Abstract
Background
Sedentary lifestyles and related morbidities are rising among adults despite existing exercise recommendations. Appealing exercise regimes yielding similar/better body composition should be sought.
Objective
We investigated the effect of moderate-intensity exercise bouts of <10 min on body composition in previously sedentary adults.
Methods
This unblinded study enrolled 53 healthy sedentary volunteers aged ≥50 years, randomised into one of two gender-balanced exercise interventions: (1) male and (2) female short-duration bouts (MS, n=14; FS, n = 13), and (3) male and (4) female long-duration bouts (ML, n=13; FL, n=13). Short-duration bouts entailed 5–10 min of jogging thrice daily; long-duration bouts, 30–60 min 3–5 days weekly. Body composition was determined at recruitment and 8-weekly thereafter, for 24 weeks.
Results
At baseline, 14.3% of MS, 38.5% of ML, 92.3% of FS and 69.2% of FL were obese, dropping to 7.1%, 15.4%, 61.5% and 30.8%, respectively. For waist:height ratio, 64.3 % of MS, 76.9% of ML, 100% of FS and 84.6.3% of FL had ratios >0.5, dropping to 42.9%, 30.8%, 92.9% and 26.2%, respectively. While baseline MS and ML waist:hip ratio (WHR) ≥0.9 were 64.3% and 69.2%, respectively, they correspondingly dropped to 23.1% and 21.4%. The FS and FL with WHR ≥0.85 dropped from 46.2% to 15.4% and from 30.8% to 7.7%, respectively. Body composition variables improved for both sexes (all p <0.05) and mean change between exercise regimes was comparable for both sexes.
Conclusion
In equal cumulative times, moderate-intensity exercise bouts lasting <10 min are comparable with current 30–60 min bouts in body composition modification for adults of ≥50 years.
Keywords: cardio-respiratory fitness, sedentary, exercise bouts, body composition
What are the new findings?
Moderate-intensity exercise bouts of <10 min have favourable effects on changing body composition in sedentary adults from either sex.
There is no difference in the magnitude of body composition change from different exercise prescriptions as long as cumulative exercise durations are equal.
How might it impact on clinical practice in the near future?
Individuals aged above 50 years could benefit from a review of current exercise recommendations.
More appealing exercise prescription options for individuals at risk of the metabolic syndrome triad will make it easier to control inactivity-related non-communicable diseases.
Introduction
Sedentary lifestyles and related morbidities are on the rise, and physical inactivity currently is the number four cause of death worldwide.1 2 Globally, 51%–79% of adults are physically inactive and do not meet their exercise recommendations.2 3 This trend has also been shown in sub-Saharan Africa, and physical inactivity has been identified as a major risk factor in the now increasing burden of lifestyle diseases here.4 5 In Eldoret, Kenya, site of the current study, 82% of adults are inactive.6 The rapid urbanisation and increasing sedentary lifestyles associated with ‘the haves’ in Kenya and indeed the larger sub-Saharan Africa today are thought to be contributory.7 This further compounds the lack of exercise time associated with daily engagements among the older populace that is variously also age-compromised for sustained exercise participation. This may be associated with the observed epidemiological transition where cardiovascular disease is on the rise among adults.8–14 Unchecked, this inactivity may lead to longer-term poor health effect on individuals and undermine the healthcare systems already overwhelmed by communicable diseases.9–11 13 15–17
Recommendations of ways to tackle such physical inactivity are in existence. Evidence that participation in exercise or physical activity improves body composition is plenty, although data among the elderly are still scanty.2 18 19 Available exercise and physical activity guidelines are, however, inadequately followed and exercise recommendations are not being achieved in adults.2 3 6 Adherence to exercise has been poor and debate on the best exercise regime that could help achieve this has not been settled for a few decades now.2 20–26
Debate on the value and effectiveness of shorter exercise bouts on body composition is unsettled. Even where studies have focused on the benefits of accumulated short bouts of moderate-intensity exercise, benefits have not been congruent.27 28 Further, observation that 30 min bouts yield poor adherence calls for further studies on the value of the shorter (but with cumulative time equalling the existing guidelines) bouts in body composition improvement. To the best of our knowledge, there has been minimal studies on the value of exercise regimes of bouts lasting <10 min in older individuals. Where some exist, they followed their participants for a short period (8 weeks) failing to answer the question of the long-term benefits.24–26 The current study compared body composition indicators among sedentary adults following different exercise regimes performed over a 24-week period to identify any distinct differences in the regimes.
