Abstract
Patients supported with implantable left ventricular assist devices (LVAD) have a significant risk of bleeding and thromboembolic complications. All patients require anticoagulation with warfarin, aiming for a target international normalised ratio (INR) of 2.5 and most patients also receive antiplatelet therapy. We found marked variation in the frequency of INR measurements and proportion of time outside the therapeutic INR range in our LVAD-supported patients. As part of a quality improvement initiative, home INR monitoring and a networked electronic database for recording INR results and treatment decisions were introduced. These changes were associated with increased frequency of INR measurement. We anticipate that changes introduced in this quality improvement project will reduce the likelihood of adverse events during long-term LVAD support.
Keywords: patient safety, quality improvement, audit and feedback
Problem
Implantable left ventricular assist device (LVAD) improves survival and quality of life in selected patients with advanced heart failure1 2 but there is a significant risk of bleeding and thromboembolic complications during LVAD support.3–6 Given these risks, tight control of anticoagulation is required and it has been shown that poorer control of anticoagulation may be associated with increased adverse events and worse outcomes.7 8 Use of antiplatelet therapy and anticoagulation is recommended for all patients implanted with an LVAD.9 10
The anticoagulation records of all patients supported with an LVAD at a single UK centre were examined. Wide variations in the frequency of international normalised ratio (INR) checks and proportion of time during which individual patients were outside the therapeutic INR range were identified. The reasons for this apparent suboptimal situation were explored and several contributory factors were identified. INR monitoring was performed in multiple locations including tertiary care, secondary care, primary care and home. There were shortcomings with communication and documentation. Patients made unscheduled telephone calls to a ventricular assist device (VAD) coordinator and expected to receive an immediate treatment recommendation. VAD coordinators frequently lacked direct access to the patient record and could neither document the INR result nor make an instant treatment recommendation.
Our aim was to increase the proportion of time that LVAD-supported patients are within the therapeutic INR range.
Background
Mechanical circulatory support with an implantable LVADs improves survival and quality of life in selected patients with advanced heart failure.1 2 Use of antiplatelet therapy and anticoagulation is recommended for patients with an LVAD, although there is no evidence to support the superiority of any anticoagulation regime.3 9 Anticoagulation is typically achieved with an oral vitamin K antagonist, aiming to achieve an INR between 2.0 and 3.0. Data from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) show that there are significant risks of bleeding, pump thrombosis and systemic thromboembolism during long periods of LVAD support.4–6 There is evidence from non-LVAD populations that increased time outside the therapeutic INR range is associated with increased risk of bleeding and thromboembolic events.11 12
Home monitoring has been shown to improve the proportion of time in therapeutic INR range, in both patients with an implantable LVAD13 and other patient groups including those with mechanical heart valves and atrial fibrillation.14 15 Home monitoring can reduce the number of adverse events and mortality.14 A system of home INR monitoring was established, supervised by VAD coordinators, using electronic communication and a cloud-based (networked) database to improve anticoagulation in LVAD patients. This system has the potential to improve patient experience and safety.
Baseline measurement
All outpatients with an implantable LVAD on 1 November 2014 were included. Patient records and electronic test results were examined. Baseline characteristics and details of anticoagulation over a period of 4 months were recorded, including INR results and instructions to patients. Categorical data are summarised as counts (percent) and continuous data are summarised as mean ±SD or median (IQR) depending on normality of data distribution. Time in the therapeutic INR range was calculated using the Rosendaal method, by which time in therapeutic range is calculated by incorporating the interval between INR measurements and the actual INR values, assuming that changes between consecutive INR measurements are linear over time.16 Statistical analysis was performed using Excel (Microsoft).
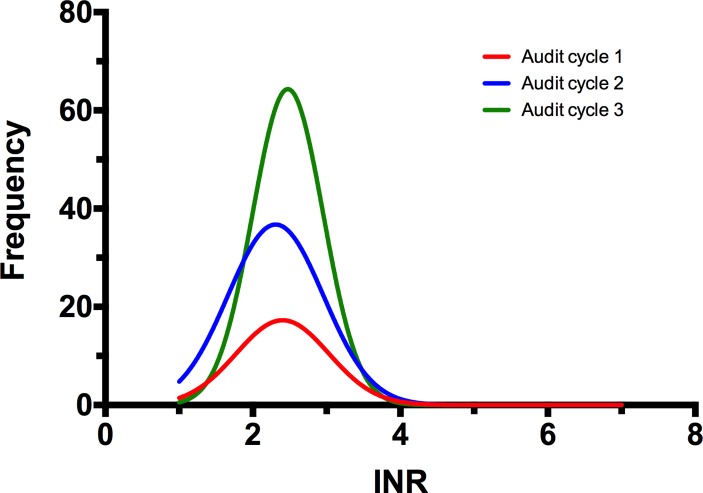
Twenty-three patients were supported with an LVAD during the first audit period with a total of 2454 patient-days of LVAD support. The target INR was 2.5 and the acceptable INR range was 2.0–3.0 during the study. Warfarin dosing changes were made by a Consultant Cardiologist, based on clinical judgement. Computerised decision support software was not used. The accuracy of anticoagulation was reasonable, with a mean INR of 2.46±0.59 during the first audit period (figure 1). However, the frequency of INR testing was lower than expected with a mean of 0.38 measurements per patient-week. There were 36 episodes where the INR was outside the therapeutic range with 16 episodes above and 20 episodes below the therapeutic range. Overall, the time in therapeutic range was 67.96% during this period.
