Abstract
The sea cucumber (Apostichopus japonicus) has become a good model organism for studying environmentally-induced aestivation by a marine invertebrate more recently. In the present study, we hypothesized that miRNA-200-3p may contribute to establish rapid biological control to regulate fatty acid metabolism during a estivation. The peroxisomal bi-functional enzyme (EHHADH) is a crucial participant of the classical peroxisomal fatty acid β-oxidation pathway, the relative mRNA transcripts and protein expressions of EHHADH were analyzed in intestine from sea cucumbers experienced long-term aestivation. Both mRNA transcripts and protein expressions of EHHADH in intestine decreased significantly during deep-aestivation as compared with non-aestivation controls. Analysis of the 3′ UTR of AjEHHADH showed the presence of a conserved binding site for miR-200-3p. Level of miR-200-3p showed an inverse correlation with EHHADH mRNA transcripts and protein levels in intestine, implicating miR-200-3p may directly targeted AjEHHADH by inducing the degradation of AjEHHADH mRNA in the aestivating sea cucumber, further dual-luciferase reporter assay validated the predicted role of miRNA-200-3p in regulating AjEHHADH. In order to further understand their regulatory mechanism, we conducted the functional experiment in vivo. The overexpression of miR-200-3p in sea cucumber significantly decreased mRNA and protein expression levels of AjEHHADH. Taken together, these findings suggested the potential contribution of miRNA-200-3p to the fatty acid metabolism by regulating AjEHHADH during aestivation in sea cucumber.
Keywords: Sea cucumber, Fatty acid metabolism, AjEHHADH, Aestivation
Introduction
The sea cucumber, Apostichopus japonicus, is an important species farmed in the Chinese aquaculture industry and recently has been embraced as a model organism for studying aestivation in marine invertebrates (Chen et al., 2013; Chen & Storey, 2014; Chen, Zhu & Storey, 2016; Chen et al., 2016). This animal becomes inactive when water temperature rises over 18 °C and is induced to aestivation when temperatures rise over 25 °C during the summer; thereafter, the animals remain in dormancy for over 100 days without feeding or locomotion (Sui et al., 1985; Sui & Liao, 1988; Yang et al., 2005). During prolonged periods of inactivity, A. japonicus undergoes a series of physiological and biochemical adaptations characterized by profound metabolic rate depression, loss of body weight, intestinal atrophy, and global redistribution of metabolic fuels between tissues (Yang et al., 2006; Yuan et al., 2007). Upon recovery from aestivation, all of these features, including intestinal atrophy, are reversed. Numerous studies completed by our group have outlined the molecular mechanisms of natural aestivation tolerance in sea cucumbers. These studies investigated the roles and regulation involved in providing global suppression of transcription and translation in order to minimize energy expenditure and protect cellular functions (Chen, Zhu & Storey, 2016; Chen et al., 2016; Chen et al., 2013; Chen & Storey, 2014). The optimal regulatory mechanisms for aestivation survival are those that can broadly control multiple metabolic processes, can be coordinated by extracellular stimuli, are easily induced and readily reversed. Among these mechanisms, post-transcription regulation of various transcripts by microRNAs (miRNAs) has become a topic of interest for many researchers.
MiRNAs are highly conserved, small non-coding RNA molecules that can regulate gene expression by binding to the 3′-untranslated regions (UTRs) of target genes, resulting in translational suppression by either causing the degradation of the corresponding mRNA transcript or move it into storage in p-bodies or stress granules for future use (Bartel, 2004; Biggar & Storey, 2011). Recently, miRNA studies have outlined the role of microRNAs in supporting hypometabolic states such as hibernation in ground squirrels and bats (Morin, Dubuc & Storey, 2008; Kornfeld, Biggar & Storey, 2012; Maistrovski, Biggar & Storey, 2012), freeze tolerance by wood frogs (Biggar, Dubuc & Storey, 2009), anoxia tolerance in turtles and marine snails (Biggar & Storey, 2012; Biggar et al., 2012) and aestivation in sea cucumbers (Chen et al., 2013; Chen & Storey, 2014). In periods where energy availability is limited, it is vitally important to rapidly and readily change the energy expenditure of the cell to provide sufficient supply for pro-survival processes while suppressing harmful or unnecessary processes in a reversible and coordinated fashion. This regulation can be achieved by miRNAs which can act to reprioritize the patterns of energy production and induce stress-specific cellular adaptations.
One major problem associated with aestivation is oxidative stress and, therefore, for an organism to withstand prolonged periods of aestivation, it must combat oxidative damage. Antioxidant defenses are important strategies used by aerobic organisms to protect macromolecules from damage by reactive oxygen species (ROS). Multiple physiological or environmental stresses can lead to oxidative damage and different combative strategies have been reported in various species that depict the role of antioxidant defenses in protecting animals when challenged by unfavorable environmental conditions (Storey, 1996). One of the problems associated with oxidative stress is lipid peroxidation. High levels of ROS can react with unsaturated bonds in membrane lipids, resulting in lipid peroxidation and causing cellular damage or potentially even leading to death (Catalá, 2009). Disruption of lipid membranes is associated with a plethora of detrimental side effects including disturbance in the fluidity and permeability of the membranes, imbalances in ion and metabolite transportation and interruption of various cellular processes (Borchman et al., 1992; Goldstein & Weissmann, 1977; Kourie, 1988; Mattson et al., 1999). Moreover, lipid peroxidation can negatively affect mitochondrial functions thereby interrupting respiration, membrane potential and calcium buffering abilities (Zhang et al., 1990; Albano et al., 1991).
A recent study has reported that lipid peroxidation is a major contributor to the loss of cellular functions, resulting in oxidative damage during aestivation (Giraud-Billoud et al., 2013). The same study concluded that an effective antioxidant response during the aestivation/recovery cycle was responsible for protecting the midgut of the South American apple snail, Pomacea canaliculata. Hence, we proposed that the regulation of key enzymes of fatty acid metabolism during estivation would contribute to the adaptive responses needed to protect under environmental conditions that can cause oxidative stress. EHHADH (enoyl-CoA hydratase and 3-hydroxyacyl CoA dehydrogenase) is a bi-functional enzyme that is a crucial participant in the classical peroxisomal fatty acid β-oxidation pathway, the primary pathway involved in lipid catabolism (Houten et al., 2012). There are four enzymatic steps in each cycle of peroxisomal β-oxidation: oxidation, hydration, dehydrogenation and thiolytic cleavage (Poirier et al., 2006). EHHADH participates in both the second and third steps of long-chain dicarboxylic fatty acid (DCA) β-oxidation (Vamecq & Draye, 1989). Recent evidence suggests that EHHADH is essential for the production of medium-chain DCAs (Houten et al., 2012) that can inhibit mitochondrial respiration by affecting the electron transport chain thereby reducing ATP production while elevating ROS levels (Passi et al., 1984; Tonsgard & Getz, 1985).
