Abstract
Purpose:
Investigate the safety of accelerated hypofractionated radiotherapy (AHRT) with concurrent chemotherapy (CT) for inoperable stage III non-small-cell lung cancer (NSCLC).
Patients and Methods:
The primary objectives were to define the maximally tolerable course of accelerated radiotherapy and describe toxicities of therapy. Total radiotherapy remained at 60 Gy. The number of once daily fractions in each successive cohort was reduced. Cohort 1: 60 Gy 27 fx, Cohort 2: 60 Gy 24 fx, Cohort 3: 60 Gy 22 fx, and Cohort 4 was 60 Gy 20 fx. Concurrent: weekly carboplatin AUC 2, paclitaxel 45 mg /m2. Consolidation: carboplatin AUC 6, paclitaxel 200 mg/m2 q3 weeks × 2 cycles. MTD: Of 6 pts/cohort, ≤ 2 pts develop ≥ grade 3 toxicity and ≤1 pt develops ≥ grade 4 toxicity.
Results:
22 patients were accrued, of which 21 patients were evaluable between July 2012 and May 2014. Grade 5 toxicity occurred in 3 patients (1 pt cohort 2 (hemoptysis), 2 pts cohort 3 (hemoptysis, pneumonitis)). The MTD was defined by Cohort 2 (60Gy in 2.5 Gy/fx). Time to grade 5 toxicity: 9 months, 6 months, and 9 months after starting treatment. Median follow-up was 23.0 (7.6-30.6) months (living patients), median overall survival 19.3 (95% CI: 9.3-34.0) months, median PFS 12.2 months (95% CI 6.1-22.5m).
Conclusion:
Only modest hypofractionation was achievable due to long-term toxicities. Nevertheless, the MTD of 60 Gy given at 2.5 Gy/fx allows completion of RT in 20% fewer treatments than conventional therapy. Further investigation of AHRT may help to better define the therapeutic index.
Keywords: clinical trial, chemoradiotherapy, hypofractionation, novel radiotherapy
Summary:
CALGB 31102 was a limited institution Phase I trial to establish a maximum tolerated dose of accelerated hypofractionated radiotherapy given concurrent with carboplatin/taxol chemotherapy in stage 3 NSCLC. The radiotherapy total dose was held constant at 60 Gy. The daily fractionation was escalated from 2.22 Gy per fraction over four planned cohorts of 6 patients to a maximum of 3.0 Gy per fraction. The MTD was reached and defined as 2.5 Gy per fraction.
INTRODUCTION:
For patients with unresectable stage IIIA and stage IIIB Non-Small-Cell Lung Cancer (NSCLC) and a good performance status, the current “standard of care” is radiation with concurrent chemotherapy.1 In recent years, much research has focused on which chemotherapeutic or targeted medications to use and how to integrate them with radiation therapy. Given the high rates of systemic disease progression, this has been a rational approach. However, failure of concurrent chemoradiotherapy to control local disease is an all too common problem. By some estimates, local regional failure as the initial site of failure occurs approximately 35-40% of the time and concomitantly with distant failure another 10% of the time.2 The relative contributions of radiation therapy versus chemotherapy to patient survival are still largely unknown, although it is fair to say that curing patients with unresectable lung cancer is not possible without local disease control.
There have been multiple prospective and retrospective experiences reported using hypofractionated radiotherapy for stage 3 lung cancer.3-6 One of the challenges of interpreting the dose escalation data from these hypofractionated trials is that all varied in both the total and daily radiation doses. This is a critical weakness in trial design as it becomes difficult to interpret whether differences in either tumor control or toxicity can be attributed to one factor or the other as both can be contributory. Other studies had simply picked a hypofractionated dose to use largely based on a review of the available literature but these shed little light on what the maximum tolerated dose (MTD) could be.7,8 The Cancer and Leukemia Group B (CALGB) had previously completed a phase I trial in stage I NSCLC using accelerated hypofractionated radiotherapy in which the total dose was held constant but the daily dose was increased, shortening the overall number of fractions.9 Therefore, by building on the techniques of CALGB 39904, we held the total dose of radiotherapy constant at 60 Gy and varied the dose per fraction as well as adding chemotherapy for this stage III population. CALGB 31102 was designed to seek an MTD for hypofractionated radiotherapy with concurrent and consolidative chemotherapy that would suggest enough of a difference from standard fractionation to prompt further study.
PATIENTS AND METHODS:
Patients eligible for study entry were required to have histologic or cytologic proven unresectable Stage IIIA or IIIB NSCLC. Patients who underwent an attempted surgical resection for stage III NSCLC were also eligible if they have residual gross disease visible on post-operative imaging. Patients with supraclavicular or contralateral hilar disease were excluded. If a pleural effusion was visible on both CT imaging and chest x-ray, a thoracentesis was required to confirm that the pleural fluid was cytologically negative. Exudative effusions made a patient ineligible regardless of cytology. Minimum age for eligibility was 18 years and performance status on the Eastern Cooperative Oncology Group (ECOG) scale was 0 or 1. Patients were required to have adequate renal, hepatic, and hematologic organ function. Pulmonary function testing was required including spirometry and diffusion capacity with a threshold FEV1 of 1.2 liters or 50% of predicted required for eligibility.
