Abstract
Background
Programmed death ligand 1 (PD-L1) targeting immunotherapies, as pembrolizumab and nivolumab, have significantly improved outcome in patients with non-small cell lung cancer (NSCLC). Tobacco smoking is the number one risk factor for lung cancer and is linked to 80%–90% of these cancers. Smoking during cancer therapy may influence on radiotherapy and chemotherapy outcome. We aimed to review the knowledge in immunotherapy.
Patients and methods
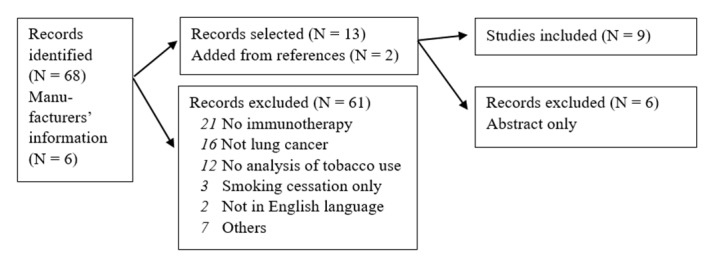
A systematic review was done. We searched for documents and articles published in English language and registered in Cochrane Library, National Health Service (NHS) Centre for Reviews and Dissemination (CRD), Embase or Medline. The search terms were (A) (Lung cancer or NSCLC) with (pembrolizumab or nivolumab) with PD-L1 with (tobacco or smoking) and (B) Lung Neoplasms and Immunotherapy and (smoking cessation or patient compliance). 68 papers were detected and two more were added during review process (references) and six based on information from the manufacturers.
Results
Nine papers were selected. High PD-L1 expression (≥50%) was correlated with current/ever smoking history in three studies. Six studies revealed a higher overall response rate (ORR) among current/former smokers. The ORR was generally (six studies) better among the current/former smoker group. So also when tumours had a molecular ‘smoking signature’ (one study). This was probably due to a higher mutational burden. In two studies, minor or no difference was revealed.
One study (KEYNOTE-024) compared former and current smokers, and documented pembrolizumab being more effective among former smokers than current smokers.
Conclusions
Tobacco smoking patients with NSCLC generally have a higher PD-L1 tumour proportion score and experience a better ORR of immunotherapy than no smokers. There is little evidence on the effect of smoking during immunotherapy, but one study (KEYNOTE-024) may indicate survival gains of smoking cessation.
Keywords: lung cancer, PD-L1, immunotherapy, tobacco, smoking, cessation
Key questions.
What is already known about this subject?
Tobacco smoking is the number one risk factor for lung cancer and is linked to 80%–90% of these cancers.
Studies have indicated the mutation burden associated with smoking predicts response to immunotherapy. This is due to a higher programmed death ligand 1 (PD-L1) tumour proportion score (TPS) among smokers.
What does this study add?
There is a better overall response rate among the current/former smoker group than the no smoker group when treated with immunotherapies. So also in patients having a molecular ‘smoking signature’. This is due to a higher PD-L1 TPS and probably a higher mutational burden due to smoking.
The situation seems to be different during therapy. The KEYNOTE-024 documented pembrolizumab being more effective among former smokers than among current smokers.
How might this impact on clinical practice?
Clinicians may encourage patients undergoing immunotherapy to quit smoking to achieve a better outcome.
There is a need to clarify the benefit achieved. When elucidated, more pressure can be put on patients continuing smoking during therapy. They may cause waste of healthcare resources spent on their therapy.