Materials and methods
We studied healthy sedentary adults aged at least 50 years (men=27; women=26) and residents of Eldoret town, Kenya. They volunteered in response to a local print advertisement. Using the WHO Global Physical Activity Questionnaire Sedentariness, sedentary individuals were those with <600 weekly metabolic equivalent (MET)-minutes of exercise. Further, only volunteers devoid of existing physical injuries and reported cardiovascular disease or treatment were included. All measurements were performed by the same researcher throughout for consistency and reproducibility.
The study was unblinded. Participants from either sex were randomly allocated into two groups per sex based on either of the two exercise prescriptions to be administered. This yielded four subgroups: (1) male short-duration bouts of exercise (MS), (2) female short-duration bouts of exercise (FS), (3) male long-duration bouts of exercise (ML) and (4) female long-duration bouts of exercise (FL). Those in the short-duration bouts of exercise, the experimental group, engaged in three daily bouts of 5–10 min of moderate-intensity jogging. Those in the long-duration bouts, the control group, involved in 30–60 min jogging bouts for 3–5 days weekly. Participants kept a record of exercise, which was objectively verified on select days using Polar Wearlink ActITrainer accelerometers (Actigraph, Pensacola, Florida, USA), monitors that detect activity and motion changes. These data were analysed weekly for confirmation that participants in the different exercise regimes had comparable MET-minutes.
The same researcher collected data from all the participants at recruitment stage and then at 8th, 16th and 24th week of exercise involvement. For each participant, height and weight without shoes but on light clothing were measured using a stadiometer and a mechanical scale (CAMRY Mechanical scale, BR9012, Shanghai, China). Waist and hip circumferences were measured with a tape measure. Skin-fold measures were taken using callipers (Harpenden Skinfold Callipers; BATY International, England). This was done from three different body sites: chest, abdomen and thigh for men; and the triceps, suprailiac and thigh for women. From these measurements, Body Mass Index (BMI), waist:hip ratio (WHR) and waist:height ratio (WHtR) were calculated. The sum of the three skin-fold measurements were used to determine body density using published generalised equations, while the fat percentage was computed from body densities using Brozek formulae.29–32
Data were analysed with STATA V.13. We used summary statistics and t-test functions, and outputs were in means and SD. Analysis was done at univariate, bivariate and multivariate levels based on sex and exercise regime adopted. Paired t-tests were conducted for exercise regime groups for each sex. Linear regressions controlling for the various predictor variables and potential confounders were further performed for the body composition outcome variables. Comparisons were evaluated at a set p value ≤0.05 for significance.
Results
At the start point of the study, male participants in the experimental arm (n=14) were aged 55.0±5.6 years while those in the control group (n=13) were aged 55.2±3.0 years. Experimental arm women (n=13) were aged 53.9±2.6 years with their control group counterparts (n=13) at 53.9±3.5 years. Sixty-four per cent (64.3%) of MS and 76.9% of ML, and 100% of FS and 76.9% of FL had acquired tertiary level of education. The rest had secondary-level training. All participants in the two experimental arms completed the 24-week exercise regime. In the control group, 61.5% of ML and 76.9% of FL completed the protocol. For BMI, 14.3% of MS, 38.5 of ML, 92.3% of FS and 69.2% of FL started as obese. Similarly, for WHtR, 64.3% of MS, 76.9% of ML, 100% of FS and 84.6.3% of FL had ratios >0.5. The MS group with baseline WHR ≥0.9 were 64.3%, with ML at 69.2%; the FS whose baseline WHR was ≥0.85 were 46.2% and their FL counterparts were 30.8%.
Mean values for these variables are presented in table 1.
Table 1.
Baseline demographic and clinical characteristics of the participants
| MS | ML | FS | FL | |
| Age (years) | 55.0±5.6 | 55.2±3.0 | 53.9±2.6 | 53.9±3.5 |
| BMI (kg/m2) | 25.8±4.0 | 28.6±4.8 | 33.3±4.8 | 32.0±5.4 |
| WHtR | 0.52±0.07 | 0.56±0.08 | 0.61±0.05 | 0.57±0.08 |
| WHR | 0.93±0.06 | 0.96±0.07 | 0.82±0.10 | 0.84±0.09 |
| Sum of 3 skin folds (mm) | 62.2±27.75 | 78.9±31.2 | 120.3±20.6 | 109.6±23.81 |
| Body density | 1.05±0.02 | 1.04±0.02 | 1.01±0.01 | 1.01±0.01 |
| Fat % | 20.7±7.3 | 24.9±7.9 | 39.8±3.6 | 37.4±5.1 |
Data presented as mean±SD.
BMI, Body Mass Index; FL, female long-duration bouts of exercise; FS, female short-duration bouts of exercise; ML, male long-duration bouts of exercise; MS, male short-duration bouts of exercise; WHR, waist:hip ratio; WHtR, waist:height ratio.
