Abstract
Objectives
Following youth concussion, objective physiological indicators are needed to corroborate changes in subjective clinical measures. The objectives of the current study were to: (1) explore the effect of concussion on heart rate variability (HRV) across days postinjury in youth athletes aged 13–18 years old, compared with healthy age-matched and sex-matched controls and (2) examine the relationship between postconcussion symptom domains (physical, cognitive, fatigue and emotional) and HRV.
Methods
Prospective, longitudinal, case-control study (N=44). This study comprised 29 concussed athletes between the ages of 13 and 18 years old (21 females, 8 males) and 15 age-matched and sex-matched controls). All participants completed baseline testing, which included demographic information (age, sex, concussion history), self-reported concussion symptoms (Post-Concussion Symptom Inventory [PCSI]) and a 24-hour heart rate recording via the Polar RS800CX system. The PCSI and HRV were collected weekly while the participant was symptomatic and then 1, 3 and 6 months following symptom resolution. HRV variables included time and frequency domain measures. Data visualisations and mixed effects modelling were used to derive parsimonious models.
Results
HRV increased across days postinjury. Concussion symptom domains (physical, cognitive, fatigue and emotional) all had a significant main effect on HRV; concussed participants who reported more symptoms had higher HRV compared with those who reported fewer symptoms. Visualisations of HRV depict the recovery trajectory as non-linear across time. No significant differences on HRV measures were found between concussed and control participants.
Conclusion
These preliminary findings provide the foundation to understand the varied trajectory and relationship between objective physiological measures and subjective symptom reporting.
Keywords: concussion, youth, heart rate variability, recovery, symptoms
What are the new findings?
This study contributes to the knowledge base in bridging traditional subjective measures with objective physiological outcomes in an understudied youth athlete population.
Concussed youth athletes demonstrated non-linear trends of change in heart rate variability (HRV) across the recovery trajectory.
HRV was significantly associated with postconcussion symptom domains (physical, cognitive, emotional and fatigue) along days postinjury.
This study demonstrated that changes in HRV were present irrespective of symptom status (symptomatic/asymptomatic) and injury status (concussed/control).
How might the findings impact clinical practice in near future?
Findings from this study are still in an early stage of interpretation and are not yet ready for clinical uptake.
Findings from this study do set the foundation in understanding that a potential stress response may be at play along the recovery trajectory following concussion in youth athletes.
Clinicians are encouraged to continue using an individualised approach to assessment and treatment, while being cognizant of the underlying stress response.
Introduction
It is estimated that 4 million children and youth present to emergency departments worldwide with concussion.1–3 In the assessment of concussion, well-established protocols have included neuropsychological testing and subjective symptom reporting to gauge clinical recovery.2–5 However, their application to youth athletes in particular presents with challenges. Elucidating the aetiology of concussion symptoms is difficult because postconcussion symptoms are non-specific.6 7 They can be found across a variety of clinical populations, including depression and anxiety8 and psychosocial stressors.9–11 Previous studies have also shown that athletes may minimise their symptoms over concerns of delayed return to play.12 13 Thus, objective physiological measures are needed to elucidate a physiological recovery trajectory that may present differently from a clinical recovery trajectory.
HRV, a measure of beat-to-beat variation in heart rate, has emerged as a non-invasive tool for the exploration of autonomic nervous system function following concussion.14–16 The exploration of HRV and concussion in sport populations has not been extensive, with few studies investigating the university athlete,17–19 and no studies examining the youth athlete population. In a recent prospective matched control study of university athletes, some perturbations in HRV (LFnu, HFnu) were found to persist beyond symptom resolution, which illustrated the potential of prolonged autonomic nervous system (ANS) disturbance following concussion.19 Similarly, Gall et al 20 found that concussed athletes who reported recent symptom resolution still demonstrated abnormal HRV during exercise compared with healthy controls. This finding was replicated by a recent study, which demonstrated that psychological systems and sleep disturbance following concussion in athletes were resolved at the return-to-play time point; however, ANS disturbances were still present (ie, decreased HRV), beyond return-to-play.17 It has been cited that more substantial follow-up will be valuable in improving the understanding of the natural history of change in ANS function following concussion.14 17 Further, it is not clear if the same trends between subjective symptom reporting and objective HRV measures would manifest similarly in the youth population.
The current consensus statement on concussion in sport3 has identified the following major gaps in assessing the value of physiological measures: (1) lack of longitudinal designs and (2) careful correlation with clinical measures, with little known on how these trends manifest within a youth population. Thus, this study aims to fill these gaps with the following exploratory research questions: (1) what is the effect of concussion on HRV across days postinjury in youth athletes aged 13–18 years old? (2) What is the relationship between postconcussion symptom domains (physical, cognitive, fatigue and emotional) and HRV? (3) What is the effect of having a concussion on HRV compared with healthy controls? As such, the hypotheses are the following: (1) HRV will decline immediately post injury and increase along the recovery trajectory; (2) HRV will have a negative relationship with postconcussion symptoms such that higher symptom reporting will be reflected in lower HRV and (3) concussed participants will demonstrate a decrease in HRV following concussion whereas controls will not demonstrate change compared with their baseline.
Methods
All participants and their parents provided informed consent prior to their participation in this study.
