Abstract
GABAergic interneurons, which are highly diverse, have long been thought to contribute to the timing of neural activity and the generation and shaping of brain rhythms. GABAergic activity is critical not only for entrainment of oscillatory activity across a neural population, but also for precise regulation of the timing of action potentials and suppression of slow timescale correlations. The diversity of inhibition provides the potential for flexible regulation of patterned activity, but also poses a challenge to identifying the elements of excitatory-inhibitory interactions underlying network engagement. This review highlights the key roles of inhibitory interneurons in spike correlations and brain rhythms, describes several scales on which GABAergic inhibition regulates timing in neural networks, and identifies potential consequences of inhibitory dysfunction.
Keywords: Interneuron, oscillation, parvalbumin, somatostatin, VIP, synchrony
Inhibitory effects on multiple timescales
Inhibitory regulation of neural activity occurs on several distinct but interacting timescales. GABAergic influences on local circuits are constrained by the intrinsic properties of interneurons, which vary across diverse populations. The postsynaptic impact of inhibitory transmission is further sculpted by short- and long-term synaptic dynamics. In particular, synaptic depression and facilitation can rapidly modulate both the excitatory synaptic recruitment of interneurons and their postsynaptic efficacy in regulating spiking in their targets on a millisecond timescale. In turn, the actions of synaptic inhibition in a neural network can have opposing impacts on correlations at fast (>30Hz) and slow (<1Hz) timescales. Although inhibition promotes fast spike synchrony between excitatory neurons, it suppresses slower noise correlations between the firing rates of those neurons, an effect that scales with network size and firing rates. On much slower timescales, integration of interneurons into local circuits is required for proper progression of circuit development. In developmentally immature circuits, interneurons organize large correlated population events. The subsequent shift from depolarizing to hyperpolarizing GABAergic inhibition is associated with a robust change in the overall timing of population activity, with large population events giving way to more decorrelated activity.
Dysregulation of inhibition can lead to loss of temporal organization, exhibited as either too much or too little correlated activity. Substantial disruption of GABAergic inhibition, whether from loss of interneurons or decreased synaptic impact, is associated with hypercorrelation and seizure. Dysfunction of inhibition associated with neurodevelopmental disorders can lead to disruption of oscillations and loss of fine spike synchrony. Despite substantial recent advances, key aspects of inhibitory function remain unclear, including the respective roles of the diverse GABAergic populations in temporal control at each timescale.
The role of synaptic inhibition in regulating network activity has largely been studied in rodents, due to the availability of genetic tools. However, both the diversity of GABAergic interneurons and their participation in temporal patterns of neural activity are also observed in other species, including cats, ferrets, and non-human primates. In this review, I focus on general principles of interneuron connectivity and the circuit-level impact of inhibitory interneurons on spike timing at the single-neuron and population levels in the neocortex and hippocampus. I further examine evidence for the roles of interneurons in brain rhythms, including theta and gamma oscillations, and highlight the consequences of developmental and disease-related dysregulation of interneuron function.
Diverse sources of GABAergic inhibition
One major challenge to identifying the function of GABAergic inhibition is the diversity of inhibitory interneurons, which can be subdivided into distinct classes with different physiology, synaptic targets, and molecular markers [1, 2]. Recent work has focused on three major classes: 1) fast-spiking basket cells that target the cell bodies of excitatory neurons and coexpress the calcium-binding protein parvalbumin (PV), 2) low-threshold spiking cells that target the distal dendrites of excitatory neurons and co-express the peptide somatostatin (SST), and 3) sparse dendrite-targeting cells that synapse on SST interneurons and the dendrites of pyramidal neurons and co-express vasoactive intestinal peptide (VIP). VIP interneurons are a subset of the larger 5HT3aR-expressing interneuron class [3].
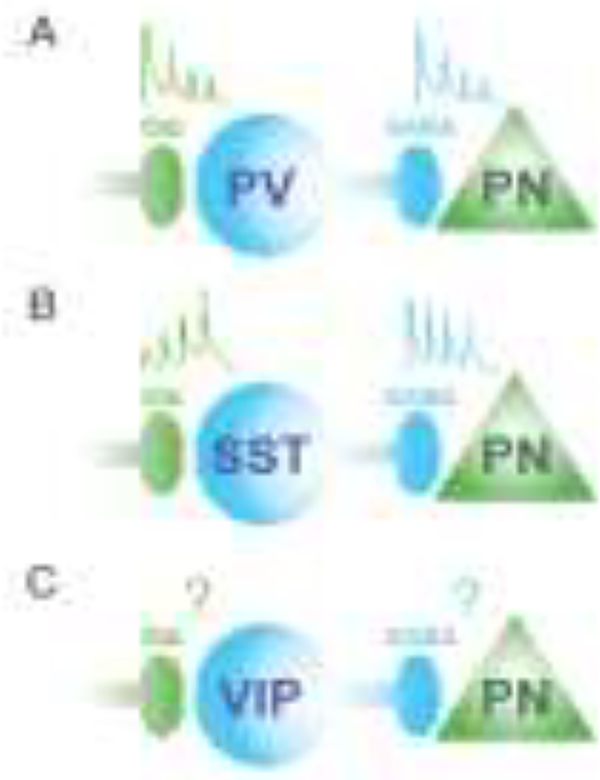
PV cells are the most abundant type of interneuron. They are rapidly activated by afferent inputs [4–8],[9] and are thought to regulate the output of excitatory neurons with millisecond-level precision via strong shunting inhibition at the cell body [10]. In contrast, SST cells require repetitive, facilitating afferent input to be activated and may regulate the dendritic integration of synaptic inputs over a longer time scale (Fig. 1). [11–15] Moreover, synaptic inhibition mediated by PV and SST interneurons exhibits distinct short-term plasticity. Inhibitory post-synaptic potentials (IPSPs) from PV synapses depress rapidly at high rates of activity [16, 17] suggesting that PV inhibition may only be effective within a short window. This brief window of PV efficacy may serve to tightly constrain the temporal precision of the first spike evoked in cortical neurons by sensory input [8, 18, 19], which encodes substantial information [20, 21]. In contrast, SST IPSPs depress only slightly with repeated activation and regulate voltage-dependent calcium signals [22, 23] [24], and may therefore exert a sustained inhibitory influence over dendritic inputs [25, 26]. PV and SST cells are thus expected to exhibit distinct temporal patterns of activity and postsynaptic impact. Indeed, computational modeling of interactions in a circuit with multiple inhibitory cell types suggests a key role for cell type-specific synaptic dynamics in PV and SST regulation of excitatory neuron activity [27]. Although synapses onto VIP interneurons and from VIP cells to their targets are less well studied, a recent report found that VIP synapses onto SST cells showed frequency-dependent facilitation [28], suggesting a potential enhancement of disinhibitory interactions in the local circuit following periods of repeated VIP interneuron activation, such as observed in sensory cortex during bouts of locomotion [29–31].
