Abstract
Ongoing studies of physiological and pathological processes have led to a corresponding need for new radiopharmaceuticals, especially when studies are limited by the absence of a particular radiolabeled target. Thus, the development of new radioactive tracers is highly relevant and can represent a significant contribution to efforts to elucidate important phenomena in biology. Currently, theranostics represents a new frontier in the fields of medicine and nuclear medicine, with the same compound being used for both diagnosis and treatment. In the human body, copper (Cu) is the third most abundant metal and it plays a crucial role in many biological functions. Correspondingly, in various acquired and inherited pathological conditions, such as cancer and Alzheimer’s disease, alterations in Cu levels have been found. Moreover, a wide spectrum of neurodegenerative disorders are associated with higher or lower levels of Cu, as well as inappropriately bound or distributed levels of Cu in the brain. In human cells, the membrane protein, hCtr1, binds Cu in its Cu(I) oxidation state in an energy-dependent manner. Copper-64 (64Cu) is a cyclotron-produced radionuclide that has exhibited physical properties that are complementary for diagnosis and/or therapeutic purposes. To date, very few reports have described the clinical development of 64Cu as a radiotracer for cancer imaging. In this review, we highlight recent insights in our understanding and use of 64CuCl2 as a theranostic agent for various types of tumors. To the best of our knowledge, no adverse effects or clinically observable pharmacological effects have been described for 64CuCl2 in the literature. Thus, 64Cu represents a revolutionary radiopharmaceutical for positron emission tomography imaging and opens a new era in the theranostic field.
Keywords: Copper-64, theranostic, cancer, radiopharmaceutical, PET
After iron and zinc, copper (Cu) is the third most abundant trace transition metal in the human body with a normal content of 1.4–2.1 mg/kg.1–3 Moreover, similar to Fe and Zn, Cu ions are essential for various biological processes, which makes Cu indispensable for life.1,4,5 The biological role of Cu coincides with the establishment of an oxygen atmospherê1.7 billion years ago. O2 facilitates the oxidation of insoluble Cu(I) to a more soluble form, Cu(II), which is also more bioavailable. An O2 atmosphere also led to the requirement for redox active metals with potentials ranging from 0 to 800 mV. Thus, for many proteins that react with O2, Cu is a key component.6
In the past few decades, great advances have been made in our understanding of the molecular and cellular mechanisms that mediate Cu homeostasis. More recently, Cu has been shown to play a key role in healthy neuronal function. Meanwhile, anomalous Cu homeostasis has been detected in many important and common diseases such as Parkinson’s disease, Alzheimer’s disease, motor neuron diseases, amyotrophic lateral sclerosis, and prion disease.6–12 In Menkes and Wilson’s diseases, systemic disproportions in Cu have also been observed, and these have been found to affect clinical and biochemical outcomes.6,11–14 For example, Camakaris et al detected resistance to Cu at the cellular level following amplification of the Menkes gene, and this was mediated via an increase in Cu efflux.15 This result supports a role for Menkes proteins to serve as Cu efflux proteins, and is also consistent with the identification of the Menkes protein as a member of the P-type ATPase family and the phenotype of cultured cells that express the Menkes protein.15
For good health, it is important for Cu ion homeostasis to be tightly regulated. It is estimated that the daily diet of a healthy adult includes 0.6–1.6 mg of Cu.5,7,13 Conversely, it is hypothesized that Cu deficiency as a chronic dietary condition contributes to osteoporosis and cardiovascular diseases.7,13 Cu imbalances have been identified in cancer patients, while Cu toxicity disorders and Cu deficiency can also be inherited or acquired.
Cu exists in two redox states relevant to biological systems, Cu+2 (cupric) and Cu+1 (cuprous). Many enzymatic systems need Cu as a cofactor, such as iron metabolism and respiratory oxidation. Cu’s capacity to donate and accept electrons is also key to its role in neurotransmitter synthesis and pigment formation.12
Cu is transported across the plasma membrane of cells before becoming accessible in cellular compartments for incorporation into Cu-dependent proteins.12,14,16,17 Studies of yeast cells were the first to identify the specific genes that encode the proteins responsible for Cu uptake. For example, two proteins that exhibit a high affinity for Cu uptake, Ctr1 and Ctr3, were originally characterized in Saccharomyces cerevisiae,14,16,17 while Ctr4 and Ctr5 were found to form heteromeric complexes in Schizosaccharomyces pombe.17 Subsequently, homology searches of databases and complementation studies of yeast mutants identified both mouse and human CTR1 homologs.16 In mammals, cellular import of Cu is primarily mediated via Ctr1 and Ctr2, and Ctr1 is the predominant transporter of dietary Cu from the intestinal lumen.12 Details regarding the mechanisms responsible for mediating Cu uptake by human cells were limited until various Cu-binding proteins, referred to as Cu chaperone proteins, were identified. When concentrations of extracellular Cu are low and the amount of free intracellular Cu is negligible, copper chaperone for SOD1 (CCS), cytochrome c oxidase copper chaperone (COX17), and antioxidant protein (ATOX1) play key roles in adjusting Cu levels. Conversely, when concentrations of Cu are elevated, the roles of these three proteins appear to become diluted due to a greater role for non-specific Cu-binding carriers.15 When Cu ions are in excess and need to be cleared from a biological system, a hepatobiliary route is preferred. For this process, Cu ions that are bound to plasma proteins, such as ceruloplasmin, transcuprein, and albumin, are carried to the cell surface. Once there, reductase enzymes reduce the Cu2+ ions to Cu+ ions prior to their uptake into cells. Reduced Cu+ ions are subsequently transported across the cell membrane by the transmembrane protein, hCTR1, a 190-amino acid protein (28 kDa) that has a high affinity for Cu.5,12
By the turn of the century, the role of systemic Cu homeostasis in both healthy and disease states had become more clear. With the subsequent development of Cu radionuclides, more localized detection of Cu in body liquids, tissues, and organs was achieved, and this provided more specific insights compared with the detection of overall systemic levels of Cu.12 Currently, the available radioisotopes of Cu include 60Cu, 61Cu, 62Cu, 64Cu, and 67Cu. The half-lives of these radioisotopes range from 9.8 minute to 61.9 hour, and this makes them compatible with radiotherapy and/or imaging protocols.18,19 In general, 62Cu is used for diagnosis and 64Cu is used for both diagnosis and therapy. Moreover, there is an improved availability of 64Cu compared to 67Cu.