Participants
A convenience sample of 553 youth athletes (ages 13–18 years old) was recruited from local sport community organisations. Participants were part of a larger preinjury baseline testing study and completed a multimodal assessment consisting of physical, neurocognitive, subjective symptom reporting and physiological (HRV) measures (Reed et al, 2014).21 This study solely focused on the relationship between subjective concussion symptom reporting and objective HRV measures. Inclusion criteria were the following: male or female; ages 13–18 years old. Participants with a diagnosis of developmental delay, neurological condition, cardiac disease, experiencing symptoms from a previous concussion or non-English speaking were excluded from the study.
Measures
Demographic collection form
Age, sex and concussion history (ie, number of previous concussions) were collected using a questionnaire. Type of primary sport (ie, sport played most often and at the highest level) and level of play (eg, representative/competitive, house-league or recreational) were also collected.
Acute Concussion Evaluation (ACE) form
The ACE form is a postinjury questionnaire on injury characteristics, symptoms, risk factors for prolonged recovery (eg, history of migraine) and red flags that would warrant going to an emergency department.22 This measure was only used for those participants who sustained a concussion.
Post-Concussion Symptom Inventory (PCSI)
The PCSI is a 22-item self-report questionnaire used to assess postconcussion symptoms with youth ages 13–18 years old. It captures severity of symptoms ranging from 0=‘not a problem’, 3=‘somewhat of a problem’ and 6=‘severe problem’. Summed domain scores were derived for the following four domains: physical domain (eg, headache, dizziness, blurred vision); cognitive domain (eg, trouble concentrating, confused, mentally foggy); emotional domain (eg, sad, irritable, nervous) and fatigue domain (eg, fatigue, drowsiness). The PCSI has been found to have good validity and reliability among this age group.23 Internal consistency has been found to be moderate to strong, with Cronbach’s alpha ranging from 0.79 to 0.93 for the symptom subscales.23
Heart rate variability (HRV)
Heart rate recording was obtained from the Polar RS800CX watch and chest strap, with a sampling rate of 1000 Hz (RS800CX; Polar Electro, Kemple, Finland). Table 1 provides a definition of the time and frequency domain measures used in this study.24 A 24-hour recording methodology was suitable to the research objectives of this study, as they are more ecologically valid.25
Table 1.
HRV variable definitions
| HRV measure | Units | Definition |
| SDNN | ms | Standard deviation of RR intervals; Total variability, calculated by the SD of interbeat intervals |
| RMSSD | ms | Root mean square of successive RR interval differences, between adjacent RRs |
| pNN50 | % | The number of pairs of successive RRs that differ by more than 50 ms, divided by total number of RRs |
| HF | ms | High frequency; Index of parasympathetic influence on heart based on rhythmic respiration cycles; power spectral analysis calculated as high-frequency (0.15–0.40 Hz) band |
| HFnu | % | High frequency normalized units; [HF/(HF + LF)] x 100; index of modulation of the parasympathetic branch of the ANS as it influences the sinoatrial node of the heart |
HRV, heart rate variability.
Procedure
On receiving their informed consent, all participants completed a baseline/preinjury assessment21 that included the completion of the demographic collection form (age [years], sex, concussion history [yes/no], number of previous concussions, type of sport and level of competition in their primary sport), PCSI, and participants were fitted with a Polar RS800CX watch and chest strap (RS800CX; Polar Electro, Kemple, Finland). Due to the group-based testing environment of the larger baseline study, individual availability and school schedules, the start and stop time of the HRV data collection varied. All participants were instructed to carry out their usual daily activities, with the exception of removing the device (and putting it back on) if they went swimming, took a bath, or played a contact sport.
Youth athletes who sustained a concussion were instructed to receive a diagnosis from a physician. Concussed participants were then followed by research personnel with health science backgrounds (occupational therapy, kinesiology); (1) weekly follow-up assessment while symptomatic; (2) 1, 3 and 6 months postsymptom resolution (asymptomatic). The first follow-up included completion of the ACE form by the research personnel and the PCSI and HRV by the participant. Within this first follow-up, all concussed participants received concussion education on management strategies according to the evidence informed ‘Concussion & You’ programme.26 Here, the following domains of concussion management strategies were addressed: (1) energy conservation; (2) sleep hygiene; (3) hydration and nutrition and (4) gradual return to school and sport. All subsequent follow-up visits included the completion of these measures (except for the ACE form). Age (±6 months) and sex-matched control participants were recruited from the baseline cohort and the same measures were collected at each follow-up with the exception of the ACE form at the first follow-up visit.
Data analysis
HRV data cleaning and processing
The RHRV package within R: A Language and Environment for Statistical Computing was used to process and clean the HRV data file. The FilterNIHR function was used to filter ectopic heart beats by using an adaptive threshold for rejecting beats whose value exceeds the cumulative mean threshold. Second, standard values were obtained by identifying minimum (25 beats per minute) and maximum (200) heart beats per minute. Regarding frequency domain measures (ie, HF and HFnu), window frames were set to 300 s, 50% overlap; thus, the measures were calculated as the mean of successive 5 min epochs. The InterpolatedNIHR function (4 Hz) created an equidistant time series. Power spectrum density, via fast Fourier transform, was used to derive the frequency domain variables: HF (0.15–0.4 Hz); LF (0.04–0.15 Hz). Recordings that were <14 hours and not continuous were excluded from the analysis as the duration of the recording has been found to influence HRV indices.27 For the concussed group (n=29), length of recording was M=19.45 hours, SD=4.37. Within the control group (n=15), length of recording was M=22.90 hours, SD=3.26.