Staging prior to registration included a CT of the chest including the adrenal glands preferably with contrast unless contraindicated, a PET/CT from the skull base to mid-thigh, and an MRI of the brain, unless contraindicated, in which case a CT with and without contrast of the head was required.
The institutional review boards of participating institutions approved this trial. Patients were required to provide signed informed consent before any study related procedures and enrollment onto this trial. Patient registration and data collection were managed by the CALGB Statistical and Data Center. Statistical analyses were performed by CALGB statisticians. Radiation quality assurance was managed by the Quality Assurance and Review Center (QARC, Lincoln RI). This trial was registered at http://ClinicalTrials.gov (NCT01486602).
Treatment:
Patients were assigned to receive an accelerated course of radiotherapy with concurrent chemotherapy using once-daily fractionation. Treatment was administered on 5 consecutive weekdays. The daily fraction size was increased and the number of fractions reduced, while the nominal total radiotherapy dose was maintained at 60 Gy, resulting in progressive acceleration of the radiotherapy course (Table 1). 3DCRT or IMRT treatment planning was mandated, and each institution was required to submit a 3DCRT and IMRT benchmark to QARC for quality review before entering patients onto the trial. This was a limited institution participation trial based on a center’s track record of participation on CALGB lung cancer trials, radiotherapy quality, and overall data quality.
Table 1.
Radiotherapy cohorts:
| Cohort | Total Dose | Fraction Size | # Fractions | Time |
|---|---|---|---|---|
| 1 | 60.0 Gy | 2.22 Gy | 27 | 5.5 weeks |
| 2 | 60.0 Gy | 2.50 Gy | 24 | 5 weeks |
| 3 | 60.0 Gy | 2.73 Gy | 22 | 4.5 weeks |
| 4 | 60.0 Gy | 3.00 Gy | 20 | 4 weeks |
Custom immobilization was required for treatment planning CT and for daily treatment. X-ray beams with nominal energy between 4 and 15 MV were used. The radiation target was defined on the treatment planning CT. The gross tumor volume (GTV) consisted of the primary lung tumor as defined on the lung windows of the planning CT scan and all lymph nodes >1cm in short diameter and/or lymph nodes on PET/CT imaging with metabolic activity above the mediastinal blood pool and clinically concerning. The clinical target volume (CTV) included the GTV without expansion. An internal target volume (ITV) was defined that included the GTV within volume of space defined by the motion of the respiratory cycle on 4-D CT. This was for both the primary tumor and lymph nodes. Strategies to limit tumor motion with respiratory gating, active breathing control (ABC), or breath hold and monitoring techniques were allowed. The planning target volume (PTV) included a uniform 0.5 cm expansion beyond the ITV. Elective irradiation of radiographically negative lymph nodes was not permitted. IGRT was used for daily set-up and could consistent of cone beam imaging, KV-KV orthogonal imaging matching, or fiducial tracking. Tissue heterogeneity factors were used in the calculations of radiation dose. The radiation dose was prescribed such that 95% of the PTV received 100% of the prescription dose. Normal tissue constraints were specified for the lung, heart, brachial plexus, spinal cord, and chest wall. Lung V20 >35% was a minor deviation and >40% a major deviation while mean lung dose was encouraged to be below 20Gy. The maximum point dose to the heart was 62 Gy and the V100 was required to be <30 Gy. The esophagus was constrained to a maximum of 105% of the prescription dose and <30% of the esophagus receiving >55 Gy. Both the brachial plexus and spinal cord were constrained based on the dose cohort such that on dose level 4 the spinal cord received <40 Gy and the brachial plexus <48 Gy. Central review of treatment plans was mandated at QARC before initiating therapy. Final review of submitted data was performed by the study chair.
Carboplatin and paclitaxel chemotherapy was given both concurrently and with two cycles of consolidation chemotherapy similar to RTOG 0617.10 The chemotherapy schedule was constant regardless of the radiation cohort. During the concurrent therapy paclitaxel 45 mg/m2 and carboplatin area under the curve (AUC) of 2 were given weekly for four weeks, and two cycles of systemic dose chemotherapy with carboplatin AUC of 6 and paclitaxel 200 mg/m2 every 3 weeks were given. The consolidative portion of treatment was scheduled to begin 4 weeks after the completion of radiotherapy and could be delayed up to 4 weeks based on recovery of the patient from the chemoradiotherapy. Standard premedication for hypersensitivity reactions and anti-emetics as well as chemotherapy dose reduction strategies were specified in the protocol.
Response and Toxicity Evaluation
Patients were assessed weekly during therapy including a physical examination and chemoradiotherapy toxicity assessment. During consolidative chemotherapy, patients were assessed on the first day of chemotherapy at each 21-day cycle. Post-treatment follow-up was obtained every 3 months for 2 years and then every 6 months for 3 years starting the interval at the completion of radiotherapy. Pulmonary function testing was performed at 6 months and 1 year following treatment.