Only a few 64Cu radioisotopes are able to be produced in a cyclotron or a nuclear reactor,1,5 and 64CuCl2 is one of those compounds. In a cyclotron, the radionuclide, 64Cu, is produced in no-carrier-added state via a 64Ni (p,n) 64Cu reaction.16 The intermediate half-life of the resulting radionuclide is 12.7 hour and decay occurs via electron capture (44%), β+ emission (17%, 0.655 MeV), and β-emission (39%, 0.573 MeV). Electron capture also leads to the release of Auger electrons, thereby facilitating its use for therapy when Cu localizes to cell nuclei.12,20 Trace amounts of β+ and γ emitting radionuclides can be detected in the human body by using positron emission tomography (PET) and single-photon emission computed tomography (SPECT), respectively. PET cameras employ opposite detectors to record simultaneous pairs of annihilation photons (511 KeV per photon), thereby detecting electronic collimation instead of mechanical collimation of incoming photons. As a result, PET sensitivity, which ranges from 10−11 to 10−12 M, is at least 1–2 orders of magnitude better than the sensitivity of single-photon imaging systems (10−10 M).21
Cu has important roles in both cancer development and cancer growth, particularly in the processes of angiogenesis and metastasis. Malignant tissues have been characterized as having higher levels of Cu accumulation, and this observation is consistent with the overexpression of human copper transporter 1 (hCTR1) in cancer cells, including melanoma, breast cancer, liver cancer, lung cancer, prostate cancer, and glioblastoma cells.5,22–24 However, the chemical form(s) of Cu ions in blood serum have not been investigated.5 Increases in Cu uptake have been confirmed in vivo with the use of 64Cu as a radiotracer.5 Thus, 64Cu, in combination with PET technology, provides a noninvasive method to assess various cancers.
Unlike the majority of conventional radiopharmaceuticals, 64CuCl2 does not require complexation with peptides, antibodies, or other expensive targeting ligands.5 Moreover, 64CuCl2 is one of the few radiocompounds that can directly serve as a probe in PET imaging of various types of cancers. Thus, the use of 64CuCl2 can be less expensive than other methods that employ other radionuclides. Generally, 64Cu is administered as a diluted solution of 64CuCl2. Dilutions are made with saline or PBS to lower the slightly acidic nature of the CuCl2 solution to achieve a pH of 6–7.5
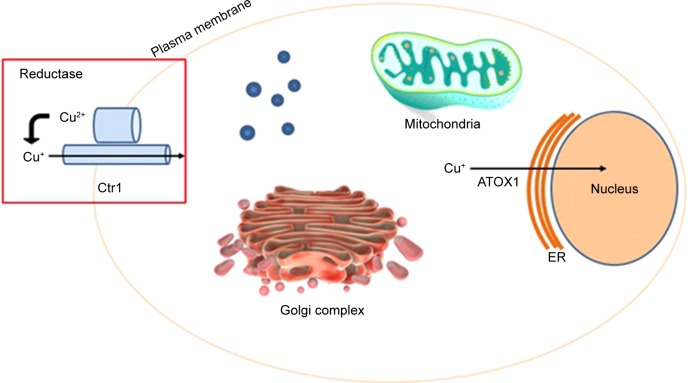
Figure 1 shows that 64Cu enters inside the cells using the enzyme Ctr1 and it is distributed in different organelles. Then, it enters inside the nucleus, inside DNA, using the enzyme ATOX1. There is a different behavior between normal and tumor cells. In normal cells 64Cu remains in the cytoplasm while in tumor cells it enters the nucleus. In this condition, 64Cu is able to produce the theranostic effect, without toxicity to normal cells.
Figure 1.
64Cu entry into the tumor cell.
Abbreviations: ATOX1, antioxidant protein; ER, endoplasmic reticulum.
Within the past ten years, numerous reports have been published regarding the use of 64Cu as a probe in PET imaging studies of experimental mouse models of prostate, breast, colorectal, hepatoma, melanoma, fibrosarcoma, ovarian, lung, head and neck, and glioblastoma cancers.5 However, only recently has 64Cu been employed as a PET imaging probe in nuclear medicine clinics for human cancers. To further explore the use of 64Cu in combination with PET imaging, we have reviewed various studies that demonstrate the importance of Cu metabolism in maintaining good health, as well as the radiochemistry of 64Cu and its applications in diagnosis and therapy.
Chelators for Cu radionuclides
The family of transmembrane Ctr Cu transporter proteins is responsible for importing Cu into eukaryotic cells as Cu+1. This family of proteins has been identified in yeast, plants, humans, and other mammals. The N- and C-terminii of these proteins are characterized by multiple methionine-rich motifs and conserved histidine and cysteine residues, respectively. More recently, Ctr proteins have been shown to mediate the uptake of platinum-based anticancer drugs.25 Cu chaperone proteins that are present in the cytosol bind soluble Cu1+ to deliver it to target proteins. It remains unclear how these chaperone proteins bind Cu1+, although it is hypothesized that direct interactions between Cu1+ and Ctr transporter proteins mediate the binding event.25 In order to develop64Cu radiopharmaceuticals, a chelator is necessary. Cu2+ can then be complexed with molecules and cells to form thermodynamically and kinetically stable systems. It has been very challenging to develop Cu chelators since there are many Cu-chelating proteins that exist in vivo (eg, metallothionein, ceruloplasmin, Cu transporters, superoxide dismutase, and chaperone proteins), and these proteins can displace a Cu ion from a chelator. Among the various oxidation states that are available to Cu, the Cu2+ form is most commonly used for 64Cu radiopharmaceuticals.4
Diethylene triamine pentaacetic acid (DTPA) and EDTA, as well as their derivatives, represent commonly used acyclic Cu chelators. Dithiocarbamates that are typically used for chelating rhenium and technetium have also been used for chelating Cu. Rosales et al26 reported a synthesis method for conjugating 64Cu-dithiocarbamatebisphosphonate to iron oxide nanoparticles. Characterization of this novel bifunctional conjugate demonstrated its ability to bind superparamagnetic iron oxide nanoparticles and 64Cu, which is relevant for magnetic resonance and PET imaging, respectively. The authors conducted further in vivo studies in mice and images of the conjugate in draining lymph nodes were obtained.26
Modified cyclen and cyclam are macrocycles that are commonly used to chelate Cu2+. Both 1,4,8,11-trazacyclodo-decane-1,4,8,11-tetraacetic acid (TETA) and 1,4,7,10-tet-raazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) have also been used as Cu chelates for PET imaging. However, the latter complexes have been reported to be unstable.4
When Pandya et al compared Cu-TETA and 1,4,8,11-tetraazabicyclo[6.6.2]hexadecane (CB-TE2A), the latter was found to be more stable.27 An animal biodistribution study of the two compounds conjugated with 64Cu further showed that 64Cu-TE2A exhibited significantly less retention in kidneys and liver after 24 hours compared with 64Cu-TETA. These results suggest that demetallation occurs at a slower rate in vivo.4,27
Cheng et al conducted studies in two melanoma models and greater retention and accumulation of 64Cu–DOTA–NAPamide was observed in the livers of both models.28 The authors attribute this observation to transchelation of the conjugated compound in the liver and/or decomposition of the conjugate and release of uncoordinated 64Cu into the bloodstream. The authors further suggest that chelated 64CuCl2 would be more stable with the use of cross-bridged cyclam ligands or TETA, and its biodistribution may be improved as well.
