Abstract
Objective
Evaluate trends in method of access (percutaneous [PERC] vs open cannulation [OPEN]) for pediatric extracorporeal membrane oxygenation (ECMO), and determine the effects of cannulation method on morbidity and mortality.
Design
Retrospective cohort study.
Setting and Subjects
The Extracorporeal Life Support Organization’s registry was queried for pediatric patients on ECMO for respiratory failure from 2007–2015.
Main Results
Of 3,501 patients identified, 77.2% underwent open cannulation, with the frequency of OPEN decreasing over the study period from approximately 80% to 70% (p<0.001). PERC patients were more commonly male (24.2% vs. 21.5%, p=0.01), older (average 7.6 years vs. 4.5 years, p<0.001), and heavier (average 33.0 kg vs 20.2 kg, p< 0.001). Subset analysis of patients on veno-venous (VV) ECMO revealed higher rates of mechanical complications due to blood clots (28.9% vs. 22.6%, p = 0.003) or cannula problems (18.9% vs. 12.7%, p < 0.001), cannula site bleeding (25.3% vs. 20.2%, p = 0.01) and increased rates of cannula site repair in the OPEN cohort. Limb related complications were not significantly different on subset analysis for VV ECMO patients stratified by access site. Logistic regression analysis revealed that method of access was not associated with a difference in mortality.
Conclusions
The proportion of pediatric patients undergoing percutaneous ECMO cannulation is increasing. Mechanical and physiological complications occur with both methods of cannulation but percutaneous cannulation appears safe in this cohort. Further analysis is needed to evaluate long-term outcomes with this technique.
Keywords: ECMO, Extracorporeal Membrane Oxygenation, ECLS, Extracorporeal Life Support, Pediatric Surgery, Pediatric Critical Care
Introduction
Extracorporeal Membrane Oxygenation (ECMO) is a form of prolonged cardiopulmonary bypass utilized to support patients with severe cardiopulmonary failure in order to avoid irreversible organ injury (1). The use of ECMO in both adult and pediatric patients has evolved secondary to technological advances and an improved understanding of the physiological effects of ECMO. Appropriate patient selection, type of ECMO (veno-venous (VV) or veno-arterial (VA)), and appropriate cannula site selection may affect outcomes in adult patients (2). In addition, novel vascular access techniques may also impact morbidity and mortality of these patients (3–5).
There is a growing body of literature describing such advances in adult patients, including the use of percutaneous cannulation techniques for the resuscitation and cardiopulmonary support of patients with cardiac arrest, cardiogenic shock, pulmonary insufficiency, and pulmonary edema (6–9). Retrospective reports demonstrate that very few complications are directly attributable to the cannulation technique with the appropriate use of ultrasound and fluoroscopic guidance (10). Despite the initial description and advances in the neonatal population, the experience with percutaneous ECMO cannulation in the pediatric population is limited (11–13).
The purpose of this study was to evaluate trends in vascular access techniques (percutaneous [PERC] vs open [OPEN] cannulation) for pediatric ECMO and the association between access type and morbidity and mortality utilizing a large, multicenter database.
Methods
The study protocol and use of the Extracorporeal Life Support Organization (ELSO) registry database were reviewed by the institutional review board of the University of Buffalo. The study received exemption from formal review and the need for patient informed consent was waived.
Data Source
ELSO is an international, non-profit consortium of providers and centers dedicated to the development and improvement of ECMO. Participating health care centers contribute detailed data to a registry of clinical information for adults and children treated with ECMO (14). As of 2015, the database included over 59,000 children who had received ECMO with pre-ECMO illness severity and support, diagnosis and procedures, details of ECMO support and equipment, as well as relevant complications and survival to hospital discharge (15, 16). The registry supports the vast majority of ECMO clinical research and is used to generate and disseminate standards and guidelines. The dataset used included select registry data from 2007 to 2015.
Patient Selection
Pediatric patients, defined as greater than 28 days and less than 18 years of age, were included for study (Supplemental Figure 1. Patient selection). A primary ELSO support type of “respiratory” as well as the International Classification of Diseases, Ninth Revision (ICD-9) codes 518.81, 518.82, 518.84, and 799.1 were used to identify patients with respiratory failure. Data extracted from the ELSO registry included patient demographics, clinical characteristics of pre-ECMO assessment, as well as ECMO run details including, but not limited to, duration of cannulation, ECMO mode, and cannulation type (percutaneous vs open). Complications and outcomes, such as mortality, were also extracted from the registry for analysis. Of these patients, 63 underwent more than one ECMO run. Individual ECMO run was used for case identification and “patients” moving forward refer to individual “ECMO run.”
Statistical Analysis
A retrospective descriptive analysis of the data was performed to evaluate characteristics of patients placed on ECMO using percutaneous [PERC] access vs. traditional [OPEN] access techniques. Categorical variables were compared using the Pearson χ2 test; continuous variables were compared using the Kruskal-Wallis test, where p < 0.05 was considered statistically significant. Cochrane Armitage test for trend was utilized to evaluate the use of PERC versus OPEN over time. Clinical and demographic variables were incorporated into logistic regression models to calculate the adjusted odds ratio (aOR) and its 95% confidence intervals (CI) for mortality and selection for percutaneous cannulation. All statistical analyses were performed using R (Version 3.3.1) and STATA® Data Analysis and Statistical Software (Version 11).
Results
A total of 3,501 patients (> 28 days and < 18 years old) with a primary respiratory diagnosis requiring pulmonary support ECMO were included for study. Of these patients, 77.2% underwent OPEN cannulation (Table 1). Patients who underwent PERC were significantly older and heavier than OPEN patients but no difference in gender or race. Mortality varied significantly between the two groups, with 63.7% of patients in the PERC group discharged alive vs. 57.6% of patients in the OPEN group (p = 0.002). Additionally, the frequency of OPEN cannulation decreased over time from approximately 80% to 70% over the study period (p < 0.001) (Figure 1a). When divided by support type, there was a significant increase in percentage of patients accessed percutaneously for VV ECMO and decrease in PERC for VA ECMO (p < 0.001) (Figure 1b).
Table 1.