Figure 1.
Histogram showing frequency of INR measurements in three consecutive audit cycles. INR, international normalised ratio.
Twelve patients had access to home INR testing using a point-of-care assay, but 11 patients needed to attend a healthcare facility to undergo INR testing. The frequency of INR measurement was 0.37 measurements per patient-week in those with home INR testing and 0.39 measurements per patient-week in those without home INR testing (p=0.709). However, home INR testing was not associated with the greater accuracy of anticoagulation. Patients with home INR testing had an average INR of 2.5±0.27 and patients without home INR testing had a mean INR of 2.3±0.57 (p=0.288).
Design
Our strategy was to increase the frequency of INR monitoring, improve communication of INR results and improve documentation relating to anticoagulation. As such, the objective was to enable VAD coordinators to make better treatment recommendations in a timely fashion.
All patients with an LVAD were issued with point-of-care INR testing equipment and taught to measure their INR at home. Patients were asked for self-test INR at least once a week and send results to the VAD coordinators using a dedicated email address. VAD coordinators were allocated time to collate INR results and make treatment recommendations. All INR results and treatment recommendations were recorded on an electronic database that was located on a secure networked drive. Patients were informed by telephone or email about changes to warfarin dose and time of next INR measurement.
Strategy
PDSA 1: first audit cycle (4-month cycle from November 2014). The first audit cycle was conducted to measure the effectiveness of anticoagulation in LVAD patients and identify areas where performance could be improved. Our performance was suboptimal, with a frequency of INR measurement lower than anticipated and proportion of time outside the therapeutic range higher than expected. The frequency of INR testing and overall INR control was similar in patients with home INR testing and those without home INR testing. Barriers to performance included inconsistent monitoring frequency and communication difficulties between patients and the LVAD team. The consensus that the system required change was reached by discussion between the Consultant Cardiologists and VAD coordinators. A number of potential changes were identified.
PDSA 2: introduction of home INR testing. Home INR testing was extended to include all LVAD-supported patients to make INR measurement as convenient as possible from their perspective. All patients were issued with a point-of-care INR testing machine and trained to take INR readings at home. Given frequency of testing was a concern; patients were encouraged to self-test INR at least once per week. A dedicated email address was established to enable patients to communicate INR results efficiently to the VAD co-ordinators.
PDSA 3: introduction of a networked database. Once home INR testing was introduced, we needed a process in which to record the extra data. Lack of a unified system for recording INR results and warfarin dosing was a barrier to efficient working. Prior to the project, each patient held their own paper-based record of INR results and warfarin dose. A networked database in Excel that could be accessed from anywhere within the hospital was established. The database was adapted from the paper-based record, with tabs for each patient and columns for INR measurements, current warfarin dose, suggested dose changes and suggested a date of next INR test. All incoming INR results were recorded. Recent INR results and warfarin dosing may be reviewed at a glance, allowing an immediate treatment recommendation to be made. The database was very simple and intuitive to use, tested by all VAD coordinators and used in clinical practice once all stakeholders were trained (figure 2). No adjustments to the design of the database were required in clinical practice, but certain VAD co-ordinators needed to be prompted to use the database during the early phase of the project.
Figure 2.
A single patient ‘sheet’ on the networked database showing how anticoagulation data is recorded.
PDSA 4: dedicated VAD coordinator session for anticoagulation management. Once home INR monitoring was introduced and patients were familiar with the system for emailing INR results, VAD coordinators were allocated dedicated time each day to process INR results, obtain treatment recommendations from cardiologists and communicate these treatment recommendations to each patient. In addition, VAD coordinators would contact patients who had not sent INR measurements to determine whether there was a problem. Previously, patients would contact the on-call VAD coordinator via the hospital switchboard with their INR results. This was often problematic, given the unpredictable work pattern of a VAD coordinator working within a busy Cardiothoracic Transplant Unit. A dedicated session was a more efficient mechanism of follow-up, reducing interruptions to other duties and gave patients certainty about when information would be provided regarding their anticoagulation dose.