Statistical analysis
The R program was used for all statistical analyses. Generalised linear mixed models were employed to explore the effect of concussion on HRV, along the recovery trajectory. Parsimonious models generated for this study resulted in a random intercept model (fixed main effects). Participant ID was the random intercept, allowing each individual participant’s trend to vary along days postinjury which is appropriate given the different variance structures across groups.28 This approach accounts for the variability in the number of follow-up assessments per participant and the variable amount of time between those time points. In terms of number of follow-up assessments, concussed group: Mdn=3, range=1–9; control: Mdn=3, range=1–7. Formal goodness of fit statistics were tested for each outcome variable with Akaike Information Criterion (AIC), in which lower values represent better fit. Sex, age and history of concussion were accounted for in these models. However, inclusion of those covariates in the models resulted in higher AIC values (lower fit) and omitted to satisfy the parsimony criteria. Baseline HRV variables were also accounted for in the models as variation exists among healthy individuals.29 The statistical threshold for significance was set to p<0.05.
Results
A total of 44 participants were included in the analysis (concussed=29; control=15). Concussed participants and matched controls were similar on all HRV variables as well as PCSI values at baseline. As well, there were no significant sex differences at baseline on HRV variables, with the exception that females had lower HFnu compared with males. Descriptives for HRV values for concussed and control participants at baseline can be found in table 2. In terms of PCSI baseline values, females reported significantly more physical symptoms, males reported significantly more fatigue symptoms and females reported more overall symptoms compared with males. Participant demographic information and injury characteristics can be found in table 3. Finally, given the variable trajectory in number of follow-ups and time between follow-ups, table 4 depicts the number of HRV measures across time since injury.
Table 2.
Descriptive HRV values for concussed (n=29) and control (n=15) participants at baseline
| HRV variable | Mean (SD) | 25th percentile | 50th percentile | 75th percentile | |
| SDNN | Concussed | 206.61 (62.04) | 156.76 | 185.85 | 245.78 |
| Control | 194.05 (55.00) | 156.42 | 198.36 | 223.63 | |
| RMSSD | Concussed | 74.48 (30.94) | 53.29 | 70.39 | 77.65 |
| Control | 78.63 (31.95) | 59.68 | 75.85 | 101.61 | |
| pNN50 | Concussed | 27.51 (10.63) | 20.36 | 25.27 | 33.43 |
| Control | 31.12 (12.88) | 26.49 | 32.51 | 37.42 | |
| HF | Concussed | 556.77 (373.28) | 211.59 | 559.71 | 604.17 |
| Control | 560.23 (352.01) | 291.40 | 546.28 | 602.64 | |
| HFnu | Concussed | 36.91 (9.60) | 29.98 | 34.21 | 45.28 |
| Control | 41.53 (9.10) | 30.22 | 37.08 | 45.2 | |
| Mean HR | Concussed | 77.01 (4.38) | 71.84 | 74.72 | 81.49 |
| Control | 75.99 (9.28) | 70.29 | 74.97 | 80.36 |
HRV, heart rate variability.
Table 3.
Participant demographic information and injury characteristics
| Concussed (n=29) | Control (n=15) | |
| Demographic Information | ||
| Age (years), Mean (SD) | 15 (1.48) | 15 (1.66) |
| Sex, N (%) | ||
| Females | 21 (72) | 11 (73) |
| Males | 8 (28) | 4 (27) |
| History of concussion, N (%) | ||
| No history | 16 (55.2) | 8 (53.3) |
| One | 7 (24.1) | 5 (33.3) |
| Two | 3 (10.3) | 1 (6.7) |
| >Three | 2 (6.9) | 1 (6.7) |
| Baseline PSCI total, median (range) | ||
| Physical | 0 (0–7) | 0 (0–2) |
| Cognitive | 0 (0–5) | 0 (0–4) |
| Emotional | 0 (0–5) | 0 (0–1) |
| Fatigue | 0 (0–4) | 0 (0–5) |
| Injury characteristics | ||
| Days from baseline to concussion, median (range) | 93 (3–325) | – |
| ACE form injury description | ||
| Blow to head, Yes, N (%) | 16 (55.2) | – |
| Cause, Sport | 26 (89.6) | – |
| Retrograde amnesia, Yes, N (%) | 2 (6.9) | – |
| Range of time, s | <5 | – |
| Anterograde amnesia, Yes, N (%) | 1 (3.4) | – |
| Range of time, min | 1–2 | – |
| Loss of consciousness, Yes, N (%) | 1 (3.4) | – |
| Range of time, s | 5–10 | |
| Headache history, Yes, N (%) | 1 (3.4) | – |
| Migraine history, Yes, N (%) | 1 (3.4) | – |
| Psychiatric history, Yes, N (%) | 2 (6.9) | – |
| Number of postconcussion follow-up visits, range | 1–9 | 1–7 |
ACE, acute concussion evaluation; PCSI, Post-Concussion Symptom Inventory.
Table 4.
Number of assessments according to HRV measure, across time since injury
| Number of assessments across time postconcussion | |||||
| 1–15 days | 16–30 days | 31–45 days | 46–60 days | 60+ days | |
| SDNN | 32 | 21 | 7 | 8 | 11 |
| RMSSD | 32 | 21 | 7 | 8 | 11 |
| pNN50 | 32 | 21 | 7 | 8 | 11 |
| HF | 16 | 11 | 3 | 3 | 6 |
| HFnu | 16 | 11 | 3 | 3 | 6 |
HRV, heart rate variability.