Our studies have demonstrated that miRNA-200-3p may play important roles in global transcriptional suppression during aestivation. Preliminary bioinformatics analysis suggested that the AjEHHADH transcript could be a target of miRNA-200-3p (Chen et al., 2013). Our present work aimed to elucidate the potential role of miRNA-200-3p in inhibiting oxidative damage during aestivation in the sea cucumber though its actions in regulating AjEHHADH gene expression. The present study is the first to report alterations in the transcriptional and translational expression of the intestinal AjEHHADH enzyme in the sea cucumber during aestivation. In vivo and in vitro analysis of the functional relationship between miR-200-3p and AjEHHADH were assessed. Our analysis depicts a preliminary relationship between the involvement of microRNAs in regulating fatty acid metabolism and the reduction or avoidance of lipid peroxidation damage in the sea cucumber during aestivation.
Materials and Methods
Animals
Adult sea cucumbers (males and females mixed) (A. japonicus), 100 ± 8g, were collected from the coast of Qingdao by diving and hand fishing (Jiaozhou Bay of the Yellow Sea, China). Two groups of sea cucumbers were sampled and dissected right after capture. Non-aestivating sea cucumbers (NA) which served as the control group were sampled in May when the seawater temperature was about 15 °C. At this time and temperature, the sea cucumbers have already recovered fully from aestivation. Animals in deep-aestivation (DA) were sampled in mid-August when seawater temperature was above 25 °C. These animals were sampled after about 15 days of continuous aestivation as indicated by cessation of feeding and locomotion, and the degeneration of the intestine into a very thin and small string (about 2 mm). At each stage (NA and DA), 10 individuals were sacrificed and intestine tissues were dissected, cleared of content and flash frozen in liquid nitrogen. All samples were kept at −80 °C for later analysis. Sea cucumbers (A. japonicus) are commercially cultured animals. The study protocol was approved by the Experimental Animal Ethics Committee of the Ocean University of China.
RNA extraction, AjEHHADH cDNA cloning, and sequence analysis
Total RNA was isolated from intestinal tissues of sea cucumbers using Trizol (Cat No. J20921; TransGen Biotech, Beijing, China) following manufacturer’s instructions. RNA concentration and quality were determined using an Agilent 2100 bioanalyzer. Gene-specific primers for cloning the full-length cDNA of AjEHHADH were designed based on the partial sequences of AjEHHADH genes obtained from our previously constructed transcriptome library of A. japonicus (Data S11). The full-length cDNA sequences were cloned using the SMART RACE cDNA Amplification kit (Cat No. ST0258; Clontech, Mountain View, USA) in accordance with the manufacturer’s instructions. The primer sets for AjEHHADH 5′/3′ RACE are listed in Table 1. Polymerase chain reaction (PCR) amplification was carried out using Advantage 2 polymerase mix (Cat No. S1798; Clontech, Mountain View, CA, USA) in a volume of 50 µL. PCR was performed under the following conditions: 95 °C for 1 min; five cycles of 94 °C for 30 s, 66 °C for 30 s, and 72 °C for 3 min; five cycles of 94 °C for 30 s, 64 °C for 30 s, and 72 °C for 3 min; five cycles of 94 °C for 30 s, 62 °C for 30 s, and 72 °C for 3 min; 20 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 3 min, followed by a final cycle of 72 °C for 10 min. The expected PCR products were eluted from a 1% agarose gel using a gel extraction kit (Cat No. D2500-02; Omega, Norcross, GA, USA) following the manufacturer’s instructions, then cloned into the pMD19-T vector (Cat No. 6013; Takara, Kusatsu, Japan) and transformed into JM109 competent cells (Cat No. 9057; Takara, Kusatsu, Japan) following the manufacturer’s instructions. Transformed cells were cultured overnight on Luria-Bertani (LB) agar plates containing 100 µg/mL ampicillin. White clones were chosen and cultured in SOC medium (Cat No. HBDC002; Hopebio, Qingdao, China) containing 100 µg/mL ampicillin for 10 h at 37 °C. Positive recombinant clones were sequenced by Sangon Biotech (Shanghai, China). The sequences were analyzed and assembled using DNAStar software (DNAStar Inc., Madison, WI, USA) to identify the open reading frame (ORF). The deduced amino acid sequence of sea cucumber AjEHHADH was analyzed using an on-site program http://www.bio-soft.net/sms/index.html. The functional sites or domains in the amino acid sequence were predicted using the simple modular architecture research tool (SMART) version 4.0 (http://smart.embl-heidelberg.de/) and Interpro (http://www.ebi.ac.uk/interpro/).
Table 1. Primers used in this study.
| Name | Primer Sequences (5′–3′) | Location | |
|---|---|---|---|
| RACE | AjEHHADH-R1 | GCGAGAATGGCGGAGGGTTTGCAAGT | 1,382–1,407 |
| R1-NEST | CAGGTATCCCACTGGCGATGAAACA | 1,125–1,149 | |
| qRT-PCR | miR-200-3p-F | TAATACTGTCTGGTGATGATG | |
| miR-200-3p-R | mRQ 3′ primer | ||
| 5.8s-F | ATCACTCGGCTCGTGCGTC | ||
| 5.8s-R | GCCATTTGCGTTCGAATAAGT | ||
| AjEHHADH-F | GCTCTTTTCTTCTCTGGCCA | 954–973 | |
| AjEHHADH-R | ACAGGTATCCCACTGCTGAT | 1,131–1,150 | |
| β- Tubulin-F | GAAAGCCTTACGACGGAACA | ||
| β- Tubulin-R | CACCACGTGGACTCAAAATG | ||
| In vivo | miR-200-3p mimics-F | UAAUACUGUCUGGUGAUGAUG | |
| miR-200-3p mimics-R | UCAUCACCAGACAGUAUUAUU | ||
| Negative control-F | UUCUCCGAACGUGUCACGUTT | ||
| Negative control-R | ACGUGACACGUUCGGAGAATT | ||
| Dual-luciferase | AjEHHADH-WT-F | CGGATCCGACCCTGTAACAACTCTTAACCTCT | 2,025–2,048 |
| AjEHHADH-WT-R | CGGATCCGCAACTGACAATTTCTTGTAA | 2,280–2,299 | |
| AjEHHADH-MUT-F | TTCACCAGGTCATAAAACGAGATTTATTTGATC | 2,133–2,165 | |
| AjEHHADH-MUT-R | TAGAGCAATTATGACGACCACTTTAAAGAA | 2,126–2,155 |
Prediction of the miR-200-3p target
Based on our previous transcriptome (Data S11) and proteome (Chen et al., 2016) data for A. japonicus, potential miR-200-3p targets were predicted using TargetScan and miRanda software. The target genes for different miRNAs were predicted by the TargetScan algorithm complying with the criteria in the seed region: no mismatch between two to eight nt on the end of miRNA (7mer-m8) The potential targets of miRNA-200-3p were also predicted using the Miranda toolbox based on the complementary region between the miRNA and the 3′-UTR of mRNA, as well as the thermodynamic stability of the miRNA-mRNA duplex. The candidate targets with S >90 (single-residue pair scores) and a minimum free energy lower than −20 kcal/mol were selected for further analysis. The TargetScan program searches for microRNA binding sites (seed matches) conserved between several organisms.