Patient characteristics receiving ECMO via percutaneous or open approach
| Characteristics | Percutaneous | Not-percutaneous | P-value |
|---|---|---|---|
| Total, n (%) | 798 (22.8) | 2703 (77.2) | |
| Age in years, mean (SD) | 7.6 (6.6) | 4.5 (5.4) | p < 0.001 |
| Median | 5.9 | 1.5 | |
| Age group | |||
| < 1 year | 188 (23.6) | 1117 (41.3) | p < 0.001 |
| 1 – 3 years | 131 (16.4) | 526 (19.5) | |
| 4 – 6 years | 80 (10.0) | 291 (10.8) | |
| 7 – 12 years | 121 (15.2) | 334 (12.4) | |
| > 12 years | 278 (34.8) | 435 (16.1) | |
| Race | p = 0.62 | ||
| White | 414 (51.9) | 1438 (53.2) | |
| Asian | 70 (8.8) | 198 (7.3) | |
| Black | 145 (18.2) | 484 (17.9) | |
| Hispanic | 115 (14.4) | 363 (13.4) | |
| Other | 40 (5.0) | 151 (5.6) | |
| Sex | |||
| Male | 447 (56.0) | 1399 (51.8) | p = 0.06 |
| Female | 350 (43.9) | 1278 (47.3) | |
| Weight (kg), mean (SD) | 33.0 (28.5) | 20.2 (22.5) | P < 0.001 |
| Median | 20.9 | 10.9 | |
| Discharged alive | p = 0.002 | ||
| Yes | 508 (63.7) | 1557 (57.6) | |
| No | 290 (36.3) | 1146 (42.4) |
Figure 1.
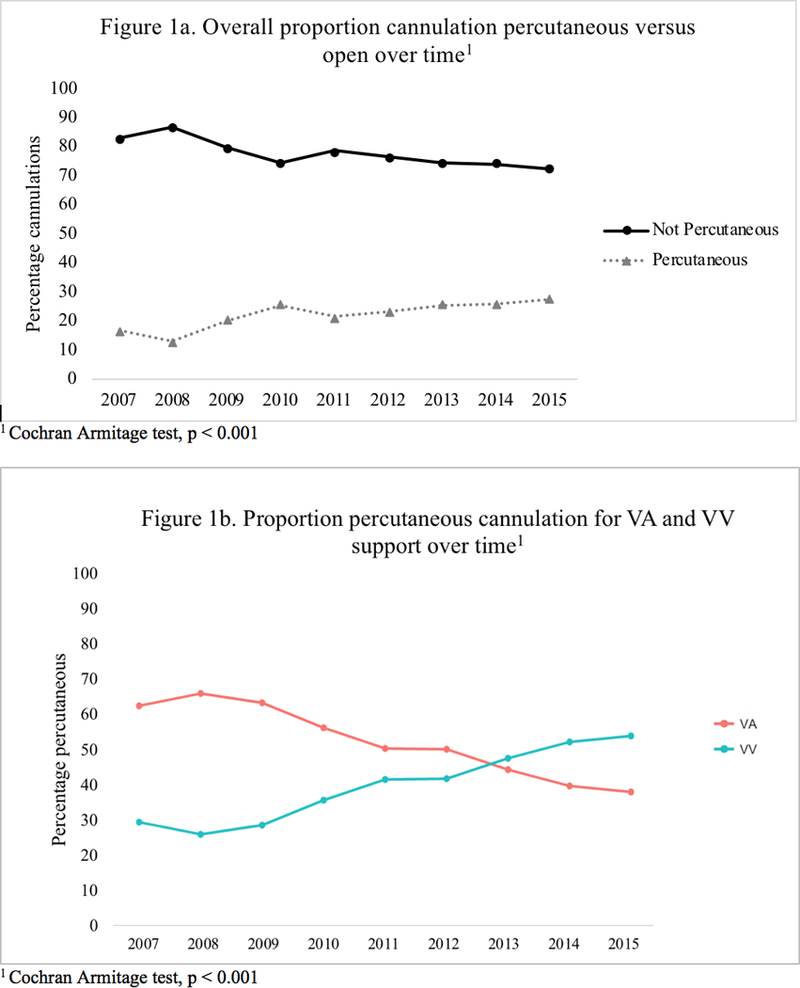
Proportion cannulation percutaneous versus open over time
There was no significant difference in mean time on ECMO for PERC vs OPEN. 45.7% received veno-venous (VV) support mode with 78.2% of PERC patients and 36.1% of OPEN patients on VV ECMO. In contrast, 19.5% of patients in the PERC group were placed on VA ECMO compared to 62.8% of OPEN patients. Excluding those classified as “other,” 53.0% of the cohort as a whole were placed on VA ECMO (Table 2). There was significant variation in ventilator type with a larger proportion of PERC patients placed on conventional ventilation and a larger proportion of OPEN patients on high-frequency oscillatory ventilation. The most common sites in both groups included the right internal jugular vein and right common carotid artery, followed by femoral vessels.
Table 2.
Extracorporeal membrane oxygenation characteristics, all patientsa
| Characteristics | Percutaneous, 798 (22.8) | Not-percutaneous, 2703 (77.2) | P-value |
|---|---|---|---|
| Time on ECMO (hours) | |||
| Mean | 272.0 | 271.5 | p = 0.42 |
| SD | 275.1 | 309.5 | |
| Median | 189.0 | 186.0 | |
| ECMO Mode, n (%) | p < 0.001 | ||
| Veno-Venousb | 624 (78.2) | 975 (36.1) | |
| Veno-Arterialc | 156 (19.5) | 1698 (62.8) | |
| Ventilator Type, n (%) | |||
| Conventional | 395 (49.5) | 1150 (42.5) | p = 0.001 |
| High-frequency oscillation | 282 (35.3) | 1114 (41.2) | |
| Other high-frequency ventilation | 19 (2.4) | 43 (1.6) | |
| Access site, n (%) | |||
| Aorta | 2 (0.3) | 95 (3.5) | p < 0.001 |
| Left atrium | 0 (0.0) | 6 (0.2) | |
| Right atrium | 2 (0.3) | 111 (4.1) | |
| Femoral vessels | 190 (23.8) | 310 (11.5) | |
| Jugular vessels | 532 (66.7) | 1420 (52.5) | |
| Carotid vessels | 56 (7.0) | 655 (24.2) | |
| Left internal jugular | 13 (1.6) | 26 (1.0) | |
| Other | 2 (0.3) | 23 (0.9) | |
| Pulmonary artery | 0 (0.0) | 6 (0.2) | |
| Right inferior vena cava (RIJVC) | 14 (1.8) | 77 (2.8) |
Column totals greater than total number of patients as patients with conversion between ECMO modes or access sites included more than once
Veno-venous includes runs categorized as: VV, VVDL, VVDL+V only
Veno-arterial includes runs categorized as: VA, VA-VV, VA+V, VVA, VV-VA
Laboratory values and initial ventilator settings varied significantly between patients on VV and patients VA ECMO within the OPEN group. When separated by support mode (VV and VA ECMO), however, only differences in initial SaO2 and Peak Inspiratory Pressure persisted in the VV group and differences in SaO2 and Mean Arterial Pressure for patients in the VA group between PERC and OPEN (Supplemental Table 1); these variables were excluded from multivariate analysis.
