotic stresses such as drought, freezing, and salt express lea genes, and biosynthesized LEA proteins are thought to play a role in protecting cells from dehydration by preventing protein aggregation.4) Gene expressions controlled by histone modification5) are also involved in plant stress tolerance.6,7) As an example of stress-responsive histone modification, hda6 (a putative class I histone deacetylase gene) was found in Arabidopsis thaliana as a drought responsive gene.8) Furthermore, the pdc1 and the aldh2b7 genes, which are responsible for acetate fermentation, are also involved in drought tolerance in the downstream hda6 gene.8) Because these gene mutants were sensitive to drought stress, activation of acetate fermentation pathway plays important roles in the plants’ acquisition of drought tolerance.8)
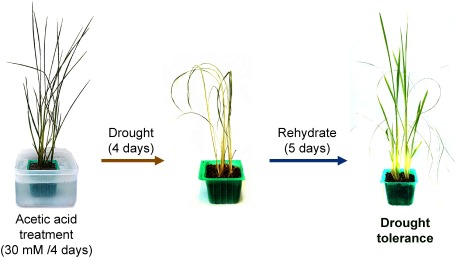
As mentioned above, the hda6 gene seems to activate the acetic acid biosynthesis pathway in response to drought stress and increases the level of acetic acid in the plant cells during drought stress. Kim and his group tried to increase the level of acetic acid in A. thaliana plants artificially by pretreating the plants with exogenous acetic acid and assessed the drought tolerance of the plants. Surprisingly, the acetic acid treatment enabled the plants to acquire drought tolerance.8) When the plants with or without the acid pre-treatment are exposed to water shortage, the former produced shoots again after being watered, while the latter withered and died (Fig. 1). Exogenous acetic acid is absorbed into the plants, converted into acetyl-CoA, and used a substrate for the histone H4 acetylation, as shown by the trace experiments using 14C-labaled acetic acid.8) This drought tolerance induced by exogenous acetic acid (hereinafter referred to as “drought resilience”) was observed in rice (Oryza sativa), maize (Zea mays), wheat (Triticum aestivum) and rapeseed (Brassica napus).8) However, it appears that the action of acetic acid on, for example, induction jasmonic acid signals is quite different between Arabidopsis and rice (unpublished data).
Fig. 1. Overview of drought tolerance induced by acetic acid treatment. The rice plants seemed to be withered and dead when they faced drought stress; however, new leaves grew after rehydration.
Plants in drought stress try to adapt by accumulating amino acids and their derivatives such as proline, betaine, and gamma-aminobutyric acid (GABA),9) and various amino acids are biosynthesized via the tricarboxylic acid (TCA) cycle. In the current study, we investigated the metabolism(s) of rice plants treated with exogenous acetic acid or 13C-labeled acetic acid in case of necessity. Hence, using LC-MS and NMR techniques, we traced the metabolites of acetic acid, focusing on amino acids and organic acids, related to the TCA cycle in rice plants.
Materials and Methods
1. Chemicals
Acetic acid and 2,6-di-t-butyl-4-methylphenol were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). [1-13C] acetic acid and [2-13C] acetic acid were purchased from Sigma-Aldrich Co. LLC (St. Louis, MO, USA). 6-Aminoquinoline, di(N-succinimidyl) carbonate, 2-nitrophenylhydrazine hydrochloride and 1-(3-dimethyl-aminopropyl)-3-ethylcarbodiimide hydrochloride were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan).
2. Plant growth conditions
Rice seeds (Oryza sativa cultivar Nipponbare) were soaked in a fungicide solution containing 0.5% (w/v) Benlate T (Sumitomo Chemical Co., Ltd., Tokyo, Japan) for 2 days. Then rice seeds were transferred to a 10 cm petri dish and soaked in tap water for 1 day at a day/night cycle of 16 hr/8 hr at 30°C. The germinated seeds were planted in soil (Bonsol No.2, Sumitomo Chemical) in plastic pots (6 cm×6 cm×4.5 cm). The seedlings were grown in a growth chamber at a day/night cycle of 14 h/10 h for 2 weeks. The 2-week-old seedlings were used for acetic acid treatment.