During the active accrual period and for 3 months after reaching the MTD, twice monthly conference calls were held with the study leadership and the active sites. These conference calls provided accurate real-time understanding of patient toxicity that was critical to the dose escalation design and the safest determination of the MTD within this multi-institutional study.
Objective response rate (ORR) and progression-free survival were assessed by the investigator using Response Evaluation Criteria for Solid Tumors (RECIST). Toxicities were assessed using National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Statistical Considerations:
The primary objective of CALGB 31102 was to determine the maximum tolerable RT dose fraction for the accelerated hypofractionated radiotherapy with concurrent chemotherapy for Stage III NSCLC. For the purpose of defining the maximum accelerated dose / fraction of the radiotherapy, toxicity related to radiation and toxicity related to chemotherapy was not be distinguished. However, grade 3 hematologic toxicities and grade ≥ 3 hypersensitivity reactions related to the paclitaxel were not considered as “dose limiting”. Also, grade 3 acute esophageal toxicity with concurrent radiotherapy is expected in 25-35% of patients and was not considered dose limiting for purposes of this protocol. These grade 3 exceptions are applicable to the definitions discussed below. Each cohort was designed to treat 6 patients at the cohort specific RT dose level with concurrent chemotherapy.
The maximum RT dose fraction was defined as the highest dose level at which ≤2 patients of the 6 treated develop ≥ grade 3 toxicity and ≤1 patient develops ≥ grade 4 toxicity. If a patient experiences multiple incidences of toxicities in different types, the toxicities of the patient will be only counted once as the maximum grade.
When escalating the dose cohorts, the decision to escalate treatment was dependent on both the outcome in previous cohorts and that observed to date in the current cohort. We used dose escalation rules similar to the prior CALGB 39904 study.9 To maintain robust momentum of accrual, escalation to the subsequent cohort was indicated if either of the following two criteria (a or b) was satisfied:
(a) By the time all 6 patients in the cohort have been treated and at least 3 patients have been followed for more than 3 months, no ≥ grade 3 toxicity has been observed.
(b) By the time all 6 patients in the cohort have been treated and followed for at least 3 months, ≤2 patients develop ≥ grade 3 toxicity and ≤1 patient develop ≥ grade 4 toxicity.
The accrual was to be suspended when both a and b were not satisfied. Additionally, the accrual was to be terminated immediately if at any time ≥3 patients develop ≥ grade 3 toxicity and ≥2 patients develop ≥ grade 4 toxicity in any given cohort. This design was undertaken to try and balance the need for an efficient dose escalation taking into account acute and relatively short-term toxicity but respecting the possibility that long-term toxicity might influence the final determination of an MTD.
Ineligible or in-evaluable patients and patients who withdrew from the study before receiving any treatment were to be excluded from all analyses. Overall survival (OS) was defined as the time from registration to death from any cause. The product limit estimator developed by Kaplan and Meier was used to characterize OS. From the product limit estimates, median OS and 28-month OS as well as the 95% confidence intervals were estimated. Progression-free survival (PFS) was defined as the time from registration to disease progression or death from any cause, whichever comes first. For tumor response, the frequency of best response (CR+PR) to the treatment was tabulated.