Despite the modest stability of 64Cu-DOTA in vivo, it is a widely used chelator.4,28 The main reasons for this may be its commercial availability, its approval by the Federal Drug Administration, and labeling conditions. However, cyclam derivatives remain the most often used chelators due to their in vivo kinetic stability that has not been surpassed by any other chelators. Furthermore, chemical modifications of the cross-bridge or pendant arms of cyclam derivatives have resulted in improved labeling conditions without compromise of in vivo stability.4
Sarcophagine (Sar) is another well-known chelator that is able to strongly bind Cu2+ to form a highly stable complex. The in vivo stabilities of Sar 64Cu complexes have been reported to be better than those of other chelators (such as DOTA), and thus, a new class of bifunctional chelators was developed based on the structure of Sar. As a result, synthesis of a cage-like hexaazamacrobicyclic Sar produced a chelator at high yields that exhibited high in vivo stability and successful radiolabeling.28–30 However, attempts to attach diamino-hexaazabicycloeicosane directly to the protein under hydrochloric acid activation have been unsuccessful due to the presence of an inert primary amine.28–30
When labeling proteins via formation of an amide bond, inactivation of biological activity is a concern. Similarly, modification of carboxylic acids or lysines near a protein’s active site has the potential to interfere with binding affinity.28–30 To prepare 64Cu radiopharmaceuticals, Liu et al29 have conjugated BaMalSar and Mal2Sar with cyclic c(RGDyC) via maleimide and free sulfhydryl reactions. Following the labeling of BaMalSar-RGD and Mal2Sar-RGD2 with 64Cu, these conjugates exhibited greater uptake by tumor tissues compared with normal tissues.
The passive uptake of 64CuCl2 by cells via pyruvaldehydebis(N4-methylthiosemicarbazone) (PTSM), a lipophilic redox-active carrier molecule, facilitates the rapid radiolabeling of cells. Moreover, PTSM exhibits greater binding affinity for divalent [(Cu II)] compared with monovalent Cu [(Cu I)]. Consequently, stable Cu2+-PTSM is reduced to the more labile Cu1+-PTSM complex, thereby allowing the charged Cu1+ ion to dissociate and undergo binding by intracellular macromolecules. Meanwhile, the neutral PTSM molecule diffuses through the plasma membrane out of the cell. As a result, a concentration gradient develops across the cell membrane and Cu-PTSM is passively taken up. In glioma cells, an influx of 64Cu-PTSM equilibrated after ~3 hours.31 Splenic accumulation of 64Cu-PTSM-labeled lymphocytes was also observed after 3 hours, although localization to the liver was the primary site.21 Furthermore, when Adonai et al obtained images following tail vein injections of labeled C6 cells, the labeled cells were found to localize to the liver and lungs of a mouse model.
During Cu homeostasis, Cu1+ is transported into cells via a Cu transporter protein, while Cu2+ is transported into cells via a divalent metal transporter protein. Upon complexation of the Cu ion to a lipophilic Cu chelator, such as PTSM, Cu can be transported into cells by passive diffusion, thereby bypassing normal cellular metabolic and excretory pathways. In normal cells, Cu2+-PTSM is reduced in mitochondria. The Cu molecule then enters the Cu metabolism pathway and remains in the cytosol bound to metallothioneins and other proteins.31
Preclinical studies
In the 1940s, early studies of Cu metabolism were conducted with radiocopper in normal and anemic rats.32 Later, blood sampling and biodistribution studies were performed with 64CuCl2 in experimental dog models to examine the uptake of radiocopper by various tissues and organs. These results established that radiocopper remains in the plasma of blood and is also taken up by blood cells.33
Many preclinical studies of 64CuCl2 have been conducted with various types of cancer. For example, Bahde et al administered 64Cu-histidine complexes to rats. In the control group, the radioactive tracer was rapidly cleared from the blood with uptake of the complexes by the liver and hepatobiliary excretion.34 Interestingly, PET imaging showed that hepatobiliary excretion of 64CuCl2 was partially restored in the rats after they underwent transplantation of healthy hepatocytes. These data support the use of PET in monitoring the effects of hepatocyte transplantation to treat Wilson’s disease.34 When 64CuCl2 was used as a tracer in human prostate cancer xenografts, PET imaging detected high levels of hCTR1 after 24 hours, yet not after 1 hour.35 Moreover, a quantitative analysis of the PET data showed that a higher concentration of 64CuCl2 was present in tumor tissues than in normal soft tissues.35 In a follow-up study by the same group,36 RNA interference-mediated knockdown of hCtr1 was performed to determine whether uptake of 64CuCl2 is mediated by hCtr1 or whether ionic Cu nonspecifically binds to tumor tissue. After 24 hours, uptake of 64CuCl2 by tumor cells that did not express hCtr1 was significantly reduced compared to the uptake of 64CuCl2 by control tumor cells that did express hCtr1.
When Liu et al investigated the stability and distribution of 64Cu-Ba3Sar in vivo, this conjugate was found to be rapidly cleared from the kidneys.30 At 5 minutes after the tail vein injection, uptake in the liver and blood was observed, and this profile was maintained up to 30 minutes post-injection. When images were acquired 24 hours post-injection, the highest uptake was observed in the liver, which indicates the in vivo stability of 64Cu-Ba3Sar.30 Liu et al also successfully tested antibodies labeled with 64CuCl2 in PET imaging studies of EphB4 expression in colorectal and breast cancers.37 Meanwhile, Kim et al detected an increase in the uptake of 64CuCl2 in PET images that were collected from xenograft mice bearing MDA-MB-231 cells that were infected with a lentiviral vector that provided exogenous expression of the hCTR1 gene.16 The latter results demonstrated that 64CuCl2 could be detected up to 48 hours after its injection in vivo, and they further suggested that hCTR1 could serve as a reporter gene in the use of PET with 64CuCl2 as a probe.
Jørgensen et al38 examined the capacity of 64CuCl2 to diagnose five different tumors, including U87MG (glioblastoma), HT29 (colorectal cancer), FaDu (head and neck cancer), A2780 (ovarian cancer), and H727 (neuroendocrine lung carcinoid). One hour after the injection of 64CuCl2, very high uptake of the radionuclide was detected in the liver. However, 21 hours later, a marked decrease in 64CuCl2 uptake was observed. A similar uptake profile was observed for 64CuCl2 in bladder and kidney. Furthermore, an initial uptake of 64CuCl2 by 4/5 of the tumor types was observed, while accumulation of 64CuCl2 was detected between 1 hour and 22 hours after injection in 3/5 of the tumor types. These results suggest that some CuCl2 is released from the liver into the blood, and then it accumulates in tumor tissue in a tumor type-dependent manner.
In a study of 64CuCl2 as a treatment for glioblastoma (U87MG), Ferrari et al39 injected animals with 64CuCl2 via cardiac puncture under anesthesia. The mice were subsequently divided into three groups: non-treated, treated with a single administration (SDG), and treated with a multiple-dose regimen (MDG) (with one injection administered daily for six consecutive days). In almost all of the cases, a good response was observed for the single- and multiple-dose treatments. In the SDG, a reduction in the volume of interest (VOI) was found to range from 68% to 94%, and tumors completely disappeared in two of the cases. In the MDG, the VOI reduction ranged from 64% to 92%, and complete disappearance of the tumor was observed in four of the cases.