3. Acetic acid treatment and drought tolerance test
In order to control the acetic acid concentration in the treatment, 2-week-old rice seedlings in plastic pots were drained and left on paper towels for 20 min. Then the pots were soaked in 30 mM acetic acid aqueous solution or tap water (control), and the seedlings were grown in a growth chamber at a day/night cycle of 14h/10h. After the treated pots were drained and dried as described above, they were kept without watering for 4 days in the growth chamber. Then the seedlings were again kept watered in the chamber for 5 days to test the restoration. After being re-watered, the seedlings from which leaves emerged were counted as the surviving seedlings.
4. Quantification of amino acids and organic acids
4.1. Extraction of amino acids and organic acids from plant materials
The soil around the roots was washed off, and the seedlings were placed in a freezer kept at −20°C. The frozen shoots and roots were cut apart, and the fresh weight of each part was recorded. The rice tissue was homogenized in 80% (v/v) aqueous methanol (5 µL/mg fresh weight) by a μT-12 bead crusher (Taitec Co., Ltd., Saitama, Japan). After centrifugation, the supernatant was filtered through a DISMIC-13HP syringe filter (0.45 µm) (Toyo Roshi Kaisha, Ltd., Tokyo, Japan). The extracts were stored at −20°C for future use.
4.2. Analysis of amino acids
A modified method of Cohen and Michaud10) was used for the derivatization and quantification of amino acids in the extracts. 6-Aminoquinolyl-N-succinimidyl carbamate (AQC) was synthesized as reported previously.10) Plant extracts (10 µL) were mixed with 10 µL of 50 µM aqueous β-alanine solution (internal standard) and 60 µL of 200 mM sodium borate buffer (pH 8.95), and the reaction was initiated by the addition of 20 µL of AQC solution (3 mg/mL in acetonitrile), followed by immediate mixing and incubation for 10 min at 55°C. One microliter of the aliquot was analyzed via LC-MS. The amount of each amino acid in the samples was estimated with calibration curves.
LC-MS was conducted using a Prominence HPLC system coupled with the LCMS-2020 (Shimadzu, Kyoto, Japan). Separations were performed with an ODS column (Mightysil RP-18 GP 50×2.0 mm I.D.; Kanto Chemical Co., Inc., Tokyo, Japan) at 30°C with 0.200 mL/min flow rate. Solvent A was H2O containing 0.1% (v/v) formic acid; solvent B was acetonitrile containing 0.1% (v/v) formic acid. The solvent program was 1% B (0–2 min), 1–15% B (2–9 min), 15–30% B (9–14 min), and 30–80% B (14–20 min). The MS operating parameters are described in detail in Supplemental Information 1A.
4.3. Analysis of organic acids
A modified method of Han et al.11) was used for derivatization and quantification of organic acids in the extracts. The extracts (10 µL) were mixed with 10 µL of 100 µM formic acid (internal standard), 20 µL of 250 mM 2-nitrophenylhydrazine hydrochloride solution (in 50% (v/v) aqueous methanol), 20 µL of 150 mM 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride solution (in methanol), and 20 µL of 7.5% (v/v) pyridine solution (in 75% (v/v) aqueous methanol). The mixture was incubated in a refrigerator at 4°C for 1 hr before the addition of 20 µL of 2,6-di-t-butyl-4-methylphenol solution (2 mg/mL in methanol) and mixing. An aliquot (1 µL) was analyzed via LC-MS. The amounts of each organic acid in the samples were estimated with calibration curves.
LC-MS was conducted with a Prominence HPLC system coupled with the LCMS-2020. Separations were performed with an ODS column (Mightysil RP-18 GP 50×2.0 mm I.D.) at 40°C with a 0.300 mL/min flow rate. Solvent A was H2O containing 0.01% (v/v) formic acid; solvent B was methanol containing 0.01% (v/v) formic acid. The solvent program was 18% B (0–2 min), 18–90% B (2–16 min), and 100% B (16–17 min). The MS operating parameters are described in detail in Supplemental Information 1B.
5. NMR measurement
The rice seedlings treated with 13C-labeled acetic acid for 4 days were used for NMR analysis. The roots of 4 ([1-13C] acetic acid) or 20 ([2-13C] acetic acid) seedlings were frozen in liquid N2, crushed by a bead crusher, and extracted with 1 mL of 80% (v/v) aqueous MeOH twice. After centrifugation, the supernatants were evaporated with a rotary evaporator and re-suspended with 2 mL of 0.1 N HCl. The sample solutions were applied to Oasis® MCX cartridges (Waters Corp., Milford, MA, USA). After adsorption of the samples, the cartridges were rinsed with 2% (v/v) aqueous formic acid and MeOH. The positive ionic compounds and dipolar ionic compounds retained on the cartridges were eluted with 4 mL of 4 N NH3 solution (in 50% (v/v) aqueous MeOH), evaporated with the rotary evaporator, and re-suspended with D2O. The sample solutions were used for 13C-NMR measurement. 13C-NMR spectra were recorded with acetone-d6 as the internal standard in D2O using a Bruker AV-III NMR spectrometer.