Results
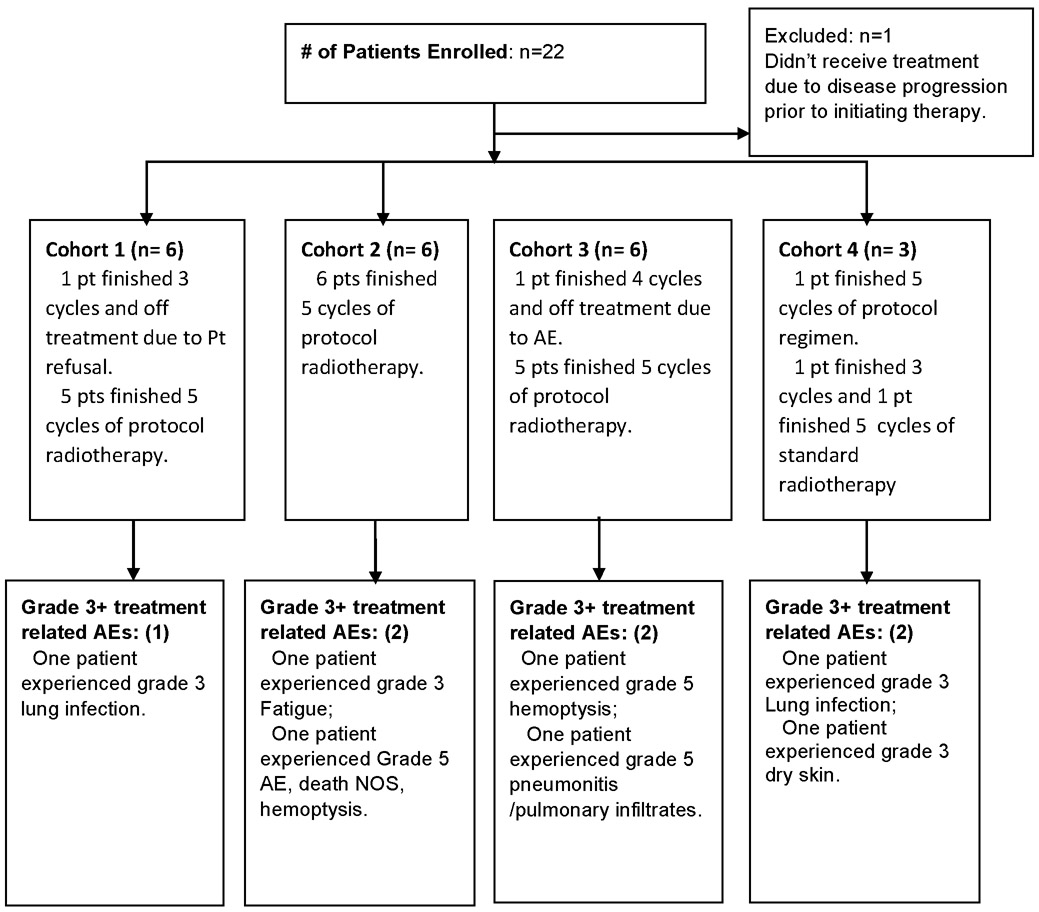
CALGB 31102 accrued twenty-two patients with unresectable stage III NSCLC between July 2012 and May 2014. (Table 2) All patients met the specified eligibility criteria. No patients underwent prior surgical resection with gross residual disease visible on post-operative imaging. Six patients were treated on each of cohorts 1 to 3 and one patient was treated on cohort 4 per protocol. Because the MTD was reached during accrual of cohort 4, we did not complete accrual to that cohort. Following the occurrence of a dose-limiting toxicity at dose level 4 of ≥grade 4, two additional patients assigned to cohort 4 who had not yet initiated study therapy were instead treated at a standard fractionation dose of 60 Gy in 30 fractions. An additional patient had been accrued to cohort 2 but was not treated due to disease progression prior to initiating therapy.
Table 2.
CALGB 31102 CONSORT Flow Diagram
 |
Fifty-seven percent of patients were male (12 of 21) (Table 3). Eighty-one percent of the patients were white (17 of 21) and the median age was 63 years. Squamous cell histology predominated in 57% of patients (12 of 21). Sixty-seven percent of patients were ECOG performance status one (14 of 21).
Table 3.
Patient and treatment characteristics
| Cohort | 1 (N=6) |
2 (N=6) |
3 (N=6) |
4 (N=3) |
Total (N=21) |
|---|---|---|---|---|---|
| Age | |||||
| N | 6 | 6 | 6 | 3 | 21 |
| Mean (SD) | 59.3 (7.2) | 63.0 (7.7) | 63.3 (7.7) | 65.3 (1.2) | 62.4 (6.9) |
| Range | (48.0–69.0) | (51.0–71.0) | (54.0–73.0) | (64.0–66.0) | (48.0–73.0) |
| Race | |||||
| White | 5 (83.3%) | 5 (83.3%) | 5 (83.3%) | 2 (66.7%) | 17 (81.0%) |
| Black or African American | 1 (16.7%) | 0 (0.0%) | 1 (16.7%) | 1 (33.3%) | 3 (14.3%) |
| Native Hawaiian or Pacific Islander | 0 (0.0%) | 1 (16.7%) | 0 (0.0%) | 0 (0.0%) | 1 (4.8%) |
| Gender | |||||
| Male | 4 (66.7%) | 4 (66.7%) | 2 (33.3%) | 2 (66.7%) | 12 (57.1%) |
| Female | 2 (33.3%) | 2 (33.3%) | 4 (66.7%) | 1 (33.3%) | 9 (42.9%) |
| HISTOLOGY | |||||
| Adenocarcinoma | 1 (16.7%) | 2 (33.3%) | 3 (50.0%) | 0 (0.0%) | 6 (28.6%) |
| Squamous | 5 (83.3%) | 4 (66.7%) | 3 (50.0%) | 0 (0.0%) | 12 (57.1%) |
| Undifferentiated NSC | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (66.7%) | 2 (9.5%) |
| Other | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (33.3%) | 1 (4.8%) |
| PS | |||||
| 0 | 1 (16.7%) | 3 (50.0%) | 3 (50.0%) | 0 (0.0%) | 7 (33.3%) |
| 1 | 5 (83.3%) | 3 (50.0%) | 3 (50.0%) | 3 (100.0%) | 14 (66.7%) |
| Stage | |||||
| IIIA | 5 (83.3%) | 3 (50.0%) | 4 (66.7%) | 3 (100.0%) | 15 (71.4%) |
| IIIB | 1 (16.7%) | 3 (50.0%) | 2 (33.3%) | 0 (0.0%) | 6 (28.6%) |
| Tumor Volumes (mean) | |||||
| GTV/ITV | 158.6 cc (10.8 – 437.0) | ||||
| PTV | 340.2 cc (82.9 – 1045.4) | ||||
| Key Mean Normal Tissue Doses (Gy) | |||||
| Mean Lung Dose | 13.73 (8.1 – 18.4) | ||||
| Mean Esophagus Dose | 22.0 (7.8 – 37.3) |
Participation on this study was limited to a core group of CALGB institutions with experience in image guided IMRT for lung cancer and accrual to CALGB trials. Ten institutions were invited to participate with accrual from 6 centers. Each center completed radiotherapy credentialing. All cases were reviewed through the NCI supported Quality Assurance Review Center for protocol compliance at the start of treatment and by the investigator team after the completion of treatment. Protocol deviations were minimal and there was excellent compliance with the target coverage requirements. There were no major protocol deviations in treatment planning. All patients completed the prescribed radiotherapy and 18 (86%) patients completed the consolidative chemotherapy. (Table 4)
Table 4.