Introduction
Cigarette smoking is the number one risk factor for lung cancer and is linked to about 80%–90% of lung cancers.1 There are more than 4000 chemical constitutes of cigarette smoke and there are several potential carcinogens, including polycyclic aromatic hydrocarbons, aromatic amines, nitrosamines and other organic and inorganic compounds, such as benzene, vinyl chloride, arsenic and chromium. The carcinogens are associated with DNA mutations. Lung cancer accounts for 21% of the total cancer deaths worldwide.1 Every year, 1.8 million people are diagnosed with lung cancer, and 1.6 million people die because of the disease. In the USA, lung cancer represents almost 27% of all cancer deaths.2 Five-year survival rates vary from 4% to 17%, depending on stage and regional differences.3
Rates have been declining due to the reduced prevalence of smoking. However, in the USA, 18% of adults still smoke cigarettes.2 4 A similar figure (19%) has been reported in Norway.5 The declining rates have, however, been absent in several economically low/middle-income countries.1
Today, lung cancer from smoking is, to a great extent, a preventable disease.6 A 62% reduction in lung cancer mortality has been reported in association with smoking cessation at age 50.7 Quitting smoking decreases the risk of dying from lung cancer and from other tobacco-related illnesses. Consequently, programmes and policies that can decrease the numbers of tobacco smokers will have significant impact on patients’ quality of life and healthcare budgets.7
During the last years, new costly drugs have been introduced for the treatment of lung cancer.8 Examples of inhibitory signalling agents approved by the Food and Drug Administration include antibodies targeting cytotoxic T-lymphocyte-associated protein 4 and programmed death 1 (PD-1)/PD-ligand 1 (L1) receptors.9 Especially, PD-1/PD-L1 blockage therapy has shown activity in lung cancer.10–12 Economic resources spent on costly new therapies could be allocated to preventive strategies. Treatment effects in various subgroups should therefore be monitored.
Smoking can cause lung cancer and then block the body from fighting it by weakening the immune system.13 Tobacco smoking during immunotherapy may influence on treatment outcome. Progression-free survival (PFS) has been documented varying, depending on whether the patient was a former or current smoker.14 Furthermore, the mutation burden associated with smoking may predict response to anti-PD-1 therapy.15 In this review, we aimed to clarify the consequences of tobacco smoking before and during immunotherapy.
Materials and methods
We performed a systematic literature search for studies on immunotherapies in lung cancer and possible effects of tobacco smoking. In February 2018, we searched for documents and articles published in English language. The following databases were used: Cochrane Library, National Health Service (NHS) Centre for Reviews and Dissemination (CRD), Embase and Medline. The following search terms were used: (A) (Lung cancer or NSCLC) with (pembrolizumab or nivolumab) with PD-L1 with (tobacco or smoking) and (B) Lung Neoplasms and Immunotherapy and (smoking cessation or patient compliance). A total of 68 papers were detected.
Initially, we screened all titles and abstracts of the articles. The selected papers were investigated and studies reporting any analysis on correlation between smoking and immunotherapy were selected (n=7). Furthermore, the references of the selected papers were screened and another two articles were added.14 16
Merck Sharp & Dohme, the manufacturer of pembrolizumab (Keytruda), Bristol Myers Squibb, the manufacturer of nivolumab (Opdivo), and Roche, the manufacturer of atezolizumab (Tecentriq) were contacted and requested for information concerning tobacco smoking and the treatment of non-small cell lung cancer (NSCLC) using pembrolizumab, nivolumab or atezolizumab. They confirmed several of the selected articles, and two more articles and four abstracts were added for further analyses.17–22
Because of low level of evidence, abstracts only, book chapters, errata, editorial comments and letters to the editor were not included. Consequently, six papers (published as abstracts only) were excluded. Furthermore, articles were excluded when they did not focus on lung cancer, immunotherapy or the use of tobacco, and/or not written in English language (61 articles rejected). We reviewed the full text of all articles twice to confirm their eligibility. Following the selection process, nine articles were included into the final study.14–18 23–26 Data were extracted from the selected papers employing Microsoft Excel V.2016 for the database. Never smoking was defined as patients who had smoked less than 100 cigarettes in their lifetime or ≤10 package-years.24 26 Study characteristics, treatment, comparator and numbers are given in table 1.
Table 1.