Results
1. Investigation of metabolites derived from exogenously applied acetic acid by LC/MS
After rice plants were treated with [1-13C] or [2-13C] acetic acid, the amounts of 13C-labeled amino acids and organic acids related to the TCA cycle were examined. Peak areas detected by monitoring at m/z [M+1]+ (Anon-labeled) and m/z [M+2]+ (Alabeled) were measured, and the 13C ratios of the metabolites were evaluated using the following equation:
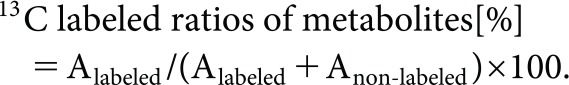 |
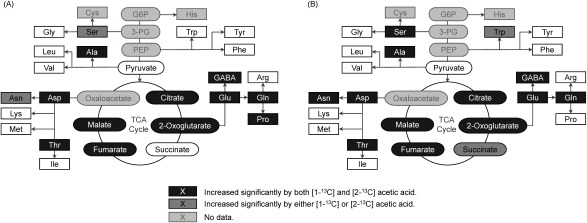
The 13C ratios of the following metabolites in both [1-13C] and [2-13C]-labeled acetic acid treatments were significantly increased compared to those in non-labeled acetic acid treatment: alanine, aspartate, citrate, fumarate, GABA, glutamine, glutamate, malate, 2-oxoglutarate, proline, and threonine in shoots and alanine, aspartate, asparagine, citrate, fumarate, GABA, glutamine, glutamate, malate, 2-oxoglutarate, serine, and threonine in roots. Most of them are categorized into metabolites downstream of acetyl CoA (Fig. 2). In contrast, the 13C ratios of metabolites upstream of acetyl CoA scarcely increased (Supplemental Figs. S1 and S2).
Fig. 2. Significant difference of 13C-labelled ratios relative to the control (non-labeled acetic acid) (A) in shoots and (B) in roots (p<0.05 using Tukey–Kramer’s multiple test, n=4).
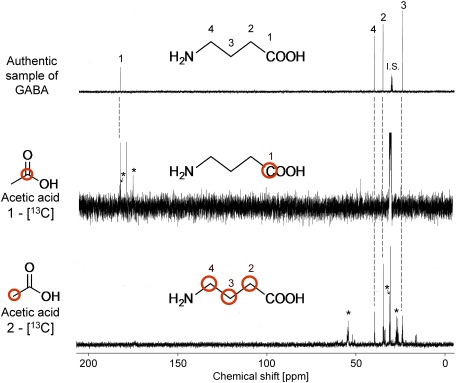
2. NMR measurement of the root extracts
The 13C-NMR spectra of root extracts of the rice plants treated with [1-13C] or [2-13C]-labeled acetic acid were compared with those of authentic GABA. The C-1 position (δ 181.6 ppm) of GABA was labeled in the former case (Fig. 3). The signals at δ 174.1 and 181.4 ppm, corresponding respectively to the C-1 and C-5 positions of glutamate, were also detected in the root extracts. In the latter case, the C-2, C-3, and C-4 positions (δ 34.5, 23.7, and 39.4 ppm, respectively) of GABA were labeled, as shown in Fig. 3. The signals observed at δ 27.2, 30.9, and 54.2 ppm corresponded with the C-3, C-4 and C-2 positions of glutamate, respectively.
Fig. 3. 13C-NMR spectra of the root extracts and authentic GABA sample (100 MHz). The signals with asterisks were assigned to those derived from glutamate.
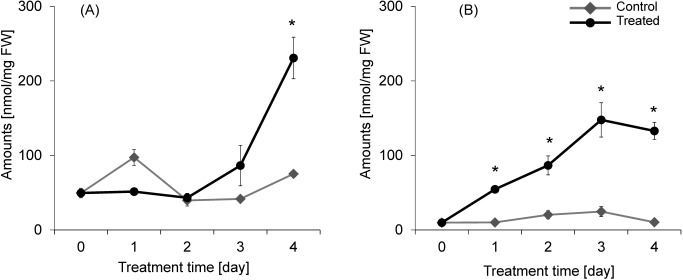
3. Correlation between the GABA quantity in plants and drought tolerance
During the acetic acid treatment, the amount of GABA in both roots and shoots increased significantly. Interestingly, the level of GABA in the roots increased from the first day of treatment, while that in the shoots increased strongly only after the third day (Fig. 4). It is not clear why the amount of GABA in the control shoots peaked on the first day.