Dual-luciferase reporter assays
Both wildtype (WT) and mutant (MT) segments of the AjEHHADH 3′-UTR (about 500 nt before and after the binding sites) were cloned into pmiR-RB-REPORT luciferase reporter vectors (Ribobio, Guangzhou, China). The primers selected are listed in Table 1. For the transfection experiment, the 293T cells were plated into 96-well white plates 24 h before transfection. Plasmids constructed with pMIR-REPORT vectors were cotransfected with a control Renilla luciferase plasmid (pRL-CMV) (Cat No. E2261; Promega, Madison, WI, USA). The ratio of experimental plasmid to control plasmid was 5:1. Then vectors with 3′ UTR of AjEHHADH (WT and MT) were cotransfected with miRNA-200-3p mimics or a negative control. Luciferase assays were performed using the Dual-Luciferase Reporter Assay System (Cat No.E1910; Promega, Madison, WI, USA). In brief, 48 h after transfection, cell lysates were prepared by incubating with 1 × passive lysis buffer for 15 min at room temperature. Cell lysates were transferred to 96-well plates and analyzed using the luciferase dual reporter assay kit (Cat No.E1910; Promega, Madison, WI, USA). The firefly luciferase values were normalized to Renilla.
qRT-PCR analysis of miRNA-200-3p and AjEHHADH, Western blotting analysis of AjEHHADH
Total RNA was isolated as described above from sea cucumber intestinal tissues of the control and estivated groups. RNA concentration and quality were determined using an Agilent 2100 bioanalyzer and then stored at −80 °C for further analysis. The Mir-X miRNA First-Strand Synthesis Kit (Cat No. 638313; Takara, Kusatsu, Japan) was used to synthesize first-strand cDNA as the qRT-PCR template. The entire mature miR-200-3p sequence was synthesized as the specific forward primer (miR-200-3p-F in Table 1) for qRT-PCR and the mRQ 3′ primer supplied with the kit was used as the reverse primer. The 5.8s rRNA was selected as the internal control (see Table 1 for primer sets). SYBR® Premix Ex TaqTM (Cat No. RR820; Takara, Kusatsu, Japan) master mix was used to determine the expression levels of miR-200-3p in both control and estivated groups using the StepOnePlus system (ABI Inc., Foster City, CA, USA). Each sample was run in triplicate along with the internal control gene. Melting-curve analysis of the amplification products was performed at the end of the PCR reaction to determine the purity of the PCR product. The 2−△△CT method was used to analyze the comparative expression levels.
Total RNA was isolated as described above from the intestine of five individuals for both control and estivated groups. First-strand cDNA was synthesized using the M-MLV reverse transcriptase enzyme (Cat No. RR047A; Takara, Kusatsu, Japan) by qRT-PCR. The AjEHHADH-specific primer pairs for qRT-PCR were designed based on the obtained open reading frame (ORF) using an online version of Primer 3.0 (Table 1). β-tubulin was previously shown to remain unchanged during aestivation in A. japonicus and therefore we used it as the internal control (Zhao et al., 2014). Gene expression levels of AjEHHADH were analyzed using SYBR® Premix Ex Taq™ master mix (Cat No. RR420; Takara, Kusatsu, Japan) and amplification was measured by the StepOnePlus system (ABI Inc., Foster, CA, USA). Each sample was run in triplicate along with the internal control. Melting-curve analysis of the amplified products was performed at the end of each PCR reaction to ensure that only one PCR product was produced. The 2−△△CT method was used to analyze the comparative expression levels.
Protein extraction and Western blot analysis were performed as previously described (Chen et al., 2016). In brief, total protein was extracted from intestinal tissues using Cell Lysis buffer (Cat No. P0013; Beyotime, Shanghai, China) following the manufacturer’s instructions. The protein concentration was measured using an enhanced BCA protein assay kit (Cat No. P0010S; Beyotime, Shanghai, China) and detected by the SpectraMax Plus system (Molecular Devices, San Jose, CA, USA). The positive control (PC) and the negative control were all set. For 10% SDS-PAGE analysis, 13 µg of protein was loaded and electrophoresed until an appropriate separation was reached. The separated proteins were then transferred to polyvinylidene fluoride membranes (PVDF membrane) (Cat No. IPVH00010; Millipore, Bedford, MA, USA) using wet transfer (BIO-RAD, Singapore). After blocking with 5% non-fat milk in TBS-Tween 20 buffer (TBST) for 2 h at room temperature on a shaker, the membranes were incubated with rabbit polyclonal AjEHHADH antibody (1:600, prepared by Abmart, Shanghai, China) or β-tubulin antibody (1:1,000, CST, Cat No. 2146S) overnight at 4 °C. The brief protocol for AjEHHADH antibody development was as follows. Two conserved fragments with antigenic determinant using MacVector version 9 (MacVector Inc., Cambridge, UK) were designed based on the ORF. Constructed plasmids containing the specific sequences were extracted and purified as previously described ( Moore et al., 2015), then injected into two healthy rabbits in vivo per antigen. Rabbits were euthanized after seven days and whole blood was withdrawn. Purified antibodies were retrieved using Protein A/G-Plus Beads (Shang Hai Yue-ke Biotechnology CO., Shanghai, China) followed by Elisa verification. The membranes were washed with TBST buffer five times for 5 min each and then incubated with goat anti-rabbit IgG labeled with HRP (1:10,000; Cat No. 7074S; CST, Danvers, MA, USA) for 1 h at room temperature. After washing with TBST for five times (5 min each), the membranes were incubated in ECL Western blot detection reagents (Cat No. P0018; Beyotime, Shanghai, China) and detected using the photo processing machine. Bands were quantified using the Image-Pro Plus 6.0 software (Media Cybernetics Inc., Rockville, MD, USA).