An overview of the study characteristics, treatment and comparator, type of evaluation, perspective, year of value and time horizon of the selected studies
| Reference | Study characteristics | Treatment and comparator | n | Key findings |
| 24 | Patients with NSCLC treated with nivolumab | Nivolumab monotherapy | 50 | Response to treatment before nivolumab associated with response to nivolumab. Smoking history had no significant influence (never vs current/former smoker ORR 5% vs 26%, p=0.1269). |
| 16 | Sequenced exons of NSCLCs | Pembrolizumab | 34 | Efficacy greater in tumours harbouring smoking signature (ORR 56% vs 17%, p=0.03) |
| 25 | EGFR and ALK rearrangements in NSCLC | PD-1/PD-L1 inhibitors | 58 | Smoking history had no significant influence (never/light vs heavy smokers ORR 4.2% vs 20.6%, p=0.123). |
| 26 | Adenocarcinoma of the lung | Testing PD-L1 tumour proportion score | 71 | Tumours with a PD-L1 TPS>50% were significantly associated with smoking status. |
| 27 | Patients with NSCLC in East Asia. 108 SCC and 221 LUAD. | PD-L1 expression/distribution | 329 | TPS>50% correlated with smoking history in both SCC (p=0.008) and adenocarcinoma (p=0.002). |
| 17 | Pembrolizumab in NSCLC. KEYNOTE-001. | Pembrolizumab10 mg/kg every 2 weeks, 2 mg/kg every 3 weeks, 10 mg/kg every 3 weeks | 495 | Current/former smoking status was associated with increased ORR (10.3% vs 22.5% in never smokers). |
| 15 | Pembrolizumab or platinum-based CT. KEYNOTE-024. | Pembrolizumab 200 mg every 3 weeks Platinum |
305 | ORR 44.8% vs 27.8%. HR for progression/death among smokers 0.68 and former smokers 0.47. |
| 18 | Pemetrexed and platinum plus pembrolizumab or placebo in advanced NSCLC. KEYNOTE-189. | Pemetrexed, platinum and pembrolizumab or placebo every 3 weeks | 616 | 88.1% current or former smokers. HR for death among current or former smokers 0.54 versus never smoker 0.23. Progression-free survival HR 0.54 and 0.43, respectively. |
| 19 | Nivolumab versus docetaxel in patients with NSCLC. Phase III study. | Nivolumab 3 mg/kg every 2 weeks versus docetaxel 75 mg/m2 every 3 weeks | 582 | 79% were current/former smokers. HR was 0.70 and 1.02 in former/current smokers and never smokers, respectively. |
ALK, anaplastic lymphoma receptor tyrosine kinase; CT, chemotherapy; EGFR, epidermal growth factor receptor; LUAD, lung adenocarcinoma; NSCLC, non-small cell lung cancer; ORR, overall response rate; PD-L1, programmed death ligand 1; SCC, squamous cell carcinoma; TPS, tumour proportion score.
Results
Literature search
The literature selection process revealed nine studies fulfilling the inclusion criteria and reporting data on tobacco use, PD-L1 expression and response on immunotherapy in NSCLC. The selection process and key findings are shown in figure 1 and table 1.
Figure 1.
The selection process.
Smoking before immunotherapy
Smoking history and its influence on the effect of immunotherapy was somewhat diverging. In three studies, high PD-L1 expression (≥50%) was correlated with current/ever smoking history.24–26 Gainor et al documented in their retrospective study (58 patients) a better (but not significant, p=0.123) overall response rate (ORR) among heavy smokers versus never or light smokers.24 The figures were 20.6% and 4.2%, respectively. Garon and colleagues published, on behalf of the KEYNOTE-001 investigators, that current or former smoking status was associated with an increased response to treatment.16 They concluded this finding was probably due to a higher mutational burden among these patients. The median PFS among current/former smokers was 4.2 months vs 2.1 months among the never smokers. The corresponding overall survival (OS) figures were 14.3 and 8.8 months, respectively.
Gandhi and associates added pembrolizumab or placebo to pemetrexed and a platinum-based regimen in first-line therapy of patients with advanced NSCLC.17 Most patients (88.1%) were former or current smokers. They revealed an HR for OS of 0.23 (95% CI 0.10 to 0.54) for never smokers and 0.54 (95% CI 0.41 to 0.71) for current/former smokers. The corresponding figures for disease progression or death were 0.43 (95% CI 0.23 to 0.81) and 0.54 (95% CI 0.43 to 0.66), respectively. However, there were only 73 never smokers among 616 patients, causing a wide CI. The data cut-off was 8 November 2017.