PDSA 5: repeated audit cycles. Second and third audit cycles (each of 4 months) were performed to determine whether changes to practice in PDSA cycles 2, 3 and 4 were associated with improved anticoagulation of LVAD patients. The frequency of INR testing and the proportion of time in the therapeutic range were examined. In addition, whether these changes were sustained over time was also assessed.
Results
A second audit cycle was performed over a 4-month period from January 2015 and a third audit cycle was performed over a 4-month period from August 2015. Both these audit cycles included 19 patients, with a total of 2206 patient-days of LVAD support in audit cycle 2 and 2275 patient-days of LVAD support in audit cycle 3. There was no difference in the INR target between the first, second and third audit cycles.
The frequency of INR testing increased (figure 1) from 0.38 measurements per patient-week in audit cycle 1 to 0.9 measurements per patient-week in audit cycle 2 (p=0.006) and was sustained at 1.24 measurements per patient-week in audit cycle 3 (cycle 2 vs cycle 3: p=0.087) (table 1). The actual INR levels achieved were similar in each of the three audit cycles (cycle 1 vs cycle 2, p=0.391; cycle 1 vs cycle 3, p=0.914; cycle 2 vs cycle 3, p=0.361). Time in therapeutic range in cycle 1 was 67.96%, in cycle 2 was 77.97% and in cycle 3 was 76.56%. However, the changes in time in therapeutic range did not reach statistical significance (cycle 1 vs cycle 2: p=0.286; cycle 1 vs cycle 3: p=0.850; cycle 2 vs cycle 3: p=0.334). Most of the improved performance was delivered by a reduction in the amount of time spent with an INR below the therapeutic range. There was marked variation in INR control between patients. The majority of patients were inside the therapeutic range for the entire audit period, but a small number of patients were outside the therapeutic range for long periods of time.
Table 1.
Summary of results from three audit cycles
| Audit cycle | INR measurements per week, n | INR, mean±SD | Time in therapeutic range, % |
| 1 | 0.38 | 2.46±0.59 | 67.96 |
| 2 | 0.9 | 2.3±0.6 | 77.97 |
| 3 | 1.24 | 2.48±0.6 | 76.56 |
Lessons and limitations
This observational study shows that it is possible to deliver measurable improvements in the anticoagulation of high-risk patients by combining simple interventions. Each intervention was designed to facilitate the ‘correct’ course of action by the patient or healthcare provider. Several lessons were learnt during this quality improvement project.
Home INR testing using a point-of-care device improves the frequency of INR testing. A patient is more likely to provide an INR result if this can be performed in the home environment within a few minutes, rather than requiring a trip to a healthcare facility for venipuncture.
Use of secure email may improve communication between patients and healthcare providers for measurements such as an INR result. A patient may be deterred from contacting their healthcare provider via telephone if this involves navigating a busy automated switchboard and waiting for a VAD coordinator to respond.
Electronic patient records improve the documentation of INR results and warfarin dosing. A healthcare provider is more likely to provide appropriate treatment recommendations if they can easily review the INR and warfarin dosing history for an individual patient. In addition, an electronic patient record including key anticoagulation data enables continuous performance monitoring.
This quality improvement project has several limitations.
The study was observational. It is not possible to determine whether the interventions implemented were the cause of an increased proportion of time within the therapeutic INR range. It is possible that other changes in practice or an awareness that an audit was being performed may have improved performance.
The study used a surrogate endpoint (time in the therapeutic range) to measure anticoagulation, rather than a clinically relevant outcome measure such as bleeding or thromboembolic events.
The interventions used in the quality improvement project require a high level of patient engagement. It is possible that the proportion of time within the therapeutic range may have increased further if attention had been focused on those patients with worse INR control or those who are less concordant with monitoring.
The sample size studied in this quality improvement project is small, but this represents the entire population of LVAD-supported patients in our centre and reflects the fact that LVAD support is a rare intervention in the UK. Analysis of a larger cohort would require a multicentre study.
Although the authors can verify that convenience has been improved from the healthcare professional’s perspective, no qualitative feedback from patients was sought.
Conclusions
Quality of anticoagulation was lower than expected, even in complex patients with an absolute need for anticoagulation such as those with implantable LVAD. Although time in therapeutic range on the baseline measurement at our centre was above that previously reported,17 18 we aimed to improve the process and ease of INR monitoring, alongside frequency of measurements and time in therapeutic range.
A combination of repeated cycles of an audit, combined with simple interventions to make anticoagulation easier for both patients and healthcare professionals, was associated with more frequent INR measurements and a trend towards an increase in the proportion of time that patients were within the therapeutic INR range. We would hope that this would reduce the likelihood of adverse events such as bleeding or thromboembolism during prolonged LVAD support. In addition, the interventions have led to a service that is more convenient for both patients and the healthcare professionals.