SDNN
No significant differences in SDNN were found when considering days postinjury, postconcussion symptom domains (physical, cognitive, emotional and fatigue), symptom status (symptomatic/asymptomatic), and concussed participants were not significantly different from controls (table 5).
Table 5.
Mixed effect model estimates
| Estimate (P values) | |||||
| SDNN | RMSSD | pNN50 | HF | HFnu | |
| Trajectory of time | |||||
| Baseline value | 0.160 (<0.001)* | 0.186 (<0.001)* | 0.196 (0.0002)* | 0.271 (0.103) | 0.238 (<0.001)* |
| Days postinjury | −0.0001 (0.712) | 0.0001 (0.020)* | 0.0009 (0.029)* | 0.001 (0.005)* | 0.0008 (<0.001)* |
| Effect of symptom domains in concussed individuals | |||||
| PCSI—Physical | NS | NS | 0.0157 (0.019)* | 0.064 (<0.001)* | 0.014 (<0.001)* |
| PCSI—Cognitive | NS | NS | 0.023 (0.012)* | 0.138 (<0.001)* | 0.033 (<0.001)* |
| PCSI—Emotional | NS | NS | 0.035 (0.101) | 0.305 (<0.001)* | 0.069 (<0.001)* |
| PCSI—Fatigue | NS | NS | 0.028 (0.030)* | 0.131 (<0.001)* | 0.022 (0.003)* |
| Symptom status† (symptomatic vs asymptomatic) | NS | NS | NS | NS | NS |
| Concussed vs controls | |||||
| Injury status‡ (concussed vs control) | −0.044 (0.524) | −0.143 (0.058) | NS | 0.101 (<0.001)* | NS |
*Denotes a significant main effect where p<0.05.
†Symptom status refers to symptomatic vs asymptomatic.
‡Injury status refers to concussed vs control.
‘NS’ denotes a non-significant effect; PCSI, Post-Concussion Symptom Inventory.
RMSSD
A decrease in RMSSD appeared to occur 15 days postinjury and decreased until day 30, followed by levelling off at approximately 50 days (B=0.0001, p=0.02; figure 1). Postconcussion symptom domains, symptom status and injury status were not significant injury variables in this model (table 5).
Figure 1.
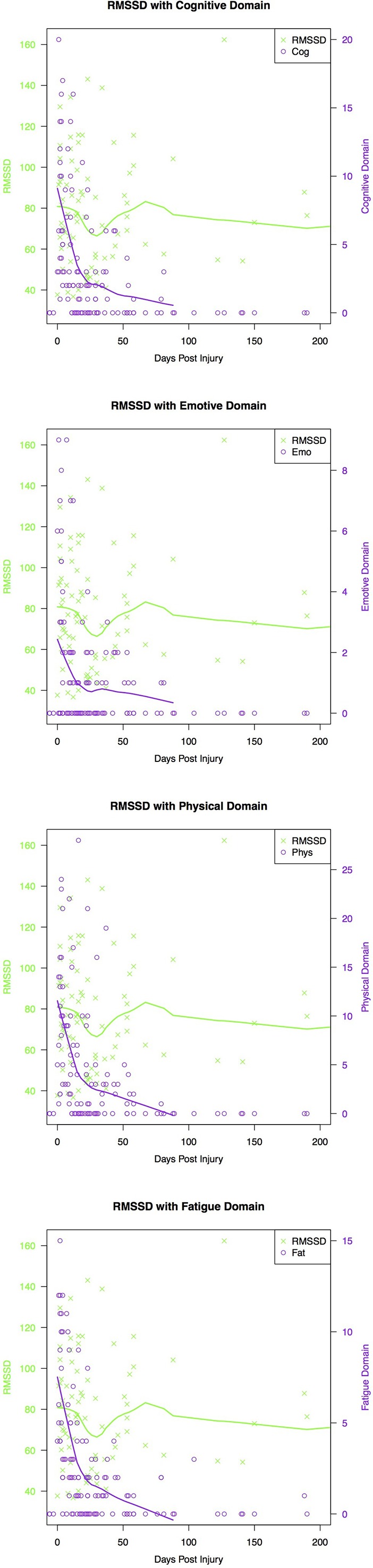
Relationship between RMSSD, concussion symptom domain and days postconcussion where day 0 marks the day of first follow-up. The green and purple trend line represents the lowess smooth line, which maps an average trajectory for all concussed individuals.
pNN50
Mixed modelling revealed a main effect of days postinjury (B =0.0009, p=0.029), whereby with increasing days post injury, pNN50 increased. Here, the visual trajectory of this finding reflected an immediate decrease from day of injury, decreasing until 30 days postinjury. PNN50 values then increase until day 65, followed by a plateau (figure 2). A positive, significant relationship was found between the physical (B =0.016, p=0.019), cognitive (B =0.023, p=0.012) and fatigue (B =0.028, p=0.030) domains and pNN50, whereby concussed participants who reported more symptoms in each domain were found to have increased pNN50. There were no significant effects of the emotional symptom domain, symptom status and injury status (table 5).
Figure 2.