Figure 1. Diversity in the temporal synaptic properties of GABAergic interneurons.
Inhibitory interneurons exhibit distinct temporal dynamics of both their synaptic inputs and outputs. A. Excitatory synaptic inputs to PV interneurons recruit these cells quickly but rapidly show synaptic depression. In turn, the inhibitory synapses from PV interneurons to excitatory pyramidal neurons (PN) likewise show synaptic depression. B. In contrast, excitatory inputs to SST interneurons require repeated activation and exhibit synaptic facilitation, resulting in delayed recruitment of spiking activity. However, SST synapses onto PNs show little synaptic depression. C. The short-term plasticity of excitatory synapses onto VIP interneurons and from VIP interneurons to PNs remain largely unexplored.
Although considerable research has focused on inhibitory innervation of excitatory neurons, recent work has highlighted inhibitory-to-inhibitory connectivity as a repeated motif in neocortical circuits (Fig. 2). SST interneurons robustly inhibit PV interneurons, potentially providing a tradeoff between somatic and dendritic inhibition [32–34]. In turn, PV interneurons provide reciprocal innervation of SST interneurons [28, 33]. VIP interneurons strongly inhibit SST interneurons [30, 33, 34], potentially disinhibiting both excitatory pyramidal neurons and PV interneurons, and receive reciprocal innervation from the SST interneurons. VIP interneurons also innervate PV interneurons and receive reciprocal inhibition from them [34, 35]. Together, these connections comprise a network of reciprocal inhibitory connections between all three populations. However, interactions between interneuron populations are not always equally weighted in each direction (Fig. 2), and the influence of these interactions on circuit activity remains poorly understood. Furthermore, it remains unclear how the extensive regulation of GABAergic interneuron activity by neuromodulators like acetylcholine, norepinephrine, and serotonin affects the efficacy of these interactions or their contributions to ongoing rhythmic activity.
Figure 2. Reciprocal connectivity between interneuron populations.
Three major subtypes of neocortical inhibitory interneurons are interconnected in a repeated motif of reciprocal inhibition. Relative strength of interactions is shown by the sizes of the circles denoting synaptic connectivity, largely based on current knowledge from in vitro electrophysiology in superficial layers of primary sensory cortex. These reciprocal interactions likely play a key role in the regulation of neural timing by inhibition. PV interneurons are unique in having both strong reciprocal synaptic connectivity with other interneurons and also robustly inhibiting other PV interneurons via chemical and electrical synapses. Strong PV-PV and PV-PN interactions promote fast oscillations and precise spike timing.
E-I interactions and spike timing
Locally recurrent networks in the hippocampus and neocortex show a typical pattern of synaptic recruitment, with feed-forward excitatory input (E) preceding locally recruited inhibition (I). This temporal pattern of E-I interactions allows for a ‘window of opportunity’ in which spikes may be evoked by excitation before further responses are quenched by the following inhibition [8]. The influence of synaptic inhibition recruited by feed-forward inputs into a network temporally restricts sensory-evoked spiking [19], and both the delay between E and I and the relative strength of excitation may shape tuning for sensory inputs [36–38]. The short delay between E and I also promotes the temporal fidelity of spiking, increasing spike timing precision and reliability [19, 36] and enhancing the temporal sensitivity of neurons to convergent inputs [5, 45–48] [18]. Previous work in vitro and in anesthetized animals focused on the role of somatargeted inhibition, arising largely from PV interneurons, in regulating spike timing. However, more recent work in awake behaving animals suggests that soma- and dendrite-targeting interneurons are recruited by sensory inputs at different latencies. Initial spike timing evoked by sensory inputs to excitatory neurons may thus be regulated by PV inhibition, but spiking later in the response period may be more strongly influenced by SST inhibition [39, 40] or delayed VIP inhibition [41].
Intriguingly, individual cell types may have distinct temporal windows of postsynaptic impact on different targets. In one example, recent work found that a population of 5HT3aR-expressing interneurons caused fast GABAAR inhibitory postsynaptic potentials (IPSPs) on target PV interneurons but slower GABAAR/GABABR IPSPs on excitatory neurons, suggesting differential temporal regulation of spiking in downstream excitatory and inhibitory populations [42]. However, it remains unknown how prevalent such differential targeting is across the different GABAergic populations. Although fast PV inhibition has been well characterized, much less is generally known about the role of non-PV interneurons in regulating spike timing. Furthermore, although the patterns and strengths of connections between interneuron populations are well established for some brain areas [33, 34], very little is known about how inhibition regulates spike timing in interneurons in vivo. In addition, because single synapses are difficult to assay in vivo, the impact of short-term synaptic dynamics at inhibitory synapses in active circuits remains largely unknown.
Inhibitory control of brain rhythms
Inhibition plays key roles in the generation of oscillations in the neocortex and hippocampus, as well as other brain areas. Gamma-band activity (30–80Hz) relies on fast inhibitory synaptic transmission by GABAergic interneurons [43]. Optogenetic activation of fast-spiking basket interneurons [44, 45] or pyramidal neurons [46] in sensory cortex evokes robust gamma oscillations that depend on both GABAergic and glutamatergic synaptic transmission. Spontaneous gamma oscillations in vivo are eliminated by optogenetic suppression of interneurons [45] and both spontaneous and optogenetically evoked cortical oscillations are abolished by application of AMPAR and NMDAR blockers [44]. Together, these data strongly suggest that temporally coordinated activity of excitatory and inhibitory neurons (E-I) is necessary for expression of neocortical gamma rhythms. In the hippocampus, both E-I and I-I mechanisms may underlie gamma activity [47, 48].