Subset analysis, VV ECMO
As the majority of patients with PERC access were placed on VV support, a subset analysis was performed of patients on VV ECMO. Complications on ECMO were divided into several categories, including mechanical complications related to equipment failure, mechanical complications secondary to blood clots, hemorrhagic complications, and complications affecting specific organ systems including neurologic, renal, cardiovascular, pulmonary, infectious, metabolic, and limb/extremity related complications (Table 3). For VV ECMO patients accessed via a femoral approach, mechanical complications secondary to blood clots and hemorrhagic complications with cannula site bleeding were more common in the OPEN group (32.5% vs 20.4%, p = 0.007; and 35.7% vs 14.9%, p < 0.001, respectively) (Supplemental Table 2). Patients in the PERC group were also less likely to undergo cannula site repair compared to OPEN patients (38% PERC vs 52.5% OPEN, p = 0.001).
Table 3.
| Complications | All patients on ECMO | VV ECMO Only | ||||
|---|---|---|---|---|---|---|
| Open, 2703 (77.2) | Percutaneous, 798 (22.8) | p-value | Open, 975 (61.0) | Percutaneous, 624 (39.0) | p-value | |
| Mechanical Failure | 299 (11.1) | 67 (8.4) | 0.08 | 102 (10.5) | 48 (7.7) | 0.06 |
| Mechanical Failure secondary to blood clots | 784 (29.0) | 188 (23.6) | 0.02 | 282 (28.9) | 141 (22.6) | 0.003 |
| Mechanical: cannula problems | 436 (16.1) | 102 (12.8) | 0.07 | 184 (18.9) | 79 (12.7) | < 0.001 |
| Hemorrhagic: cannulation site bleeding | -- | -- | -- | 247 (25.3) | 126 (20.2) | 0.01 |
| Metabolic: pH < 7.20 | -- | -- | -- | 79 (8.1) | 72 (11.5) | 0.02 |
| Metabolic: pH > 7.60 | 98 (3.6) | 19 (2.4) | 0.13 | -- | -- | -- |
| Limb complication | 15 (0.6) | 10 (1.3) | 0.03 | 2 (0.2) | 5 (0.8) | 0.08 |
| Mortality (not discharged alive) | 203 (32.5) | 327 (33.5) | 0.68 | 327 (33.5) | 203 (32.5) | 0.68 |
| Neurologic: other (seizures, CNS infarction) | 409 (15.1) | 95 (11.9) | 0.07 | -- | -- | -- |
| Renal complication | 1262 (46.7) | 313 (39.2) | 0.005 | -- | -- | -- |
| Cannula site repair, n (%) | ||||||
| None | 1496 (55.3) | 477 (59.8) | 0.03 | 577 (59.2) | 401 (64.3) | 0.04 |
| Carotid artery | 66 (2.4) | 5 (0.6) | 0.001 | 1 (0.1) | 1 (0.2) | 0.75 |
| Jugular vein | 132 (4.9) | 48 (6.0) | 0.20 | 104 (10.7) | 46 (7.4) | 0.03 |
| Both carotid and jugular | 247 (9.1) | 47 (5.9) | 0.004 | 30 (3.1) | 15 (2.4) | 0.43 |
| Other | 170 (6.3) | 31 (3.9) | 0.01 | 50 (5.1) | 16 (2.6) | 0.01 |
Complications tested include: Mechanical failure from blood clots, from air in circuit, or cracks in pigtail connectors or cannula problems; Hemorrhagic problems including GI hemorrhage, cannula site, or surgical site bleeding or coagulopathy/DIC; Neurological problems including clinically determine drain death, seizures or CNS infarction or CNS hemorrhage; Cardiovascular complications or cardiac tamponade; pulmonary or infections complications; and metabolic derrangements including acidosis (pH < 7.20), alkalosis (pH > 7.60), hyper-/or hypoglycemia and hyperbilirubinemia. Complications excluded for p > 0.20 for difference between percutaneous and not-percutaneous approach
Patients with more than one complication counted per complication
For VV ECMO patients undergoing jugular access, mechanical complications including blood clots and cannula problems were more common with OPEN compared to PERC techniques (28.6% vs. 22.8%, p = 0.009; and 19.3% vs. 12.8%, p < 0.001, respectively) (Table 3b). Hemorrhagic complications at the cannula site were also more common in the OPEN group (25.4% vs. 20.4%, p = 0.02), with fewer cannula site repairs performed in the PERC group. There was no statistical difference in likelihood of mortality at discharge based on access type for all patients on VV ECMO.
Logistic Regression Analysis
Logistic regression was performed to evaluate factors predictive of PERC rather than OPEN cannulation for patients on VV ECMO. Only increasing age and weight were associated with the choice of PERC technique (Table 4). When considered as a continuous variable, increasing weight (kg) was associated with statistically significant (although miniscule) increases in the odds of PERC as opposed to OPEN (aOR 1.03, 95% C.I. 1.01–1.07, p = 0.04). Compared to age < 1 year, patients aged > 12 years were significantly more likely to undergo PERC cannulation. Compared to continuous mandatory ventilation, patients on high frequency ventilation/oscillator were more likely to undergo percutaneous cannulation (aOR 0.69, 95% C.I. 0.50–0.94, p = 0.02). For patients on VA ECMO, age 4 – 6 years (compared to age < 1 year) and weight change were age for patients age 4 – 6 years were associated with increased odds of PERC compared to OPEN.
Table 4.