Cycles received.
| Cohort | 1 (N=6) |
2 (N=6) |
3 (N=6) |
4 (N=3) |
Total (N=21) |
|---|---|---|---|---|---|
| # of cycle completed | |||||
| 2 cycles | 1 (16.7%) | 0 (0.0%) | 0 (0.0%) | 1 (33.3%) | 2 (9.5%) |
| 4 cycles | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) | 0 (0.0%) | 1 (4.8%) |
| 5 cycles | 5 (83.3%) | 6 (100.0%) | 5 (83.3%) | 2 (66.7%) | 18 (85.7%) |
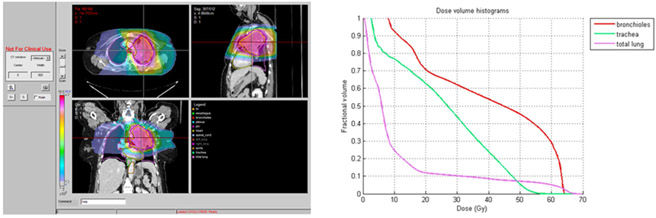
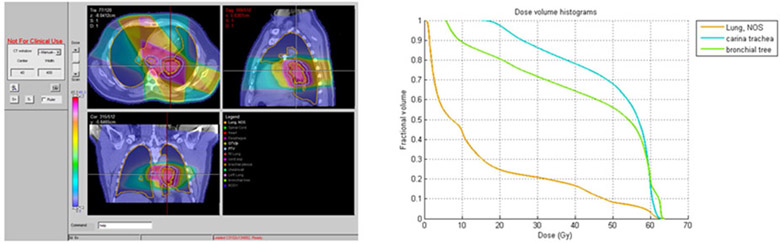
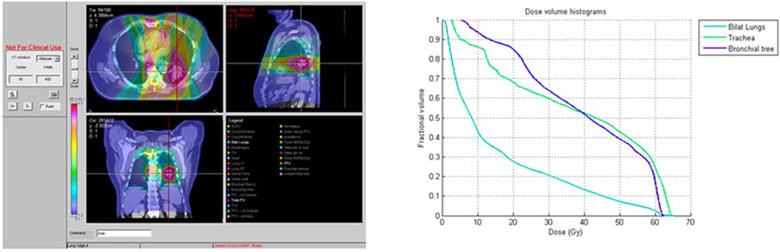
Five of 19 (26%) patients who received accelerated radiotherapy experienced grade 3 or higher treatment related toxicity. Of these, two patients had grade 3 toxicities attributed to protocol treatment by the investigator assessment. Three cases of grade 5 toxicity were observed: One case of fatal hemoptysis occurred at 9 months after starting treatment on cohort 2. On cohort 3; 9 and 6 months after starting treatment cases of fatal hemoptysis and pneumonitis occurred. Figures 1,2, and 3 show the representative planning images and DVHs for each of the patients with grade 5 toxicity. Based on the toxicity reported on cohort 3, the maximally tolerated dose was reached and determined to be 60 Gy in 2.5 Gy/fx which was cohort 2. This represents a 25% increase in the daily dose of radiotherapy.
Figure 1.
Patient on Cohort 2 with Grade 5 pulmonary hemorrhage
Figure 2.
Patient on Cohort 3 with Grade 5 pulmonary hemorrhage
Figure 3.
Patient on Cohort 3 with Grade 5 pneumonitis
After a median follow-up of 23.0 (range 7.6-30.6) months for living patients, local progression occurred in 24% of patients and distant progression occurred in 29% of patients as well. Local failure occurred within the PTV. There were no isolated regional nodal failures outside the PTV. (Table 5) Most patients (76.2%) had a partial response or stable disease as their best response to therapy and only 2 patients (9.5%) had progression as their best recorded response. (Table 6) The median progression free survival was 12.2 months (95% CI=6.1-22.5m). The median overall survival was 19.3 months (95% CI=9.3-34.0) and the one year overall survival rate was 50% (95% CI=29-74%).
Table 5.