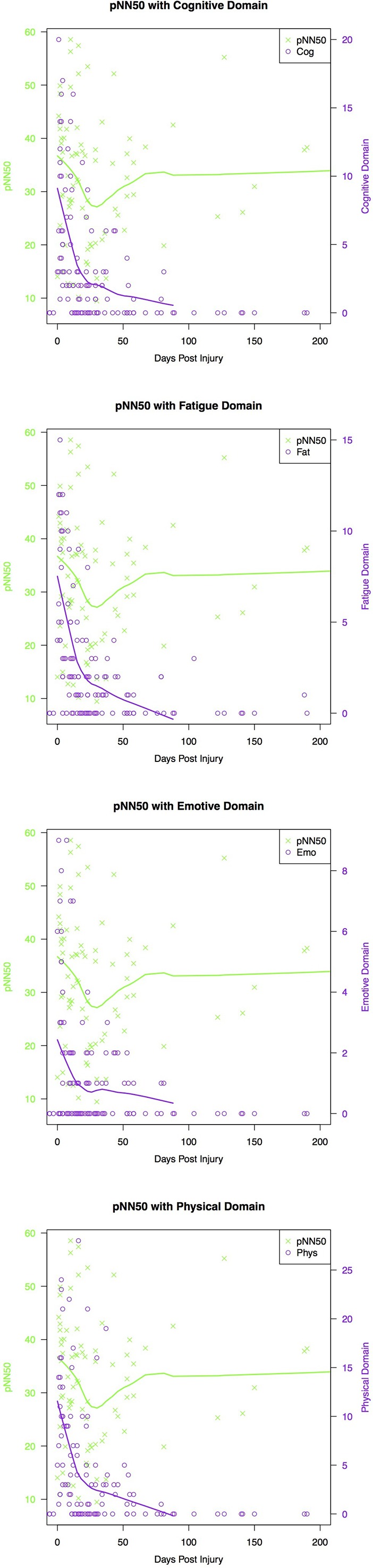
Relationship between pNN50, concussion symptom domain and days postconcussion where day 0 marks the day of first follow-up. The green and purple trend line represents the lowess smooth line, which maps an average trajectory for all concussed individuals.
HF
Mixed modelling revealed a main effect of days postinjury (B =0.001, p=0.005), whereby concussed participants were found to have increasing values of HF as days postinjury increased. All four postconcussion symptom domains, physical (B =0.064, p<0.001), cognitive (B =0.138, p<0.001), fatigue (B =0.131, p<0.001) and emotional (B =0.305, p<0.001) were also found to have significant main effect on HF, whereby those who reported more symptoms in each of those domains displayed increased HRV. A main effect of concussion status was also found, in which concussed participants were found to have significantly higher HF compared with controls (B =0.101, p<0.001). Within the concussed participants, symptomatic vs asymptomatic participants did not significantly differ in their values of HF (table 5).
HFnu
Mixed modelling revealed a main effect of days postinjury (B =0.0008, p<0.001), whereby concussed participants were found to have increasing values of HFnu as days postinjury increased. All four postconcussion symptom domains were also found to have a positive and significant main effect on HFnu. With increased report of concussion symptoms in the physical (B =0.014, p<0.001), cognitive (B =0.033, p<0.001), emotional (B =0.069, p<0.001) and fatigue domain (B =0.022, p<0.001), significant increases in HFnu were found. Within the concussed participants, symptomatic vs asymptomatic participants did not significantly differ in their values of HFnu (table 5).
Discussion
Findings from this study suggest a general trend of increasing HRV along the recovery trajectory. The relationship between concussion symptoms and HRV revealed differential trends along days postinjury, which reflects a non-linear physiological pattern in concussion recovery. Age and sex differences were not present in this study; however, the current study’s sample was predominantly female. History of concussion did not appear to play a role in the findings. With a larger sample size and adequate control, these potential demographic differences may be elucidated in future studies.
HRV along days postinjury
This study found a general increase over days postinjury in concussed youth athletes across all HRV variables except SDNN. However, in the initial 30–40 days of concussion, HRV is shown to decrease (14%–25% decrease was observed in this time period). Following this period, increases are observed until approximately day 75 (8%–26% increase across HRV measures) and returns to baseline thereafter. This trend should be interpreted with caution, as there are fewer data as days postinjury increases. These findings are somewhat in contrast to a prospective cohort study of varsity athletes, which revealed significant differences in the acute phase of concussion (days 1–7), but these were only found in RMSSD compared with other HRV measures.30 It is important to note that looking at HRV during an isolated period of time (acute injury) may depict changes in HRV as linear, that is, an expectation that HRV may be linearly decreasing or increasing. The strength of this repeated measures study is in the visualisation where the trajectory of time revealed trends of decreasing HRV, followed by trends of increasing HRV. While the mechanism of change is still unknown, it is hypothesised that an ‘uncoupling’ exists between the ANS and cardiovascular systems whereby dysregulated shifts occur between the parasympathetic and sympathetic branches of the ANS.31 Finally, no real differences were found when comparing concussed youth athletes to controls and further investigation is needed to explore this difference.