Borghaei and colleagues compared nivolumab and docetaxel in 582 patients with advanced non-squamous NSCLC and concluded an OS benefit in favour of nivolumab (12.2 months vs 9.4 months).18 A total of 79% were current or former smokers. When comparing OS between current/former smokers versus never smoked, they revealed smokers having a greater benefit of nivolumab therapy. The unstratified HRs (95% CI) were 0.70 (95% CI 0.56 to 0.86) vs 1.02 (95% CI 0.64 to 1.61), respectively. However, the interpretation of the results was somewhat limited by the wide CI in a small subgroup of patients (118 out of 582 had never smoked).
Based on the majority of studies, we concluded, there is a correlation between smoking history and higher PD-L1 tumour proportion score.16 18 25 26
Molecular signature of smoking and immunotherapy
Rizvi and colleagues identified the molecular signature of smoking to clarify the efficacy of pembrolizumab in patients with NSCLCs harbouring the smoking signature.15 A previously validated binary classifier was applied.27 The ORR was significantly higher in tumours with smoking signature versus never smoking signature (56% vs 17%, p=0.03).15 Similar findings were detected in PFS with median survival not reached versus 3.5 months (p=0.0001). Whereas smoking signature significantly correlated with efficacy, self-reported smoking status did not. Kobayashi et al did also conclude similarly.23 In their study, smoking history (never vs current or former smoker) did not influence on response rate of nivolumab monotherapy, but the study included only 50 patients and 31 out of them were current smoker or ever smoker.
Smoking during immunotherapy
There was only one study comparing former smokers with current smokers.14 The categorisation was based on patients’ smoking status at study entry and the investigators documented a better effect of pembrolizumab therapy among former smokers (216 patients) compared with current smokers (65 patients). The HRs for disease progression or death were for current smokers 0.68 (95% CI 0.17 to 0.71) and for former smokers 0.47 (95% CI 0.33 to 0.67). Brahmer et al updated these data (data cut-off 10 July 2017) in an abstract version.28 The paper indicated a better response among those being former smokers at the initiation of immunotherapy. No study compared smoking habits in terms of whether the patient actually stopped smoking or continued during immunotherapy.
Discussion
We conclude most studies revealed a correlation between tobacco smoking and higher PD-L1 tumour proportion score. This was probably due to a higher mutational burden. There was little evidence on the effect of tobacco smoking during immunotherapy. However, one major study revealed better outcome for former smokers than for the current ones.14 This indicates that smoking cessation should be encouraged before and during immunotherapy.
Smoking status, response rate and survival
Some discrepancy between studies may be due to various numbers of patients and the fact that smoking status was based on patients’ self-reports.15 23 24 There were no follow-ups and none of the studies did actually measure blood level of nicotine and no studies did split data between former and current smokers.29 Consequently, there is a need for studies on the possible effect of continuing smoking during immunotherapy.
Smoking status among patients with NSCLC has been reported varying between real world and clinical trials.30 However, we could not confirm this statement. Khozin et al mentioned a real-world current/former smoker figure (88%) similar to that of the KEYNOTE-024 study (92%).14 30 Similar figures have also been reported by others (79%–88%).17 18
The proportion score of PD-L1 expression of at least 50% was associated with a higher ORR, and longer PFS and OS.31 This effect was hypothesised to be due to a higher mutational burden.32–34 Consequently, differences in ORR between various studies might be due to variations in the percentage of smokers. This was also argued by Kobayashi et al. 23
In our review, most data were on pembrolizumab and nivolumab. However, other drugs have also been tested and data published in abstract forms. Such an example is the MPDL3280A.35 This is an engineered IgG anti-PD-L1 antibody with modified Fc domain that prevents antibody-dependent cell-mediated cytotoxicity in other immune cells expressing PD-L1. The MPDL3280A achieved a better ORR among former and current smokers (25%) than among never smokers (16%).35 This was also confirmed by others.36
Pneumonitis is one of the potentially serious side effects of immunotherapy. Ahn and colleagues reported pneumonitis grades 3–5 in 3.8% of patients with NSCLC undergoing pembrolizumab therapy.37 Smoking history did however not influence the risk of pneumonitis. Leighl et al recently published an abstract updating the KEYNOTE-001 study.19 Three years of survival in previously treated patients was better among ever (21.9%; 95% CI 17.1 to 27.2) than never (11.9%; 95% CI 6.3 to 19.5) smokers. Looking at treatment-naïve patients, the 3 years of OS was in favour of those who had never smoked (24.0%; 95% CI 11.7 to 38.7 vs 45.5%; 95% CI 16.7 to 70.7). Consequently, the never smoked group seems to have the best prognosis in the treatment-naïve setting. However, the number of patients and the number of adverse events were too small to draw any conclusions.