Progression data summary
| Relapse type | Total # of patient |
# of progress patients |
# of this type progression |
% on total # patients (/21) |
% on progressed patients (/10) |
|---|---|---|---|---|---|
| Local* | 21 | 10 | 5 | 24% | 50% |
| Distant* | 21 | 10 | 6 | 29% | 60% |
There were total of 10 patients had PD, of which 1 patient had both local and distant progression. Distant sites include contra lateral lung, bone, CNS, spine metastasis.
Table 6.
Response data summary
| Cohort | 1 (N=6) |
2 (N=6) |
3 (N=6) |
4 (N=3) |
Total (N=21) |
|---|---|---|---|---|---|
| Best Response | |||||
| Complete Response | 0 (0.0%) | 1 (16.7%) | 2 (33.3%) | 0 (0.0%) | 3 (14.3%) |
| Partial Response | 4 (66.7%) | 3 (50.0%) | 4 (66.7%) | 0 (0.0%) | 11 (52.4%) |
| Stable | 1 (16.7%) | 1 (16.7%) | 0 (0.0%) | 3 (100.0%) | 5 (23.8%) |
| Progression | 1 (16.7%) | 1 (16.7%) | 0 (0.0%) | 0 (0.0%) | 2 (9.5%) |
| Response | |||||
| No response | 2 (33.3%) | 2 (33.3%) | 0 (0.0%) | 3 (100.0%) | 7 (33.3%) |
| CR or PR | 4 (66.7%) | 4 (66.7%) | 6 (100.0%) | 0 (0.0%) | 14 (66.7%) |
DISCUSSION
CALGB 31102 met its’ pre-specified goal of determining a maximally tolerated dose of accelerated hypofractionated radiotherapy combined with concurrent and consolidative chemotherapy. Based on our review of the literature in designing this study we suspected this dose to be between 2.25 and 3.5 Gy per fraction when treating to a total dose of 60 Gy. On cohort 3, we had grade 5 toxicity in 2 patients. Based on our trial design, this establishes 60 Gy in 24 fractions at 2.5 Gy/fx as the maximally tolerated dose with concurrent and consolidative chemotherapy. Dosimetry for each of these cases was extensively reviewed and none of the protocol specific dose volume limitations were violated. As would be expected in stage III lung cancer, the hilum and mediastinum were included in the treatment fields.
We suggest that results of this Phase I trial are particularly robust due to the stringent quality assurance and safety monitoring. Each patient’s radiotherapy plan was submitted to the NCI supported Quality Assurance Review Center for pre-treatment review of the plan with feedback provided to the treating physician. Stringent adherence to radiotherapy protocol guidelines has been shown to relate to outcome.11 Additionally, all treating institutions participated in a twice monthly conference call with the Study Chair and key personnel. The purpose of this call was to maintain tight control of patient safety data in as near real-time a method as possible. Each patient was “at risk” for toxicity analysis throughout the entire study accrual period and for several months thereafter to facilitate capturing any late radiotherapy event. If the study had been designed to only consider more acute toxicity it is likely that these events would have been missed and dose escalation would have continued. We believe that late toxicity events are often under-reported in the literature particularly as they relate to hypofractionation. Our twice monthly conference call was particularly helpful as we began to see toxicity in cohorts 2 and 3 several months after treatment. As these patient events occurred, stringent reviews about the safety of therapy took place with appropriate attribution of toxicity based on the team’s consensus. This hopefully helped to eliminate any potential individual or investigator bias. The challenge with attribution of grade 5 toxicity was particularly difficult in the two cases of hemoptysis. It is conceivable that these fatal events were related to tumor progression but our conservative review of the treatment of these patients suggests that the treatment itself could be responsible. As a side benefit, we were able to hold and adjust the treatment in near real time of the last two enrolled patients enabling us to avoid overtreatment of any patients beyond our known toxicity envelope. In addition to management of the maximally tolerated dose, we also utilized the conference call to address specific study related eligibility and planning questions. In our opinion, these frequent discussions helped ensure a high degree of protocol compliance.
There are multiple challenges in interpreting the dose escalation data from the reported hypofractionated trials and institutional series. As we outlined, all of them either varied both the total dose of radiation therapy and the daily dose administered, tested only one dose level, or did not combine radiotherapy with concurrent chemotherapy.3-8 These are all critical weakness in trial design as it becomes impossible to interpret whether differences in either tumor control or toxicity can be attributed to one factor or the other as clearly any can be contributory. Therefore, our technique holding the total dose radiotherapy constant and varying the dose per fraction will at least isolate the dose per fraction question.