GABAergic interneurons in the neocortex and hippocampus are highly diverse, but converging evidence points to fast-spiking, PV-expressing basket cells as an important source of synaptic inhibition for generating gamma oscillations. PV interneurons are heavily connected to each other via chemical and electrical synapses [17, 49–54] and exhibit extensive reciprocal synaptic connectivity with nearby excitatory neurons, allowing them to synchronize and respond to excitatory spiking [16, 55]. Basket cells fire at high rates, have intrinsic resonance in the gamma range, and are robustly entrained to endogenous gamma oscillations [56, 57]. Furthermore, the time course of GABAA receptor-mediated IPSPs is optimal for generating a 40Hz oscillation cycle [58, 59], enhancing the entrainment of excitatory neurons. Theoretical and computational work suggests that these specialized synaptic and firing properties promote gamma oscillations [49, 50, 58, 60–62].
The interaction between synaptic inhibition and temporal patterns of neural activity can be spatiotemporally complex. In the dentate gyrus, inhibitory interactions among distant interneurons show a distance-dependent variation in synaptic strength and duration of inhibitory events. These interactions promote the emergence of complex temporal patterns, generating focal bursts of gamma-range activity correlated with exploratory behavior and action selection [63] [64]. In addition to dynamic regulation of gamma rhythms, inhibition plays a key role in the hippocampal theta rhythm [65]. In the hippocampus, optogenetic activation of PV interneurons specifically amplifies theta frequency resonance in pyramidal neurons, whereas activation of pyramidal neurons increases power in a broad frequency range [66]. Suppression of PV cell activity alters the phase relationship between pyramidal neuron spiking and the theta rhythm [67].
Although soma-targeting inhibition from PV interneurons has been relatively well characterized, less is known about the roles of non-basket interneuron populations in directly generating oscillations. In the neocortex, PV cells receive innervation from other interneuron populations, including SST and VIP interneurons, and their firing is strongly regulated by these inputs [32–34]. Gamma activity could thus also be strongly modulated by synaptic inhibition of PV cells from multiple sources. Computational modeling of the emergence of gamma oscillations from neural networks suggests that synaptic inhibition of PV basket cells may promote the flexible expression of gamma oscillations with varying frequencies [61].
Recent work identified SST interneurons as a regulator of beta/low gamma (20–30Hz) oscillations in the neocortex. Suppression of SST cells in primary visual cortex reduced beta/low gamma activity evoked by large stimuli, and optogenetic stimulation of these cells augmented activity in this frequency range, whereas PV cell manipulation had little to no impact [68]. Further work suggests that SST activity may preferentially promote cortical low-frequency oscillations (5–30Hz), whereas PV activity selectively promotes fast frequencies (>30Hz) in behaving animals, with cooperative activation of both populations giving rise to beta (20–30Hz) oscillations [69] [70, 71]. Together, these findings suggest multiple streams of temporal control by inhibition in cortical networks (Fig. 3). The activation of SST interneurons may further entrain local and distant ensembles of neurons, enhancing long-range coherence in the beta/low gamma range [72]. Because SST cells robustly inhibit PV cells [32, 33], interactions between these channels of inhibitory influence are likely to be dynamic according to their recruitment by bottom-up and top-down inputs or in a stimulus-dependent manner.
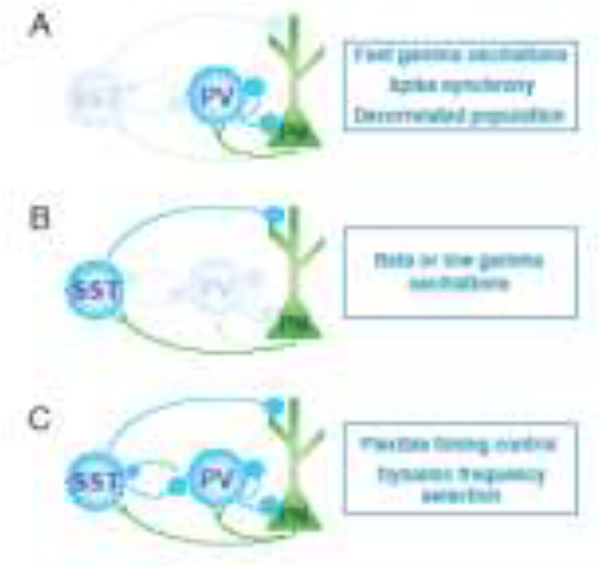
Figure 3. Distinct interneuron populations promote different cortical rhythms.
Recent work has highlighted the respective roles of PV and SST interneurons in shaping oscillations in cortical networks. A. Reciprocal interactions between PV interneurons and PNs generate ~40Hz gamma oscillations as a result of fast firing by the PV cells and strong reciprocal connections. B. SST interneurons likewise exhibit reciprocal connectivity with PNs, and their activity may underlie the generation of rhythmic activity at slower frequencies. They may generate activity at 5–30Hz and are necessary for sensory-evoked cortical beta/low gamma oscillations in the visual cortex. C. One intriguing possibility is that the simultaneous interactions of these two circuit motifs allows for the flexible selection of neural timing in low or high frequency bands as demand changes. Such interactions may be mediated by the relative occurrence of bottom-up or top-down inputs that recruit PV and SST interneuron spiking in the active circuit in vivo.
Interactions among inhibitory interneurons may be enhanced by extensive electrical synaptic connectivity. Both PV and SST interneurons are connected, mainly to other interneurons of the same class, by networks of gap junctional coupling [17, 52, 70, 73, 74]. Electrical connectivity via gap junctions may enhance synchrony between interneurons of the same type and facilitate phase coupling to ongoing oscillations [70, 75]. Loss of electrical synapses through deletion of the gene for the gap junction protein Connexin 36 selectively impairs gamma oscillations in the hippocampus, as well as theta-gamma phase coupling [76, 77]. However, recent work in vitro found that loss of Connexin 36 does not affect the synchrony of gamma-frequency inhibition in the neocortex [78, 79], suggesting potentially differential contributions to circuit activity in hippocampus and neocortex. The precise role of interneuron electrical connectivity in patterned network activity is thus not fully understood
Computational models of networks based on multiple cell types highlight the potential impact of interactions between interneurons in regulating the temporal pattern of neural activity. Inclusion of both SST and PV interneurons may widen the oscillatory behavior of the cortical network and replicates the impact of SST inhibition on PV cells, supporting the possibility of multiple rhythmic influences within the local cortical circuit [68]. Models of the hippocampal network likewise identify varying interneuron-interneuron interactions as a key element of theta oscillation generation in different activity regimes [80]. In a recently developed full-scale hippocampal network model, theta rhythms were observed only under conditions of interneuron diversity [81]. Computational modeling of cortical networks further suggests that behavioral or neuromodulatory context may dynamically adjust the functional connectivity among interneurons [82], providing more flexible control of temporal interactions within the local circuit.
