Abstract
Ichthyosis is a heterogeneous group of skin disorders characterized by abnormal epidermal scaling. Occasionally, extracutaneous features are associated. A novel autosomal recessive ichthyosis syndrome is described here with scalp hypotrichosis, scarring alopecia, sclerosing cholangitis, and leukocyte vacuolization in two inbred kindreds of Moroccan origin. We also report the mapping of the diseased gene to a21.2 cM interval of chromosome 3q27–q28. Homo-zygosity for polymorphic markers has enabled us to reduce the genetic interval to a 16.2 cM region. Furthermore, comparison of mutant chromosomes in the two families has suggested a common ancestral mutant haplotype. This linkage disequilibrium has reduced the genetic interval encompassing the diseased gene to less than 9.5 cM maximum. Further study of additional families from the same geographic area will hopefully reduce the genetic interval as well as help in the cloning of the gene involved in this rare disorder.
Keywords: consanguinity, cholestasis, founder effect
IIchthyosis is the common name for a highly heterogeneous group of inherited skin disorders characterized by scaling over the whole body. Classification of ichthyoses remains difficult, and is usually based on clinical, histologic, and electronic criteria as well as associated features. Clinically, two major groups are described: (i) primary ichthyoses limited to the skin, and (ii) syndromic ichthyoses, in which associated features are present. In the first group, lamellar ichthyosis (MIM146750) and nonbullous congenital ichthyosiform erythroderma are the major forms. Several syndromes are listed in the second group such as Sjögren–Larsson syndrome (MIM270200), which is characterized by spastic paraplegia, mental retardation, and retinopathy, in addition to ichthyosis, Refsum disease with retinitis pigmentosa (MIM266500; Jansen et al 1997), Dorfman–Chanarin syndrome with cholestasis and cataract (MIM275630; Dorfman et al 1974; Chanarin et al 1975; Wollenberg et al 2000), or biliary atresia (MIM275630; Gould, 1854).
On the other hand, different forms of congenital ichthyoses may also be distinguished by the modes of inheritance namely autosomal dominant (ichthyosis vulgaris, MIM146700, rare forms of lamellar ichthyosis), X-linked recessive ichthyosis (MIM308100), and autosomal recessive ichthyosis (ARCI). ARCI belongs to both primary and syndromic ichthyoses. In the first clinical group, approximately one-third of ARCI cases are ascribed to a mutation in the gene of transglutaminase 1 (TGM1) on chromosome 14q11 (Russel et al 1995); however, four other loci for ARCI have been mapped (Krebsova et al 2001). Syndromic ichthyosis are almost always autosomal recessive. Four genes have been identified, namely, phytanic acid oxidase for Refsum disease, SPINK5 for Netherton syndrome (MIM256500), and FALDH in Sjögren– Larsson disease (De Laurenzi et al 1996; Jansen et al 1997; Mihalik et al 1997; Chavanas et al 2000) and CGI-58 in Dorfman– Chanarin syndrome (Lefevre et al 2001); however, in most syndromic ichthyoses, the underlying genetic mechanisms remain unknown.
In this study, we describe a novel variant of syndromic ARCI associated with sclerosing cholangitis, hypotrichosis of the frontal scalp, and vacuolated leukocytes and keratinocytes in two kindred of Moroccan ancestry, and we show that the disease gene maps to chromosome 3q27–q28.
MATERIALS AND METHODS
Patients
Family 1 Patient 1 (V.3) is a 14 y old boy born to second cousin healthy parents of Moroccan ancestry (Fig 1). Pregnancy, labor, and delivery were uneventful, with no evidence of collodion membrane at birth. Six weeks after birth, moderate jaundice, hepatomegaly without splenomegaly, and unexplained cholestasis was diagnosed. Mild diffuse ichthyosis predominant in skin folds with small regular scales on the trunk and limbs was noted. At 12 y of age, mild hypotrichosis was found associated with short dystrophic and thick hair, cicatricial frontoparietal alopecia, sparse eyelashes with loss of the outer third of the eyebrows. Palms, soles, and nails were normal. Oligodontia and hypodontia were noted. Microscopic skin examination showed normal epidermis and dermis. Blood smears showed intracytoplasmic vacuoles in the eosinophils only (Table I). Clinical neuromuscular evaluation showed no abnormality and muscle enzymes were normal.