Fig. 4. GABA quantity in the rice plants during acetic acid treatment (30 mM, 4 days; A, in shoots; B, in roots). Data are the mean±S.E. Asterisks indicate significant differences relative to the control (p<0.05 using Welch’s t-test, n=3–4). FW: fresh weight.
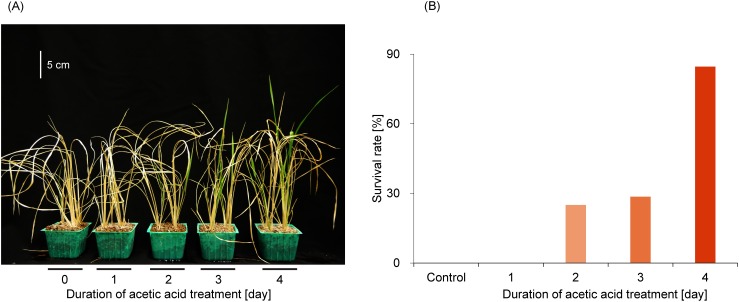
We tested whether the duration of acetic acid treatment affects the plant survival rate. The survival rate against drought stress increased with prolonged acetic acid treatment, and the plants treated for 4 days with acetic acid showed the highest survival rate against drought stress (Fig. 5).
Fig. 5. Effect of the duration of acetic acid treatment on rice plants. (A) Picture of rehydrated rice plants treated differently with acetic acid. (B) Survival rates and duration of acetic acid treatment (n=8–14).
Discussion
As far as we know, exogenous acetic acid is converted to acetyl CoA mainly by acetyl CoA synthetase (ACS) in the plastid of A. thaliana.12,13) In the current study, when rice plants were treated with 13C-labeled acetic acid, only metabolites downstream of acetyl CoA were labeled with 13C. This result indicated that exogenous acetic acid was also absorbed into rice plants and was metabolized to acetyl CoA. In addition, the metabolism of acetic acid is thought to be controlled by the availability of exogenous acetic acid rather than by the ACS activity.12,14) Arabidopsis thaliana plants treated with a lower concentration (1 mM) of acetic acid did not show drought resilience in a previous report.8) Thus, the synthesis of acetyl CoA derived from acetic acid was considered to be promoted by high concentrations (10, 20, or 30 mM) of treated acetic acid. As mentioned previously, it is clear that 14C-labeled acetic acid is absorbed into the plants and incorporated to the histone H4 protein in A. thaliana.8) Acetyl CoA is required as a substrate for histone acetylation12); therefore, conversion from exogenous acetic acid to acetyl CoA seems to be meaningful for acetate-mediated drought resilience.
When rice plants were treated with [2-13C]-labeled acetic acid, the C-2, C-3, and C-4 positions of GABA were clearly labeled (Fig. 3). Considering the metabolic pathway in the TCA cycle, it is understandable that the labeled methyl-carbon of acetyl CoA was incorporated into the C-4 position of 2-ketoglutarate in the first round, and then into the C-2 position of GABA via [4-13C] glutamate by glutamic acid decarboxylase (GAD),15,16) as shown in Fig. 6A. Furthermore, the labeled methyl-carbon was also transferred into the C-2 or C-3 positions of oxaloacetate, which is biosynthesized via the symmetric metabolites succinate and fumarate. In the second and later rounds, consequently, it is suggested that the labeled methyl-carbon of acetyl CoA was also incorporated into the C-3 or C-4 positions of GABA (Fig. 6B). In the present study, it was observed that the labeled methyl-carbon was clearly detected in the C-2, C-3, and C-4 positions of GABA. Furthermore, as mentioned in the Result section, it was reasonable that the labeled methyl-carbon was also detected in the C-2, C-3, and C-4 positions of glutamate, which is a precursor of GABA. When [1-13C]-labeled acetic acid was used, GABA was labeled only at the C-1 position (Supplemental Fig. S3) with the release of 13CO2 gas.
Fig. 6. The expected 13C-labeled position of metabolites by [2-13C] acetic acid (A) in the first round and (B) in the second and later rounds.