At the end of the 24-week follow-up, MS, who had started as obese, reduced to 7.1% compared with a drop to 15.4% for ML. Similarly, obese FS dropped to 61.5% versus a drop to 30.8% for FL. The percentage MS whose WHtR was above 0.5 dropped to 42.9 compared with a drop to 30.8% for ML. For FS, it dropped to 92.3% versus a drop to 26.2% for FL. The MS group whose baseline WHR was ≥0.9 dropped to 23.1% while ML dropped to 21.4%, and for the FS participants whose baseline WHR was ≥0.85, it dropped to 15.4% with the FL counterparts dropping to 7.7%.
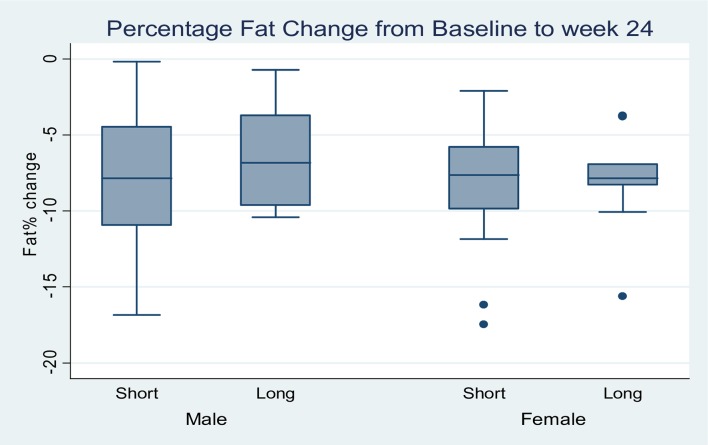
Generally, all variables associated with body composition were found to decrease in measured values for both sexes. There were significant drops in body weight, BMI, WHtR, WHR and percentage body fat (all p<0.05). However, the mean change difference for each variable was not different between the two exercise regimes for both sexes as tabulated for the overall change from baseline values in table 2, and shown by the p values derived from the comparison of the mean change difference between the regimes. On controlling for sex, table 3 presents a summary of linear regressions for these variables. The change in percentage body fat for the two sexes and groups showed a similar drop as plotted in figure 1.
Table 2.
Body composition measurements
| Variable | Group | Baseline | Week 8 | Week 16 | Week 24 | Mean change (week 24–week 0) | P values |
| Men | |||||||
| Weight |
Short | 76.4±14.7 | 75.2±14.5 | 74.9±14.0 | 72.3±14.2 | −4.07±5.8 | 0.74 |
| Long | 82.8±16.2 | 82.3±15.8 | 81.4±15.3 | 79.4±15.8 | −3.31±3.0 | ||
| BMI |
Short | 25.8±4.0 | 25.4±4.0 | 25.3±3.8 | 24.4±4.0 | −1.37±2.0 | 0.75 |
| Long | 28.2±5.0 | 27.9±4.6 | 27.7±4.7 | 27.0±4.1 | −1.13±1.01 | ||
| WHtR |
Short | 0.52±0.07 | 0.51±0.06 | 0.50±0.06 | 0.49±0.05 | −0.03±0.02 | 0.63 |
| Long | 0.55±0.08 | 0.54±0.09 | 0.53±0.08 | 0.51±0.07 | −0.04±0.02 | ||
| WHR |
Short | 0.93±0.06 | 0.92±0.06 | 0.90±0.05 | 0.87±0.05 | −0.05±0.04 | 0.44 |
| Long | 0.96±0.08 | 0.94±0.08 | 0.91±0.08 | 0.89±0.07 | −0.06±0.03 | ||
| Fat % |
Short | 20.7±7.3 | 15.6±5.9 | 14.2±5.5 | 12.4±4.8 | −8.32±5.0 | 0.36 |
| Long | 21.6±8.5 | 19.3±7.5 | 17.3±6.3 | 15.2±5.8 | −6.43±3.8 | ||
| Women | |||||||
| Weight |
Short | 85.3±13.7 | 84.2±13.9 | 83.0±14.1 | 81.7±13.2 | −3.61±1.54 | 0.22 |
| Long | 78.1±11.0 | 75.0±9.6 | 73.9±9.6 | 72.5±9.9 | −5.65±5.53 | ||
| BMI |
Short | 33.3±4.8 | 32.9±4.8 | 32.4±4.9 | 31.9±4.6 | −1.41±0.60 | 0.25 |
| Long | 30.5±5.1 | 29.3±4.6 | 28.9±4.9 | 28.4±5.1 | −2.15±2.13 | ||
| WHtR |
Short | 0.605±.049 | 0.573±.054 | 0.565±.049 | 0.557±.050 | −0.05±0.02 | 0.21 |
| Long | 0.55±.085 | 0.534±.081 | 0.527±.085 | 0.518±.082 | −0.04±0.02 | ||
| WHR |
Short | 0.822±.095 | 0.822±.065 | 0.811±.066 | 0.801±.066 | −0.02±0.05 | 0.84 |
| Long | 0.803±.078 | 0.794±.085 | 0.783±.081 | 0.785±.079 | −0.02±0.04 | ||
| Fat % |
Short | 39.8±3.6 | 35.0±5.1 | 32.3±5.3 | 31.1±5.6 | −8.69±4.41 | 0.67 |
| Long | 35.8±4.4 | 30.8±4.6 | 29.3±4.9 | 27.8±4.9 | −7.96±3.34 |
Values are means±SD.