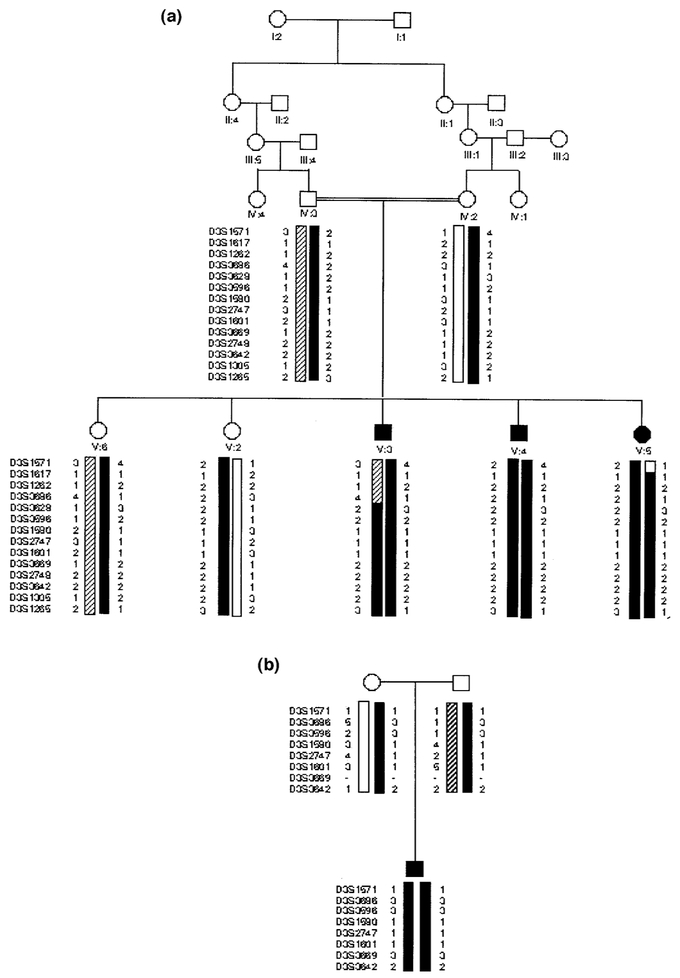
Figure 1. Family pedigrees.
The polymorphic DNA haplotypes are presented for mutant (solid columns) and normal chromosomes (open and hatched columns). (a) Family 1; (b) family 2.
Table I.
Clinical features in the reported family 1 as compared with related syndromes with ichthyosisa
| This report | Syndromes with ichthyosis | |||||
|---|---|---|---|---|---|---|
| VI | V2 | V3 | Dorfman-Chanarin (MIM275630) |
Bilary atresia (MIM242400) |
Refsum (MIM266510) |
|
| Physical features | ||||||
| Skin | ||||||
| White scaly ichthyosis | + | + | + | + | + | + |
| Hypotrichosis/alopecia | + | + | + | − | + | − |
| Eyelashes/eyebrows | + | + | + | − | − | − |
| anomalies | ||||||
| Collodion baby, NBIE | − | − | − | + | − | + |
| Palmoplantar hyperkeratosis | − | − | − | + | − | + |
| Dystrophic nails | − | − | − | + | − | − |
| Pruritis | + | − | − | + | − | − |
| Extracutaneous | ||||||
| Hepatic involvement | + | + | + | + | + | − |
| Teeth anomalies | + | + | + | − | ND | − |
| Cataract | ND | − | − | + | ND | +, RP |
| Muscular weakness | − | − | − | + | ND | − |
| Mental retardation | + | − | − | + | ND | + |
| Histologic findings | ||||||
| Skin | ND | ND | ||||
| Acanthosis | − | + | + | |||
| Vacuoled keratinocytes | − | + | + | + | ||
| Oil red O staining | − | − | + | − | ||
| Liver | ND | ND | ||||
| Fatty infiltration | − | + | − | + | ||
| Fibrosis | + | − | +/− | |||
| Sclerotic cholangitis | + | − | − | − | ||
| Laboratory results | ND | |||||
| Muscular enzymes | N | N | N | Elevated | N | |
| Lipid droplets | Eo | Eo | Eo | All leukocytes | − | |
| Lipidogram | ND | N | N | N | Low cholesterol | |
| Electromyography | ND | N | ND | Myogenic changes | Neurogenic changes | |
+, present; −, absent; ND, not determined; N, normal; NBIE, nonbullous ichthyosiform erythroderma; RP, retinitis pigmentosa; Eo, eosinophilic granulocytes.