Functional analysis of miRNA-200-3p in vivo
Mimics and negative controls for miR-200-3p were synthesized by GenePharma (Shanghai, China) as shown in Table 1. MiR-200-3p mimics were dissolved in RNase-free water to obtain a working solution of 20 mM. Aliquots of 10 µL of mimics or negative control were mixed with an equal volume of transfection reagent (Beyotime, Shanghai, China) and 80 µL of PBS to serve as the working solution (Lu et al., 2015). Eighteen sea cucumbers (100 ± 8 g) were injected with 100 µl of miR-200-3p mimics or the negative control and a booster injection was given after 24 h. After a further 24 h, treated and control animals were sacrificed and intestine tissues were collected and stored in liquid nitrogen for expression analysis using the qRT-PCR and Western blot protocols described above. The assays were performed with three biological and three technical replicates.
Statistics
qRT-PCR data are given as mean ± S.E. (N = 5) and were analyzed using one-way analysis of variance (ANOVA) followed by a Tukey’s post hoc test (SPSS 17.0 software, Chicago, IL, USA). The level of statistical significance was set at P < 0.05 unless otherwise stated. Relative protein abundance was analyzed using a t-test (SPSS 17.0) (Chicago, IL, USA) and all the results are given as mean ± S.E. (N = 4) .
Dual-luciferase reporter data are given as mean ± S.E. (N = 6), qRT-PCR data are given as mean ± S.E. (N = 5) and relative protein abundance is given as mean ± S.E. (N = 3). These data were used to demonstrate the interaction between miRNA-200-3p and the AjEHHADH mRNA transcript. All data were analyzed using an independent sample t-test by SPSS 17.0 software. The level of statistical significance was set at P < 0.05. P < 0.001 indicated an extremely significant difference.
Results
Sequence characterization of AjEHHADH and target identification of miR-200-3p
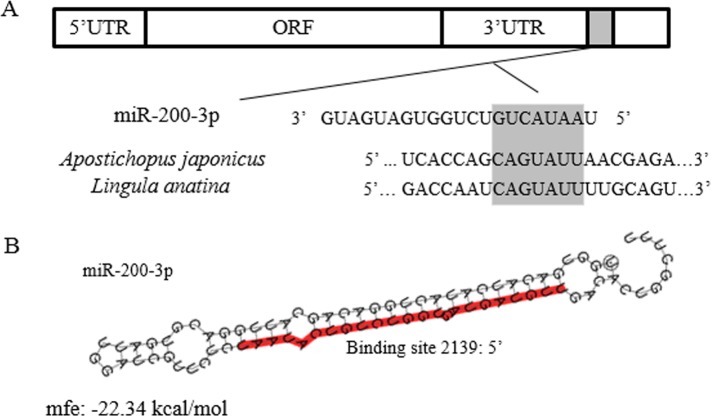
The full-length cDNA of AjEHHADH spanned 2,352 bp, including a 179 bp 5′-UTR and a 1,048 bp 3′ UTR. The open reading frame (ORF) contained 1,125 bp and encoded a 375 long amino acid polypeptide with a calculated molecular weight of 67 kDa (Fig. 1). SMART analysis indicated that the sea cucumber AjEHHADH protein contained two enoyl-CoA hydratase/isomerase (ECH) domains and a 3-hydroxyacyl-CoA dehydrogenase NAD binding domain (Fig. 1) (Gene accession No. KY328747KY). Analysis of the putative miR-200-3p binding sites was then performed using TargetScan 5.2 and Miranda 3.3a programs. Results showed that the 3′ UTR of sea cucumber AjEHHADH contained binding sequences for miR-200-3p starting from residue 2,139 within the 3′ UTR sequence. Importantly, the binding site was also identified and found to be conserved in Lingula anatine, a member of another marine invertebrate group, the brachiopods (Fig. 2). The theoretical minimum free energy of binding (kcal/mol) was also calculated using the program Miranda. Theoretical prediction of thermodynamic binding parameters for miRNA-200-3p binding to AjEHHADH was calculated to be −22.34 kcal/mol (below the given theoretical parameters −20 kcal/mol), suggesting that AjEHHADH is a likely target of miRNA-200-3P in sea cucumbers (Fig. 2).
Figure 1. The complete cDNA sequence and deduced amino acid sequence of AjEHHADH from A. japonicus.

Amino acids are numbered starting with the N-terminal Met residue. The asterisk indicates the translational termination codon. The open reading frame (ORF) from the initiation codon (ATG) to the termination codon (TAG) is notated by uppercase letters. At the bottom of the page is the schematic diagram of domains and characteristic motifs.
Figure 2. Theoretical binding of miR-200-3p to a conserved region in the 3′ UTR of the AjEHHADH gene.
(A) Conservation analysis of the miR-200-3p binding site in the EHHADH gene from sea cucumber A. japonicus and Lingula anatina. The seed region sequence (shaded) shows 100% conservation between these two sequences. (B) Predicted binding structure of miR-200-3p when binding to the 3′ UTR of AjEHHADH, as determined from the TargetScan and miRanda program.
The expression of miR-200-3p and AjEHHADH during aestivation
The mRNA transcript levels of AjEHHADH decreased significantly in the intestine by 0.34 ± 0.11-fold at the DA stage compared to the NA stage (P < 0.05) (Fig. 3A). Moreover, the protein expression levels of AjEHHADH also showed a significant decrease by 0.72 ± 0.08-fold at the DA compared with NA stage (P < 0.05) (Fig. 3B). The expression of miR-200-3p was assessed in the intestine of A. japonicus from non-aestivation and deep-aestivation stages. MiR-200-3p expression levels showed an inverse correlation with AjEHHADH transcript and protein levels in the intestine. Expression levels of miR-200-3p increased significantly in the intestine by 2.53 ± 0.3-fold in the deep-estivated group compared to controls (P < 0.05) (Fig. 4).
Figure 3. Effect of estivation on the relative expression levels of AjEHHADH.