BMI, Body Mass Index; WHR, waist:hip ratio; WHtR, waist:height ratio.
Table 3.
Linear regressions for body composition (controlling for sex)
| Mean change, long | Coefficient | SE | P value>|t| | 95% CI |
| Weight | −0.71 | 1.35 | 0.60 | −3.44 to 2.02 |
| BMI | −0.27 | 0.49 | 0.58 | −1.25 to 0.71 |
| WHtR | 0.004 | 0.01 | 0.53 | 0.01 to 0.09 |
| WHR | −0.004 | 0.01 | 0.75 | 0.03 to 0.02 |
| Fat% | 1.28 | 1.29 | 0.33 | 1.33 to 3.89 |
BMI, Body Mass IndexWHR, waist:hip ratio; WHtR, waist:height ratio.
Figure 1.
Percentage fat change between week 0 (baseline) and week 24 (endpoint).
Discussion
Body composition at start point
Men were overweight while women were obese (class I) at recruitment. Such BMI classes are associated with unfavourable health outcomes.33–35 The high baseline BMI of our participants suggests that they were at an increased risk for cardiovascular disease and, further, other pathologies associated with the metabolic syndrome. A few studies, however, propose that values within the 23–33 kg/m2 range have lower mortality risks among the elderly, so that the higher BMI values as observed in the current study may actually be associated with better cardiovascular disease epidemiology in this cohort.36–39 Based on this, women in the current study are especially safer.
Recently, studies have suggested that WHtR is superior to BMI in assessing risks associated with metabolic syndrome pathologies among different races and ages.40–45 Using the proposed WHtR cut-off of 0.5, participants in the current study were at cardiovascular disease risk at recruitment.42–44 46 In fact, considering proposals this ratio is superior to BMI in evaluating health risks and outcomes among apparently healthy populations as it particularly considers fat distribution, participants in this study had a considerably high risk for metabolic syndrome.40 41 47
A comparison of the baseline WHR against WHO cut-off guidelines of ≥0.90 (men) and ≥0. 85 (women) associated with substantially increased metabolic complications showed four in every five men and two in every five women had central/abdominal obesity with mean value above their respective WHO cut-offs.48 This indicates that at the start point, men in this study had a relatively higher risk for metabolic syndrome and other health problems, and bore a higher mortality risk.35 They had twice the risk portrayed by their female counterparts based on WHR alone.
Body composition reference data for the elderly African population with which comparison of the present study would be done are unavailable. While data abound elsewhere, these are based on non-African populations, and comparison may be affected by racial differences. Men and women in the current study had baseline percentage fat mass comparable with those found in an Indian population, although that study covered a wider age range.49 The high baseline percentage body fat for the women in the current study, making well over one-third of their body mass, and with a concurrent lower body density, is consistent with the baseline blood chemistry findings from another of our study on the same sample where the women had higher total cholesterol (TC) values.50 Based on Jackson and Pollock equations, men in this study had average baseline body fat, but their female counterparts were obese at the start point.29–31
These baseline findings indicated a potentially poor future health outcome for this cohort as such clinical features have been shown to get worse with advancing age and adoption of sedentary lifestyles, a finding consistent with other measures associated with cardiovascular disease independently and, broadly, the conglomeration of metabolic syndrome pathologies in the East-African region.51–53 Our exercise intervention and follow-up investigation improved these health parameters for the participants.
Changes in body composition
Previous works have shown that 6–12 weeks of exercise is sufficient to cause body composition changes across all ages.54–56 Sustained exercise involvement is necessary to maintain any such gains, which is why the current study measurements were done 8-weekly for a follow-up period that allowed at least four measurements. Over the 24 weeks, all values on variables associated with body composition decreased in a similar manner for both men and women in the two exercise regimes. Although this drop was not to the recommended levels in some variables, it was significant nevertheless, and there was no demonstrable difference in the magnitude of change between regimes.