Jin et al40 evaluated the therapeutic potential of 64Cu-cyclam-RAFT-c(-RGDfK-)4 in xenograft models of human glioblastoma cells in mice. Administration of 37 MBq or 74 MBq of 64Cu-cyclam-RAFT-c(-RGDfK-)4 to mice carrying an αVβ3-positive U87MG tumor led to a dose-dependent delay in tumor growth, and this was independent of any considerable toxicities.
Qin et al41 demonstrated the potential of 64CuCl2 to serve as a theranostic agent for malignant melanoma. Briefly, the uptake and efflux of 64CuCl2 by two melanoma cell lines expressing high levels of CTR1 (A375M and B16F10) were examined. Tumor growth was found to be reduced in both of the models that received 64CuCl2 treatment compared to the control group.
Another group of diseases that have been studied with 64Cu include those related to development, aging, and function in the brain. To study changes in Cu metabolism with aging, various groups have used a mouse model of Wilson’s disease that develops an inherited human Cu metabolism disorder due to a mutation present in the ATP7B gene.42–44 Age-dependent changes in mouse brains were observed following the oral administration of 64CuCl2 to mice. To our knowledge, these experiments were the first to demonstrate the potential of cerebral uptake of 64CuCl2 to serve as a biomarker in noninvasive assessments of brain aging and age-related disorders.
Clinical studies
Advances in our understanding of physiological and/or pathological processes maintain an ongoing need for new radiopharmaceuticals. However, these studies are often limited by the absence of a particular radiopharmaceutical. Thus, the development of new radioactive tracers is highly relevant and it can significantly contribute to efforts to elucidate important biological phenomena.
Theranostics is a new frontier in the fields of medicine and nuclear medicine. Furthermore, the possibility of using the same compound, such as 64CuCl2, for diagnosis and treatment opens a new era in theranostics. To date, studies of PET imaging with 64CuCl2 as a radiotracer are in a proof-of-concept phase. However, the potential of this system to be applied to personalized cancer management is extremely attractive, and thus, is an active area of research.5
The first study of 64CuCl2 in humans was carried out by Schubert and Riezler in 1947.33 They analyzed blood samples from healthy volunteers following oral, subcutaneous, and intravenous injections of 64CuCl2. Catalogna et al23 characterized the combined effect of 64CuCl2 and SI113 on human glioblastoma multiforme cell lines with variable p53 expression. They support evidence to underline the theranostic potential of 64CuCl2 in this tumor.
Currently, there are very few clinical cancer imaging studies with 64CuCl2 used as a radiotracer. In addition, all of these studies have produced no carrier added (NCA)-64− CuCl2 in a cyclotron. Capasso et al45 authored the first study of 64CuCl2 as a PET probe with PET-CT imaging used to stage patients confirmed to have prostate cancer according to histology and MRI.5,45
While it is well recognized that Cu is vital for good health, overexposure to Cu ions can lead to a wide spectrum of side effects. One potential side effect is the production of radical species due to a Fenton-type reaction. Oxidative stress that is caused by these species can lead to downstream damage to proteins, nucleic acids, and lipids.5,31 However, cytotoxic effects have only been reported for Cu ions at concentrations ≥7.42 mg/L. For PET imaging of a tumor with a reasonably good tumor-to-background ratio, only 5–10 mCi (185–370 MBq) of 64CuCl2 is generally administered. This clinical dose translates into few nanogram of Cu2+ ions when cyclotron-produced NCA 64Cu is used. Therefore, the amount of Cu2+ ions administered in a clinical setting would not be predicted to induce cytotoxic effects.5
The use of 64CuCl2 for PET imaging was first reported by Panichelli et al46 for glioblastoma patients. PET-CT imaging exhibited good concurrence with MRI in this study, and no adverse effects were observed following the administration of 64CuCl2.5 These results support the use of 64CuCl2 as a diagnostic agent for tumors of the central nervous system. However, to our knowledge, this is the only clinical study of 64CuCl2 for PET imaging that has been published to date.
Recently, Righi et al24 evaluated the kinetics and dosimetry of 64CuCl2 in 50 patients with prostate cancer. They suggest that the reported therapeutic effect of 64CuCl2 is mainly due to Auger electron emission than to the beta radiation. Avila-Rodriguez et al47 reported the first-in-human study on biodistribution and radiation dosimetry of 64CuCl2 in six healthy volunteers. They showed that the liver has the highest uptake, followed by intestine and pancreas while the urinary excretion is negligible. Although they are critical organs, the authors suggest that the liver, intestine, and pancreas would support therapeutic activities of up to hundreds of mCi (several GBq) without compromising their functions.
Alzheimer’s disease
The World Health Organization has reported that among the cases of dementia, Alzheimer’s accounts for 60%–70% of these cases. Approximately 47 million people worldwide are estimated to be suffering from dementia, and each year approximately 10 million new cases are registered. Age is considered the main risk factor for dementia, although the disease is not an inevitable consequence of aging and it does not exclusively affect older people. For example, approximately 9% of Alzheimer’s patients present with symptoms prior to the age of 65.48,49 A link between cognitive impairment and lifestyle-related risk factors has been observed in cases of Alzheimer’s disease, with sedentary lifestyle, unbalanced diet, smoking, obesity, excessive alcohol consumption, diabetes, and hypertension representing potential risk factors. Other risk factors include depression, low education level, social isolation, and cognitive inactivity.48,49
Alzheimer’s is a disease that is characterized by progressive loss of synaptic activity and neuron cell death. A hallmark of Alzheimer’s progression is the development of extracellular senile plaques in brain tissue.50 These plaques develop in the hippocampal and cortical regions of the brain and are composed of a 39–43 amino acid peptide derived from amyloid-β protein precursor. The presence of this amyloid-β peptide contributes to the disruption of trace metal homeostasis and intracellular neurofibrillary tangles. Correspondingly, copper metabolism has been found to be altered in patients with Alzheimer’s, and it is hypothesized that amyloid-β protein precursor may contribute to the mechanism of Cu transport. If Cu accumulates in extracellular plaques, this could aggravate Cu deficiency in neurons.14,50,51 It is also hypothesized that Cu metabolism has roles in Huntington’s, prion, and Parkinson’s diseases.14
Uptake, distribution, and removal of Cu from the brain are regulated by enzymes and proteins that specifically bind or transport Cu. When Cu is abnormally distributed in the brain, when it is inaccurately bound, or when it is present at higher or lower levels, neurodegenerative disorders may develop. Premortem studies of Alzheimer’s disease that used Cu radionuclides supported the role of Cu in Alzheimer’s disease pathology.12