Patient 2 (V.4), the 11 y old brother of patient 1, presented with similar clinical features, including neonatal jaundice, hepatomegaly, cholestasis, and ichthyosis (Fig 1, Table I). Cystic fibrosis and α1-anti-trypsin deficiency were excluded. At 4 mo of age, a liver biopsy showed extensive fibrosis with neither fatty infiltration nor ductular proliferation, but did show a combination of intracellular and extracellular cholestasis. Transparietal cholangiography showed sclerotic cholangitis that was complicated by portal hypertension at 5 y of age. When evaluated at the age of 11, the skin was dry and xerotic, with large scaling ichthyosis predominant on the limbs and abdomen but sparing the skin folds. Thick, short and sparse hair was associated with scarring and scaling alopecia of the frontal and parietal areas of the scalp and eyebrows. The patient also had an infraorbital crease of Dennie–Morgan, follicular keratosis of the knees, and enamel dysplasia without other tooth abnormalities (Fig 2). Nails, palms, soles, and eyelashes were normal. Hepatomegaly, splenomegaly, and jaundice were still present with elevated liver enzymes. A skin biopsy showed orthokeratosis and parakeratosis, acanthosis, papillomatosis, and granular layer hyperplasia. Intracytoplasmic vacuoles were present in some basal keratinocytes but oil red O-staining was negative. A mild upper dermal infiltrate and atrophic hair follicles were also noted. Ultrastructural analysis showed split anchoring plaques of desmosomes in the granular layer (Fig 3). Intracytoplasmic vacuoles were present in eosinophils, neutrophils, and lymphocytes on the blood smear. Psychomotor development, neuromuscular ophthalmologic evaluation (including electromyography and muscular enzymes), as well as cardiac ultrasound were normal.
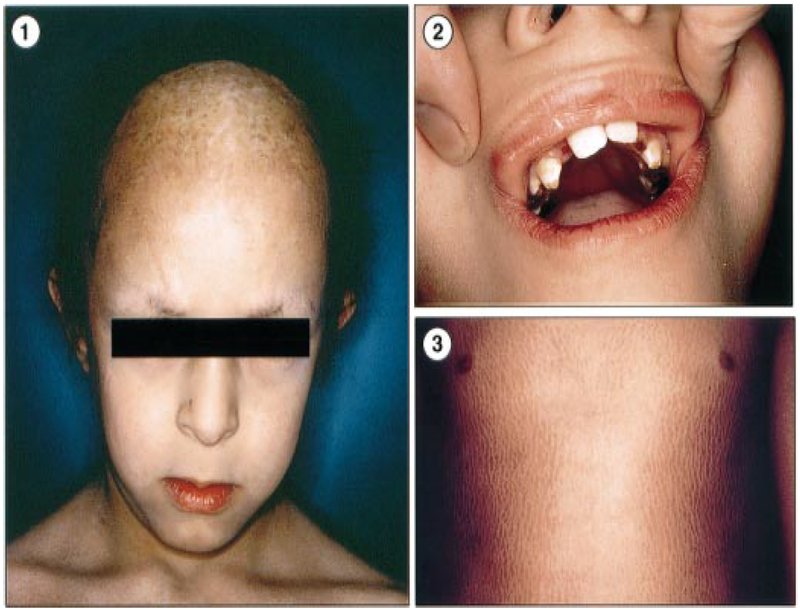
Figure 2. Clinical features of patient 2 (family 1).
(1) Thick, short and sparse hair with scarring and scaling alopecia of the frontal and parietal areas of the scalp and eyebrows, as well as infraorbital crease of Dennie–Morgan. (2) Oligodontia and enamel dysplasia. (3) Dry and xerotic skin, with large scaling ichthyosis predominant on limbs and abdomen but sparing the skin folds.
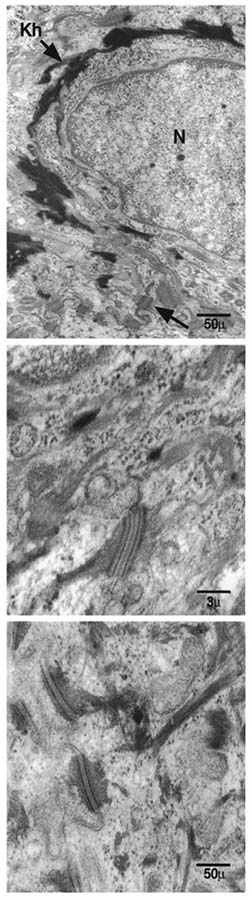
Figure 3. Microscopic dermatologic and hematologic features (patient 2, family 2).
(1) Intracytoplasmic vacuoles in eosinophils. (2) Ultrastructural skin biopsy showing splitted anchoring plaques of desmosomes in the granular layer. N, nucleus, Kh, keratohyalin. (3) Ultrastructural skin biopsy showing normal desmosomes of the granular layer.
Patient 3 (V.5), the 3 y old sister of patients 1 and 2, had a large scaly ichthyosis of the trunk and limbs. She presented with diffuse jaundice and hepatomegaly (Fig 1, Table I). Major hypotrichosis on parietal and occipital areas were noted. Nails, palms, and soles were normal (as in the patients). Tooth enamel dysplasia was also noted. Ophthalmologic examination showed bilateral anterior uveal synechiae. Intracytoplasmic vacuoles were found in blood eosinophils only. Blood lipids, muscular evaluation, and cardiac ultrasound were normal. Finally, the complete clinical examination and peripheral blood smear of both parents and several relatives were normal.