Given that BMI drop among the elderly to normal values as provided by the international WHO classification is a huge challenge, the percentage drop for our participants with BMI >24.9 kg/m2 was a significant achievement.33 Short bouts’ regime halved the percentage of obese men with the longer-bouts’ regime dropping the same by one-third. The longer bouts for the women had a marginally higher proportion of the drop for the obese when compared with the shorter. The finding, however, still resonates with a recent suggestion that shorter-frequent exercise bouts are efficient in weight management in obese and overweight women.28 Effectively, the reduction in the BMI for both men and women in the short bouts’ regime was similar to that observed in their longer bouts’ counterparts, with the comparison of the mean change for different regimes within each sex showing no significant difference over the 24 weeks.
Lower values of WHtR are associated with lesser cardiovascular disease risk and indeed other pathologies related to metabolic syndrome.42 43 In this study, there was a drop in the percentage of both men and women on short bouts whose WHtR remained >0.5 cut-off at the end of the 24-week follow-up. Similarly, the mean values for those whose ratios remained >0.5 dropped. Regardless of the exercise regime and whether or not participants attained a mean value <0.5, however, all participants in the current study portrayed a drop in their WHtR. While this drop was significant from the baseline to the endpoint, a similar trend of no demonstrable difference in the rate of the change based on the exercise regime was noticed. No previous work we are aware of has compared the effect of intermittent and traditional regimes with WHtR in such a follow-up as our current work in this regard. It is safe to argue that the two exercise regimes have similar modification of central obesity and body composition at large given that no difference was noticed in this aspect for the two regimes on controlling for sex.
A similar picture was observed for WHR. There was a more than half reduction in the percentage of both men and women whose WHR remained above the WHO cut-off of ≥0.9 and ≥0.85, respectively, in both the short and the long bouts’ regimes of exercise. The difference between the two bout types was, however, indistinct.48 For participants whose values remained above the WHO threshold, short bouts were found to nonetheless decrease the absolute values in a similar manner as the long bouts. Thus, no difference was demonstrable in the manner the two exercise regimes affected WHR. The short bouts were as effective as the long ones in reducing abdominal obesity and therefore the risk associated with high WHR values.35 On controlling for sex, no differences would yet be found between the short and the long regimes in this regard, depicting their similarity in effect.
Participants in this study had a drop in the percentage body fat, regardless of the exercise regime adopted. However, only the men had a drop to within their recommended levels based on the ideal body fat percentage with widest reference.30 The women had a significant drop as well regardless of their regime of exercise but could not attain their recommended ranges.29 This could be explained by fact that at the baseline, they had a higher baseline percentage body fat so that although there was a drop, only a longer follow-up period would have helped attain the recommended ideal levels. By a direct comparison of the two exercise regimes, the shorter bouts had a slightly although statistically insignificant advantage by causing an absolute higher mean drop of the fat percentage, in both men and women. Recent works on this area have found bouts lasting <10 min to be equally effective in lowering body fat in men and women when cumulative intermittent bouts are considered.28 57 That our study found no difference between the two exercise regimes on controlling for sex in this variable further shows that the short bouts’ regime of moderate-intensity exercise is at least as good as the traditional longer bouts in the regulation of body fat across sexes.
Limitations
Generalisability of our findings may be affected by participants having been volunteers following a local print advert. Further, blinding was not done, and some lifestyle factors such as diet that would affect physical activity and cardiorespiratory fitness were not tracked given the logistical follow-up difficulties with this lengthy home-based trial, both of which may have confounded our results. That we used activity monitors on select days to verify the individually filled exercise logs may also have affected our results.
Conclusions
As long as the aggregated exercise time and intensity be equivalent, bouts lasting up to 10 min are as effective as the traditional longer bouts of 30–60 min in modification of body composition among sedentary Kenyan adults of 50 years and above.
Footnotes
Contributors: KM helped in designing the protocol, data collection, analysis and writing. Both KT and NBP helped in designing the protocol and writing the manuscript.