The present study demonstrated that GABA was not only biosynthesized but also accumulated in the rice plants with acetic acid treatment. GABA accumulation has not been observed in Arabidopsis thaliana treated with acetic acid.8) Therefore, the role of exogenous acetic acid in inducing “drought resilience” in rice plants might be different from that in A. thaliana, where histone acetylation occurred.8) To speculate the mechanism of drought resilience induced by acetic acid, we focus on the time course of GABA accumulation. In previous studies, GABA accumulated in various kinds of plants when they were challenged by stress.16,17) Some reports showed that GABA accumulation in shoots and roots occurred almost at the same time.18,19) In contrast to these reports, however, we found that the GABA accumulation in roots occurred about two days earlier than that in shoots for rice plants treated with acetic acid. As explained above, GABA derived from exogenous acetic acid was estimated to be biosynthesized by GAD, which is reported to be essential for the GABA accumulation in plants.16) The GAD activity is stimulated by acidic pH in cytosol or calcium/calmodulin binding to protein,17) but the correlation between GAD activity and soil pH remains unknown. When plants try to cope with stress, they activate calcium/calmodulin-mediated signaling20) and make their “preparation for stress”; thus we hypothesize that the exogenous acetic acid treatment induces the “preparation for drought stress” in rice plants, and the delay of GABA accumulation in shoots might be explained by the difference in the “preparation for drought stress” between shoots and roots. In the current study, the survival rate of plants treated with acetic acid seemed to correlate with the GABA level of the shoots, especially from the third to the fourth day of treatment. Thus, that “preparation” level of the shoots was seemingly important according to this hypothesis. Plants require a shoot apical meristem for shoot growth.21,22) The appropriate “preparation” in shoots might include effects on the preservation of essential elements for shoot growth, such as a shoot apical meristem. Furthermore, no drought resilience was observed in rice plants treated with the naturally occurring weak acids in soil (citric acid, phosphoric acid, and lactic acid) (30 mM), although GABA accumulation was not investigated (unpublished data). Therefore, we concluded that “preparation for drought stress” in rice plants was induced by the treatment with acetic acid itself, not by its weak acidity.
In this paper, we reported strong indications that acetic acid treatment was converted to GABA by GAD and demonstrated that GABA accumulation in shoots seems to have a relationship with drought resilience induced by acetic acid in rice plants. However, the role of accumulated GABA remains unknown. There are several studies on accumulated GABA during plant stress, and some roles, such as an osmoregulator23) and a scavenger of reactive oxygen species,24) have been suggested. In addition, Mekonenn and his group recently reported that gad1/2 mutant A.thaliana plants that accumulated less GABA were oversensitive to drought with the inhibition of stomata closure.25) Because a GABA-regulated aluminum-activated malate transporter (ALMT) has been identified26) and ALMT homologues were found on guard cells of A.thaliana,27,28) GABA might work as a signaling molecule in plants’ defense against stress, including drought stress.29) On the other hand, at present, it is unclear whether exogenous acetic acid is used as a carbon source or as a signaling molecule in rice plants. To reveal the mechanism of the drought resilience induced by acetic acid, the roles of accumulated GABA in the resilience in rice plants need to be studied.
Acknowledgments
This study was supported by Grants-In-Aids for Scientific research (No. 17K07770) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and a grant from Japan Science and Technology Agency to YH (CREST, No. JPMJCR132B4).