HRV and postconcussion symptom domains
A consistent result was found across some of the HRV measures (pNN50, HF, HFnu) whereby those who reported more concussion symptoms displayed increased HRV and those who reported fewer concussion symptoms had lower HRV. This finding differs significantly from research to date. In concussed adult samples, physiological markers of increased sympathetic output have been found (eg, decreased HRV).32–34 As well, increases in symptom reporting or psychological stress have been associated with decreased HRV.17 19 35 It is worth noting that these cited studies were conducted in more controlled and short-term data collection environments (eg, assessing change in HRV following positional changes); thus, their direct comparison to this study warrants caution. Nonetheless, a potential hypothesis for this opposite trend includes the role of consistent clinical education follow-ups within this study. All concussed participants received one-on-one concussion management strategies regarding graduated return to activity and symptom limited activity. It may be possible that this served as a clinically meaningful interaction for the concussed youth athletes. For example, those with increased symptoms may have followed the management strategy to rest and graduate their level of activity. This in turn may have served as modulatory feedback to the ANS and influenced homeostasis (increased HRV).18 The implication of this novel finding is that changes in HRV may have the potential to be altered based on support and consistent clinical education. While this was not the intent of the study protocol, it behoves the concussion community to further investigate external factors, which may alter the recovery trajectory for youth athletes.
Limitations
Overall, there appears to be large within-group variability in examining HRV following concussion. It has been postulated that autonomic dysregulation may present as a subclinical phenomena for those who have sustained a concussion36 and a physiological threshold may exist for those differences to be detectable.25 It is still unclear if the findings in this study represent a typical response to a stressful event (having a concussion) or if autonomic dysregulation is occurring above and beyond this response. Within the context of a large preinjury baseline study, it was not feasible to collect other meaningful variables such as physical activity repertoires. This is important to consider as changes in exercise training (frequency, duration and intensity) have been shown to significantly alter vagal tone (increase HRV over time).27 37
This study employed a non-controlled, 24-hour recording protocol, in which participants were able to go about their daily lives. This is in contrast to the majority of HRV concussion studies, which employ resting state position protocols or observe change in HRV according to exercise exertion. Restrictions on physical activity in this study were not feasible or appropriate for long-term recordings, given the diverse activity repertoires of youth (school, sport, extracurricular activity). This is an important consideration as changes in exercise training have been shown to significantly alter vagal tone (increase HRV over time).27 37 In the present study, the physical activity levels of concussed and control participants were likely different due to concussed participants’ inability to engage in physical activity and due to the training phase of each youth (ie, competitive, training or off season). Specific qualifications to physical activity (ie, frequency, duration and intensity) across the various follow-up time points will be important to consider for future study and likely will contribute valuable information to assist in the interpretation of change in HRV.
Other factors related to cognitive load and sleep-wake cycles are also important to consider. The role of cognitive demand/activity in everyday life (ie, academic demands, extracurricular activities) was not considered in this study. This study did not collect information in the form of a cognitive diary or employ other forms of cognitive activity tracking, and it is important to consider these clinical measures to allow a fulsome interpretation of the autonomic nervous system. Further, a potentially confounding effect may have been the inability to control for sleep-wake cycles. Sleep factors can significantly impact how an individual carries out their daily activities and can serve as a feedback loop to the ANS to regulate stress responses.38 Thus, a standardised assessment (ie, sleep questionnaire) to quantify these sleep factors is needed.
According to the most recent consensus statement, a single ‘physiological time window’ for concussion recovery does not exist due to methodological differences in assessing physiological measures and study design.3 Multiple studies have suggested physiological dysfunction that outlasts clinical measures of recovery,33 34 39 but this has not been conclusively established. The challenge present in this study was the inability to directly compare results to other similar concussion studies given the difference in recording protocol. However, regarding the baseline values, normative studies in adolescents examining 24-hour recordings show similarity across the measures collected in this study. For example, Faulkner, Hathaway, and Tolley40 examined healthy adolescents (15 years old ±1.6) and reported similar baseline values and ranges on pNN50, RMSSD, SDNN. Nonetheless, the finding that baseline values between concussed and control groups were similar and that baseline values compare to analogous studies may indicate promise in using the 24-hour recording as an ecologically valid methodology to observe change in the ANS. This assessment protocol may be the initial first step in using a dynamic protocol over a sufficient length of time to gauge how the ANS fluctuates in response to concussion over time. Taken together, while this 24-hour protocol introduces variability and noise within the HRV signal, the ability of trends to be seen despite this, is indicative of a potentially salient signal, and one that may be more ecologically valid41 42 when gauging the physiological response to concussive injury.
Finally, while robust hierarchical modelling was employed in this study to address the variability in data points across time since injury, the data were heavily concentrated within acute periods following concussion and more sparse within the chronic period. The power of the analysis approach to detect reliable differences may be influenced and thus skewed to reflect earlier time points in recovery. However, the authors present the visualisation of these recovery trajectories in an effort to be transparent about the distribution of data.
Acknowledgments
The authors thank the Rehabilitations Sciences Institute at the University of Toronto for their support throughout the doctoral program. We would also like to acknowledge the efforts of the members of the CIHR ‘NeuroCare’ Team and the members of the Concussion Centre (Bloorview Research Institute), specifically Talia Dick, James Murphy and Katherine Mah. We are grateful to the youth and families for their participation in this research. Jordan Collins is also thanked for his work and expertise in statistical analysis.