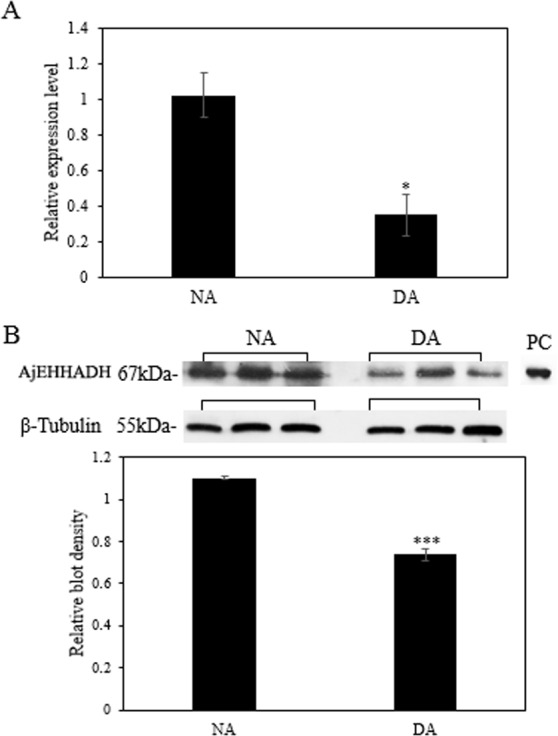
(A) Relative mRNA expression levels of AjEHHADH in the intestine of NA and DA groups respectively. Values were normalized against β-Tubulin. “*” indicates significant statistical differences (P < 0.05). Values are means ± SE (N = 5). (B) Relative protein expression level of AjEHHADH at the NA and DA stages in intestine by Western blot. Representative bands show blot intensity for NA and DA groups. Histograms show normalized expression levels for NA and DA. β-Tubulin was chosen as the internal control. “***” indicates significant differences for NA and DA groups (P < 0.001). Values are means ± SE (N = 4).
Figure 4. Effect of estivation on the relative expression of miRNA-200-3p.
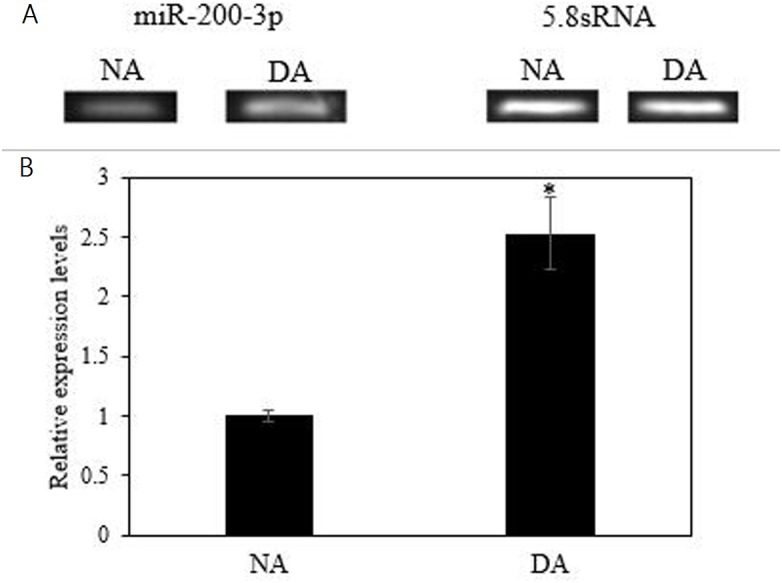
Representative bands show RNA transcript levels amplified by RT-PCR. Band intensities from the RT-PCR samples were normalized to either 5.8S rRNA (microRNA) band amplified from the same sample. Data are means ± SE (N = 5 independent trials on tissue from different animals). “*” Indicates a significant difference from the corresponding control (p < 0.05).
Validation of the interaction between 3′ UTR of AjEHHADH and miR-200-3p by dual-luciferase reporter assays
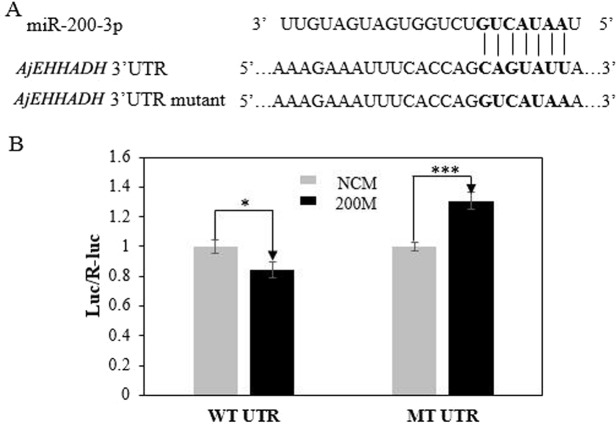
To further validate the predicted role of miR-200-3P in regulating AjEHHADH, plasmids containing the putative binding site for miRNA-200-3p and the mutation site (Fig. 2) of the 3′ UTR of AjEHHADH were constructed and used in the classical reporter assay. Information concerning the binding and mutation sites of miRNA-200-3p in the 3′ UTR of AjEHHADH is shown in Fig. 5A. A significant reduction (11.9%, P = 0.044) was observed in luciferase activity transfected with WT UTR compared with control (Fig. 5B).
Figure 5. Identification and characterization of the miRNA-200-3p binding sites in the 3′ UTR of AjEHHADH and functional effect of miR-200-3p on AjEHHADH.
(A) Schematic representation of the putative miRNA-200-3p targeting sites in AjEHHADH mRNA and the respective mutant sites. (B) HEK-293T cells were co-transfected with the pMIRREPORT-BHMT-WT vector, carrying the wild-type and the mutated AjEHHADH 3′-UTR, pRLCMV-Renilla-luciferase, and control miR-200-3p mimics as indicated. “*”indicates significant differences (P < 0.05). WT, Wild-type; MT, Mutant type; 200M, miR-200-3p mimics; NCM, negative control without miR-200-3p.
MiR-200-3p modulates AjEHHADH at the post-transcriptional level in vivo
To fully understand the potential roles of miR-200-3p in regulating AjEHHADH, gain-of-function assays for miR-200-3p were performed in vivo. As shown in Fig. 6A, the qRT-PCR results indicated that overexpression of miR-200-3p decreased the transcript levels of AjEHHADH, although it was not significant in statistical analysis (P > 0.05). Western blot analysis revealed that AjEHHADH protein levels were also reduced significantly upon miR-200-3p overexpression (Fig. 6B), consistent with the AjEHHADH transcript expression levels (Fig. 3). These results indicate that miR-200-3p may directly target AjEHHADH gene expression by inducing the degradation of the AjEHHADH mRNA transcripts.