Logistic regression for percutaneous access instead of open for patients on VV ECMO
| VV ECMO Only | VA ECMO Only | |||||
|---|---|---|---|---|---|---|
| Characteristics | OR PERC | 95% C.I. | p-value | OR PERC | 95% C.I. | p-value |
| Race | ||||||
| White | Ref. | -- | -- | Ref. | -- | -- |
| Asian | 1.22 | (0.77, 1.92) | 0.40 | 0.71 | (0.26, 1.62) | 0.45 |
| Black | 1.15 | (0.85, 1.56) | 0.37 | 0.84 | (0.45, 1.49) | 0.56 |
| Hispanic | 1.31 | (0.92, 1.85) | 0.13 | 1.37 | (0.76, 2.38) | 0.28 |
| Other | 0.92 | (0.51, 1.61) | 0.78 | 1.49 | (0.64, 3.17) | 0.32 |
| Sex | ||||||
| Female | Ref. | -- | -- | Ref. | -- | -- |
| Male | 1.10 | (0.86, 1.39) | 0.45 | 1.33 | (0.88, 2.04) | 0.18 |
| Weight change per age group | ||||||
| < 1 year | Ref. | -- | -- | Ref. | -- | -- |
| 1 – 3 years | 0.99 | (0.89, 1.09) | 0.78 | 0.96 | (0.85, 1.04) | 0.38 |
| 4 – 6 years | 0.96 | (0.88, 1.09) | 0.22 | 0.78 | (0.61, 0.94) | 0.02 |
| 7 – 12 years | 0.97 | (0.93, 1.00) | 0.13 | 0.91 | (0.82, 0.99) | 0.05 |
| > 12 years | 0.97 | (0.93, 1.00) | 0.06 | 0.97 | (0.89, 1.02) | 0.25 |
| Weight (kg) | 1.03 | (1.01, 1.07) | 0.04 | 1.05 | (1.00, 1.13) | 0.10 |
| Age group | ||||||
| < 1 year | Ref. | -- | -- | Ref. | -- | -- |
| 1 – 3 years | 1.29 | (0.39, 4.24) | 0.67 | 2.43 | (0.88, 8.77) | 0.11 |
| 4 – 6 years | 2.30 | (0.68, 8.45) | 0.19 | 39.22 | (2.13, 1082.04) | 0.02 |
| 7 – 12 years | 2.20 | (0.97, 5.00) | 0.06 | 5.00 | (0.54, 46.69) | 0.15 |
| > 12 years | 3.13 | (1.38, 7.13) | 0.006 | 2.16 | (0.61, 7.61) | 0.23 |
| Ventilator Type | ||||||
| CMV | Ref. | -- | -- | |||
| HFOV | 0.69 | (0.50, 0.94) | 0.02 | 1.17 | (0.67, 2.03) | 0.58 |
| Other | 1.56 | (0.73, 3.36) | 0.25 | 0.84 | (0.13, 3.33) | 0.83 |
| HFOV (support code)1 | ||||||
| No | Ref. | -- | -- | Ref. | -- | -- |
| Yes | 1.09 | (0.79, 1.51) | 0.59 | 0.60 | (0.34, 1.05) | 0.08 |
| Nitric Oxide | ||||||
| No | Ref. | -- | -- | Ref. | -- | -- |
| Yes | 1.05 | (0.82, 1.35) | 0.69 | 1.05 | (0.68, 1.65) | 0.81 |
| CVVH | ||||||
| No | Ref. | -- | -- | Ref. | -- | -- |
| Yes | 2.17 | (0.98, 5.08) | 0.06 | 5.91 | (n/a, 3.84e12) | 0.98 |
| Year of cannulation | ||||||
| 2007 | Ref. | -- | -- | -- | ||
| 2008 | 0.70 | (0.35, 1.41) | 0.32 | 0.63 | (0.21, 1.81) | 0.38 |
| 2009 | 0.94 | (0.51, 1.75) | 0.84 | 1.65 | (0.72, 4.05) | 0.25 |
| 2010 | 1.06 | (0.58, 1.95) | 0.86 | 2.24 | (0.98, 5.58) | 0.07 |
| 2011 | 0.97 | (0.54, 1.77) | 0.92 | 1.79 | (0.75, 4.57) | 0.20 |
| 2012 | 1.37 | (0.78, 2.47) | 0.28 | 0.53 | (0.17, 1.58) | 0.26 |
| 2013 | 1.35 | (0.77, 2.39) | 0.30 | 0.87 | (0.32, 2.41) | 0.79 |
| 2014 | 1.10 | (0.63, 1.95) | 0.73 | 0.39 | (0.10, 1.29) | 0.14 |
| 2015 | 1.25 | (0.73, 2.20) | 0.42 | 0.97 | (0.36, 2.65) | 0.94 |
Abbreviations: VV (veno-venous); VA (veno-arterial); ECMO (Extracorporeal Membrane Oxygenation); CMV (Continuous Mandatory Ventilation); HFOV (High Frequency Ventilation/oscillation); CVVH (Continuous Veno-Venous Hemofiltration); OR (Odds Ratio); PERC (percutaneous cannulation)
Support code for HFOV included to compare use of high frequency ventilation compared to not; ventilator type compares use of HFOV to traditional continuous mandatory ventilation
Logistic regression analysis to identify factors associated with being discharged alive for all patients showed that type of access (PERC vs OPEN) was not associated with a significant difference in mortality for all patients or within the subset of patients on VV ECMO only or VA ECMO only. Similarly, gender, weight, and weight change per age group, were not associated with a decreased likelihood of hospital discharge. VV ECMO was also associated with increased likelihood of being discharged alive compared to VA ECMO (aOR 1.82, 95% C.I. 1.53–2.16, p < 0.001) (Table 5). Age greater than 12 years, as compared to age less than 1 year, and Asian or Hispanic race (as compared to White) were associated with a decreased likelihood of being discharged alive when evaluating the cohort as a whole.
Table 5.