To date, the mechanistic details of a possible role of Cu in neurological pathologies remain unclear. Furthermore, to our knowledge, there is currently no clinical data available regarding the use of 64CuCl2 in Alzheimer’s disease. However, studies of Cu metabolism among the large population of affected patients is an active area of research.12
Hypoxia in tumor tissue
Carcinogenesis in solid tumors is characterized by a rapid proliferation of tumor cells that outpaces the process of angiogenesis. As a result, a hypoxic region is often observed in cells that are at a distance from blood vessels (generally >100 mm) due to inefficient tumor vascularization.52 Consequently, hypoxia is often considered a hallmark of cancer, and in a number of tumor types, it is a predictor of tumor progression, resistance to therapeutic agents, and poor prognosis via multiple mechanisms.53 Biochemists define hypoxia as limited electron transport of O2− in mitochondria, while physiologists define hypoxia as a reduction in O2 availability due to decreased O2 partial pressure. The clinical aspect of hypoxia involves limited oxygen delivery to aerobic stromal and neoplastic cells, with the partial pressure for oxygen in several tumor types being <5 mmHg vs 40–60 mmHg in normal tissues.54
Over the past ten years, hypoxia has been shown to affect gene expression and alter tumor malignancy to promote a more aggressive phenotype. Thus, it has been difficult to treat hypoxic cancers with cytotoxic chemotherapy, photodynamic therapy, or radiation. To counteract the effects of hypoxia, radiation-sensitizing drugs and hyperbaric oxygen have been applied. However, the application of hypoxia-specific cytotoxins has been more encouraging. To evaluate hypoxia-directed therapies in clinical trials, noninvasive detection and monitoring of hypoxia are needed. In addition, the ability to image hypoxia could help identify patients who would most benefit hypoxia-targeted therapies.55
A number of promising PET radiopharmaceuticals have been tested for their ability to measure pO2. [18F]-fluoromisonidazole is the classical reference for nitroimidazole compounds to date and has been used to measure pO2. ATSM [diacetylbis(N4-methylthiosemicarbazone)] has also been used to image hypoxia and can be labeled with different Cu isotopes (eg, 60/61/62/64Cu). When the metabolism and pharmacology of ATSM-Cu complexes are the same and are independent of the isotope used, then the physical properties of the isotopes used should be considered.52 The first human use of 64Cu-ATSM was published in 2000 in a cohort of lung cancer, and all patients presented with positive exams.
For PET imaging of hypoxia, an injection of 925 MBq of 64Cu-ATSM has been performed. For a diagnostic protocol, this is a relatively high dosimetric value. However, it is consistent with liver radiation detected during multidetector CT scans of the abdomen (11.5 mSv for a tissue weighting factor of 0.04) and the magnitude of total body dosimetry observed for thallium-201 during cardiac imaging of stress and rest conditions.56
In clinical PET studies with 64Cu-ATSM, a disparity between normoxic and hypoxic tissues has been observed 10–15 minute post-injection, consistent with radio-/chemoresistance findings for various tumors, including lung, rectal, and cervical carcinomas.52 Imaging uniformity may be related to the site of uptake (eg, by mitochondria or in the cytosol) or the biochemical pathways involved (eg, glycolysis or oxidative pathways). Furthermore, cellular accumulation of 64Cu-ATSM may be related to cancer cell phenotypes, and this could affect patient outcome.52
Severe and chronic hypoxia can lead to a decrease in intracellular reactive oxygen species and reduced oxidative phosphorylation in mitochondria. While it has been observed that uptake of Cu-ATSM is enhanced under these conditions, it remains unclear whether there is a direct correlation between these conditions and Cu-ATSM uptake. However, clinical studies have demonstrated that uptake of Cu-ATSM was associated with a worse prognosis in cases involving cancer of the lung, uterine cervix, and rectum.54
The ability to produce and deliver 64Cu has been established by several companies. For example, ACOM (Italy), MDS Nordion (Canada), IBA Molecular (US and Europe), IsoTrace (US), and Trace Life Sciences (US) currently sell 64CuCl2 for the preparation of radiopharmaceuticals. In particular, 64Cu-ATSM is a preferred reagent for PET imaging of tumor hypoxia based on the simple method for its synthesis, its straightforward quantification, and its more rapid clearance rate from normoxic tissue that facilitates a short period of time between its injection and imaging.54,55
Neuroendocrine tumors (NETs)
It remains challenging to diagnose NETs due to their highly variable symptoms, their relatively small size, and their ability to develop anywhere in the body. Generally, there is a 5–7 year delay between the initial presentation of symptoms and the diagnosis of patients with NETs. Unfortunately, during this time, 20%–50% of patients will develop metastatic disease. Therefore, there is a great need to obtain an early diagnosis with a sensitive and easily accessible diagnostic imaging method. Sensitive imaging modalities would also be useful for long-term surveillance of NETs in order to detect progression/recurrence in its early stages and modify treatment strategy accordingly.57
Overexpression of somatostatin receptors distinguishes NETs from other tumors.57–59 Consequently, analogs of somatostatin receptors have been used both for imaging and pharmacological treatment. In addition, radiolabeling of peptides has been used to provide receptor-targeted radionuclide therapy.59
Pfeifer et al58 conducted a study in which patients with a history of NETs underwent SPECT/CT with 111In-DTPA-octreotide and PET/CT with 64Cu-labeled-(DOTATATE). The latter produced images with high spatial resolution and excellent quality. 64Cu-DOTATATE PET also detected additional lesions in 6/14 patients. In five of these patients, the lesions were localized to organ systems and organs that had not previously been characterized as possible sites of metastasis.
Similarly, in a prospective study of 64Cu-DOTATATE and 68Ga-labeled–(DOTATOC) that was conducted by Johnbeck et al,57 the former exhibited a markedly improved lesion detection rate in NET patients (n=59). Furthermore, additional lesions were detected by 64Cu-DOTATATE during the follow-up period, and most of these lesions were true positives. This result is attributed to the lower detection range of 64Cu compared to 68Ga. In addition, the shelf life of 64Cu is greater than 24 hours and it has a flexible scan window (eg, >3 hour), which makes 64Cu-DOTATATE very attractive for use in a clinical routine. Further studies are needed to confirm whether reliable detection of NETs translates into better patient management and improved outcome.
64Cu-labeled antibodies
Accumulating evidence supports a role for ephrin type B receptor 4 (EphB4) in the progression of various cancers. In one of these studies, 64Cu-labeled anti-EphB4 antibodies (hAb47 and hAb131) were used to characterize the distribution of EphB4 both in vitro and in vivo in models of breast and colorectal cancers.37 This approach could facilitate evaluations of dose optimization, pharmacokinetics, dose interval, and tumor-targeting efficacy of hAb47 and hAb131 antibody-based cancer therapeutics. Furthermore, these probes could be applied to other cancer types that overexpress EphB4.