Family 2 Patient 1, a 12 y old boy born to healthy nonconsanguineous Moroccan parents, presented with similar clinical features, including neonatal jaundice, hepatomegaly, cholestasis, and ichthyosis. Major hypotrichosis predominated on parietal and occipital areas. At 1 y of age, growth (–3.7 SD) and height retardation were noted (–4 SD). At 2 y of age, a liver biopsy showed extensive fibrosis with intracellular and extracellular cholestasis but neither fatty infiltration nor ductular proliferation. Transparietal cholangiography showed sclerotic cholangitis complicated with portal hypertension at 6 y of age. Interestingly, a liver transplantation performed at 8 y of age, and immunosuppression with FK506, led to a dramatic improvement of development and the complete regression of skin lesions and alopecia (Fig 1).
Genotyping
Genomic DNA was extracted according to standard procedures after informed consent of the parents. A genome-wide search on the three affected individuals of family 1 was performed, using 400 fluorescent microsatellite markers of the ABI PRISM Linkage Mapping Set Version II (Perkin Elmer Cetus/Applied Biosystems, Foster City, CA), which covers the entire human genome with an average spacing of 10 cM (Dib et al 1996). Genomic DNA (50 ng per μl) was amplified in a ready-made buffer with deoxyribonucleoside triphosphates and AmpliTaq Gold DNA polymerase (True Allele PCR Premix ABI PRISM) according to the manufacturer’s instructions. Polymerase chain reaction products from all markers (2 μl) were pooled with 2 μl of a mixture containing bromophenol blue (0.5 μl), formamide (1.25 μl), and Genescan 400D [ROX] size standard ladder (0.25 μl), denatured and loaded on to a 3% acrylamide gel (Long Ranger Gel Solution, FMCR, Bio Whittaker Molecular Applications, ME). The data were analyzed using computer programs Genescan version 3.0 and Genotyper version2.5 (Applied Biosystems) on an automated DNA sequencer (ABI PRISM 377 system). When homozygosity was found, the corresponding markers were further genotyped in all family members. Twelve additional flanking markers of the 3q26–q27 region were then selected from the Marshfield map and studied for two-point linkage analysis (Broman et al 1998). The patient of family 2 was genotyped for markers of the 3q27 region only.
Statistical analysis
LOD score calculations were performed with the computer program package LINKAGE version 5.2 under the assumption of a fully penetrant autosomal recessive mode of inheritance, with no phenocopy, a disease allele frequency of 0.001 and an equal marker informativity, apart from allele 1 for marker AFM308yf1 at locus D3S1601, which was found to be present in 5% chromosomes in the control Moroccan population tested. The recombination fraction was assumed to be equal for males and females. The marker order used was taken from the Center for Medical Genetics at Marshfield Medical Research Foundation. Recombination events were recognized on the basis of LOD scores values and segregation of haplotypes. Multipoint linkage analysis was conducted using the MLINK program of the FASTLINK computer program (Cottingham et al 1993). Allele frequencies at the D3S1601 and D3S2747 loci were compared between mutant chromosomes (n = 4) and 84 control chromosomes from 40 unrelated and unselected Moroccan individuals and the two parents of family 1. Significance of linkage disequilibrium was tested using the standard χ2 test.
Sequencing
We designed intronic primers to amplify the 12 coding exons of IL1-RacP gene by aligning the cDNA and the genomic sequences. We selected primers and polymerase chain reaction conditions using the Amplify 1.2 program (primer sequences available on request). Polymerase chain reaction-amplified DNA was sequenced on both strands using the fluorochrome PRISM krt (PE Biosystems). Electrophoresis was carried out for 12 h on a 373 automated DNA sequencer (ABI).
Electronic database information
Accession numbers and URL for data in this article are as follows:
Online Mendelian Inheritance in Man (OMIM), www.ncbi.nlm.nih.gov/Omim/for ARCI
Généthon, http://www.genethon.fr (for markers)
Whitehead Institute for Biomedical Research/MIT Center for Genome Research (http://www-genome.wi.mit.edutel (for markers)
Center for Medical Genetics, Marshfield Medical Research Foundation, http://www.marshmed.org/genetics (for genetic interval)
CEPH-Généthon database, http://www.cephb.frcephdb/(for genetic interval).
RESULTS
Homozygosity in all three affected individuals of family 1 was found at 20 loci among the 400 polymorphic markers tested. These markers were then further genotyped in all family members and tested for linkage using the MLINK program of the FASTLINK computer package. Only marker AFM308yf1 at locus D3S1601 proved to be homozygous in affected individuals and heterozygous in parents and unaffected sibs. Two-point linkage analysis gave a maximum LOD score at this locus (Zmax of 2.61 at θ = 0%; Table II). It should be noted that the maximum pairwise LOD score value expected for this pedigree was 3.25 assuming a maximum informativity, a unique mutant allele at the disease locus and a recombination fraction of 0 between the disease locus and the marker.