Supplementary Data
References
- 1).Q. Schiermeier: Nature 452, 270–273 (2008). [DOI] [PubMed] [Google Scholar]
- 2).C. Lesk, P. Rowhani and N. Ramankutty: Nature 529, 84–87 (2016). [DOI] [PubMed] [Google Scholar]
- 3).L. Xiong, K. S. Schumaker and J. K. Zhu: Plant Cell 14(Suppl), S165–S183 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).R. Imai: Chem. Biol. (Tokyo, Jpn.) 34, 294–303 (1996), in Japanese. [Google Scholar]
- 5).B. D. Strahl and C. D. Allis: Nature 403, 41–45 (2000). [DOI] [PubMed] [Google Scholar]
- 6).A. Boyko and I. Kovalchuk: Environ. Mol. Mutagen. 49, 61–72 (2008). [DOI] [PubMed] [Google Scholar]
- 7).M. Jaskiewicz, U. Conrath and C. Peterhänsel: EMBO Rep. 12, 50–55 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).J. M. Kim, T. K. To, A. Matsui, K. Tanoi, N. I. Kobayashi, F. Matsuda, Y. Habu, D. Ogawa, T. Sakamoto, S. Matsunaga, K. Bashir, S. Rasheed, M. Ando, H. Takeda, K. Kawaura, M. Kusano, A. Fukushima, T. A. Endo, T. Kuromori, J. Ishida, T. Morosawa, M. Tanaka, C. Torii, Y. Takebayashi, H. Sakakibara, Y. Ogihara, K. Saito, K. Shinozaki, A. Devoto and M. Seki: Nat. Plants 3, 17097 (2017). [DOI] [PubMed] [Google Scholar]
- 9).E. A. Bray: Plant Physiol. 103, 1035–1040 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).S. A. Cohen and D. P. Michaud: Anal. Biochem. 211, 279–287 (1993). [DOI] [PubMed] [Google Scholar]
- 11).J. Han, S. Gagnon, T. Eckle and C. H. Borchers: Electrophoresis 34, 2891–2900 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).D. J. Oliver, B. J. Nikolau and E. S. Wurtele: Plant Sci. 176, 597–601 (2009). [Google Scholar]
- 13).M. Lin and D. J. Oliver: Plant Physiol. 147, 1822–1829 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).R. H. Behal, M. Lin, S. Back and D. J. Oliver: Arch. Biochem. Biophys. 402, 259–267 (2002). [DOI] [PubMed] [Google Scholar]
- 15).N. Bouché and H. Fromm: Trends Plant Sci. 9, 110–115 (2004). [DOI] [PubMed] [Google Scholar]
- 16).A. Fait, H. Fromm, D. Walter, G. Galili and A. R. Fernie: Trends Plant Sci. 13, 14–19 (2008). [DOI] [PubMed] [Google Scholar]
- 17).A. M. Kinnersley and F. J. Turano: Crit. Rev. Plant Sci. 19, 479–509 (2000). [Google Scholar]
- 18).M. C. Bolarín, A. Santa-Cruz, E. Kayuela and F. Pérez-Alfocea: J. Plant Physiol. 147, 463–468 (1995). [Google Scholar]
- 19).N. Aurisano, A. Bertani and R. Reggiani: Phytochemistry 38, 1147–1150 (1995). [Google Scholar]
- 20).T. Yang and B. W. Poovaiah: Trends Plant Sci. 8, 505–512 (2003). [DOI] [PubMed] [Google Scholar]
- 21).K. Yamazaki: Jpn. J. Crop. Sci. 31, 371–378 (1963), in Japanese. [Google Scholar]
- 22).Rural Culture Association Japan (eds.): “Inasaku Dai Hyakka 〈1〉 Sousetsu/Keitai/Hinshu/Hinshitsukanri”, Rural Culture Association Japan, Tokyo, p. 162, 2004. (in Japanese).
- 23).R. Schwacke, S. Grallath, K. E. Breitkreuz, E. Stransky, H. Stransky, W. B. Frommer and D. Rentsch: Plant Cell 11, 377–391 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).N. Bouché, A. Fait, D. Bouchez, S. G. Møller and H. Fromm: Proc. Natl. Acad. Sci. U.S.A. 100, 6843–6848 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).D. W. Mekonenn, U.-I. Flügge and F. Ludewig: Plant Sci. 245, 25–34 (2016). [DOI] [PubMed] [Google Scholar]
- 26).A. W. Bown and B. J. Shelp: Trends Plant Sci. 21, 811–813 (2016). [DOI] [PubMed] [Google Scholar]
- 27).T. Sasaki, I. C. Mori, T. Furuichi, S. Munemasa, K. Toyooka, K. Matsuoka, Y. Murata and Y. Yamamoto: Plant Cell Physiol. 51, 354–365 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).M. Hashimoto-Sugimoto, J. Negi, K. Kusumoto and K. Iba: Chem. Biol. (Tokyo, Jpn.) 51, 831–839 (2013), in Japanese. [Google Scholar]
- 29).S. A. Ramesh, S. D. Tyerman, M. Gilliham and B. Xu: Cell. Mol. Life Sci. 74, 1577–1603 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



![Fig. 6. The expected 13C-labeled position of metabolites by [2-13C] acetic acid (A) in the first round and (B) in the second and later rounds.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/8cb2/6173134/47d3bf41a2a2/jps-43-3-D18-036-figure06.jpg)