Multivariate regression for odds of being discharged alive for pediatric patients on ECMO
| Characteristics | All patients on ECMO | Patients on VV ECMO Only | Patients on VA ECMO Only | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR for discharge alive | 95% C.I. | p-value | OR for discharge alive | 95% C.I. | p-value | OR for discharge alive | 95% C.I. | p-value | |
| Percutaneous | 1.03 | (0.83, 1.26) | 0.82 | 1.03 | (0.80, 1.34) | 0.81 | 0.90 | (0.59, 1.36) | 0.62 |
| Race | |||||||||
| White | Ref. | -- | -- | Ref. | -- | -- | Ref. | -- | -- |
| Asian | 0.72 | (0.53, 0.97) | 0.03 | 0.89 | (0.55, 1.44) | 0.62 | 0.58 | (0.37, 0.89) | 0.01 |
| Black | 0.99 | (0.81, 1.23) | 0.96 | 1.03 | (0.75, 1.43) | 0.84 | 0.98 | (0.72, 1.34) | 0.91 |
| Hispanic | 0.75 | (0.59, 0.95) | 0.02 | 0.87 | (0.61, 1.26) | 0.46 | 0.68 | (0.48, 0.96) | 0.03 |
| Other | 0.94 | (0.66, 1.34) | 0.72 | 1.23 | (0.69, 2.27) | 0.50 | 0.79 | (0.48, 1.32) | 0.37 |
| Sex | |||||||||
| Female | Ref. | -- | -- | Ref. | -- | -- | Ref. | -- | -- |
| Male | 0.98 | (0.83, 1.14) | 0.76 | 0.91 | (0.71, 1.17) | 0.48 | 1.08 | (0.86, 1.36) | 0.52 |
| Days on ECMO | 0.98 | (0.97, 0.99) | < 0.001 | 0.96 | (0.95, 0.97) | < 0.001 | 0.99 | (0.98, 1.00) | 0.04 |
| Mode of ECMO | |||||||||
| VA1 | Ref. | -- | -- | -- | -- | -- | -- | -- | -- |
| VV2 | 1.82 | (1.53, 2.16) | < 0.001 | -- | -- | -- | -- | -- | -- |
| Other | 2.24 | (1.03, 5.18) | 0.05 | -- | -- | -- | -- | -- | -- |
| Weight change per age group | |||||||||
| < 1 year | Ref. | -- | -- | Ref. | -- | -- | Ref. | -- | -- |
| 1 – 3 years | 0.96 | (0.90, 1.02) | 0.19 | 1.02 | (0.92, 1.14) | 0.68 | 0.92 | (0.83, 1.00) | 0.11 |
| 4 – 6 years | 1.00 | (0.95, 1.05) | 0.94 | 1.05 | (0.97, 1.17) | 0.30 | 0.97 | (0.90, 1.04) | 0.45 |
| 7 – 12 years | 1.00 | (0.97, 1.02) | 0.75 | 1.00 | (0.97, 1.03) | 0.90 | 0.98 | (0.92, 1.03) | 0.44 |
| > 12 years | 1.01 | (0.99, 1.03) | 0.34 | 1.01 | (0.98, 1.03) | 0.37 | 0.99 | (0.94, 1.03) | 0.66 |
| Weight (kg) | 1.00 | (0.98, 1.02) | 0.95 | 1.00 | (0.98, 1.02) | 0.96 | 1.01 | (0.97, 1.07) | 0.51 |
| Age group | |||||||||
| < 1 year | Ref. | -- | -- | Ref. | -- | -- | Ref. | -- | -- |
| 1 – 3 years | 1.94 | (1.04, 4.01) | 0.05 | 0.94 | (0.27, 3.23) | 0.91 | 2.98 | (1.18, 9.24) | 0.04 |
| 4 – 6 years | 1.05 | (0.47, 2.31) | 0.90 | 0.59 | (0.10, 2.50) | 0.52 | 1.23 | (0.41, 3.82) | 0.72 |
| 7 – 12 years | 1.28 | (0.69, 2.35) | 0.44 | 1.37 | (0.56, 3.33) | 0.49 | 1.22 | (0.43, 3.51) | 0.71 |
| > 12 years | 0.36 | (0.20, 0.67) | 0.001 | 0.48 | (0.19, 1.16) | 0.11 | 0.45 | (0.17, 1.15) | 0.09 |
| Ventilator Type | |||||||||
| CMV | Ref. | -- | -- | Ref. | -- | -- | Ref. | -- | -- |
| HFOV | 1.05 | (0.85, 1.30) | 0.68 | 0.94 | (0.68, 1.29) | 0.69 | 1.16 | (0.85, 1.59) | 0.34 |
| Other | 0.98 | (0.56, 1.75) | 0.95 | 1.11 | (0.49, 2.67) | 0.81 | 0.88 | (0.35, 2.13) | 0.77 |
| HFOV support code | |||||||||
| No | Ref. | -- | -- | Ref. | -- | -- | Ref. | -- | -- |
| Yes | 0.98 | (0.79, 1.22) | 0.84 | 1.05 | (0.76, 1.47) | 0.76 | 0.91 | (0.66, 1.26) | 0.58 |
| Nitric Oxide | |||||||||
| No | Ref. | -- | -- | Ref. | -- | -- | Ref. | -- | -- |
| Yes | 0.94 | (0.79, 1.12) | 0.48 | 0.95 | (0.73, 1.24) | 0.70 | 0.97 | (0.76, 1.25) | 0.84 |
| CVVH | |||||||||
| No | Ref. | -- | -- | Ref. | -- | -- | Ref. | -- | -- |
| Yes | 0.54 | (0.29, 1.02) | 0.06 | 0.59 | (0.26, 1.39) | 0.21 | 0.59 | (0.17, 1.86) | 0.38 |
| Year of cannulation | |||||||||
| 2007 | Ref. | -- | -- | Ref. | -- | -- | Ref. | -- | -- |
| 2008 | 0.99 | (0.67, 1.44) | 0.94 | 1.15 | (0.60, 2.21) | 0.68 | 0.86 | (0.51, 1.42) | 0.55 |
| 2009 | 1.12 | (0.78, 1.60) | 0.54 | 1.30 | (0.71, 2.38) | 0.39 | 1.01 | (0.62, 1.64) | 0.95 |
| 2010 | 1.05 | (0.73, 1.51) | 0.80 | 1.11 | (0.61, 2.00) | 0.73 | 0.95 | (0.57, 1.57) | 0.83 |
| 2011 | 1.14 | (0.79, 1.65) | 0.49 | 1.85 | (1.01, 3.38) | 0.04 | 0.70 | (0.42, 1.17) | 0.17 |
| 2012 | 1.48 | (1.02, 2.15) | 0.04 | 1.30 | (0.72, 2.34) | 0.38 | 1.49 | (0.88, 2.51) | 0.14 |
| 2013 | 1.18 | (0.82, 1.69) | 0.37 | 1.14 | (0.64, 2.02) | 0.65 | 1.09 | (0.65, 1.84) | 0.75 |
| 2014 | 1.27 | (0.89, 1.82) | 0.19 | 1.26 | (0.72, 2.21) | 0.41 | 1.19 | (0.70, 2.03) | 0.52 |
| 2015 | 1.23 | (0.86, 1.76) | 0.26 | 1.35 | (0.77, 2.35) | 0.29 | 0.92 | (0.54, 1.55) | 0.75 |
VA ECMO: (VA-VV, VA+V, VV-VA, VVA)
VV ECMO: (VV, VVDL, VVDL+V)
Abbreviations: VV (veno-venous); ECMO (Extracorporeal Membrane Oxygenation); CMV (Continuous Mandatory Ventilation); HFOV (High Frequency Ventilation/oscillation); CVVH (Continuous Veno-Venous Hemofiltration); OR (Odds Ratio); PERC (percutaneous cannulation)
Within the cohort of patients on VV ECMO, the only factors associated with decreasing odds of discharge alive was increasing time on ECMO (days) with increased offs of discharge alive for cannulation in 2011 compared to 2007. For patients on VA ECMO, however, Asian and Hispanic race (as compared to White) and increasing days on ECMO were associated with decreased odds of discharge alive. In contrast, age 1 – 3 years, compared to age < 1 year, was associated with increased odds of discharge alive (aOR 2.98, 95% C.I. 1.18–2.94, p = 0.04) (Table 5).