Table II.
Two-point LOD scores between the ichthyosis syndrome locus and chromosome 3q27–q28 polymorphic DNA locia
| Locus | Genetic distance (cM) |
Recombination fraction (θ) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.0 | 0.05 | 0.10 | 0.15 | 0.20 | 0.30 | 0.40 | ||
| D3S1571 | 4.93 | −∞ | −1.821 | −1.093 | −0.722 | −0.487 | −0.204 | −0.054 |
| D3S1617 | 2.46 | 0.851 | 0.733 | 0.620 | 0.512 | 0.408 | 0.218 | 0.069 |
| D3S1262 | 5.29 | −∞ | −0.542 | −0.320 | −0.211 | −0.142 | −0.057 | −0.011 |
| D3S3628 | 1.3 | −0.774 | 0.710 | 0.768 | 0.703 | 0.586 | 0.303 | 0.070 |
| D3S3596 | 0 | 1.754 | 1.517 | 1.285 | 1.058 | 0.836 | 0.425 | 0.115 |
| D3S1580 | 4.88 | 1.936 | 1.677 | 1.422 | 1.172 | 0.929 | 0.480 | 0.139 |
| D3S2747 | 1.84 | 1.936 | 1.677 | 1.422 | 1.172 | 0.929 | 0.480 | 0.139 |
| D3S1601 | 1.39 | 2.61 | 2.31 | 2.01 | 1.71 | 1.40 | 0.81 | 0.30 |
| D 3 S3 6 69 | 1.4 | 1.754 | 1.517 | 1.285 | 1.058 | 0.836 | 0.425 | 0.115 |
| D3S2748 | 0 | 0.851 | 0.733 | 0.620 | 0.512 | 0.408 | 0.218 | 0.069 |
| D3S3642 | 5.59 | 0.851 | 0.733 | 0.620 | 0.512 | 0.408 | 0.218 | 0.069 |
| D3S1305 | 0 | 1.936 | 1.677 | 1.422 | 1.172 | 0.929 | 0.480 | 0.139 |
| D3S1265 | - | −0.774 | 0.710 | 0.768 | 0.703 | 0.586 | 0.303 | 0.070 |
Homozygosity was found in affected individuals of family 1 for the loci shown in italics. The disease locus maps to a 13.4 cM region.
Studying 12 additional markers of the 3q26–q27 region confirmed these data (Table I). Multipoint LOD score analysis gave a maximum value of 3.0 across the region defined by the following loci: D3S3596, D3S1580, D3S2747, D3S1601, and D3S3669 (data not shown). Homozygosity mapping, suggested that the disease locus maps to a 16.2 cM interval of chromosome 3q27–q28 defined by loci D3S3628 and D3S1265 where the affected individuals of family 1 were homozygotes.
Interestingly, the proband 1 of family 2 was also homozygous for the same markers between loci D3S3596 and D3S1305 despite the absence of consanguinity. Comparison of mutant chromosomes in the two families originating from Morocco suggested a common ancestral mutant haplotype. Indeed, the four patients shared a common allele at each of the noncontiguous polymorphic loci: 1(D3S1580)–1(D3S2747)–1(D3S1601), suggesting a founder effect. In order to support this hypothesis, we have estimated the frequency of polymorphic alleles in a series of 40 chromosomes from unrelated controls of the same ethnic background. The distribution of alleles 1 at loci D3S2747 and D3S1601, respectively, was significantly different in affected Moroccan individuals as compared with controls. In particular, allele 1 at the D3S1601 and allele 1 at D3S2747 loci displayed strong linkage disequilibrium with the disease mutant allele (100% in mutant chromosomes vs 5% in control Moroccan chromosomes p < 0.001, and 100% vs 16%, p < 0.001 at loci D3S1601 and D3S2747, respectively). This linkage disequilibrium allowed us to reduce the genetic interval encompassing the disease gene to only 9.5 cM.