Inhibitory influences that regulate oscillatory patterns are not limited to local circuit interactions. Long-range inhibitory projections, such as the population of PV-expressing GABAergic neurons in the basal forebrain that project to the frontal cortex, can also entrain cortical activity and robustly promote gamma oscillations [83]. In the hippocampus, both a subset of SST cells that project to the medial septum and retrohippocampal areas and a population of non-SST GABAergic neurons in the stratum radiatum that project to subiculum and cortex show strong rhythmic activity in the theta band that is reflected at target sites [84, 85] [65]. Locally recorded oscillations may thus represent a mixture of local and long-range inhibitory influences on circuitbased rhythms.
Inhibitory regulation of correlated spiking
In addition to regulating spike timing, synaptic inhibition promotes synchrony of spiking among interneurons and between groups of excitatory neurons. Synchrony among PV interneurons is enhanced by extensive synaptic interconnectivity [86] and gap junctions [52]. Spike synchrony among interneurons can be observed in extracellular recordings of neocortical fast-spiking putative PV interneurons in vivo [29] and appears to promote millisecond timescale synchrony in the hippocampus both between local pairs of interneurons [87] and between more distant interneurons in CA1 and CA3 [88]. Individual GABAergic interneurons may further synchronize the activity of multiple local pyramidal neurons [89]. During oscillations, the entrainment of excitatory spiking by rhythmic inhibition promotes synchrony among pyramidal neurons. Although individual neurons do not participate in every cycle, pairwise synchrony is enhanced by restricting spiking to a narrow range of phases within the oscillation cycle [90]. Rhythmic activation of inhibitory interneurons increases spike timing precision and narrows the window for spiking to promote synchronous sensory-evoked spikes [44]. Increased excitatory drive to inhibitory interneurons thus enhances excitatory synchrony [91].
Although synaptic inhibition can increase pairwise synchrony between neurons on a short timescale, previous work has also highlighted a role for inhibition in reducing slow-timescale relationships among large populations of neurons, sometimes called ‘noise correlations’. By being temporally coupled to excitation, inhibitory feedback may suppress pairwise correlations that promote shared population fluctuations in firing rate [92–94]. Synaptic activity with both excitatory and inhibitory components modulates the relative amount of fast and slow timescale correlations in a rate-dependent manner [95], with low input rates promoting slow timescale correlations and high rates promoting fast spike synchrony. The impact of inhibition on fast and slow correlations may vary dynamically with overall synaptic input rates and changes in the relative balance of excitation and inhibition [96]. However, modulation of noise correlation strength is not always coupled to changes in fast correlations in the neocortex [97, 98].
Inclusion of recurrent inhibition in network models reduces noise correlations [99], thereby enhancing the fidelity of stimulus encoding [100–102]. Physiologically, blockade of inhibitory synaptic transmission enhances noise correlations [103]. Recent work further suggests that topdown modulation of inhibition reduces endogenous slow-timescale correlated activity in cortical networks [104]. However, the actions of inhibition at fast and slow timescales are not mutually exclusive. In the olfactory bulb, inhibition simultaneously enhances fast-timescale correlations, such as synchronous spikes, while decreasing slow timescale pairwise firing rate correlations [105]. Both increased synchrony and decreased noise correlations are thought to enhance encoding of information, suggesting that inhibition may promote network function in multiple ways. Of note, the contributions of non-PV interneurons to regulating correlations at either fast or slow scales remain unclear.
Developmental role of inhibition in timing of circuit activity
The overall temporal profile of neural activity is shaped by early developmental events. In rodents, GABA is depolarizing during the first postnatal week of life, and synaptic connectivity has not yet matured, giving rise to large bouts of activity coordinated by inhibitory interneurons [106]. After the developmental shift to hyperpolarizing GABA, mediated by a change in expression of the Cl− extruder KCC2, synaptic inhibition begins to shape network activity in a more temporally constrained manner [107]. Although the last interneurons migrate into the cortex and hippocampus by the end of the first postnatal week in mice [108], very little is known about the role of different interneuron populations in regulating the timing of neural activity in the early postnatal period.
The intrinsic properties of GABAergic interneurons mature during the juvenile and adolescent periods. In the neocortex, both PV cells and principal neurons are innervated by thalamocortical terminals by mouse postnatal day 6–7 and PV interneurons begin to exhibit fast-spiking properties by postnatal day 18, setting up the core components of the recurrent local circuit and allowing somatic inhibition to begin to regulate spike timing [109–111]. During the adolescent period, PV inhibition is required for normal refinement of synaptic connectivity and critical period plasticity [112]. In contrast, much less is known about the development of synaptic dynamics of other interneuron populations in the immature brain. However, the activity of non-PV interneurons appears to be critical for the proper development of circuit architecture. Loss of VIP interneuron activity early in postnatal life results in loss of temporal organization of excitatory spiking [29]. Early activity of SST interneurons is critical for the development of thalamocortical connections to PV interneurons, and loss of SST inhibition disrupts normal feedback inhibitory circuit formation [113]. These findings highlight the importance of inhibitory-inhibitory interactions in the development and function of temporal structure of local circuit activity.
Disruption of inhibition and abnormal timing
Dysregulation of inhibition is linked to altered timing of neural activity on several timescales. Profound disruption of GABAergic synaptic transmission or loss of major interneuron populations has long been thought to contribute to the emergence of hypercorrelated activity and seizures [114–116]. However, the specific contributions of different interneuron populations to seizure initiation and resulting pathophysiology remain unclear. Developmental loss of interneuron activity reduces seizure thresholds [117, 118], whereas overall reductions in interneuron numbers results in epilepsy [119]. Optogenetic suppression of PV interneuron activity causes cortical networks to produce highly correlated population spikes [68], and developmental impairment of synaptic transmission from PV interneurons results in spike-wave seizures [120]. Loss of SST interneurons in early postnatal life is likewise associated with development of epileptiform activity [121].