Relatively early experiences are catalogued in the RTOG 8312 and the EORTC experiences of 08912 and 08972-22973. Each of these used 2.75 Gy/fx targeted at gross disease with large elective fields.5,6,12 More recently, the Korean Radiation Oncology Group in a 49 patient Phase II study employed a concomitant boost technique with concurrent carboplatin/taxol where the gross tumor volume received 60 Gy in 25 fractions and the planning volume receiving 45 Gy over that same treatment period.7 The median survival was 28.1 months. This represents one of the best results for survival reported in the literature for this patient population. Similar results were obtained in a randomized Phase II trial including 130 patients randomized to induction versus concurrent chemotherapy using cisplatin and vinorelbine and a consistent radiotherapy program of 55 Gy in 20 fractions (2.75 Gy/fx).8 Median survival for the concurrent arm was 27.6 months and 18.8 months for the sequential arm. Grade 4 esophagitis did not occur on the study. A small Phase I study was reported in which 3 Gy per fraction was given with concurrent chemotherapy.13 They determined the MTD was 69 Gy at 3 Gy per fraction with no treatment related deaths reported. Another experience published in 2013, used a dose of 60 Gy in 3 Gy daily fractions for stage III NSCLC.14 Toxicity was modest in their series with only 3 patients developing grade 3 late adverse effects. The 2 year OS and PFS were 38% and 36%, respectively.
Of particular relevance to our study because they reached a nearly identical maximally tolerated dose, Cannon and colleagues at the University of Wisconsin reported a 79 patient Phase I trial of hypofractionated radiotherapy without chemotherapy for stage III NSCLC.4 They escalated the dose based on an estimated risk of radiation pneumonitis. They varied both the dose per fraction as well as the total dose of radiation given. Based on their schema, they concluded that the MTD was 63.25 Gy in 25 fractions at 2.53 Gy per fraction. Four (2%) cases of grade 4-5 toxicity occurred greater than 6 months after treatment and all of these patients received a total dose of at least 75 Gy.
Taken in context, the results outlined above in general suggest a trend to improving outcomes as patients moved from the earliest experiences with hypofractionated lung radiotherapy in the 1980’s to the more contemporary reports.
CALGB 31102 utilized advanced radiotherapy techniques including 4D imaging to take into account target motion, image guidance (IGRT) at the linear accelerator to ensure accurate delivery, and IMRT. We utilized as minimal a series of expansions from the gross tumor volume as possible to help minimize the treated volume. We accomplished this by eliminating any CTV expansion beyond the ITV which only provides a motion envelope of the GTV. This technique has been clinically used and recently described in a large single institution report with rare failure in the a CTV expansion region.15 Tightening dose constraints was explored by Kelsey et al in a study of accelerated radiotherapy using a 6 fraction per week regimen and with tight constraints around mediastinal structures. They did use a narrowing CTV expansion (3mm in mediastinum) and only a 3 mm PTV, thus only a 1mm increased total expansion compared to 31102. Although they achieved their Phase I goals, survival was not different than most other Stage 3 experiences.16 This paradigm is being additionally tested in the current photon versus proton trial (RTOG 1308) in stage III disease which hypothesizes that the improved normal tissue sparing of proton therapy will allow for higher total doses and less toxicity than conventional x-ray based therapy resulting in improved survival.17
The shortened course of therapy may also allow for increased compliance with systemic therapies. On this trial we had 86% of patients complete the consolidative cycles of chemotherapy. Although our sample size is small, this is better than has been seen on other recent studies using consolidative chemotherapy in which 70% of patients completed the consolidative therapy.10,18
We ultimately contend that hypofractionation may have inherent advantages over extending the course of radiotherapy as was tested in RTOG 0617/NCCTGN0628/CALGB 30609. The lack of consistency apparent in other recent papers on hypofractionation for stage III lung cancer points to the need for additional data prior to being able to move forward with a suggested dose for general clinical practice. As a starting point, the relative alignment of our result with the recent results from the Wisconsin group,4 suggests 60 Gy given in 24 fractions is likely a sound dose choices for future investigation.
Conclusion:
Only modest hypofractionation was achievable in a multi-institutional experience combining accelerated hypofractionated radiotherapy with concurrent and consolidative chemotherapy (carboplatin and paclitaxel) for stage III NSCLC. Nevertheless, the MTD of 60 Gy given at 2.5 Gy/fx allows completion of RT in 20% fewer treatments than conventional therapy. Further investigation of AHRT may help define the therapeutic index.
Acknowledgments
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA021060, U10CA041287, U10CA047559, U10CA011789, U10CA180836, and U10CA180838. Radiation quality assurance was supported by National Cancer Institute of the National Institutes of Health under Award Numbers CA29511 for the Quality Assurance Review Center CA180803 for the Imaging and Radiation Oncology Core. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The following institutions participated in this study:
Mayo Clinic LAPS, Rochester, MN, Steven Alberts, U10CA180790; State University of New York Upstate Medical University, Syracuse, NY, Stephen L. Graziano; (U10CA011789); UC San Diego Moores Cancer Center, La Jolla, CA, Barbara A. Parker, (U10CA011789); UNC Lineberger Comprehensive Cancer Center LAPS, Chapel Hill, NC, Thomas C. Shea, U10CA180838 (U10CA047559); University of Chicago Comprehensive Cancer Center LAPS, Chicago, IL, Hedy L. Kindler, U10CA180836; (U10CA041287); and Wake Forest University Health Sciences, Winston-Salem, NC, Heidi Klepin (David D. Hurd), (U10CA003927).