Discussion
Extracorporeal assist systems are increasingly used for the treatment of severe cardiopulmonary failure in patients of all ages. In adults, the advent of percutaneous cannulation has contributed to a resurgence in ECMO use for respiratory failure and thus linked to decreased complication rates (17). While small reports have described experiences with PERC access for ECMO in pediatrics, these are often limited to case series or VV ECMO only (18). This paper addresses an increasing trend towards percutaneous cannulation for ECMO in pediatrics and highlights several differences in patient characteristics based on the method of cannulation.
The traditional or open surgical approach to ECMO is the standard of care in pediatric patients where cannula size has limited the adoption of percutaneous approaches. Percutaneous access, developed in part to address high complication rates, offered a faster means of cannulation that is readily performed by intensivists or ECMO providers other than surgeons, thus increasing its accessibility (19) (17). In parallel to increased use of percutaneous cannulation, there has been a rise in the use of VV ECMO in respiratory failure further supporting the importance of readily available, easy, and safe cannulation techniques (20, 21). The rise in use of ECMO for respiratory support is particularly relevant in pediatrics with 102% increase in annual number of runs for patients between 28 days and 18 years from 2007 to 2015 (16).
Differences in the characteristics of patients undergoing PERC and open cannulation were observed in this study with patients in the PERC access group generally larger and older (i.e. > 12 years of age) and patients under 1 year of age more likely to undergo OPEN cannulation. While circuits are becoming smaller and amenable for transport, there is still a limited arsenal of cannulae available for ECMO cannulation making these results not surprising. For example, the most frequently used cannula sizes for percutaneous access in adults (17 Fr arterial cannula and 21 Fr venous cannula), are much too large for most pediatric patients (22, 23). In addition to the use of new cannula technology, percutaneous cannulation in older or adult patients is frequently performed through femoral access while the internal jugular vein and common carotid artery remain the preferred cannulation sites in neonates and children weighing less than 15 kg (24). In support of this, we identified higher rates of femoral access in PERC patients despite the right internal jugular vein and right common carotid artery accessed most frequently overall.
ECMO complications reported in the ELSO registry were divided into several categories for evaluation where mechanical failure secondary to blood clots and cardiovascular complications, for example, were more common in the OPEN group. In contrast, limb complications were more common in the PERC group overall but the differences disappeared on subset analysis of patients on VV ECMO. When further divided by access site, there was no significant differences in incidence of limb or neurologic complications. These results should be interpreted with caution as data on vessel patency following decannulation was not available from the registry. Early experiences with techniques for managing distal limb ischemia, such as the insertion of distal perfusion catheters with PERC or OPEN access in pediatrics, demonstrate feasibility but have not demonstrated definitive improvement in clinical outcomes (25, 26). More granular data, as may be obtained through multicenter retrospective reviews outside of registry data, is needed for detailed analyses of the use of distal perfusion techniques and more importantly, for evaluation of vessel patency and usability following decannulation (27, 28).
Thrombotic and thromboembolic complications during ECMO occur secondary to several mechanisms including the exposure of blood to foreign, non-endothelial materials, high flow rates and shear stress resulting in red blood cell hemolysis, as well as increased plasma viscosity due to protein denaturation while on ECMO (29–31). In this study, mechanical complications secondary to blood clots were more common in patients undergoing OPEN rather than PERC access. Unfortunately this study was unable to differentiate patients by factors known to influence mechanical complications such as anticoagulation and comorbidities. Cardiac complications, including cardiac wall perforation and tamponade, which are described as both real and theoretical risks in single center studies, were not significantly different between percutaneous and open access groups, regardless of ECMO support mode (10, 32). Need for inotrope support, presence of cardiac arrhythmia, hypertension requiring vasodilators, and hemodynamically significant patent ductus arteriosus, were more common in patients on VA ECMO but given the small sample size for PERC patients on VA ECMO, further analysis including access site and type of cannulation could not be performed. The increased occurrence of these complications in patients with open access for the whole cohort may be related to blood loss or transfusion requirements, though further data is needed to identify causative factors (30).
In contrast to thrombotic complications which are associated with immediate and long term risk with either access type, initial experience with percutaneous access aimed to demonstrate decreased rates of bleeding complications often observed during open cannulation (33). In our study, evaluation of the VV ECMO subgroup demonstrated that cannula site bleeding was more common for patients undergoing an open technique. Clinical relevance of these bleeds, however, could not be established without data on transfusion requirements. Along similar lines, increased rates of cannulation site bleeding and performance of cannula site repair were identified in the open cannulation group, and was likely due to the larger incisions made at time of cannulation which placed surrounding structures at risk of injury and in need of vascular repair (34). Further research is needed to compare practices, but these findings are consistent with the present study demonstrating decreased cannulation site complications.
Finally, mortality was compared between the PERC and OPEN groups through multivariate logistic regression in order to identify factors independently associated with increased mortality. Method of access did not impact the odds of being discharged alive despite a significant difference in mortality on univariate analysis (36.3% and 42.4%, respectively). The only factor consistently associated with a significant decrease in the odds of discharge alive was increased duration of time on ECMO supporting the notion that sicker patients, or patients requiring more time on ECMO, are less likely to survive their hospitalization (35). Factors such as racial differences associated with decreased odds of discharge alive on VA ECMO warrant further investigation into the potential role of socioeconomic and demographic variables in outcomes for critically ill neonatal patients. Though it did not retain significance on multivariate regression, the higher mortality in patients undergoing open cannulation is likely associated with the inherent differences in patients in the two groups.
Limitations
There are inherent limitations to this study as it is a retrospective review of a large database. The study is limited by the accuracy and completeness of data reported to the ELSO registry – which is a voluntary registry – as well as the ability to extract only de-identified data. Data related to centers where cannulation was performed and transport of patients cannulated by a traveling ECMO teams are not available, thus limiting the ability to evaluate the effect of center or physician specific characteristics on outcome. More specifically, the inability to identify center where ECMO was performed limited our ability to consider within-center correlation of the patients and evaluation of percutaneous cannulation adoption over time versus annual center volume and outcomes, a topic of increasing interest in contemporary literature. Additionally, no long-term outcome data are available in the ELSO registry; these would provide valuable insight into common complications of ECMO cannulation such as neurodevelopmental outcomes and vessel patency following decannulation.