Developmental impairment of inhibitory interneuron activity or synaptic inhibition has been identified as a potential mechanism underlying cognitive and psychiatric disorders, including autism and schizophrenia. Patients with schizophrenia exhibit reduced numbers of PVexpressing cells in cortical tissue and impaired gamma band synchronization [122–124]. Mouse genetic models of neurodevelopmental disorders have likewise highlighted disruption of inhibition as a key element of underlying pathophysiology. Mutation of the interneuron-specific gene ErbB4 in PV cells leads to altered PV firing patterns, changes in gamma oscillations, and disrupted temporal coherence between hippocampal and frontal brain regions [125]. Similarly, mice with a mutation in Disc1, a gene associated with several human psychiatric diseases, exhibit deficits in PV interneuron activity along with reduced hippocampal theta and gamma oscillations [126]. Mutations in the autism-associated genes CNTNAP2 and Fmr1 lead to reduced synaptic inhibition and altered synchrony [127, 128]. In particular, Fmr1-KO mice exhibit hypersynchrony in the theta and gamma ranges [129], along with abnormal patterns of hippocampal theta-gamma phase coupling [130] and elevated pairwise spike synchrony in the neocortex [128].
Although PV interneuron deficits have received particular attention for their potential role in altered neural activity patterns in disease, dysregulation of other interneuron populations may also impair the timing of neural activity. Early postnatal disruption of VIP interneuron activity in sensory cortex leads to a near-complete loss of pairwise spike synchrony between excitatory neurons and loss of phase coupling of spiking to both low and high frequency rhythms [29], suggesting these sparse interneurons may represent a point of developmental vulnerability for neocortical circuits.
Concluding remarks and future perspectives
Inhibition plays varied roles in regulating neural timing on several scales. Inhibitory cell types vary in their intrinsic and synaptic properties, and inhibitory interneuron properties, even within the same cell type, can differ depending on developmental stage, neuromodulation and brain region. Excitatory synaptic recruitment of GABAergic interneuron activity is modulated by cell type-specific short term synaptic plasticity, as is the impact of synaptic inhibition onto excitatory neurons. In turn, rhythmic synaptic inhibition robustly entrains the firing of excitatory neurons and promotes the generation of oscillations and pairwise spiking synchrony between excitatory neurons. Inhibition also suppresses pairwise correlations on slower timescales, potentially mediating a two-pronged enhancement of neural encoding. Inhibitory interneurons and synaptic inhibition also modulate network activity on a much slower timescale during development, with interneurons serving as organizers of correlated population activity. Loss or dysregulation of interneurons during development gives rise to long-term dysfunction of oscillations and spike timing in local and long-range circuits, reducing synchrony and enhancing slow correlations.
Much of our current knowledge about the inhibitory regulation of timing in the brain comes from work examining fast-spiking, parvalbumin-expressing interneurons or studies on somatic inhibition. Findings from recent work suggest that in addition to somatic inhibition, dendritetargeting inhibition has potential for a rich and dynamic contribution to both generating patterned activity [68, 69] and regulating the development of proper circuit architecture [29]. In addition, available data on synaptic dynamics at synapses onto inhibitory interneurons, and from interneurons to their targets, point to a complex temporal series of local circuit interactions. These intriguing findings point to many unanswered questions about the developmental and mature roles for the diversity of GABAergic interneurons in regulating neural timing (see Outstanding Questions). In particular, the roles of non-PV GABAergic cell types in temporal control at the timescales of synaptic dynamics, synchrony, slow correlations, and developmental coordination remain to be explored.
Outstanding Questions.
Parvalbumin-expressing, fast-spiking interneurons play a well-characterized role in restricting the timing of excitatory spiking. What are the roles of non-PV interneurons, such as SST and VIP cells, in regulating spike timing of excitatory neurons?
How does synaptic inhibition onto interneurons regulate their spike timing?
PV interneurons promote gamma oscillations, whereas SST interneurons promote beta/low gamma oscillations. Notably, SST interneurons powerfully inhibit PV cells. How do interactions among these different interneuron types regulate the expression of beta/gamma rhythms in the brain? How are those interactions regulated by neuromodulatory influences?
Do 5HT3aR-expressing and/or VIP interneurons promote specific rhythmic activity in local brain circuits?
What are the characteristics of short-term plasticity at synapses between interneuron populations (VIP-SST, SST-PV, etc)?
Inhibition regulates both fast spike synchrony and slow timescale noise correlations, typically enhancing the former and suppressing the later. The GABAergic interneurons that provide this synaptic inhibition are highly diverse. Do all sources of synaptic inhibition promote fast timescale synchrony and suppress noise correlations?
What is the developmental profile of short-term plasticity at inhibitory synapses onto dendrites?
What are the roles of dendrite-targeting interneurons in the postnatal development of appropriate E-I temporal interactions?
Highlights.
Intrinsic and synaptic properties of GABAergic interneurons shape their impact on temporal patterns in the local circuit.
Synaptic inhibition enhances short timescale correlations in spiking, such as spike synchrony, but suppresses long timescale correlations, such as noise correlations.
Different inhibitory interneuron populations, including PV and SST cells, may engage distinct rhythms in the cortex.
The emergence of circuit timing characteristics is shaped on the developmental timescale by multiple interneuron populations.