The candidate region contains several genes and anonymous expressed sequences. We have tested the IL1-RacP gene as IL1-RacP participates in IL-1 signaling as a co-receptor for IL-1, a cytokine that plays a major part in skin homeostasis (Maas-Szabowski et al 2000). Additionally, it has recently been reported that a soluble form of IL1-RacP is produced in the liver, resulting from a tissue-specific differential splicing (Jensen et al 2000). Because these two compartments appear affected in this ichthyosis– sclerosing cholangitis syndrome, we also analyzed IL-1 signaling in patient V.4 fibroblasts. As IL-1 activates the transcription factor NF-kB through degradation of the inhibitory molecule IkBa, we incubated patient V.4 fibroblasts with IL-1 at various times, then prepared cytoplasmic extracts and analyzed the fate of IkBa by western blotting. IkBa degradation appeared normal apart from a defect in the IL-1 signaling pathway (data not shown). Moreover, sequencing of all the 12 exons of mIL1-RacP and of the alternative exon 9 for the soluble IL-1RacP showed no deleterious mutation. Nevertheless, we detected two polymorphisms: in exon 11 (G1308A) and in the alternative exon 9 of the soluble IL-1RacP isoform (nt 216 after the end of 3’ UTR). The homozygous exon 11 polymorphism was detected in both an affected boy and his healthy father. Homozygous polymorphism of exon 9 was detected in two of 10 healthy individuals from the same ethnic background.
DISCUSSION
Here we report on a novel form of syndromic ichthyosis and the mapping of the disease causing gene to 3q27–q28 in an inbred family of Moroccan origin (Fig 2). To the best of our knowledge, this form of nonlamellar nonerythrodermic diffuse ichthyosis, with severe scarring alopecia and sclerosis of the bile ducts has not been previously described. More than a century ago, however, a case of ichthyosis congenita with bilary atresia (MIM242400) was reported. In our patients, however, biliary ducts were histologically normal (patient 2). This novel syndrome shares similarities with a syndrome of ichthyosis with neutral lipid storage disease, Dorfman–Chanarin syndrome (MIM275630), which is related to mutations of the CGI-58 gene located on chromosome 3p21. Yet this diagnosis may be excluded on the basis of following features: (i) the absence of muscular or ocular involvement; (ii) the hepatic involvement with hepatomegaly related to cholestasis and sclerosing cholangitis, but not to the fatty infiltration as in Dorfman– Chanarin syndrome; and (iii) the presence of small-sized leukocytes and keratinocyte vacuoles that were not stained with usual lipid staining and were absent in unaffected relatives. Thus, whereas this disorder appears clearly distinct from Dorfman–Chanarin syndrome, clinical overlap may reflect a common genetic basis or metabolic pathway, although the nature of the vacuoles is not known (Scheimberg et al 1996). Both the classical and infantile form of Refsum disease (MIM266500; Bader et al 2000), another syndrome with ichthyosis and vacuolated keratinocytes, could also be excluded as cataract, retinitis pigmentosa, cerebellar ataxia, facial dysmorphism, polyneuropathy, and mental impairment were absent in our families. Finally, ichthyosis syndromes with hepatic involvement such as KID (keratitis, ichthyosis, deafness) syndrome with micronodular cirrhosis (Wilson et al 1991), ichthyosis with hepatomegaly and cerebellar ataxia (Harper et al, 1980), or hepatomegaly and hypogonadism (Arnold et al 1992) have been excluded.
Ultrastructural analysis of skin biopsy showed split anchoring plaques of desmosomes in the granular layer (Fig 3). Desmosomes are highly organized intercellular epithelial adhesions complexes formed of two compartments: a central core of desmosomal cadherins (desmogleins and desmocollins), and a submembrane plaque (plakoglobin, desmoplakin, and accessory proteins such as plakophilin 1). Another group is represented by cytoskeleton associating proteins such as envoplakin and periplakin (Kowalczyk et al 1997). So far desmosomes have been involved in three groups of disorders: (i) autoimmune diseases; (ii) hereditary diseases of intracellular calcium channels (such as Hailey–Hailey and Darier diseases); and (iii) hereditary diseases of desmosomal structural components (McMillan and Shimizu, 2001). Whereas our data support the involvement of epidermal desmosome components in keratinization physiology, electron microscopy features in our observations differ from these disorders (Keith et al 1999; McGrath et al 1999; Rickman et al 1999; McKoy et al 2000; Norgett et al 2000). Moreover, among the genes and anonymous expressed sequences located in the 3q27 region none seems to be involved in desmosomal function. It may suggest the implication of non-structural proteins of desmosomes.
Using conventional mapping, linkage analysis allowed us to narrow the region encompassing the disease gene to the 21.2 cM interval defined by locus D3S686 and the telomere of chromosome 3. Further homozygosity mapping allowed us to place the disease gene in the 16.2 cM interval defined by loci D3S3628 and D3S1265. Finally, linkage disequilibrium further reduced the genetic interval to a 9.5 cM region defined by loci D3S3596 and D3S3669. Linkage disequilibrium mapping takes advantage of ancestral recombinational events that have occurred during the history of a population (Hastbacka et al 1992; Jorde, 1995; de la Chapelle and Wright, 1998). As most of the mutant chromosomes in a small inbred population carry a single mutation, the smaller the region with linkage disequilibrium, the older the mutation. In fact, a linkage disequilibrium was detected between the disease locus and two polymorphic DNA markers loci in the two unrelated Moroccan kindred tested. This finding supports the hypothesis of a unique mutation resulting in this syndrome in Morocco.