Molecular signature and immunotherapy
Rizvi and colleagues employed a previously validated binary classifier to identify the molecular signature of smoking.15 27 The classifier applied differentiated transversion-high (smoking signature) from transversion-low (never smoking signature) tumours.27 This classification was based on the fact that past or present smoking has been shown to be associated with cytosine to adenine (C>A) nucleotide transversions both in individual genes and genomic-wide.38 Furthermore, the C>A nucleotide transversion has been shown inversely correlated with cytosine to thymidine (C>T) transition frequency.38 39
It could be questioned whether smoker tumours should be defined by the genetic signature rather than by self-reported smoking status. However, we did not reveal large-scale studies that could answer this question and the study by Rizvi et al did only include 30 patients.15
On the other hand, we revealed one study showing a correlation between amounts of smoking and genetic alterations.40 The puff volume was indicated a more powerful objective phenotype of smoking behaviour than self-reported cigarettes per day and nicotine dependence.
Should patients quit smoking during immunotherapy?
Patients frequently ask what they can do themselves to improve treatment outcome. Smoking cessation may be a key action in this setting.41 Smoking not only causes cancer, but continued smoking may alter cancer biology, leading to tumours that are resistant to treatment and thereby increase mortality.42 Smoking cessation may also improve cardiovascular status. Consequently, we argue that oncologists and pulmonologists should encourage smoking cessation during immunotherapy.
O’Malley and colleagues did a review of the literature on metabolism and effectiveness of systemic therapy for lung cancer.43 They revealed that smokers might exhibit a more rapid clearance, requiring a higher dose compared with non-smokers. However, no studies have shown the influence of continuous smoking on the clearance of immunotherapies. A detailed smoking history should be part of future clinical evaluations in NSCLC. At least three levels of smoking status should be ascertained (never smoker, former smoker and current smoker) and the number of pack-years should be calculated.
Smoking cessation is not easy and the success rate has been disappointing.44 Therefore, patients should be offered assistance during and after smoking cessation.45
Conclusion
Tobacco smoking patients with NSCLC generally have a higher PD-L1 tumour proportion score and experience a higher response rate in immunotherapy than non-smokers. There is little evidence on the effect of smoking during immunotherapy, but one study indicates better outcome for former smokers than for the current ones. Further studies are necessary to elucidate the negative effects of smoking during immunotherapy.
Acknowledgments
The support from Ronald Sivertsen at the UiT–The Arctic University of Norway, Hammerfest Campus, was appreciated. He did the literature search. We also thank the Library at the UiT–The Arctic University of Norway for its service.
Footnotes
Contributors: Both authors have taken part in the review process and the writing of the article.