Acknowledgements
This work was supported by NIH R01 MH102365, NIH R01 EY022951, NIH R01 MH113852, a Smith Family Award for Excellence in Biomedical Research, a Klingenstein Fellowship Award, an Alfred P. Sloan Fellowship, a NARSAD Young Investigator Award, a McKnight Fellowship, and a grant from the Ludwig Family Foundation to J.A.C. The author thanks Drs. M.J. Higley and B. Doiron for insightful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fishell G and Rudy B (2011) Mechanisms of inhibition within the telencephalon: “where the wild things are”. Annu Rev Neurosci 34, 535–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelkey KA et al. (2017) Hippocampal GABAergic Inhibitory Interneurons. Physiol Rev 97 (4), 1619–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudy B et al. (2011) Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol 71 (1), 45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Csicsvari J et al. (1998) Reliability and state dependence of pyramidal cell-interneuron synapses in the hippocampus: an ensemble approach in the behaving rat. Neuron 21 (1), 179–89. [DOI] [PubMed] [Google Scholar]
- 5.Hu H et al. (2011) Submillisecond firing synchrony between different subtypes of cortical interneurons connected chemically but not electrically. J Neurosci 31 (9), 3351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klausberger T et al. (2003) Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421 (6925), 844–8. [DOI] [PubMed] [Google Scholar]
- 7.Klausberger T et al. (2004) Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci 7 (1), 41–7. [DOI] [PubMed] [Google Scholar]
- 8.Pouille F and Scanziani M (2001) Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293 (5532), 1159–63. [DOI] [PubMed] [Google Scholar]
- 9.Beierlein M et al. (2003) Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol 90 (5), 2987–3000. [DOI] [PubMed] [Google Scholar]
- 10.Cruikshank SJ et al. (2007) Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. [DOI] [PubMed] [Google Scholar]
- 11.Kapfer C et al. (2007) Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat Neurosci 10 (6), 743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma WP et al. (2010) Visual representations by cortical somatostatin inhibitory neurons--selective but with weak and delayed responses. J Neurosci 30 (43), 14371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silberberg G and Markram H (2007) Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron 53 (5), 735–46. [DOI] [PubMed] [Google Scholar]
- 14.Tan Z et al. (2008) Robust but delayed thalamocortical activation of dendritic-targeting inhibitory interneurons. Proc Natl Acad Sci U S A 105 (6), 2187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiang Z et al. (1998) GABAA receptor-mediated currents in interneurons and pyramidal cells of rat visual cortex. J Physiol 506 ( Pt 3), 715–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofer SB et al. (2011) Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nat Neurosci 14 (8), 1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson JR et al. (1999) Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402 (6757), 75–9. [DOI] [PubMed] [Google Scholar]
- 18.Cardin JA et al. (2010) Cellular mechanisms of temporal sensitivity in visual cortex neurons. J Neurosci 30 (10), 3652–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higley MJ and Contreras D (2006) Balanced excitation and inhibition determine spike timing during frequency adaptation. J Neurosci 26 (2), 448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panzeri S et al. (2001) The role of spike timing in the coding of stimulus location in rat somatosensory cortex. Neuron 29 (3), 769–77. [DOI] [PubMed] [Google Scholar]
- 21.Resulaj A et al. (2018) First spikes in visual cortex enable perceptual discrimination. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang Z et al. (2002) Synaptic inhibition of pyramidal cells evoked by different interneuronal subtypes in layer v of rat visual cortex. J Neurophysiol 88 (2), 740–50. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y et al. (2012) Short-Term Plasticity of Unitary Inhibitory-to-Inhibitory Synapses Depends on the Presynaptic Interneuron Subtype. J Neurosci 32 (3), 983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higley MJ (2014) Localized GABAergic inhibition of dendritic Ca(2+) signalling. Nat Rev Neurosci 15 (9), 567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovett-Barron M et al. (2012) Regulation of neuronal input transformations by tunable dendritic inhibition. Nat Neurosci. [DOI] [PubMed] [Google Scholar]
- 26.Murayama M et al. (2009) Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature 457 (7233), 1137–41. [DOI] [PubMed] [Google Scholar]
- 27.Phillips EAK et al. (2017) Cortical Interneurons Differentially Regulate the Effects of Acoustic Context. Cell Rep 20 (4), 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker F et al. (2016) Parvalbumin- and vasoactive intestinal polypeptide-expressing neocortical interneurons impose differential inhibition on Martinotti cells. Nat Commun 7, 13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batista-Brito R et al. (2017) Developmental Dysfunction of VIP Interneurons Impairs Cortical Circuits. Neuron 95 (4), 884–895 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu Y et al. (2014) A cortical circuit for gain control by behavioral state. Cell 156 (6), 1139–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pakan JM et al. (2016) Behavioral-state modulation of inhibition is context-dependent and cell type specific in mouse visual cortex. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cottam JC et al. (2013) Target-specific effects of somatostatin-expressing interneurons on neocortical visual processing. J Neurosci 33 (50), 19567–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfeffer CK et al. (2013) Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci 16 (8), 1068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pi HJ et al. (2013) Cortical interneurons that specialize in disinhibitory control. Nature 503 (7477), 521–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.David C et al. (2007) The innervation of parvalbumin-containing interneurons by VIP-immunopositive interneurons in the primary somatosensory cortex of the adult rat. Eur J Neurosci 25 (8), 2329–40. [DOI] [PubMed] [Google Scholar]
- 36.Wehr M and Zador AM (2003) Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426 (6965), 442–6. [DOI] [PubMed] [Google Scholar]
- 37.Wilent WB and Contreras D (2005) Dynamics of excitation and inhibition underlying stimulus selectivity in rat somatosensory cortex. Nat Neurosci 8 (10), 1364–70. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y et al. (2012) Generation of spike latency tuning by thalamocortical circuits in auditory cortex. J Neurosci 32 (29), 9969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Boustani S and Sur M (2014) Response-dependent dynamics of cell-specific inhibition in cortical networks in vivo. Nat Commun 5, 5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li LY et al. (2014) A feedforward inhibitory circuit mediates lateral refinement of sensory representation in upper layer 2/3 of mouse primary auditory cortex. J Neurosci 34 (41), 13670–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mesik L et al. (2015) Functional response properties of VIP-expressing inhibitory neurons in mouse visual and auditory cortex. Front Neural Circuits 9, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takesian AE et al. (2018) Inhibitory circuit gating of auditory critical-period plasticity. Nat Neurosci 21 (2), 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardin JA (2016) Snapshots of the Brain in Action: Local Circuit Operations through the Lens of gamma Oscillations. J Neurosci 36 (41), 10496–10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cardin JA et al. (2009) Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459 (7247), 663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sohal VS et al. (2009) Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459 (7247), 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adesnik H and Scanziani M (2010) Lateral competition for cortical space by layer-specific horizontal circuits. Nature 464 (7292), 1155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Vreeswijk C et al. (1994) When inhibition not excitation synchronizes neural firing. J Comput Neurosci 1 (4), 313–21. [DOI] [PubMed] [Google Scholar]