The first Phase I/II clinical study of a 64Cu-labeled antibody was conducted by Philpott et al.60 In this study, uptake of the monoclonal antibody, 64Cu-BAT-2IT-1A3, and 18F-FDG was detected in patients that were suspected to have metastatic, or advanced primary, colorectal cancer (n=36). 64Cu-BAT-2IT-1A3 exhibited greater specificity in detecting colorectal tumors compared with 18F-FDG. The therapeutic potential of 64Cu-BAT-2IT-1A3 and 67Cu-BAT-2IT-1A3 was also evaluated in additional preclinical studies. Taken together, these results demonstrate that 64Cu should be further developed as a radioimmunotherapy agent.37,60,61
64Cu-prostate-specific membrane antigen (PSMA)
In the US, prostate cancer is the second leading cause of cancer-related death and the most common cancer diagnosed in men.62 Radiation or surgery is typically the first line of treatment. However, tumor recurrence frequently occurs and it is difficult to detect in its early stages since CT and MRI imaging frequently provide unsatisfactory specificity and sensitivity.63,64 Hence, there is an ongoing need to develop better imaging techniques for prostate cancer. PSMA has been identified as a promising target for endoradiotherapy and scintigraphic imaging of prostate cancer.63,64 This type II transmembrane protein is also not only overexpressed in androgen-dependent and androgen-independent advanced and metastatic prostate cancer, but also in schwannoma, in the tumor neovasculature of many solid tumors, and in certain subtypes of bladder carcinoma.63 To date, the function of PSMA remains unknown, although it has been observed to relocalize from the apical membrane to the luminal surface of ducts during dysplastic and neoplastic transformations of prostate tissues.63
PSMA-617 was the first 64Cu-labeled ligand to be used for PET imaging in a clinical study. This study included 29 patients (mean age, 71 years) from two centers in Germany and Austria.64 The patients received an injection of 64Cu-PSMA-617 (synthesized by ACOM, Italy) and no pharmacological or adverse effects were subsequently observed. The images obtained showed a high lesion contrast with a reasonable effective dose compared to the images obtained with administration of 18F-choline PET/CT or 68Ga-PSMA PET/CT. Moreover, all of the patients with local disease that was confirmed histologically were identified with 64Cu-PSMA-617 PET imaging. There were two cases of suspected lymph node metastasis that were confirmed by MRI or they had been suspected based on previous PET imaging that employed different radiopharmaceuticals. These results support the labeling of PSMA ligands with radionuclides that have a longer half-life (eg, 64Cu) for use by PET centers that otherwise lack radiochemistry facilities and 68Ga generators (satellite concept).64
The studies highlighted in this review demonstrate that 64CuCl2 is a unique radiopharmaceutical with physiological properties that are compatible with its use as a diagnostic and therapeutic agent. To date, this dual capacity has only previously been described for radioiodine. Thus, 64CuCl2 represents a revolutionary radiopharmaceutical for theranostic purposes and its complex chemistry is outweighed by its imaging advantages compared with other radiopharmaceuticals, such as 68Ga (short half-life [68 minutes] and lower resolution due to higher positron energy). Furthermore, based on the initial results obtained with use of 64Cu as a therapeutic, it is anticipated that further applications of 64CuCl2 will continue this new era in the field of theranostics.
Acknowledgments
This work was supported by the National Council for Scientific and Technological Development (CNPq) and International Atomic Energy Agency (IAEA), CRP F22067.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.IAEA Radioisotopes and radiopharmaceuticals Reports No.1 Cyclotron produced radionuclides: Emerging positron emitters for medical applications: 64Cu and 124I. 2016. Available from: https://www-pub.iaea.org/books/IAEABooks/10791/Cyclotron-Produced-Radionu-clides-Emerging-Positron-Emitters-for-Medical-Applications-64Cu-and-124I.
- 2.Osredkar J, Sustar N. Copper and zinc, biological role and significance of copper/zinc imbalance. [Accessed July 3, 2018];J Clin Toxicol. 2011 S3:001. [Google Scholar]
- 3.Chellan P, Sadler PJ. The elements of life and medicines. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2015;373(2037):20140182. doi: 10.1098/rsta.2014.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai Z, Anderson CJ. Chelators for copper radionuclides in positron emission tomography radiopharmaceuticals. J Labelled Comp Radiopharm. 2014;57(4):224–230. doi: 10.1002/jlcr.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakravarty R, Chakraborty S, Dash A. 64Cu2+ Ions as PET Probe: An Emerging Paradigm in Molecular Imaging of Cancer. Mol Pharm. 2016;13(11):3601–3612. doi: 10.1021/acs.molpharmaceut.6b00582. [DOI] [PubMed] [Google Scholar]
- 6.Mercer JF, Camakaris J. Copper comes of age in Melbourne. Metallomics. 2016;8(9):816–823. doi: 10.1039/c6mt90022d. [DOI] [PubMed] [Google Scholar]
- 7.Burlando B, Evangelisti V, Dondero F, Pons G, Camakaris J, Viarengo A. Occurrence of Cu-ATPase in Dictyostelium: possible role in resistance to copper. Biochem Biophys Res Commun. 2002;291(3):476–483. doi: 10.1006/bbrc.2002.6463. [DOI] [PubMed] [Google Scholar]
- 8.Montes S, Rivera-Mancia S, Diaz-Ruiz A, Tristan-Lopez L, Rios C. Copper and copper proteins in Parkinson’s disease. Oxid Med Cell Longev. 2014;2014:147251. doi: 10.1155/2014/147251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boll MC, Alcaraz-Zubeldia M, Montes S, Rios C. Free copper, ferroxidase and SOD1 activities, lipid peroxidation and NO(x) content in the CSF. A different marker profile in four neurodegenerative diseases. Neurochem Res. 2008;33(9):1717–1723. doi: 10.1007/s11064-008-9610-3. [DOI] [PubMed] [Google Scholar]
- 10.Eum WS, Kang JH. Release of copper ions from the familial amyotrophic lateral sclerosis-associated Cu, Zn-superoxide dismutase mutants. Mol Cells. 1998;9(1):110–114. [PubMed] [Google Scholar]
- 11.Bellingham SA, Lahiri DK, Maloney B, La Fontaine S, Multhaup G, Camakaris J. Copper depletion down-regulates expression of the Alzheimer’s disease amyloid-beta precursor protein gene. J Biol Chem. 2004;279(19):20378–20386. doi: 10.1074/jbc.M400805200. [DOI] [PubMed] [Google Scholar]