Figure 6. Functional analysis of miR-200-3p and AjEHHADH in vivo.
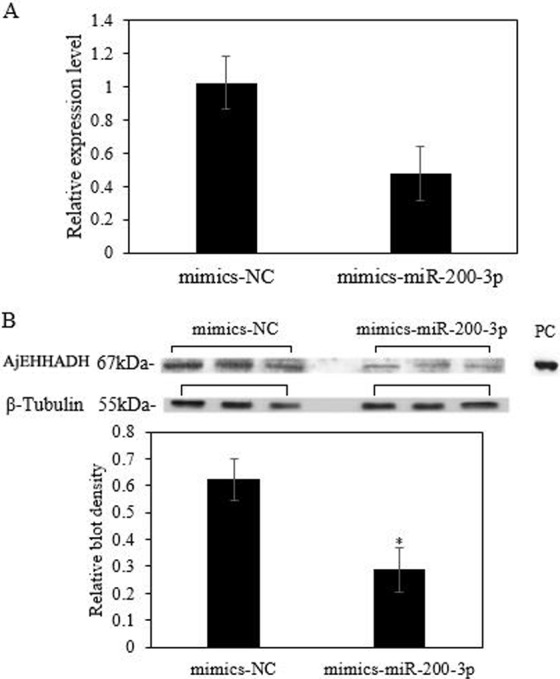
(A) Relative AjEHHADH mRNA transcripts expression after transfection with miRNA modified mimics. (B) Western blot analysis after transfection with miRNA modified mimics. “*” indicates significant differences (P < 0.05). Values are means ± SE (N = 3 for mRNA and protein analysis).
Discussion
Recent studies have shown that miRNA-mediated gene silencing is a widespread regulatory mechanism influencing many processes during periods of environmental stress including the cell cycle, apoptosis, signal transduction, muscle atrophy and fatty acid metabolism (Kornfeld, Biggar & Storey, 2012; Wu, Biggar & Storey, 2014; Biggar, Dubuc & Storey, 2009; Biggar & Storey, 2012). Accumulated evidence shows that miRNAs play a significant role in metabolic rate depression where they can modulate the availability of corresponding transcripts, thereby providing a method for maintaining energy expenditure in check.
To date, very little is known about the potential stress-responsive roles of miRNAs in targeting specific genes in non-model marine invertebrates like the estivating sea cucumber. Using RNA-seq and microRNA microarrays, we have previously shown the involvement of miR-200-3P in the intestine of the sea cucumber whereby expression levels increased significantly during deep aestivation compared to the non-estivated control group (Chen et al., 2013). To further understand the regulatory influence of miR-200-3p in the estivating sea cucumber, potential target genes were predicted. Our prediction showed that the AjEHHADH gene, corresponding to a protein involved in lipid peroxidation could be targeted by miR-200-3p; therefore, we aimed to investigate this prediction further.
MicroRNAs are known to regulate translation by regulating the availability of mRNA to the translational machinery, thereby providing a finely tuned mechanism for regulating protein translation. The present study identified AjEHHADH as a novel target of miR-200-3p using bioinformatics analysis. To confirm our predictions, we explored the expression patterns of AjEHHADH gene and protein during aestivation using qRT-PCR and Western blot in the sea cucumber. The results indicated a negative relationship between miR-200-3p and AjEHHADH expression at both the transcriptional and the translational levels, suggesting that AjEHHADH could be regulated by miR-200-3p. Dual-luciferase reporter assays further identified the interaction between miRNA-200-3p and AjEHHADH. To further examine our claims, we performed in vivo gain-of-function assays to overexpress miR-200-3p and again explored the gene and protein levels of sea cucumber AjEHHADH by qRT-PCR and Western blot, respectively. The results support the claim that miR-200-3p is responsible for regulating the degradation of AjEHHADH mRNA transcripts, thereby causing a sharp decline in the abundance of the corresponding protein. This finding further supports our claim and shows that miR-200-3p does indeed play a significant role in inhibiting the expression of AjEHHADH.
The EHHADH enzyme has been previously identified as a key player in the classical peroxisomal β-oxidation pathway, and is crucial in the formation of the medium-chain DCAs, adipic and suberic acid, as well as their carnitine esters during fasting (Houten et al., 2012). Earlier studies showed that medium-chain DCAs can inhibit mitochondrial respiration thus favoring ROS production (Passi et al., 1984; Tonsgard & Getz, 1985) which can be further exacerbated by peroxisomal β-oxidation (Curzio, Esterbauer & Dianzani, 1985). These two findings that are consistent with our observations in this study. Intestinal degeneration and cessation of feeding for prolonged periods of time are well-known characteristics of in sea cucumber aestivation (Li et al., 1996). Such events generally lead to oxidative stress which can damage macromolecules and cause the accumulation of lipid peroxidation products, leading to cellular stress and inflammation (Bikman & Summers, 2011). Here, we propose that miRNA-200-3p may act as a regulatory mechanism to protect cells from oxidative damage by inhibiting the translation of the AjEHHADH transcripts when sea cucumbers enter an aestivation-induced hypometabolic state.
In summary, the present study provides the first demonstration of EHHADH regulation during aestivation in sea cucumbers. Our results suggest that aestivation-responsive suppression of AjEHHADH mRNA and protein levels in the intestine may be subjected to regulation at the post-transcriptional level by miRNA-200-3p. This also indicates β that miRNA-200-3p could play a role in regulating fatty acid metabolism, thereby offering cytoprotection to cells and organelles during aestivation in sea cucumbers. Further studies are required to confirm the roles of miRNAs in other aspects of fatty acid metabolism in animals that undergo hypometabolism to deal with environmental stress.
Supplemental Information
Representative sequencing chromatogram of AjEHHADH gene, different colors of evenly-spaced peak represent different nucleotide bases.
Representative sequencing chromatogram of AjEHHADH gene, different colors of evenly-spaced peak represent different nucleotide bases.
Raw data of mRNA expression levels of AjEHHADH and β-Tubulin in the intestine of NA and DA groups respectively.
The gel photo of protein expression level of AjEHHADH and β-Tubulin at the NA and DA stages in intestine.
Raw data of RNA transcript levels of miRNA-200-3p in the intestine of NA and DA groups respectively.
1% agarose gel photos of 5.8 sRNA and miR-200-3p.
Original fluorescence values of Luc and R-Luc in the WT and MT.
Raw data of relative mRNA expression level of AjEHHADH and β-Tubulin in the intestine of miR-200-3p mimics groups.