- 48.Whittington MA et al. (2000) Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol 38 (3), 315–36. [DOI] [PubMed] [Google Scholar]
- 49.Bartos M et al. (2001) Rapid signaling at inhibitory synapses in a dentate gyrus interneuron network. J Neurosci 21 (8), 2687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bartos M et al. (2002) Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci U S A 99 (20), 13222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cobb SR et al. (1997) Synaptic effects of identified interneurons innervating both interneurons and pyramidal cells in the rat hippocampus. Neuroscience 79 (3), 629–48. [DOI] [PubMed] [Google Scholar]
- 52.Galarreta M and Hestrin S (1999) A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature 402 (6757), 72–5. [DOI] [PubMed] [Google Scholar]
- 53.Tamas G et al. (2000) Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci 3 (4), 366–71. [DOI] [PubMed] [Google Scholar]
- 54.Tamas G et al. (1998) Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. J Neurosci 18 (11), 4255–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Packer AM and Yuste R (2011) Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci 31 (37), 13260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klausberger T and Somogyi P (2008) Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321 (5885), 53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tukker JJ et al. (2007) Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci 27 (31), 8184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang XJ and Buzsaki G (1996) Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci 16 (20), 6402–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whittington MA et al. (1995) Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373 (6515), 612–5. [DOI] [PubMed] [Google Scholar]
- 60.Bartos M et al. (2007) Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8 (1), 45–56. [DOI] [PubMed] [Google Scholar]
- 61.Borgers C et al. (2008) Gamma oscillations mediate stimulus competition and attentional selection in a cortical network model. Proc Natl Acad Sci U S A 105 (46), 18023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rinzel J et al. (1998) Propagating activity patterns in large-scale inhibitory neuronal networks. Science 279 (5355), 1351–5. [DOI] [PubMed] [Google Scholar]
- 63.Struber M et al. (2017) Distance-dependent inhibition facilitates focality of gamma oscillations in the dentate gyrus. Nat Commun 8 (1), 758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamamoto J et al. (2014) Successful execution of working memory linked to synchronized high-frequency gamma oscillations. Cell 157 (4), 845–57. [DOI] [PubMed] [Google Scholar]
- 65.Allen K and Monyer H (2015) Interneuron control of hippocampal oscillations. Curr Opin Neurobiol 31, 81–7. [DOI] [PubMed] [Google Scholar]
- 66.Stark E et al. (2013) Inhibition-induced theta resonance in cortical circuits. Neuron 80 (5), 1263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Royer S et al. (2012) Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci 15 (5), 769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Veit J et al. (2017) Cortical gamma band synchronization through somatostatin interneurons. Nat Neurosci 20 (7), 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen G et al. (2017) Distinct Inhibitory Circuits Orchestrate Cortical beta and gamma Band Oscillations. Neuron 96 (6), 1403–1418 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beierlein M et al. (2000) A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci 3 (9), 904–10. [DOI] [PubMed] [Google Scholar]
- 71.Deans MR et al. (2001) Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron 31 (3), 477–85. [DOI] [PubMed] [Google Scholar]
- 72.Hakim R et al. (2018) A neural circuit for gamma-band coherence across the retinotopic map in mouse visual cortex. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gibson JR et al. (2005) Functional properties of electrical synapses between inhibitory interneurons of neocortical layer 4. J Neurophysiol 93 (1), 467–80. [DOI] [PubMed] [Google Scholar]
- 74.Mancilla JG et al. (2007) Synchronization of electrically coupled pairs of inhibitory interneurons in neocortex. J Neurosci 27 (8), 2058–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blatow M et al. (2003) A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron 38 (5), 805–17. [DOI] [PubMed] [Google Scholar]
- 76.Buhl DL et al. (2003) Selective impairment of hippocampal gamma oscillations in connexin-36 knock-out mouse in vivo. J Neurosci 23 (3), 1013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hormuzdi SG et al. (2001) Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron 31 (3), 487–95. [DOI] [PubMed] [Google Scholar]
- 78.Neske GT and Connors BW (2016) Synchronized gamma-frequency inhibition in neocortex depends on excitatory-inhibitory interactions but not electrical synapses. J Neurophysiol 116 (2), 351–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salkoff DB et al. (2015) Synaptic Mechanisms of Tight Spike Synchrony at Gamma Frequency in Cerebral Cortex. J Neurosci 35 (28), 10236–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferguson KA et al. (2015) Network models provide insights into how oriens-lacunosum-moleculare and bistratified cell interactions influence the power of local hippocampal CA1 theta oscillations. Front Syst Neurosci 9, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bezaire MJ et al. (2016) Interneuronal mechanisms of hippocampal theta oscillations in a full-scale model of the rodent CA1 circuit. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dipoppa M et al. (2018) Vision and Locomotion Shape the Interactions between Neuron Types in Mouse Visual Cortex. Neuron 98 (3), 602–615 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim T et al. (2015) Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc Natl Acad Sci U S A 112 (11), 3535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jinno S et al. (2007) Neuronal diversity in GABAergic long-range projections from the hippocampus. J Neurosci 27 (33), 8790–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Katona L et al. (2016) Sleep and Movement Differentiates Actions of Two Types of Somatostatin-Expressing GABAergic Interneuron in Rat Hippocampus. Neuron 91 (5), 1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Galarreta M and Hestrin S (2002) Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proc Natl Acad Sci U S A 99 (19), 12438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.English DF et al. (2017) Pyramidal Cell-Interneuron Circuit Architecture and Dynamics in Hippocampal Networks. Neuron 96 (2), 505–520 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Diba K et al. (2014) Millisecond timescale synchrony among hippocampal neurons. J Neurosci 34 (45), 14984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cobb SR et al. (1995) Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 378 (6552), 75–8. [DOI] [PubMed] [Google Scholar]