Footnotes
ClinicalTrials.gov Identifier: NCT (NCT01486602)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Bezjak A, Temin S, Franklin G, et al. Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. J Clin Oncol 2015;33(18):2100–2105. [DOI] [PubMed] [Google Scholar]
- 2.Socinski MA, Rosenman JG, Halle J, et al. Dose-escalating conformal thoracic radiation therapy with induction and concurrent carboplatin/paclitaxel in unresectable stage IIIA/B nonsmall cell lung carcinoma: a modified phase I/II trial. Cancer. 2001;92(5):1213–1223. [DOI] [PubMed] [Google Scholar]
- 3.Bral S, Duchateau M, Versmessen H, et al. Toxicity report of a phase 1/2 dose-escalation study in patients with inoperable, locally advanced nonsmall cell lung cancer with helical tomotherapy and concurrent chemotherapy. Cancer. 2010;116(1):241–250. [DOI] [PubMed] [Google Scholar]
- 4.Cannon DM, Mehta MP, Adkison JB, et al. Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol 2013;31(34):4343–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uitterhoeve AL, Belderbos JS, Koolen MG, et al. Toxicity of high-dose radiotherapy combined with daily cisplatin in non-small cell lung cancer: results of the EORTC 08912 phase I/II study. European Organization for Research and Treatment of Cancer. Eur J Cancer. 2000;36(5):592–600. [DOI] [PubMed] [Google Scholar]
- 6.Graham MV, Pajak TE, Herskovic AM, Emami B, Perez CA. Phase I/II study of treatment of locally advanced (T3/T4) non-oat cell lung cancer with concomitant boost radiotherapy by the Radiation Therapy Oncology Group (RTOG 83-12): long-term results. Int J Radiat Oncol Biol Phys 1995;31(4):819–825. [DOI] [PubMed] [Google Scholar]
- 7.Cho KH, Ahn SJ, Pyo HR, et al. A Phase II study of synchronous threedimensional conformal boost to the gross tumor volume for patients with unresectable Stage III non-small-cell lung cancer: results of Korean Radiation Oncology Group 0301 study. Int J Radiat Oncol Biol Phys 2009;74(5):1397–1404. [DOI] [PubMed] [Google Scholar]
- 8.Maguire J, Khan I, McMenemin R, O’Rourke N, McNee S, Kelly V, Peedell C, Snee M. : SOCCAR: A randomised phase II trial comparing sequential versus concurrent chemotherapy and radical hypofractionated radiotherapy in patients with inoperable stage III Non-Small Cell Lung Cancer and good performance status. Eur J Cancer. 2014. November;50(17):2939–49. [DOI] [PubMed] [Google Scholar]
- 9.Bogart JA, Hodgson L, Seagren SL, et al. : Phase I study of accelerated conformal radiotherapy for stage I non-small-cell lung cancer in patients with pulmonary dysfunction: CALGB 39904. J Clin Oncol 28:202–206, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16(2):187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohri N, Shen X, Dicker AP, Doyle LA, Harrison AS, Showalter TN. Radiotherapy protocol deviations and clinical outcomes: a meta-analysis of cooperative group clinical trials. J Natl Cancer Inst 2013;105(6):387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belderbos J, Uitterhoeve L, van ZN, et al. Randomised trial of sequential versus concurrent chemo-radiotherapy in patients with inoperable nonsmall cell lung cancer (EORTC 08972-22973). Eur J Cancer. 2007;43(1):114–121. [DOI] [PubMed] [Google Scholar]
- 13.Lin Q, Liu YE, Ren XC, et al. Dose escalation of accelerated hypofractionated three-dimensional conformal radiotherapy (at 3 Gy/fraction) with concurrent vinorelbine and carboplatin chemotherapy in unresectable stage III non-small-cell lung cancer: a phase I trial. Radiat Oncol 2013;8(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osti MF, Agolli L, Valeriani M, et al. Image guided hypofractionated 3-dimensional radiation therapy in patients with inoperable advanced stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;85(3):e157–163. [DOI] [PubMed] [Google Scholar]
- 15.Kilburn JM, Lucas JT, Soike MH, et al. Is a Clinical Target Volume (CTV) Necessary in the Treatment of Lung Cancer in the Modern Era Combining 4-D Imaging and Image-guided Radiotherapy (IGRT)? Cureus. 2016;8(1):e466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelsey CR, Das S, Gu L, Dunphy FR 3rd, Ready NE, Marks LB. Phase 1 Dose Escalation Study of Accelerated Radiation Therapy With Concurrent Chemotherapy for Locally Advanced Lung Cancer. Int J Radiat Oncol Biol Phys 2015;93(5):997–1004. [DOI] [PubMed] [Google Scholar]
- 17.Giaddui T, Chen W, Yu J, et al. Establishing the feasibility of the dosimetric compliance criteria of RTOG 1308: phase III randomized trial comparing overall survival after photon versus proton radiochemotherapy for inoperable stage II-IIIB NSCLC. Radiat Oncol 2016;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol 2008;26(35):5755–5760. [DOI] [PubMed] [Google Scholar]