Gel photo of western blot of AjEHHADH after transfection with miRNA modified mimics.
Gel photo of western blot of β-Tubulin after transfection with miRNA modified mimics.
Acknowledgments
We sincerely thank the reviewers for their critique and suggestions.
Funding Statement
This research was supported by the National Natural Science Foundation of China (No. 41676124 and No. 31472257). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Kenneth B. Storey is an Academic Editor for PeerJ.
Author Contributions
Muyan Chen conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Shanshan Wang and Xingke Li performed the experiments, analyzed the data, prepared figures and/or tables.
Kenneth B. Storey authored or reviewed drafts of the paper.
Xiumei Zhang contributed reagents/materials/analysis tools.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
The sequence described is accessible via GenBank accession number KY328747.
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.
References
- Albano et al. (1991).Albano E, Bellomo G, Parola M, Carini R, Dianzani MU. Stimulation of lipid peroxidation increases the intracellular calcium content of isolated hepatocytes. Biochimica et Biophysica Acta/General Subjects. 1991;1091:310–316. doi: 10.1016/0167-4889(91)90194-3. [DOI] [PubMed] [Google Scholar]
- Bartel (2004).Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Biggar, Dubuc & Storey (2009).Biggar KK, Dubuc A, Storey K. MicroRNA regulation below zero: differential expression of miRNA-21 and miRNA-16 during freezing in wood frogs. Cryobiology. 2009;59:317–321. doi: 10.1016/j.cryobiol.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Biggar et al. (2012).Biggar KK, Kornfeld SF, Maistrovski Y, Storey KB. MicroRNA regulation in extreme environments: differential expression of microRNAs in the intertidal snail Littorina littorea during extended periods of freezing and anoxia. Genomics, Proteomics & Bioinformatics. 2012;10:302–309. doi: 10.1016/j.gpb.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggar & Storey (2011).Biggar K, Storey KB. The emerging roles of microRNAs in the molecular responses of metabolic rate depression. Journal of Molecular Cell Biology. 2011;3:167–175. doi: 10.1093/jmcb/mjq045. [DOI] [PubMed] [Google Scholar]
- Biggar & Storey (2012).Biggar KK, Storey KB. Evidence for cell cycle suppression and microRNA regulation of cyclin D1 during anoxia exposure in turtles. Cell Cycle. 2012;11:1705–1713. doi: 10.4161/cc.19790. [DOI] [PubMed] [Google Scholar]
- Bikman & Summers (2011).Bikman BT, Summers SA. Ceramides as modulators of cellular and whole-body metabolism. Journal of Clinical Investigation. 2011;121:4222–4230. doi: 10.1172/JCI57144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchman et al. (1992).Borchman D, Lamba OP, Salmassi S, Lou M, Yappert MC. The dual effect of oxidation on lipid bilayer structure. Lipids. 1992;27:261–265. doi: 10.1007/BF02536472. [DOI] [PubMed] [Google Scholar]
- Catalá (2009).Catalá A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chemistry & Physics of Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2016).Chen M, Li X, Zhu A, Storey KB, Sun L, Gao T, Wang T. Understanding mechanism of sea cucumber Apostichopus japonicus aestivation: insights from TMT-based proteomic study. Comparative Biochemistry and Physiology—Part D: Genomics and Proteomics. 2016;19:78–89. doi: 10.1016/j.cbd.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Chen & Storey (2014).Chen M, Storey KB. Large-scale identification and comparative analysis of miRNA expression profile in the respiratory tree of the sea cucumber Apostichopus japonicus during aestivation. Marine Genomics. 2014;13:39–44. doi: 10.1016/j.margen.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2013).Chen M, Zhang X, Liu J, Storey KB. High-throughput sequencing reveals differential expression of miRNAs in intestine from sea cucumber during aestivation. PLOS ONE. 2013;8(10):e76120. doi: 10.1371/journal.pone.0076120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Zhu & Storey (2016).Chen M, Zhu A, Storey KB. Comparative phosphoproteomic analysis of intestinal phosphorylated proteins in active versus aestivating sea cucumbers. Journal of Proteomics. 2016;135:141–150. doi: 10.1016/j.jprot.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Curzio, Esterbauer & Dianzani (1985).Curzio M, Esterbauer H, Dianzani MU. Chemotactic activity of hydroxyalkenals on rat neutrophils. International Journal of Tissue Reactions. 1985;7:137–142. [PubMed] [Google Scholar]
- Giraud-Billoud et al. (2013).Giraud-Billoud M, Vega IA, Tosi MRE, Abud MA, Calderón ML, Castro-Vazquez A. Antioxidant and molecular chaperone defences during estivation and arousal in the South American apple snail Pomacea canaliculata. Journal of Experimental Biology. 2013;216:614–622. doi: 10.1242/jeb.075655. [DOI] [PubMed] [Google Scholar]
- Goldstein & Weissmann (1977).Goldstein RM, Weissmann G. Effects of the generation of superoxide anion on permeability of liposomes. Biochemical and Biophysical Research Communications. 1977;75:604–609. doi: 10.1016/0006-291X(77)91515-7. [DOI] [PubMed] [Google Scholar]
- Houten et al. (2012).Houten SM, Denis S, Argmann CA, Jia Y, Ferdinandusse S, Reddy JK, Wanders RJ. Peroxisomal L-bifunctional enzyme (Ehhadh) is essential for the production of medium-chain dicarboxylic acids. Journal of Lipid Research. 2012;53(7):1296–1303. doi: 10.1194/jlr.M024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld, Biggar & Storey (2012).Kornfeld SF, Biggar KK, Storey KB. Differential expression of mature microRNAs involved in muscle maintenance of hibernating little brown bats, Myotis lucifugus: a model of muscle atrophy resistance. Genomics, Proteomics & Bioinformatics. 2012;10:295–301. doi: 10.1016/j.gpb.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourie (1988).Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. American Journal of Physiology. 1988;275:1–24. doi: 10.1152/ajpcell.1998.275.1.C1. [DOI] [PubMed] [Google Scholar]