Footnotes
Contributors: MP conceptualised the study objectives, data collection and analysis and drafted manuscript. LV contributed to the conceptualisation of the study with particular expertise in methodology and data processing, contributed to literature review and manuscript draft. SGT participated in the methodology and data processing design and editing manuscript. TT participated in the conceptual design with expertise in data processing, editing manuscript. MK conceptualised study objectives and edited manuscript. KEW participated in data collection, literature review and editing manuscript. NR is the principal investigator on this manuscript with a broader role in supervising the proposal, data collection and analysis, methodology, diligent manuscript draft review and editing for submission.
Funding: This is work was funded by the Canadian Institutes of Health Research (#127048), the Ontario Neurotrauma Foundation and the Ontario Brain Institute. The Ontario Brain Institute is an independent non-profit corporation, funded partially by the Ontario government.
Disclaimer: This work was originally featured within the first author’s doctoral thesis, as part of a larger body of work on neurophysiological variation (Paniccia, 2018 (University of Toronto, published doctoral thesis). The opinions, results, and conclusions are those of the authors and no endorsement by the Ontario Brain Institute is intended or should be inferred.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: Holland Bloorview Research Ethics Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Aubry M, Cantu R, Dvorak J, et al. . Summary and agreement statement of the first international conference on concussion in sport, vienna 2001. Recommendations for the improvement of safety and health of athletes who may suffer concussive injuries. Br J Sports Med 2002;36:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCrory P. Summary and agreement statement of the 2nd international conference on concussion in sport, prague 2004. Br J Sports Med 2005;39):78–86. 10.1136/bjsm.2005.018614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCrory P, Meeuwisse W, Dvorak J, et al. . Consensus statement on concussion in sport—the 5 th international conference on concussion in sport held in Berlin. Br J Sports Med 2016;26:097699. [DOI] [PubMed] [Google Scholar]
- 4. Giza CC, Kutcher JS, Ashwal S, et al. . Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the guideline development subcommittee of the american academy of neurology. Neurology 2013;80:2250–7. 10.1212/WNL.0b013e31828d57dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harmon KG, Drezner JA, Gammons M, et al. . American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med 2013;47:15–26. 10.1136/bjsports-2012-091941 [DOI] [PubMed] [Google Scholar]
- 6. Bigler ED. Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J Int Neuropsychol Soc 2008;14:1–22. 10.1017/S135561770808017X [DOI] [PubMed] [Google Scholar]
- 7. Hunt AW, Paniccia M, Reed N, et al. . Concussion-like symptoms in child and youth athletes at baseline: what Is "typical"? J Athl Train 2016;51:749–57. 10.4085/1062-6050-51.11.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meares S, Shores EA, Taylor AJ, et al. . The prospective course of postconcussion syndrome: the role of mild traumatic brain injury. Neuropsychology 2011;25:454–65. 10.1037/a0022580 [DOI] [PubMed] [Google Scholar]
- 9. Iverson GL, Lange RT. Examination of "postconcussion-like" symptoms in a healthy sample. Appl Neuropsychol 2003;10:137–44. 10.1207/S15324826AN1003_02 [DOI] [PubMed] [Google Scholar]
- 10. Iverson GL, Lange RT, Franzen MD. Effects of mild traumatic brain injury cannot be differentiated from substance abuse. Brain Inj 2005;19:11–18. 10.1080/02699050410001720068 [DOI] [PubMed] [Google Scholar]
- 11. Stancin T, Kaugars AS, Thompson GH, et al. . Child and family functioning 6 and 12 months after a serious pediatric fracture. J Trauma 2001;51:69–76. 10.1097/00005373-200107000-00011 [DOI] [PubMed] [Google Scholar]
- 12. Bailey CM, Echemendia RJ, Arnett PA. The impact of motivation on neuropsychological performance in sports-related mild traumatic brain injury. J Int Neuropsychol Soc 2006;12:475–84. 10.1017/S1355617706060619 [DOI] [PubMed] [Google Scholar]
- 13. Echemendia RJ, Putukian M, Mackin RS, et al. . Neuropsychological test performance prior to and following sports-related mild traumatic brain injury. Clin J Sport Med 2001;11:23–31. 10.1097/00042752-200101000-00005 [DOI] [PubMed] [Google Scholar]
- 14. Blake TA, McKay CD, Meeuwisse WH, et al. . The impact of concussion on cardiac autonomic function: a systematic review. Brain Inj 2016;30:132–45. 10.3109/02699052.2015.1093659 [DOI] [PubMed] [Google Scholar]
- 15. Nunan D, Sandercock GR, Brodie DA. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin Electrophysiol 2010;33:1407–17. 10.1111/j.1540-8159.2010.02841.x [DOI] [PubMed] [Google Scholar]
- 16. Sandercock GR, Brodie DA. The use of heart rate variability measures to assess autonomic control during exercise. Scand J Med Sci Sports 2006;16:302–13. 10.1111/j.1600-0838.2006.00556.x [DOI] [PubMed] [Google Scholar]
- 17. Hutchison MG, Mainwaring L, Senthinathan A, et al. . Psychological and physiological markers of stress in concussed athletes across recovery milestones. J Head Trauma Rehabil 2017;32:E38–E48. 10.1097/HTR.0000000000000252 [DOI] [PubMed] [Google Scholar]