- 12.Hueting R. Radiocopper for the imaging of copper metabolism. J Labelled Comp Radiopharm. 2014;57(4):231–238. doi: 10.1002/jlcr.3155. [DOI] [PubMed] [Google Scholar]
- 13.Mercer JF, Llanos RM. Molecular and cellular aspects of copper transport in developing mammals. J Nutr. 2003;133(5 Suppl 1):1481S–1484S. doi: 10.1093/jn/133.5.1481S. [DOI] [PubMed] [Google Scholar]
- 14.Camakaris J, Voskoboinik I, Mercer JF. Molecular mechanisms of copper homeostasis. Biochem Biophys Res Commun. 1999;261(2):225–232. doi: 10.1006/bbrc.1999.1073. [DOI] [PubMed] [Google Scholar]
- 15.Camakaris J, Petris MJ, Bailey L, et al. Gene amplification of the Menkes (MNK; ATP7A) P-type ATPase gene of CHO cells is associated with copper resistance and enhanced copper efflux. Hum Mol Genet. 1995;4(11):2117–2123. doi: 10.1093/hmg/4.11.2117. [DOI] [PubMed] [Google Scholar]
- 16.Kim KI, Jang SJ, Park JH, et al. Detection of increased 64Cu uptake by human copper transporter 1 gene overexpression using PET with 64CuCl2 in human breast cancer xenograft model. J Nucl Med. 2014;55(10):1692–1698. doi: 10.2967/jnumed.114.141127. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Peña MM, Nose Y, Thiele DJ. Biochemical characterization of the human copper transporter Ctr1. J Biol Chem. 2002;277(6):4380–4387. doi: 10.1074/jbc.M104728200. [DOI] [PubMed] [Google Scholar]
- 18.Jalilian AR, Rowshanfarzad P, Sabet M. Preparation of [61Cu]pyruvaldehyde-bis (N4-methylthiosemicarbazone) complex as a possible PET radiopharmaceutical. Radiochim Acta. 2006;94:113–117. [Google Scholar]
- 19.Bhargava KK, Gupta RK, Nichols KJ, Palestro CJ. In vitro human leukocyte labeling with 64Cu: an intraindividual comparison with 111In-oxine and 18F-FDG. Nucl Med Biol. 2009;36(5):545–549. doi: 10.1016/j.nucmedbio.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou M, Zhao J, Tian M, et al. Radio-photothermal therapy mediated by a single compartment nanoplatform depletes tumor initiating cells and reduces lung metastasis in the orthotopic 4T1 breast tumor model. Nanoscale. 2015;7(46):19438–19447. doi: 10.1039/c5nr04587h. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Adonai N, Adonai N, Nguyen KN, et al. Ex vivo cell labeling with 64Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone) for imaging cell trafficking in mice with positron-emission tomography. Proc Natl Acad Sci U S A. 2002;99(5):3030–3035. doi: 10.1073/pnas.052709599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang L, Tu Y, Hu X. Pilot study of 64Cu(I) for PET imaging of melanoma. Sci Rep. 2017;31(7):2574. doi: 10.1038/s41598-017-02691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catalogna G, Talarico C, Dattilo V, et al. The SGK1 kinase inhibitor SI113 sensitizes theranostic effects of the 64CuCl2 in human glioblas-toma multiforme cells. Cell Physiol Biochem. 2017;43(1):108–119. doi: 10.1159/000480328. [DOI] [PubMed] [Google Scholar]
- 24.Righi S, Ugolini M, Bottoni G. Biokinetic and dosimetric aspects of 64CuCl2 in human prostate cancer: possible theranostic implications. EJNMMI Res. 2018;8(1):18. doi: 10.1186/s13550-018-0373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boal AK, Rosenzweig AC. Structural biology of copper trafficking. Chem Rev. 2009;109(10):4760–4779. doi: 10.1021/cr900104z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres Martin de Rosales R, Tavaré R, Paul RL, et al. Synthesis of 64Cu(II)-bis(dithiocarbamatebisphosphonate) and its conjugation with superparamagnetic iron oxide nanoparticles: in vivo evaluation as dual-modality PET-MRI agent. Angew Chem Int Ed Engl. 2011;50(24):5509–5513. doi: 10.1002/anie.201007894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandya DN, Kim JY, Park JC, et al. Revival of TE2A; a better chelate for Cu(II) ions than TETA? Chem Commun. 2010;46(20):3517–3519. doi: 10.1039/b925703a. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Z, Xiong Z, Subbarayan M, Chen X, Gambhir SS. 64Cu-labeled alpha-melanocyte-stimulating hormone analog for microPET imaging of melanocortin 1 receptor expression. Bioconjug Chem. 2007;18(3):765–772. doi: 10.1021/bc060306g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Li D, Huang CW. The efficient synthesis and biological evaluation of novel bi-functionalized sarcophagine for 64cu radiopharmaceuticals. Theranostics. 2012;2(6):589–596. doi: 10.7150/thno.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S, Li Z, Conti PS. Development of multi-functional chelators based on sarcophagine cages. Molecules. 2014;19(4):4246–4255. doi: 10.3390/molecules19044246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J, Lee CC, Sutcliffe JL, Cherry SR, Tarantal AF. Radiolabeling rhesus monkey CD34+ hematopoietic and mesenchymal stem cells with 64Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone) for microPET imaging. Mol Imaging. 2008;7(1):7290. [PubMed] [Google Scholar]
- 32.Schultze MO, Simmons SJ. The use of radioactive copper in studies on nutritional anemia of rats. J Biol Chem. 1942;142:97–106. [Google Scholar]
- 33.Schubert G, Riezler W. Indicator-Untersuchungen mit Radiokupfer beim Menschen; die Absorption parenteral und enteral zugeführter Kupferdosen, zugleich Untersuchungen über die Kreislaufzeit [Indicator examinations with radio-ligands in humans; the absorption of parenterally and enterally supplied copper doses, at the same time studies on the circulation time] Klin Wochenschr. 1947;24:304–306. doi: 10.1007/BF01734531. German. [DOI] [PubMed] [Google Scholar]
- 34.Bahde R, Kapoor S, Bhargava KK, Schilsky ML, Palestro CJ, Gupta S. PET with 64Cu-histidine for noninvasive diagnosis of biliary copper excretion in Long-Evans cinnamon rat model of Wilson disease. J Nucl Med. 2012;53(6):961–968. doi: 10.2967/jnumed.111.092361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng F, Lu X, Janisse J, Muzik O, Shields AF. PET of human prostate cancer xenografts in mice with increased uptake of 64CuCl2. J Nucl Med. 2006;47(10):1649–1652. [PubMed] [Google Scholar]
- 36.Cai H, Wu JS, Muzik O, Hsieh JT, Lee RJ, Peng F. Reduced 64Cu uptake and tumor growth inhibition by knockdown of human copper transporter 1 in xenograft mouse model of prostate cancer. J Nucl Med. 2014;55(4):622–628. doi: 10.2967/jnumed.113.126979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S, Li D, Park R, et al. PET imaging of colorectal and breast cancer by targeting EphB4 receptor with 64Cu-labeled hAb47 and hAb131 antibodies. J Nucl Med. 2013;54(7):1094–1100. doi: 10.2967/jnumed.112.116822. [DOI] [PubMed] [Google Scholar]