Funding: The publication charges for this article have been funded by a grant from the publication fund of UiT–The Arctic University of Norway.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Brawley OW. Avoidable cancer deaths globally. CA Cancer J Clin 2011;61:67–8. 10.3322/caac.20108 [DOI] [PubMed] [Google Scholar]
- 2. Bates SE. Quit early, quit often. Clin Cancer Res 2015;21:2212 10.1158/1078-0432.CCR-14-2749 [DOI] [PubMed] [Google Scholar]
- 3. Hirsch FR, Scagliotti GV, Mulshine JL, et al. . Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299–311. 10.1016/S0140-6736(16)30958-8 [DOI] [PubMed] [Google Scholar]
- 4. Arteaga CL, Adamson PC, Engelman JA, et al. . AACR Cancer progress report 2014. Clin Cancer Res 2014;20: S1–112. 10.1158/1078-0432.CCR-14-2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Norway S. Smoking habits in Norway. Oslo: Statistics Norway, 2018. [Google Scholar]
- 6. DeVita VT, Rosenberg SA. Two hundred years of cancer research. N Engl J Med 2012;366:2207–14. 10.1056/NEJMra1204479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Emmons KM, Colditz GA. Realizing the potential of cancer prevention - The role of implementation science. N Engl J Med 2017;376:986–90. 10.1056/NEJMsb1609101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Norum J, Antonsen MA, Tollåli T, et al. . Pembrolizumab as second-line therapy in non-small cell lung cancer in northern Norway: budget impact and expected gain-a model-based analysis. ESMO Open 2017;2:e000222 10.1136/esmoopen-2017-000222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Somasundaram A, Burns TF. The next generation of immunotherapy: keeping lung cancer in check. J Hematol Oncol 2017;10:87 10.1186/s13045-017-0456-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56–61. 10.1126/science.aaa8172 [DOI] [PubMed] [Google Scholar]
- 11. Herbst RS, Baas P, Kim DW, et al. . Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 12. Lynch TJ, Bondarenko I, Luft A, et al. . Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012;30:2046–54. 10.1200/JCO.2011.38.4032 [DOI] [PubMed] [Google Scholar]
- 13. U.S. Department of Health and Human Services. A report of the surgeon general. How tobacco smoke causes disease: what it means to you. Atlanta: U.S, 2010. [Google Scholar]
- 14. Reck M, Rodríguez-Abreu D, Robinson AG, et al. . Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 15. Rizvi NA, Hellmann MD, Snyder A, et al. . Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124–8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garon EB, Rizvi NA, Hui R, et al. . Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 17. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. . Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med Overseas Ed 2018;378:2078–92. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 18. Borghaei H, Paz-Ares L, Horn L, et al. . Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leighl NB, Hellmann MD, Hui R, et al. . KEYNOTE-001: 3-year overall survival for patients with advanced NSCLC treated with pembrolizumab. Journal of Clinical Oncology 2017;35(15_suppl):9011 10.1200/JCO.2017.35.15_suppl.9011 [DOI] [Google Scholar]
- 20. Gandhi L, Rodgríguez-Abreu D, Gadgeel S, et al. . Abstract CT075: KEYNOTE-189: Randomized, double-blind, phase 3 study of pembrolizumab (pembro) or placebo plus pemetrexed (pem) and platinum as first-line therapy for metastatic NSCLC. Cancer Res 2018;78(13 Suppl):CT075 10.1158/1538-7445.AM2018-CT075 [DOI] [Google Scholar]
- 21. Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. . Progression after the next line of therapy (PFS2) and updated OS among patients (pts) with advanced NSCLC and PD-L1 tumor proportion score (TPS) ≥50% enrolled in KEYNOTE-024. J Clin of Oncol 2017;35(15_suppl):9000 10.1200/JCO.2017.35.15_suppl.9000 [DOI] [Google Scholar]
- 22. Reck M, Rodriguez-Abreu D, Robinson AG. KEYNOTE-024: Pembrolizumab vs platinum-based chemotherapy as first-line therapy for advanced NSCLC with a PD-L1 TPS > 50%. Copenhagen, Denmark, 2016. [Google Scholar]
- 23. Kobayashi H, Omori S, Nakashima K, et al. . Response to the treatment immediately before nivolumab monotherapy may predict clinical response to nivolumab in patients with non-small cell lung cancer. Int J Clin Oncol 2017;22:690–7. 10.1007/s10147-017-1118-x [DOI] [PubMed] [Google Scholar]