The geographic distribution of carriers of the mutation in a restricted geographic area, namely the Rif mountain in north Morocco, and the size of the common ancestral haplotype suggest that the disease causing mutation in this population is old; however, identification of affected patients of other North African countries should help in dating the mutation and identifying the disease causing gene.
At present, five ARCI genes have been identified, namely, the transglutaminase in lamellar ichthyosis, phytanic acid oxidase for Refsum disease, SPINK5 for Netherton syndrome, FALDH in Sjögren-Larsson disease, and CGI-58 in Dorfman–Chanarin syndrome (Russel et al 1995; De Laurenzi et al 1996; Jansen et al 1997; Mihalik et al 1997; Chavanas et al 2000; Lefévre et al 2001). Four other ARCI loci have been mapped to chromosomes 2q33, 19p12, and 17p (lamellar ichthyosis types 2 and 3; Fischer et al 2000; Parmentier et al 1996; Virolainen et al 2000; Krebsova et al 2001). Most of these genes are likely to be involved in epidermal differentiation; however, to the best of our knowledge, the ichthyosis–cholangitis locus described here is the Ærst involved in both skin and hepatic development.
The study of additional families, from the same geographic area, will hopefully reduce the genetic interval and help in cloning the gene involved in this rare disorder.
Acknowledgments
We thank Anna Pelet and Hatem El Shanti for their support and Sylvie Fraitag and Catherine Prost for electronic microscopy. This work was supported by grants from the 1nstitut National de la Santé et de la Recherche (INSERM), the Fondation pour la Recherche Médicale (FRM) and the CNCPRST (Morocco).
REFERENCES
- Arnold ML, Anton-Lamprecht I, Albrecht-Nebe H: Congenital ichthyosis with hypogonadism and growth retardation—a new syndrome with peculiar ultrastructural features. Arch Dermatol Res 284:198–208, 1992 [DOI] [PubMed] [Google Scholar]
- Bader PI, Dougherty S, Cangany N, Raymond G, Jackson CE: Infantile Refsum disease in four Amish sibs. Am J Med Genet 90:110–114, 2000 [PubMed] [Google Scholar]
- Broman KW, Murray JC, Sheffield VC, White RL, Weber JL: Comprehensive human genetic maps. individual and sex-specific variation in recombination. Am J Hum Genet 63:861–869, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanarin I, Patel A, Slavin G, Wills EJ, Andrews TM, Stewart G: Neutral-lipid storage disease. a new disorder of lipid metabolism. Br Med J 1:553–555, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Chapelle A, Wright FA: Linkage disequilibrium mapping in isolated populations. the example of Finland revisited. Proc Natl Acad Sci USA 95:12416–12423, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavanas S, Bodemer C, Rochat A, et al. : Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet 25:141–142, 2000 [DOI] [PubMed] [Google Scholar]
- Cottingham RW Jr, Idury RM, Schaffer AA: Faster sequential genetic linkage computations. Am J Hum Genet 53:252–263, 1993 [PMC free article] [PubMed] [Google Scholar]
- De Laurenzi V, Rogers GR, Hamrock DJ, et al. : Sjögren–Larsson syndrome is caused by mutations in the fatty aldehyde dehydrogenase gene. Nat Genet 12:52–57, 1996 [DOI] [PubMed] [Google Scholar]
- Dib C, Faure S, Fizames C, et al. : A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380:152–154, 1996 [DOI] [PubMed] [Google Scholar]
- Dorfman ML, Hershko C, Eisenberg S, Sagher F: Ichthyosiform dermatosis with systemic lipidosis. Arch Dermatol 10:261–266, 1974 [PubMed] [Google Scholar]
- Fischer J, Faure A, Bouadjar B, et al. : Two new loci for autosomal recessive ichthyosis on chromosomes 3p21 and 19p12-q12 and evidence for further genetic heterogeneity. Am J Hum Genet 66:904–913, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould AA: Ichthyosis in an infant: hemorrhage from umbilicus: death. Am J Med Sci 27:356, 1854 [Google Scholar]
- Harper PS, Marks R, Dykes PJ, Young ID: Ichthyosis, hepatosplenomegaly, and cerebellar degeneration in a sibship. J Med Genet 17:212–215, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastbacka J, de la Chapelle A, Kaitila I, Sistonen P, Weaver A, Lander E: Linkage disequilibrium mapping in isolated founder populations: diastrophic dysplasia in Finland. Nat Genet 2:204–211, 1992 [DOI] [PubMed] [Google Scholar]