- 90.Fries P et al. (2007) The gamma cycle. Trends Neurosci 30 (7), 309–16. [DOI] [PubMed] [Google Scholar]
- 91.Buia C and Tiesinga P (2006) Attentional modulation of firing rate and synchrony in a model cortical network. J Comput Neurosci 20 (3), 247–64. [DOI] [PubMed] [Google Scholar]
- 92.Graupner M and Reyes AD (2013) Synaptic input correlations leading to membrane potential decorrelation of spontaneous activity in cortex. J Neurosci 33 (38), 15075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Helias M et al. (2014) The correlation structure of local neuronal networks intrinsically results from recurrent dynamics. PLoS Comput Biol 10 (1), e1003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tetzlaff T et al. (2012) Decorrelation of neural-network activity by inhibitory feedback. PLoS Comput Biol 8 (8), e1002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Litwin-Kumar A et al. (2011) Balanced synaptic input shapes the correlation between neural spike trains. PLoS Comput Biol 7 (12), e1002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Doiron B et al. (2016) The mechanics of state-dependent neural correlations. Nat Neurosci 19 (3), 383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cohen MR and Maunsell JH (2009) Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci 12 (12), 1594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kohn A and Smith MA (2005) Stimulus dependence of neuronal correlation in primary visual cortex of the macaque. J Neurosci 25 (14), 3661–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Renart A et al. (2010) The asynchronous state in cortical circuits. Science 327 (5965), 587–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Franke K et al. (2017) Inhibition decorrelates visual feature representations in the inner retina. Nature 542 (7642), 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ly C et al. (2012) Cellular and circuit mechanisms maintain low spike co-variability and enhance population coding in somatosensory cortex. Front Comput Neurosci 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Middleton JW et al. (2012) Neural correlation is stimulus modulated by feedforward inhibitory circuitry. J Neurosci 32 (2), 506–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sippy T and Yuste R (2013) Decorrelating action of inhibition in neocortical networks. J Neurosci 33 (23), 9813–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang C et al. (2018) Circuit-based models of shared variability in cortical networks. bioRxiv 10.1101/217976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Giridhar S et al. (2011) Timescale-dependent shaping of correlation by olfactory bulb lateral inhibition. Proc Natl Acad Sci U S A 108 (14), 5843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Griguoli M and Cherubini E (2017) Early Correlated Network Activity in the Hippocampus: Its Putative Role in Shaping Neuronal Circuits. Front Cell Neurosci 11, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ben-Ari Y (2002) Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci 3 (9), 728–39. [DOI] [PubMed] [Google Scholar]
- 108.Miyoshi G et al. (2015) Prox1 Regulates the Subtype-Specific Development of Caudal Ganglionic Eminence-Derived GABAergic Cortical Interneurons. J Neurosci 35 (37), 12869–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Daw MI et al. (2007) Coordinated developmental recruitment of latent fast spiking interneurons in layer IV barrel cortex. Nat Neurosci 10 (4), 453–61. [DOI] [PubMed] [Google Scholar]
- 110.Goldberg EM et al. (2011) Rapid developmental maturation of neocortical FS cell intrinsic excitability. Cereb Cortex 21 (3), 666–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pangratz-Fuehrer S and Hestrin S (2011) Synaptogenesis of electrical and GABAergic synapses of fast-spiking inhibitory neurons in the neocortex. J Neurosci 31 (30), 10767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takesian AE and Hensch TK (2013) Balancing plasticity/stability across brain development. Prog Brain Res 207, 3–34. [DOI] [PubMed] [Google Scholar]
- 113.Tuncdemir SN et al. (2016) Early Somatostatin Interneuron Connectivity Mediates the Maturation of Deep Layer Cortical Circuits. Neuron 89 (3), 521–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ben-Ari Y and Holmes GL (2005) The multiple facets of gamma-aminobutyric acid dysfunction in epilepsy. Curr Opin Neurol 18 (2), 141–5. [DOI] [PubMed] [Google Scholar]
- 115.Cossart R et al. (2005) Multiple facets of GABAergic neurons and synapses: multiple fates of GABA signalling in epilepsies. Trends Neurosci 28 (2), 108–15. [DOI] [PubMed] [Google Scholar]
- 116.Steriade M (2005) Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends Neurosci 28 (6), 317–24. [DOI] [PubMed] [Google Scholar]
- 117.Lau D et al. (2000) Impaired fast-spiking, suppressed cortical inhibition, and increased susceptibility to seizures in mice lacking Kv3.2 K+ channel proteins. J Neurosci 20 (24), 9071–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tai C et al. (2014) Impaired excitability of somatostatin- and parvalbumin-expressing cortical interneurons in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A 111 (30), E3139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cobos I et al. (2005) Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci 8 (8), 1059–68. [DOI] [PubMed] [Google Scholar]
- 120.Rossignol E et al. (2013) CaV 2.1 ablation in cortical interneurons selectively impairs fastspiking basket cells and causes generalized seizures. Ann Neurol 74 (2), 209–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Close J et al. (2012) Satb1 is an activity-modulated transcription factor required for the terminal differentiation and connectivity of medial ganglionic eminence-derived cortical interneurons. J Neurosci 32 (49), 17690–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Spencer KM et al. (2003) Abnormal neural synchrony in schizophrenia. J Neurosci 23 (19), 7407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Uhlhaas PJ and Singer W (2010) Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11 (2), 100–13. [DOI] [PubMed] [Google Scholar]
- 124.Woo TU et al. (2010) Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv Rev Psychiatry 18 (3), 173–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Del Pino I et al. (2013) Erbb4 Deletion from Fast-Spiking Interneurons Causes Schizophrenia-like Phenotypes. Neuron 79 (6), 1152–68. [DOI] [PubMed] [Google Scholar]
- 126.Sauer JF et al. (2015) Impaired fast-spiking interneuron function in a genetic mouse model of depression. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jurgensen S and Castillo PE (2015) Selective Dysregulation of Hippocampal Inhibition in the Mouse Lacking Autism Candidate Gene CNTNAP2. J Neurosci 35 (43), 14681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goncalves JT et al. (2013) Circuit level defects in the developing neocortex of Fragile X mice. Nat Neurosci 16 (7), 903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Arbab T et al. (2018) Abnormal hippocampal theta and gamma hypersynchrony produces network and spike timing disturbances in the Fmr1-KO mouse model of Fragile X syndrome. Neurobiol Dis 114, 65–73. [DOI] [PubMed] [Google Scholar]
- 130.Radwan B et al. (2016) Impaired cognitive discrimination and discoordination of coupled theta-gamma oscillations in Fmr1 knockout mice. Neurobiol Dis 88, 125–38. [DOI] [PMC free article] [PubMed] [Google Scholar]