- 18. Leddy JJ, Kozlowski K, Fung M, et al. . Regulatory and autoregulatory physiological dysfunction as a primary characteristic of post concussion syndrome: implications for treatment. NeuroRehabilitation 2007;22:199–205. [PubMed] [Google Scholar]
- 19. Senthinathan A, Mainwaring LM, Hutchison M. Heart rate variability of athletes across concussion recovery milestones: a preliminary study. Clin J Sport Med 2017;27:288–95. 10.1097/JSM.0000000000000337 [DOI] [PubMed] [Google Scholar]
- 20. Gall B, Parkhouse W, Goodman D. Heart rate variability of recently concussed athletes at rest and exercise. Med Sci Sports Exerc 2004;36:1269–74. 10.1249/01.MSS.0000135787.73757.4D [DOI] [PubMed] [Google Scholar]
- 21. Reed N, Murphy J, Dick T, et al. . A multi-modal approach to assessing recovery in youth athletes following concussion. J Vis Exp 2014;(91):51892 10.3791/51892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gioia G, Collins M. Acute Concussion Evaluation. Heads Up: Brain Injury in Your Practice, 2006. [Google Scholar]
- 23. Sady MD, Vaughan CG, Gioia GA. Psychometric characteristics of the postconcussion symptom inventory in children and adolescents. Arch Clin Neuropsychol 2014;29:348–63. 10.1093/arclin/acu014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heart rate variability. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996;93:1043–65. [PubMed] [Google Scholar]
- 25. Biswas AK, Scott WA, Sommerauer JF, et al. . Heart rate variability after acute traumatic brain injury in children. Crit Care Med 2000;28:3907–12. 10.1097/00003246-200012000-00030 [DOI] [PubMed] [Google Scholar]
- 26. Reed N, Provvidenza C. Concussion & You A handbook for parents and kids Internet. Holland Bloorview Kids Rehabilitation Hospital, 2014. [Google Scholar]
- 27. Aubert AE, Seps B, Beckers F. Heart rate variability in athletes. Sports Med 2003;33:889–919. 10.2165/00007256-200333120-00003 [DOI] [PubMed] [Google Scholar]
- 28. Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med 1997;16:2349–80. [DOI] [PubMed] [Google Scholar]
- 29. Paniccia M, Verweel L, Thomas S, et al. . Heart rate variability in healthy non-concussed youth athletes: exploring the effect of age, sex, and concussion-like symptoms. Front Neurol 2017;8:753 10.3389/fneur.2017.00753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berkoff D, Boggess B, Bytoniski J, et al. . Difference in heartrate variability changes over time in concussed versus non-concussed athletes. Clin J Sport Med Off J Can Acad Sport Med 2008;18:197–8. [Google Scholar]
- 31. Hinds A, Leddy J, Freitas M, et al. . The effect of exertion on heart rate and rating of perceived exertion in acutely concussed individuals. J Neurol Neurophysiol 2016;7 10.4172/2155-9562.1000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ellis MJ, Cordingley D, Vis S, et al. . Vestibulo-ocular dysfunction in pediatric sports-related concussion. J Neurosurg Pediatr 2015;16:248–55. 10.3171/2015.1.PEDS14524 [DOI] [PubMed] [Google Scholar]
- 33. Ellis MJ, Leddy J, Willer B. Multi-disciplinary management of athletes with post-concussion syndrome: an evolving pathophysiological approach. Front Neurol 2016;7:136 10.3389/fneur.2016.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leddy J, Baker JG, Haider MN, et al. . A physiological approach to prolonged recovery from sport-related concussion. J Athl Train 2017;52:299–308. 10.4085/1062-6050-51.11.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paniccia M, Paniccia D, Thomas S, et al. . Clinical and non-clinical depression and anxiety in young people: a scoping review on heart rate variability. Auton Neurosci 2017;208:1–14. 10.1016/j.autneu.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 36. Hilz MJ, DeFina PA, Anders S, et al. . Frequency analysis unveils cardiac autonomic dysfunction after mild traumatic brain injury. J Neurotrauma 2011;28:1727–38. 10.1089/neu.2010.1497 [DOI] [PubMed] [Google Scholar]
- 37. Gutin B, Howe C, Johnson MH, et al. . Heart rate variability in adolescents: relations to physical activity, fitness, and adiposity. Med Sci Sports Exerc 2005;37:1856–63. 10.1249/01.mss.0000175867.98628.27 [DOI] [PubMed] [Google Scholar]
- 38. Kim HS, Yoon KH, Cho JH. Diurnal heart rate variability fluctuations in normal volunteers. J Diabetes Sci Technol 2014;8:431–3. 10.1177/1932296813519013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hutchison MG, Mainwaring L, Senthinathan A, et al. . Psychological and physiological markers of stress in concussed athletes across recovery milestones. J Head Trauma Rehabil 2016;1. [DOI] [PubMed] [Google Scholar]
- 40. Faulkner MS, Hathaway D, Tolley B. Cardiovascular autonomic function in healthy adolescents. Heart & Lung: The Journal of Acute and Critical Care 2003;32:10–22. 10.1067/mhl.2003.6 [DOI] [PubMed] [Google Scholar]
- 41. Eckberg DL. Parasympathetic cardiovascular control in human disease: a critical review of methods and results. Am J Physiol 1980;239:581–93. 10.1152/ajpheart.1980.239.5.H581 [DOI] [PubMed] [Google Scholar]
- 42. Kleiger RE, Stein PK, Bigger JT. Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol 2005;10:88–101. 10.1111/j.1542-474X.2005.10101.x [DOI] [PMC free article] [PubMed] [Google Scholar]