As a voluntary registry we are required to accept the data entered as true and reliable. One area of particular difficulty is in determination of mode of ECMO. While a variable does exist in the database for specification, it does not easily allow for identification of patients who were transitioned from one mode to another. It has been proposed that cannulas be used to obtain a more accurate picture of VV versus VA-ECMO but this was not felt to be necessary in this study. Detailed analysis of cannula type was not performed given the inability to account for confounding variables such as institution or provider preference and experience which may affect cannula choice.
Conclusions
The proportion of pediatric patients undergoing percutaneous ECMO cannulation is increasing. Variable rates of mechanical and physiological complications were observed between the two methods of cannulation including higher rates of mechanical cannula failure and renal or cardiovascular complications with open access and higher rates of limb complications with percutaneous access prior to subgroup analysis. Further research is needed to evaluate for any causative relationship between outcomes and method of cannulation with stratification for severity of illness and other patient related factors not currently available through the registry.
Supplementary Material
Study population selection
Initial ventilator settings and laboratory values by support mode and cannulation type
Complications on VV ECMO by access site
Acknowledgements:
The following members of the American Pediatric Surgical Association Critical Care Committee are non-author contributors.
Funding Source: Statistical support was made possible through the grant award: NIH 1UL1TR001412–01 Buffalo Clinical and Translational Research Center.
Copyright form disclosure: Dr. Arbuthnot disclosed government work (surgical resident at a military residency program). Dr. Ricca disclosed government work (military service member). Dr. Yu�s institution received funding from Clinical and Translational Science Institute, and he received support for article research from the National Institutes of Health. The remaining authors have disclosed that they do not have any other conflicts.
Abbreviations:
- ECMO
extracorporeal membrane oxygenation
- ELSO
Extracorporeal Life Support Organization
- APSA
American Pediatric Surgical Association
Footnotes
Financial Disclosure: The authors have no potential, perceived, or real conflict of interest nor financial relationships relevant to this article to disclose.
References:
- 1.Maslach-Hubbard A, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: History, development and current status. World J Crit Care Med. 2013;2(4):29–39. Epub 2013/11/04. doi: 10.5492/wjccm.v2.i4.29. PubMed PMID: ; PubMed Central PMCID: PMCPMC3953872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghodsizad A, Koerner MM, Brehm CE, El-Banayosy A. The role of extracorporeal membrane oxygenation circulatory support in the ‘crash and burn’ patient: from implantation to weaning. Curr Opin Cardiol. 2014;29(3):275–80. doi: 10.1097/HCO.0000000000000061. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 3.Kar S Percutaneous Mechanical Circulatory Support Devices for High-Risk Percutaneous Coronary Intervention. Curr Cardiol Rep. 2018;20(1):2. Epub 2018/01/19. doi: 10.1007/s11886-018-0946-2. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 4.Sandoval Y, Burke MN, Lobo AS, Lips DL, Seto AH, Chavez I, et al. Contemporary Arterial Access in the Cardiac Catheterization Laboratory. JACC Cardiovasc Interv. 2017;10(22):2233–41. doi: 10.1016/j.jcin.2017.08.058. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 5.Ternus BW, Jentzer JC, El Sabbagh A, Eleid MF, Bell MR, Murphy JG, et al. Percutaneous Mechanical Circulatory Support for Cardiac Disease: Temporal Trends in Use and Complications Between 2009 and 2015. J Invasive Cardiol. 2017;29(9):309–13. Epub 2017/07/15. PubMed PMID: . [PubMed] [Google Scholar]
- 6.Matsubayashi T, Miwa Y, Takeda S, Koide M, Enoki H, Mizukami A, et al. Percutaneous cardiopulmonary support in a child with enterovirus 71 encephalitis. Pediatr Int. 2006;48(3):327–9. doi: 10.1111/j.1442-200X.2006.02213.x. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 7.Verma S, Burkhoff D, O’Neill WW. Avoiding hemodynamic collapse during high-risk percutaneous coronary intervention: Advanced hemodynamics of impella support. Catheter Cardiovasc Interv. 2017;89(4):672–5. Epub 2016/09/23. doi: 10.1002/ccd.26795. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 8.Spratt JR, Raveendran G, Liao K, John R. Novel percutaneous mechanical circulatory support devices and their expanding applications. Expert Rev Cardiovasc Ther. 2016;14(10):1133–50. Epub 2016/08/02. doi: 10.1080/14779072.2016.1214573. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 9.Abnousi F, Yong CM, Fearon W, Banerjee D. The evolution of temporary percutaneous mechanical circulatory support devices: a review of the options and evidence in cardiogenic shock. Curr Cardiol Rep. 2015;17(6):40. doi: 10.1007/s11886-015-0594-8. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 10.Burns J, Cooper E, Salt G, Gillon S, Camporota L, Daly K, et al. Retrospective Observational Review of Percutaneous Cannulation for Extracorporeal Membrane Oxygenation. ASAIO J. 2016;62(3):325–8. doi: 10.1097/MAT.0000000000000339. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 11.Moscatelli A, Buratti S, Gregoretti C, Lampugnani E, Salvati P, Marasini M, et al. Emergency percutaneous, bicaval double-lumen, ECMO cannulation in neonates and infants: insights from three consecutive cases. Int J Artif Organs. 2015;38(9):517–21. Epub 2015/09/29. doi: 10.5301/ijao.5000432. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 12.Kurkluoglu M, Hynes CF, Alfares FA, El-Sayed Ahmed MM, Peer SM, Zurakowski D, et al. Choice of Peripheral Venoarterial Extra-Corporeal Membrane Oxygenation Cannulation Site in Patients Above 15 kilograms. J Card Surg. 2015;30(5):461–5. Epub 2015/03/19. doi: 10.1111/jocs.12538. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 13.Inoue Y, Kaneko H, Yoshizawa Y, Morikawa A. Rescue of a child with fulminant myocarditis using percutaneous cardiopulmonary support. Pediatr Cardiol. 2000;21(2):158–60. doi: 10.1007/s002469910026. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 14.Extracorporeal Life Support Organization. Extracorporeal life support organization- ECMO and ECLS > registry. 2017. [10 May 2017]. Available from: https://www.elso.org/Registry.aspx.