Footnotes
Contributors: WS and AG: collected data, analysed the data and drafted and revised the manuscript. PL: aided in design of the data collection tools and revised the manuscript. STLT: aided in conception of the project and revised the manuscript. SJP: initiated the project, designed data collection tools, monitored data collection throughout the project, analysed the data and drafted and revised the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Rose EA, Gelijns AC, Moskowitz AJ, et al. . Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435–43. 10.1056/NEJMoa012175 [DOI] [PubMed] [Google Scholar]
- 2. Slaughter MS, Rogers JG, Milano CA, et al. . Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241–51. 10.1056/NEJMoa0909938 [DOI] [PubMed] [Google Scholar]
- 3. Baumann Kreuziger LM, Kim B, Wieselthaler GM. Antithrombotic therapy for left ventricular assist devices in adults: a systematic review. J Thromb Haemost 2015;13:946–55. 10.1111/jth.12948 [DOI] [PubMed] [Google Scholar]
- 4. Kirklin JM, Pagani FD, Kormos RL, et al. . Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant 2014;33:555–64. [DOI] [PubMed] [Google Scholar]
- 5. Whitson BA, Eckman P, Kamdar F, et al. . Hemolysis, pump thrombus, and neurologic events in continuous-flow left ventricular assist device recipients. Ann Thorac Surg 2014;97–2103. 10.1016/j.athoracsur.2014.02.041 [DOI] [PubMed] [Google Scholar]
- 6. Uriel N, Han J, Morrison KA, et al. . Device thrombosis in HeartMate II continuous-flow left ventricular assist devices: a multifactorial phenomenon. J Heart Lung Transplant 2014;33:51–9. 10.1016/j.healun.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 7. Boehme AK, Pamboukian SV, George JF, et al. . Anticoagulation control in patients with ventricular assist devices. ASAIO Journal 2017;63:759–65. 10.1097/MAT.0000000000000592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Connors JM. On target: optimum international normalized ratio for left ventricular assist device patients. Circ Heart Fail 2016;9:e003166 10.1161/CIRCHEARTFAILURE.116.003166 [DOI] [PubMed] [Google Scholar]
- 9. Feldman D, Pamboukian SV, Teuteberg JJ, et al. . The 2013 international society for heart and lung transplantation guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013;32:157–87. 10.1016/j.healun.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 10. Rossi M, Serraino GF, Jiritano F, et al. . What is the optimal anticoagulation in patients with a left ventricular assist device? Interact Cardiovasc Thorac Surg 2012;15:733–40. 10.1093/icvts/ivs297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wallentin L, Yusuf S, Ezekowitz MD, et al. . Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 2010;376:975–83. 10.1016/S0140-6736(10)61194-4 [DOI] [PubMed] [Google Scholar]
- 12. Connolly SJ, Pogue J, Eikelboom J, et al. . Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008;118:1938 10.1161/CIRCULATIONAHA.107.750000 [DOI] [PubMed] [Google Scholar]
- 13. Bishop MA, Streiff MB, Ensor CR, et al. . Pharmacist-managed international normalized ratio patient self-testing is associated with increased time in therapeutic range in patients with left ventricular assist devices at an academic medical center. Asaio J 2014;60:193–8. 10.1097/MAT.0000000000000047 [DOI] [PubMed] [Google Scholar]
- 14. Heneghan C, Ward A, Perera R, et al. . Self-Monitoring Trialist Collaboration). Self-monitoring of oral anticoagulation: Systematic review and meta-analysis of individual patient data. Lancet 2012;379:322–34. [DOI] [PubMed] [Google Scholar]
- 15. Bloomfield HE, Krause A, Greer N, et al. . Meta-analysis: effect of patient self-testing and self-management of long-term anticoagulation on major clinical outcomes. Ann Intern Med 2011;154:472–82. 10.7326/0003-4819-154-7-201104050-00005 [DOI] [PubMed] [Google Scholar]
- 16. Rosendaal FR, Cannegieter SC, van der Meer FJ, et al. . A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 1993;69:236–9. [PubMed] [Google Scholar]
- 17. Halder LC, Richardson LB, Garberich RF, et al. . Time in therapeutic range for left ventricular assist device patients anticoagulated with warfarin: a correlation to clinical outcomes. Asaio J 2017;63:37–40. 10.1097/MAT.0000000000000449 [DOI] [PubMed] [Google Scholar]
- 18. Kuyumjian YM, Miyares MA, Leverock J, et al. . A multidisciplinary team-based process improves outpatient anticoagulation quality with continuous-flow left-ventricular assist devices. Int J Cardiol 2016;218:118–9. 10.1016/j.ijcard.2016.05.030 [DOI] [PubMed] [Google Scholar]