- Li et al. (1996).Li F, Liu Y, Song B, Sun H, Gu B, Zhang X. Study on aestivating habit of sea cucumber Apostichopus japonicus Selenka: the factors relating to aestivation. Journal of Fishery Sciences of China. 1996;3:49–57. doi: 10.1152/ajpcell.1998.275.1.C1. [DOI] [Google Scholar]
- Lu et al. (2015).Lu M, Zhang P, Li C, Zhang W, Jin C, Han Q. MiR-31 modulates coelomocytes ROS production via targeting p105 in Vibrio splendidus challenged sea cucumber Apostichopus japonicus in vitro and in vivo. Fish & Shellfish Immunology. 2015;45(2):293–299. doi: 10.1016/j.fsi.2015.04.024. [DOI] [PubMed] [Google Scholar]
- Maistrovski, Biggar & Storey (2012).Maistrovski Y, Biggar KK, Storey KB. HIF-1α regulation in mammalian hibernators: role of non-coding RNA in HIF-1α control during torpor in ground squirrels and bats. Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology. 2012;182:849–859. doi: 10.1007/s00360-012-0662-y. [DOI] [PubMed] [Google Scholar]
- Mattson et al. (1999).Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer’s and Parkinson’s diseases. Annals of the New York Academy of Sciences. 1999;893:154–175. doi: 10.1111/j.1749-6632.1999.tb07824.x. [DOI] [PubMed] [Google Scholar]
- Moore et al. (2015).Moore MJ, Scheel TKH, Luna JM, Park CY, Fak JJ, Nishiuchi E, Rice CM, Darnellb RB. Mirna–target chimeras reveal mirna 3′-end pairing as a major determinant of argonaute target specificity. Nature Communications. 2015;6:8864. doi: 10.1038/ncomms9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin, Dubuc & Storey (2008).Morin PJ, Dubuc A, Storey KB. Differential expression of microRNA species in organs of hibernating ground squirrels: a role in translational suppression during torpor. BBA Gene Regulatory Mechanisms. 2008;1779:628–633. doi: 10.1016/j.bbagrm.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Passi et al. (1984).Passi S, Picardo M, Nazzaro-Porro M, Breathnach A, Confaloni AM, Serlupi-Crescenzi G. Antimitochondrial effect of saturated medium chain length (C8-C13) dicarboxylic acids. Biochemical Pharmacology. 1984;33:103–108. doi: 10.1016/0006-2952(84)90376-9. [DOI] [PubMed] [Google Scholar]
- Poirier et al. (2006).Poirier Y, Antonenkov VD, Glumoff T, Hiltunen JK. Peroxisomal β-oxidation—a metabolic pathway with multiple functions. BBA Molecular Cell Research. 2006;1763(12):1413–1426. doi: 10.1016/j.bbamcr.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Storey (1996).Storey KB. Oxidative stress: animal adaptations in nature. Brazilian Journal of Medical and Biological Research. 1996;29:1715–1733. [PubMed] [Google Scholar]
- Sui & Liao (1988).Sui X, Liao Y. Culture and proliferation of Apostichopus japonicus. China Agriculture Publishing House; Beijing: 1988. [Google Scholar]
- Sui et al. (1985).Sui X, Liu Y, Liu Y, Shang L, Hu Q. A study on reproductive rhythm of Apostichopus japonicus. Journal of Fishery of Sciences of China. 1985;9:303–310. [Google Scholar]
- Tonsgard & Getz (1985).Tonsgard JH, Getz GS. Effect of Reye’s syndrome serum on isolated chinchilla liver mitochondria. Journal of Clinical Investigation. 1985;76:816–825. doi: 10.1172/JCI112039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamecq & Draye (1989).Vamecq J, Draye JP. Peroxisomal and mitochondrial beta-oxidation of monocarboxylyl-CoA, omega-hydroxymonocarboxylyl-CoA and dicarboxylyl-CoA esters in tissues from untreated and clofibrate-treated rats. Journal of Biochemistry. 1989;106:216–222. doi: 10.1093/oxfordjournals.jbchem.a122835. [DOI] [PubMed] [Google Scholar]
- Wu, Biggar & Storey (2014).Wu CW, Biggar KK, Storey KB. Expression profiling and structural characterization of microRNAs in adipose tissues of hibernating ground squirrels. Genomics, Proteomics & Bioinformatics. 2014;12:284–291. doi: 10.1016/j.gpb.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2005).Yang H, Yuan X, Zhou Y, Mao Y, Zhang T, Liu Y. Effects of body size and water temperature on food consumption and growth in the sea cucumber Apostichopus japonicus (Selenka) with special reference to aestivation. Aquaculture Research. 2005;36:1085–1092. doi: 10.1111/j.1365-2109.2005.01325.x. [DOI] [Google Scholar]
- Yang et al. (2006).Yang H, Zhou Y, Zhang T, Yuan X, Li X, Liu Y, Zhang F. Metabolic characteristics of sea cucumber Apostichopus japonicus (Selenka) during aestivation. Journal of Experimental Marine Biology and Ecology. 2006;330:505–510. doi: 10.1016/j.jembe.2005.09.010. [DOI] [Google Scholar]
- Yuan et al. (2007).Yuan X, Yang H, Wang L, Zhou Y, Zhang T, Liu Y. Effects of aestivation on the energy budget of sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea) Acta Ecologica Sinica. 2007;27:3155–3161. doi: 10.1016/S1872-2032(07)60070-5. [DOI] [Google Scholar]
- Zhang et al. (1990).Zhang Y, Marcillat O, Giulivi C, Ernster L, Davies KJ. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. Journal of Biological Chemistry. 1990;265:16330–16336. [PubMed] [Google Scholar]
- Zhao et al. (2014).Zhao Y, Chen M, Wang T, Sun L, Xu D, Yang H. Selection of reference genes for qRT-PCR analysis of gene expression in sea cucumber Apostichopus japonicus during aestivation. Chinese Journal of Oceanology and Limnology. 2014;32:1248–1256. doi: 10.1007/s00343-015-4004-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative sequencing chromatogram of AjEHHADH gene, different colors of evenly-spaced peak represent different nucleotide bases.
Representative sequencing chromatogram of AjEHHADH gene, different colors of evenly-spaced peak represent different nucleotide bases.
Raw data of mRNA expression levels of AjEHHADH and β-Tubulin in the intestine of NA and DA groups respectively.
The gel photo of protein expression level of AjEHHADH and β-Tubulin at the NA and DA stages in intestine.
Raw data of RNA transcript levels of miRNA-200-3p in the intestine of NA and DA groups respectively.
1% agarose gel photos of 5.8 sRNA and miR-200-3p.
Original fluorescence values of Luc and R-Luc in the WT and MT.
Raw data of relative mRNA expression level of AjEHHADH and β-Tubulin in the intestine of miR-200-3p mimics groups.
Gel photo of western blot of AjEHHADH after transfection with miRNA modified mimics.
Gel photo of western blot of β-Tubulin after transfection with miRNA modified mimics.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.