- 15.Barbaro RP, Paden ML, Guner YS, Raman L, Ryerson LM, Alexander P, et al. Pediatric Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017;63(4):456–63. doi: 10.1097/MAT.0000000000000603. PubMed PMID: ; PubMed Central PMCID: PMCPMC5626007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thiagarajan RR, Barbaro RP, Rycus PT, Mcmullan DM, Conrad SA, Fortenberry JD, et al. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017;63(1):60–7. doi: 10.1097/MAT.0000000000000475. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 17.Conrad SA, Grier LR, Scott LK, Green R, Jordan M. Percutaneous cannulation for extracorporeal membrane oxygenation by intensivists: a retrospective single-institution case series. Crit Care Med. 2015;43(5):1010–5. doi: 10.1097/CCM.0000000000000883. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 18.Foley DS, Swaniker F, Pranikoff T, Bartlett RH, Hirschl RB. Percutaneous cannulation for pediatric venovenous extracorporeal life support. J Pediatr Surg. 2000;35(6):943–7. doi: 10.1053/jpsu.2000.6933. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 19.Roussel A, Al-Attar N, Alkhoder S, Radu C, Raffoul R, Alshammari M, et al. Outcomes of percutaneous femoral cannulation for venoarterial extracorporeal membrane oxygenation support. Eur Heart J Acute Cardiovasc Care. 2012;1(2):111–4. doi: 10.1177/2048872612449417. PubMed PMID: ; PubMed Central PMCID: PMCPMC3760531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ham PB, Hwang B, Wise LJ, Walters KC, Pipkin WL, Howell CG, et al. Venovenous Extracorporeal Membrane Oxygenation in Pediatric Respiratory Failure. Am Surg. 2016;82(9):787–8. PubMed PMID: . [PubMed] [Google Scholar]
- 21.Fan E, Gattinoni L, Combes A, Schmidt M, Peek G, Brodie D, et al. Venovenous extracorporeal membrane oxygenation for acute respiratory failure : A clinical review from an international group of experts. Intensive Care Med. 2016;42(5):712–24. Epub 2016/03/23. doi: 10.1007/s00134-016-4314-7. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 22.Ganslmeier P, Philipp A, Rupprecht L, Diez C, Arlt M, Mueller T, et al. Percutaneous cannulation for extracorporeal life support. Thorac Cardiovasc Surg. 2011;59(2):103–7. Epub 2011/03/07. doi: 10.1055/s-0030-1250635. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 23.Takayama H, Landes E, Truby L, Fujita K, Kirtane AJ, Mongero L, et al. Feasibility of smaller arterial cannulas in venoarterial extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 2015;149(5):1428–33. Epub 2015/02/07. doi: 10.1016/j.jtcvs.2015.01.042. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 24.Sabik JF, Lytle BW, McCarthy PM, Cosgrove DM. Axillary artery: an alternative site of arterial cannulation for patients with extensive aortic and peripheral vascular disease. J Thorac Cardiovasc Surg. 1995;109(5):885–90; discussion 90-1. doi: 10.1016/S0022-5223(95)70312-8. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 25.Schad CA, Fallon BP, Monteagudo J, Okochi S, Cheung EW, Morrissey NJ, et al. Routine Use of Distal Arterial Perfusion in Pediatric Femoral Venoarterial Extracorporeal Membrane Oxygenation. Artif Organs. 2017;41(1):11–6. doi: 10.1111/aor.12861. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 26.Yeo HJ, Yoon SH, Jeon D, Kim YS, Cho WH, Kim D, et al. The Utility of Preemptive Distal Perfusion Cannulation During Peripheral Venoarterial Extracorporeal Membrane Oxygenation Support. J Interv Cardiol. 2016;29(4):431–6. Epub 2016/06/21. doi: 10.1111/joic.12309. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 27.Kurkluoglu M, Badia S, Peer SM, Jonas R, Shankar V, Sinha P. Patency of common carotid artery and internal jugular vein after a simple vessel sparing cannulation for extracorporeal membrane oxygenation support. J Pediatr Surg. 2017;52(11):1806–9. Epub 2017/08/08. doi: 10.1016/j.jpedsurg.2017.08.001. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 28.Duggan EM, Maitre N, Zhai A, Krishnamoorthi H, Voskresensky I, Hardison D, et al. Neonatal carotid repair at ECMO decannulation: patency rates and early neurologic outcomes. J Pediatr Surg. 2015;50(1):64–8. Epub 2014/12/07. doi: 10.1016/j.jpedsurg.2014.10.029. PubMed PMID: ; PubMed Central PMCID: PMCPMC5285515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho HJ, Kim DW, Kim GS, Jeong IS. Anticoagulation Therapy during Extracorporeal Membrane Oxygenator Support in Pediatric Patients. Chonnam Med J. 2017;53(2):110–7. Epub 2017/05/25. doi: 10.4068/cmj.2017.53.2.110. PubMed PMID: ; PubMed Central PMCID: PMCPMC5457945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Werho DK, Pasquali SK, Yu S, Donohue J, Annich GM, Thiagarajan RR, et al. Hemorrhagic complications in pediatric cardiac patients on extracorporeal membrane oxygenation: an analysis of the Extracorporeal Life Support Organization Registry. Pediatr Crit Care Med. 2015;16(3):276–88. doi: 10.1097/PCC.0000000000000345. PubMed PMID: ; PubMed Central PMCID: PMCPMC4668708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalton HJ, Reeder R, Garcia-Filion P, Holubkov R, Berg RA, Zuppa A, et al. Factors Associated with Bleeding and Thrombosis in Children Receiving Extracorporeal Membrane Oxygenation. Am J Respir Crit Care Med. 2017;196(6):762–71. doi: 10.1164/rccm.201609-1945OC. PubMed PMID: ; PubMed Central PMCID: PMCPMC5620676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Speggiorin S, Robinson SG, Harvey C, Westrope C, Faulkner GM, Kirkland P, et al. Experience with the Avalon® bicaval double-lumen veno-venous cannula for neonatal respiratory ECMO. Perfusion. 2015;30(3):250–4. Epub 2014/06/27. doi: 10.1177/0267659114540020. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 33.Lamb KM, Hirose H, Cavarocchi NC. Preparation and technical considerations for percutaneous cannulation for veno-arterial extracorporeal membrane oxygenation. J Card Surg. 2013;28(2):190–2. Epub 2013/02/05. doi: 10.1111/jocs.12058. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 34.Sarioglu A, McGahren ED, Rodgers BM. Effects of carotid artery repair following neonatal extracorporeal membrane oxygenation. Pediatr Surg Int. 2000;16(1–2):15–8. PubMed PMID: [DOI] [PubMed] [Google Scholar]
- 35.Bokman CL, Tashiro J, Perez EA, Lasko DS, Sola JE. Determinants of survival and resource utilization for pediatric extracorporeal membrane oxygenation in the United States 1997–2009. J Pediatr Surg. 2015;50(5):809–14. Epub 2015/02/19. doi: 10.1016/j.jpedsurg.2015.02.042. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study population selection
Initial ventilator settings and laboratory values by support mode and cannulation type
Complications on VV ECMO by access site