Funding: This research was supported by the Consortium for Advanced Research Training in Africa (CARTA). CARTA is jointly led by the African Population and Health Research Center (APHRC) and the University of the Witwatersrand and funded by the Wellcome Trust (UK) (grant no. 087547/Z/08/Z), the Department for International Development (DfID) under the Development Partnerships in Higher Education (DelPHE), the Carnegie Corporation of New York (grant no. B 8606), the Ford Foundation (grant no. 1100–0399), Google.org (grant no. 191994), Sida (grant no. 54100029), MacArthur Foundation (grant no. 10-95915-000-INP) and British Council.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Joint institutional research ethics body at Moi Teaching and Referral Hospital/Moi University (approval no. IREC 0001242).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Steele RM, Brage S, Corder K, et al. . Physical activity, cardiorespiratory fitness, and the metabolic syndrome in youth. J Appl Physiol 2008;105:342–51. 10.1152/japplphysiol.00072.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global recommendations on physical activity for health, 2010. [PubMed] [Google Scholar]
- 3. Ward BW, Clarke TC, Nugent CN. Early release of selected estimates based on data from the 2015 National Health Interview Survey. National Center for Health Statistics, 2016. [Google Scholar]
- 4. Mensah GA. Descriptive epidemiology of cardiovascular risk factors and diabetes in sub-Saharan Africa. Prog Cardiovasc Dis 2013;56:240–50. 10.1016/j.pcad.2013.10.014 [DOI] [PubMed] [Google Scholar]
- 5. Mensah GA, Roth GA, Sampson UKA, et al. . Mortality from cardiovascular diseases in sub-Saharan Africa, 1990–2013: a systematic analysis of data from the Global Burden of Disease Study 2013: cardiovascular topic. Cardiovasc J Afr 2015;26:S6–S10. 10.5830/CVJA-2015-036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nambaka JE, Kamau J, Amusa LO, et al. . Factors influencing participation in physical exercise by the elderly in Eldoret West District, Kenya. AJPHERD 2011;17:462–72. 10.4314/ajpherd.v17i3.71096 [DOI] [Google Scholar]
- 7. Moran A, Forouzanfar M, Sampson U, et al. . The epidemiology of cardiovascular diseases in sub-Saharan Africa: the global burden of diseases, injuries and risk factors 2010 study. Prog Cardiovasc Dis 2013;56:234–9. 10.1016/j.pcad.2013.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. BeLue R, Okoror TA, Iwelunmor J, et al. . An overview of cardiovascular risk factor burden in sub-Saharan African countries: a socio-cultural perspective. Global Health 2009;5:10 10.1186/1744-8603-5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikem I, Sumpio BE. Cardiovascular disease: the new epidemic in sub-Saharan Africa. Vascular 2011;19:301–7. 10.1258/vasc.2011.ra0049 [DOI] [PubMed] [Google Scholar]
- 10. van der Sande MA. Cardiovascular disease in sub-Saharan Africa: a disaster waiting to happen. Neth J Med 2003;61:32–6. [PubMed] [Google Scholar]
- 11. Hendriks ME, Wit FW, Roos MT, et al. . Hypertension in sub-Saharan Africa: cross-sectional surveys in four rural and urban communities. PLoS One 2012;7:e32638 10.1371/journal.pone.0032638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paterson DH, Jones GR, Rice CL. Ageing and physical activity: evidence to develop exercise recommendations for older adults. Can J Public Health 2007;98(Suppl 2):S69–108. [PubMed] [Google Scholar]
- 13. Yach D, Hawkes C, Gould CL, et al. . The global burden of chronic diseases: overcoming impediments to prevention and control. JAMA 2004;291:2616–22. 10.1001/jama.291.21.2616 [DOI] [PubMed] [Google Scholar]
- 14.Kenya National Bureau of Statistics (KNBS), ICF Macro. Kenya Demographic and Health Survey 2008–09. Cavelton, Maryland: KNBS and ICF Micro, 2010. [Google Scholar]
- 15. Dalal S, Beunza JJ, Volmink J, et al. . Non-communicable diseases in sub-Saharan Africa: what we know now. Int J Epidemiol 2011;40:885–901. 10.1093/ije/dyr050 [DOI] [PubMed] [Google Scholar]
- 16. Hassinen M, Lakka TA, Hakola L, et al. . Cardiorespiratory fitness and metabolic syndrome in older men and women: the dose responses to Exercise Training (DR’s EXTRA) study. Diabetes Care 2010;33:1655–7. 10.2337/dc10-0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hassinen M, Lakka TA, Savonen K, et al. . Cardiorespiratory fitness as a feature of metabolic syndrome in older men and women: the Dose-Responses to Exercise Training study (DR’s EXTRA). Diabetes Care 2008;31:1242–7. 10.2337/dc07-2298 [DOI] [PubMed] [Google Scholar]