- Jansen GA, Ofman R, Ferdinandusse S, et al. : Refsum disease is caused by mutations in the phytanoyl-CoA hydroxylase gene. Nat Genet 17:190–193, 1997 [DOI] [PubMed] [Google Scholar]
- Jensen LE, Muzio M, Mantovani A, Whitehead AS: IL-1 signaling cascade in liver cells and the involvement of a soluble form of the IL-1 receptor accessory protein. J Immunol 164:5277–5286, 2000 [DOI] [PubMed] [Google Scholar]
- Jorde LB: Linkage disequilibrium as a gene-mapping tool. Am J Hum Genet 56:11–14,1995 [PMC free article] [PubMed] [Google Scholar]
- Keith D, Armstrong DK, McKenna KE, et al. : Haploinsufficiency of desmoplakin causes a striate subtype of palmoplantar keratoderma. Hum Mol Genet 8:143–148, 1999 [DOI] [PubMed] [Google Scholar]
- Kowalczyk AP, Bornslaeger EA, Norvell SM, Palka HL, Green KJ: Desmosomes: intercellcular adhesive junctions specialized for attachment of intermediate filaments. Int Rev Cytol 185:237–302, 1997 [DOI] [PubMed] [Google Scholar]
- Krebsova A, Kuster W, Lestringant GG, et al. : Identification, by homozygosity mapping, of a novel locus for autosomal recessive congenital ichthyosis on chromosome 17p, and evidence for further genetic heterogeneity. Am J Hum Genet 69:216–222, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre C, Jobard F, Caux F, et al. : Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin–Dorfman syndrome. Am J Hum Genet 69:1002–1012, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas-Szabowski N, Stark HJ, Fusenig NE: Keratinocyte growth regulation in defined organotypic cultures through IL-1-induced keratinocyte growth factor expression in resting fibroblasts. J Invest Dermatol 114:1075–1084, 2000 [DOI] [PubMed] [Google Scholar]
- McGrath JA, McMillan JR, Shemanko CS, et al. : Mutations in the plakophilin 1 gene result in ectodermal dysplasia/skin fragility syndrome. Nat Genet 17:240–244, 1997 [DOI] [PubMed] [Google Scholar]
- McKoy G, Protonotarios N, Crosby A, et al. : Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet 355:2119–2124, 2000 [DOI] [PubMed] [Google Scholar]
- McMillan JR, Shimizu H: Desmosomes. structure and function in normal and diseased epidermis. J Dermatol 28:291–298, 2001 [DOI] [PubMed] [Google Scholar]
- Mihalik SJ, Morrell JC, Kim D, Sacksteder KA, Watkins PA, Gould SJ: Identification of PAHX, a Refsum disease gene. Nat Genet 17:185–189, 1997 [DOI] [PubMed] [Google Scholar]
- Norgett EE, Hatsell SJ, Carvajal-Huerta L, et al. : Recessive mutation in desmoplakin disrupts desmoplakin–intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet 9:2761–2766, 2000 [DOI] [PubMed] [Google Scholar]
- Parmentier L, Lakhdar H, Blanchet-Bardon C, Marchand S, Dubertret L, Weissenbach J: Mapping of a second locus for lamellar ichthyosis to chromosome 2q33–35. Hum Mol Genet 5:555–559, 1996 [DOI] [PubMed] [Google Scholar]
- Rickman L, Simrak D, Stevens HP, et al. : N-terminal deletion in a desmosomal cadherin causes the autosomal dominant skin disease striate palmoplantar keratoderma. Hum Mol Genet 8:971–976, 1999 [DOI] [PubMed] [Google Scholar]
- Russell LJ, Di Giovanna JJ, Rogers GR, et al. : Mutations in the gene for transglutaminase 1 in autosomal recessive lamellar ichthyosis. Nat Genet 9:279–283, 1995 [DOI] [PubMed] [Google Scholar]
- Scheimberg I, Harper JI, Malone M, Lake BD: Inherited ichthyoses: a review of the histology of the skin. Pediatr Pathol Laboratory Med 16:359–378, 1996 [DOI] [PubMed] [Google Scholar]
- Virolainen E, Wessman M, Hovatta I, et al. : Assignment of a novel locus for autosomal recessive congenital ichthyosis to chromosome 19p13.1-p13.2. Am J Hum Genet 66:1132–1137, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GN, Squires RH Jr, Weinberg AG: Keratitis, hepatitis, ichthyosis, and deafness: report and review of KID syndrome. Am J Med Genet 40:255–259, 1991 [DOI] [PubMed] [Google Scholar]
- Wollenberg A, Geiger E, Schaller M, Wolff H: Dorfman–Chanarin syndrome in a Turkish kindred. conductor diagnosis requires analysis of multiple eosinophils. Acta Derm Venereol 80:39–43, 2000 [DOI] [PubMed] [Google Scholar]