- 24. Gainor JF, Shaw AT, Sequist LV, et al. . EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: A retrospective analysis. Clin Cancer Res 2016;22:4585–93. 10.1158/1078-0432.CCR-15-3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rangachari D, VanderLaan PA, Shea M, et al. . Correlation between classic driver oncogene mutations in EGFR, ALK, or ROS1 and 22C3-PD-L1 ≥50% expression in lung adenocarcinoma. J Thorac Oncol 2017;12:878–83. 10.1016/j.jtho.2016.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pan Y, Zheng D, Li Y, et al. . Unique distribution of programmed death ligand 1 (PD-L1) expression in East Asian non-small cell lung cancer. J Thorac Dis 2017;9:2579–86. doi:10.21037/jtd.2017.08.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543–50. 10.1038/nature13385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brahmer JR, Rodriguez-Abreu D, Robinson A. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced NSCLC with PD-L1 TPS >50%. Yokohama, Japan, 2017. [DOI] [PubMed] [Google Scholar]
- 29. Hellman M, Garon E, Gandhi L. Efficacy of pembrolizumab in key subgroups of patients with advanced NSCLC. Denver, Colorado, USA., 2015. [Google Scholar]
- 30. Khozin S, Abernethy AP, Nussbaum NC, et al. . Characteristics of real-world metastatic Non-small cell lung cancer patients treated with nivolumab and pembrolizumab during the year following approval. Oncologist 2018;23:328–36. 10.1634/theoncologist.2017-0353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. NIHR. University of Birmingham. Pembrolizumab (Keytruda) for PD-L1 strong-positive metastatic non-small cell lung cancer – first line, 2016. [Google Scholar]
- 32. Alexandrov LB, Nik-Zainal S, Wedge DC, et al. . Signatures of mutational processes in human cancer. Nature 2013;500:415–21. 10.1038/nature12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. D’Incecco A, Andreozzi M, Ludovini V, et al. . PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer 2015;112:95–102. 10.1038/bjc.2014.555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rades D, Setter C, Schild SE, et al. . Effect of smoking during radiotherapy, respiratory insufficiency, and hemoglobin levels on outcome in patients irradiated for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;71:1134–42. 10.1016/j.ijrobp.2007.11.006 [DOI] [PubMed] [Google Scholar]
- 35. Soria J, Cruz C, Bahleda R. Clinical activity, safety and biomarkers of PD-L1 blockade in non-small cell lung cancer (NSCLC): additional analyses from a clinical study of the engineered antibody MPDL3280A (anti-PDL1), 2013. [Google Scholar]
- 36. Sundar R, Soong R, Cho BC, et al. . Immunotherapy in the treatment of non-small cell lung cancer. Lung Cancer 2014;85:101–9. 10.1016/j.lungcan.2014.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahn M-J, Gandhi L, Hamid O, et al. . 459PRisk of pneumonitis in patients with advanced NSCLC treated with pembrolizumab in KEYNOTE-001. Annals of Oncology 2015;26(suppl 9):ix140.3–ix140. 10.1093/annonc/mdv532.43 [DOI] [Google Scholar]
- 38. Imielinski M, Berger AH, Hammerman PS, et al. . Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 2012;150:1107–20. 10.1016/j.cell.2012.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Govindan R, Ding L, Griffith M, et al. . Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 2012;150:1121–34. 10.1016/j.cell.2012.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Macqueen DA, Heckman BW, Blank MD, et al. . Variation in the α 5 nicotinic acetylcholine receptor subunit gene predicts cigarette smoking intensity as a function of nicotine content. Pharmacogenomics J 2014;14:70–6. 10.1038/tpj.2012.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Norum J. Pembrolizumab and other immunotherapies in patients with extensive-stage small-cell lung cancer—are we entering a new era? Transl Cancer Res 2018;7(S5):S553–S557. doi:10.21037/tcr.2018.04.12 [Google Scholar]
- 42. Tsao AS, Scagliotti GV, Bunn PA, et al. . Scientific Advances in Lung Cancer 2015. J Thorac Oncol 2016;11:613–38. 10.1016/j.jtho.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 43. O’Malley M, King AN, Conte M, et al. . Effects of cigarette smoking on metabolism and effectiveness of systemic therapy for lung cancer. J Thorac Oncol 2014;9:917–26. 10.1097/JTO.0000000000000191 [DOI] [PubMed] [Google Scholar]
- 44. Dobson Amato KA, Hyland A, Reed R, et al. . Tobacco cessation may improve lung cancer patient survival. J Thorac Oncol 2015;10:1014–9. 10.1097/JTO.0000000000000578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schachter EN, Neuman T. Targeted therapies for the prevention of lung cancer. Drugs Today 2007;43:897–936. 10.1358/dot.2007.43.12.1088822 [DOI] [PubMed] [Google Scholar]