- 18. Gremeaux V, Drigny J, Nigam A, et al. . Long-term lifestyle intervention with optimized high-intensity interval training improves body composition, cardiometabolic risk, and exercise parameters in patients with abdominal obesity. Am J Phys Med Rehabil 2012;91:941–50. 10.1097/PHM.0b013e3182643ce0 [DOI] [PubMed] [Google Scholar]
- 19. Colip L, Burge MR, Sandy P. Exercise intervention improves the metabolic profile and body composition of Southwestern American Indian adolescents. J Diabetes Obes 2016;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DeBusk RF, Stenestrand U, Sheehan M, et al. . Training effects of long versus short bouts of exercise in healthy subjects. Am J Cardiol 1990;65:1010–3. 10.1016/0002-9149(90)91005-Q [DOI] [PubMed] [Google Scholar]
- 21. LaFontaine T, Robbins L. Do multiple short bouts of exercise really produce the same benefits as single long bouts? Am J Cardiol 1991;67:325–6. 10.1016/0002-9149(91)90583-7 [DOI] [PubMed] [Google Scholar]
- 22. Pate RR, Pratt M, Blair SN, et al. . Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 1995;273:402–7. [DOI] [PubMed] [Google Scholar]
- 23. Blair SN, Kohl HW, Gordon NF, et al. . How much physical activity is good for health? Annu Rev Public Health 1992;13:99–126. 10.1146/annurev.pu.13.050192.000531 [DOI] [PubMed] [Google Scholar]
- 24. Murphy MH, Blair SN, Murtagh EM. Accumulated versus continuous exercise for health benefit: a review of empirical studies. Sports Med 2009;39:29–43. [DOI] [PubMed] [Google Scholar]
- 25. Linke SE, Gallo LC, Norman GJ. Attrition and adherence rates of sustained vs. intermittent exercise interventions. Ann Behav Med 2011;42:197–209. 10.1007/s12160-011-9279-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Macfarlane DJ, Taylor LH, Cuddihy TF. Very short intermittent vs continuous bouts of activity in sedentary adults. Prev Med 2006;43:332–6. 10.1016/j.ypmed.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 27. Jakicic JM, Wing RR, Butler BA, et al. . Prescribing exercise in multiple short bouts versus one continuous bout: effects on adherence, cardiorespiratory fitness, and weight loss in overweight women. Int J Obes Relat Metab Disord 1995;19:893–901. [PubMed] [Google Scholar]
- 28. Alizadeh Z, Kordi R, Rostami M, et al. . Comparison between the effects of continuous and intermittent aerobic exercise on weight loss and body fat percentage in overweight and obese women: a randomized controlled trial. Int J Prev Med 2013;4:881–8. [PMC free article] [PubMed] [Google Scholar]
- 29. Jackson AS, Pollock ML, Ward ANN. Generalized equations for predicting body density of women. Med Scie Sports & Exer 1980;12:182–81. 10.1249/00005768-198023000-00009 [DOI] [PubMed] [Google Scholar]
- 30. Jackson AS, Pollock ML. Generalized equations for predicting body density of men. 1978. Br J Nutr 2004;91:161–8. [PubMed] [Google Scholar]
- 31. Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr 1978;40:497–504. 10.1079/BJN19780152 [DOI] [PubMed] [Google Scholar]
- 32. Brozek J, Grande F, Anderson JT, et al. . Densitometric analysis of body composition: revision of some quantitative assumptions. Ann N Y Acad Sci 1963;110:113–40. 10.1111/j.1749-6632.1963.tb17079.x [DOI] [PubMed] [Google Scholar]
- 33.WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. WHO Technical Report Series 854. 1995 Geneva: World Health Organization, 1995. [PubMed] [Google Scholar]
- 34. Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. . Body-mass index and mortality among 1.46 million white adults. N Engl J Med 2010;363:2211–9. 10.1056/NEJMoa1000367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Myint PK, Kwok CS, Luben RN, et al. . Body fat percentage, body mass index and waist-to-hip ratio as predictors of mortality and cardiovascular disease. Heart 2014;100:1613–9. 10.1136/heartjnl-2014-305816 [DOI] [PubMed] [Google Scholar]
- 36. Winter JE, MacInnis RJ, Wattanapenpaiboon N, et al. . BMI and all-cause mortality in older adults: a meta-analysis. Am J Clin Nutr 2014;99:875–90. 10.3945/ajcn.113.068122 [DOI] [PubMed] [Google Scholar]
- 37. Veronese N, Cereda E, Solmi M, et al. . Inverse relationship between body mass index and mortality in older nursing home residents: a meta-analysis of 19,538 elderly subjects. Obes Rev 2015;16:1001–15. 10.1111/obr.12309 [DOI] [PubMed] [Google Scholar]