- 38.Jørgensen JT, Persson M, Madsen J, Kjær A. High tumor uptake of (64) Cu: implications for molecular imaging of tumor characteristics with copper-based PET tracers. Nucl Med Biol. 2013;40(3):345–350. doi: 10.1016/j.nucmedbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Ferrari C, Asabella AN, Villano C, et al. Copper-64 Dichloride as Theranostic Agent for Glioblastoma Multiforme: A Preclinical Study. Biomed Res Int. 2015;2015:1–6. doi: 10.1155/2015/129764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin ZH, Furukawa T, Degardin M, et al. αVβ3 Integrin-Targeted Radionuclide Therapy with 64Cu-cyclam-RAFT-c(-RGDfK-)4. Mol Cancer Ther. 2016;15(9):2076–2085. doi: 10.1158/1535-7163.MCT-16-0040. [DOI] [PubMed] [Google Scholar]
- 41.Qin C, Liu H, Chen K, et al. Theranostics of malignant melanoma with 64CuCl2. J Nucl Med. 2014;55(5):812–817. doi: 10.2967/jnumed.113.133850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie F, Xi Y, Pascual JM, Muzik O, Peng F. Age-dependent changes of cerebral copper metabolism in Atp7b −/− knockout mouse model of Wilson’s disease by [64Cu]CuCl2-PET/CT. Metab Brain Dis. 2017;32(3):717–726. doi: 10.1007/s11011-017-9956-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng F, Lutsenko S, Sun X, Muzik O. Imaging copper metabolism imbalance in Atp7b (−/−) knockout mouse model of Wilson’s disease with PET-CT and orally administered 64CuCl2. Mol Imaging Biol. 2012;14(5):600–607. doi: 10.1007/s11307-011-0532-0. [DOI] [PubMed] [Google Scholar]
- 44.Peng F, Xie F, Muzik O. Alteration of Copper Fluxes in Brain Aging: A Longitudinal Study in Rodent Using 64CuCl2-PET/CT. Aging Dis. 2018;9(1):109–118. doi: 10.14336/AD.2017.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Capasso E, Durzu S, Piras S, et al. Role of (64)CuCl 2 PET/CT in staging of prostate cancer. Ann Nucl Med. 2015;29(6):482–488. doi: 10.1007/s12149-015-0968-4. [DOI] [PubMed] [Google Scholar]
- 46.Panichelli P, Villano C, Cistaro A, et al. Imaging of brain tumors with copper-64 chloride: early experience and results. Cancer Biother Radiopharm. 2016;31(5):159–167. doi: 10.1089/cbr.2016.2028. [DOI] [PubMed] [Google Scholar]
- 47.Avila-Rodriguez MA, Rios C, Carrasco-Hernandez J, et al. Biodistribution and radiation dosimetry of [64Cu]copper dichloride: first-in-human study in healthy volunteers. EJNMMI Res. 2017;7(1):98. doi: 10.1186/s13550-017-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization (WHO) Dementia: a public health priority. Geneva: World Health Organization – Alzeheimer’s Disease International; 2012. [Google Scholar]
- 49.Alzheimer’s Disease International The global voice on dementia. Policy brief for heads of government. The global impact of dementia 2013–2050. 2013 [Google Scholar]
- 50.Fodero-Tavoletti MT, Villemagne VL, Paterson BM, et al. Bis(thiosemicarbazonato) Cu-64 complexes for positron emission tomography imaging of Alzheimer’s disease. J Alzheimers Dis. 2010;20(1):49–55. doi: 10.3233/JAD-2010-1359. [DOI] [PubMed] [Google Scholar]
- 51.Acevedo KM, Hung YH, Dalziel AH, et al. Copper promotes the trafficking of the amyloid precursor protein. J Biol Chem. 2011;286(10):8252–8262. doi: 10.1074/jbc.M110.128512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bourgeois M, Rajerison H, Guerard F, et al. Contribution of [64Cu]-ATSM PET in molecular imaging of tumour hypoxia compared to classical [18F]-MISO – a selected review. Nucl Med Rev Cent East Eur. 2011;14(2):90–95. doi: 10.5603/nmr.2011.00022. [DOI] [PubMed] [Google Scholar]
- 53.Hicks KO, Siim BG, Jaiswal JK, et al. Pharmacokinetic/pharmacodynamic modeling identifies SN30000 and SN29751 as tirapazamine analogues with improved tissue penetration and hypoxic cell killing in tumors. Clin Cancer Res. 2010;16(20):4946–4957. doi: 10.1158/1078-0432.CCR-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Colombié M, Gouard S, Frindel M, et al. Focus on the controversial aspects of 64Cu-ATSM in tumoral hypoxia mapping by PET imaging. Front Med. 2015;2:58. doi: 10.3389/fmed.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Padhani AR, Krohn KA, Lewis JS, Alber M. Imaging oxygenation of human tumours. Eur Radiol. 2007;17(4):861–872. doi: 10.1007/s00330-006-0431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis JS, Laforest R, Dehdashti F, Grigsby PW, Welch MJ, Siegel BA. An imaging comparison of 64Cu-ATSM and 60Cu-ATSM in cancer of the uterine cervix. J Nucl Med. 2008;49(7):1177–1182. doi: 10.2967/jnumed.108.051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnbeck CB, Knigge U, Loft A, et al. Head-to-head comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: a prospective study of 59 patients with neuroendocrine tumors. J Nucl Med. 2017;58(3):451–457. doi: 10.2967/jnumed.116.180430. [DOI] [PubMed] [Google Scholar]
- 58.Pfeifer A, Knigge U, Mortensen J, et al. Clinical PET of neuroendocrine tumors using 64Cu-DOTATATE: first-in-humans study. J Nucl Med. 2012;53(8):1207–1215. doi: 10.2967/jnumed.111.101469. [DOI] [PubMed] [Google Scholar]
- 59.Johnbeck CB, Knigge U, Kjær A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol. 2014;10(14):2259–2277. doi: 10.2217/fon.14.139. [DOI] [PubMed] [Google Scholar]
- 60.Philpott GW, Schwarz SW, Anderson CJ, et al. Radioimmuno-PET: detection of colorectal carcinoma with positron-emitting copper-64-labeled monoclonal antibody. J Nucl Med. 1995;36(10):1818–1824. [PubMed] [Google Scholar]
- 61.Holland JP, Williamson MJ, Lewis JS. Unconventional nuclides for radiopharmaceuticals. Mol Imaging. 2010;9(1):7290. [PMC free article] [PubMed] [Google Scholar]
- 62.Mease RC, Foss CA, Pomper MG. PET imaging in prostate cancer: focus on prostate-specific membrane antigen. Curr Top Med Chem. 2013;13(8):951–962. doi: 10.2174/1568026611313080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Afshar-Oromieh A, Hetzheim H, Kratochwil C, et al. The theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesions. J Nucl Med. 2015;56(11):1697–1705. doi: 10.2967/jnumed.115.161299. [DOI] [PubMed] [Google Scholar]
- 64.Grubmüller B, Baum RP, Capasso E, et al. 64Cu-PSMA-617 PET/CT imaging of prostate adenocarcinoma: first in-human studies. Cancer Biother Radiopharm. 2016;31(8):277–286. doi: 10.1089/cbr.2015.1964. [DOI] [PubMed] [Google Scholar]