- 38. Ahmadi SF, Streja E, Zahmatkesh G, et al. . Reverse epidemiology of traditional cardiovascular risk factors in the geriatric population. J Am Med Dir Assoc 2015;16:933–9. 10.1016/j.jamda.2015.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu CY, Chou YC, Huang N, et al. . Association of body mass index with all-cause and cardiovascular disease mortality in the elderly. PLoS One 2014;9:e102589 10.1371/journal.pone.0102589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hsieh SD, Muto T. The superiority of waist-to-height ratio as an anthropometric index to evaluate clustering of coronary risk factors among non-obese men and women. Prev Med 2005;40:216–20. 10.1016/j.ypmed.2004.05.025 [DOI] [PubMed] [Google Scholar]
- 41. Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev 2012;13:275–86. 10.1111/j.1467-789X.2011.00952.x [DOI] [PubMed] [Google Scholar]
- 42. Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutr Res Rev 2010;23:247–69. 10.1017/S0954422410000144 [DOI] [PubMed] [Google Scholar]
- 43. Ashwell M, Gibson S. A proposal for a primary screening tool: ‘Keep your waist circumference to less than half your height’. BMC Med 2014;12:207 10.1186/s12916-014-0207-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ashwell M. Plea for simplicity: use of waist-to-height ratio as a primary screening tool to assess cardiometabolic risk. Clin Obes 2012;2(1-2):3–5. 10.1111/j.1758-8111.2012.00037.x [DOI] [PubMed] [Google Scholar]
- 45. Savva S, Lamnisos D, Kafatos A. Predicting cardiometabolic risk: waist-to-height ratio or BMI. A meta-analysis. Diab, Met Syndr & Obes: Targets and Therapy 2013;6:403–19. 10.2147/DMSO.S34220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ashwell M, Cole TJ, Dixon AK. Ratio of waist circumference to height is strong predictor of intra-abdominal fat. BMJ 1996;313:559–60. 10.1136/bmj.313.7056.559d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhu Q, Shen F, Ye T, et al. . Waist-to-height ratio is an appropriate index for identifying cardiometabolic risk in Chinese individuals with normal body mass index and waist circumference. J Diabetes 2014;6:527–34. 10.1111/1753-0407.12157 [DOI] [PubMed] [Google Scholar]
- 48.WHO. Waist circumference and waist-hip ratio: report of a WHO expert consultation. Geneva, 2008: 8–11. [Google Scholar]
- 49. Marwaha RK, Tandon N, Garg MK, et al. . Normative data of body fat mass and its distribution as assessed by DXA in Indian adult population. J Clin Densitom 2014;17:136–42. 10.1016/j.jocd.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 50. Magutah K, Patel NB, Thairu K. Majority of elderly sedentary Kenyans show unfavorable body composition and cardio-metabolic fitness. J Aging Sci 2016;04:160 10.4172/2329-8847.1000160 [DOI] [Google Scholar]
- 51. Jackson AS, Sui X, Hébert JR, et al. . Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med 2009;169:1781–7. 10.1001/archinternmed.2009.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lakka TA, Laaksonen DE, Lakka HM, et al. . Sedentary lifestyle, poor cardiorespiratory fitness, and the metabolic syndrome. Med Sci Sports Exerc 2003;35:1279–86. 10.1249/01.MSS.0000079076.74931.9A [DOI] [PubMed] [Google Scholar]
- 53. Njelekela MA, Mpembeni R, Muhihi A, et al. . Gender-related differences in the prevalence of cardiovascular disease risk factors and their correlates in urban Tanzania. BMC Cardiovasc Disord 2009;9:30 10.1186/1471-2261-9-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nikbakht H, Mirmostafaie S, Ghazalian F. The effect of eight weeks of whole body vibration training on body composition and adipose tissue localized in overweight women. Intl J Basic Sci & Appl Res 2013;2:1034–7. [Google Scholar]
- 55. Lee JS, Kim CG, Seo TB, et al. . Effects of 8-week combined training on body composition, isokinetic strength, and cardiovascular disease risk factors in older women. Aging Clin Exp Res 2015;27:179–86. 10.1007/s40520-014-0257-4 [DOI] [PubMed] [Google Scholar]
- 56. McGuigan MR, Tatasciore M, Newton RU, et al. . Eight weeks of resistance training can significantly alter body composition in children who are overweight or obese. J Strength Cond Res 2009;23:80–5. 10.1519/JSC.0b013e3181876a56 [DOI] [PubMed] [Google Scholar]
- 57. Jefferis BJ, Parsons TJ, Sartini C, et al. . Does duration of physical activity bouts matter for adiposity and metabolic syndrome? A cross-sectional study of older British men. Int J Behav Nutr Phys Act 2016;13:36 10.1186/s12966-016-0361-2 [DOI] [PMC free article] [PubMed] [Google Scholar]